Esophageal cancer is a common digestive tract cancer
worldwide and ranks seventh in morbidity and sixth in mortality
rates among all cancer types. Approximately 604,000 new cases were
recorded in 2020 worldwide (1).
According to differences in histopathology, esophageal cancer can
be divided into two subtypes: Esophageal adenocarcinoma (EAC) and
esophageal squamous cell carcinoma (ESCC). ESCC is the most common
type of esophageal cancer worldwide, which is primarily distributed
in the Asian esophageal cancer belt, including Japan, northern
China, Iran, and parts of Africa (2). With a 5-year survival rate ranging
from 15 to 25% in China, there is an urgent need to improve patient
survival by improving early diagnosis and treatment (3). Therefore, it is vital to study the
pathogenesis of ESCC, to identify prognostic markers and effective
drugs for patients.
To date, several genome-wide profiles of ESCC have
been performed by whole genome, exome, and targeted sequencing.
Compared with EAC, the copy number variations (CNVs) landscape of
ESCC is more akin to that of other types of squamous carcinomas,
such as head and neck squamous cell carcinoma (HNSCC) and cervical
squamous cell carcinoma (CESC) (4–6). In
addition to well-established cancer genes (EGFR, CDKN2A/2B,
CCND1), researchers have also revealed that other genes
(CBX4, ZNF750, CDCA7, FAM84B) may play important roles in
ESCC (7–10). Moreover, generous focal CNV segments
(amplifications and deletions) have been identified. Certain gene
signatures in these segments can lead to metastasis or poor
prognosis (11). Integrated gene
enrichment analysis of somatic mutations and CNVs has found pivotal
cancer pathways involved in ESCC progression, including the
receptor tyrosine kinase (RTK)-Ras-PI3K, JAK-STAT, Akt, NOTCH, Wnt,
cell cycle, and p53 signaling pathways (6,7,12). All
these findings highlight potential therapeutic molecular targets
for the management of ESCC (13).
Although certain reports have shed light on specific
genes related to the molecular carcinogenic mechanism or targeted
treatment of ESCC (6,12), a systematic review concerning CNVs
has not been conducted yet to the best of our knowledge. The
present review aimed to summarize the implications of CNVs on the
formation and progression of ESCC; to describe the identified genes
that are amplified or deleted in ESCC; and to discuss their roles
as potential biomarkers. A summary of the present review is
presented in Table I. The current
review may help to understand the genomic characteristics and
variations in ESCC, thus providing a guideline for targeted
therapy.
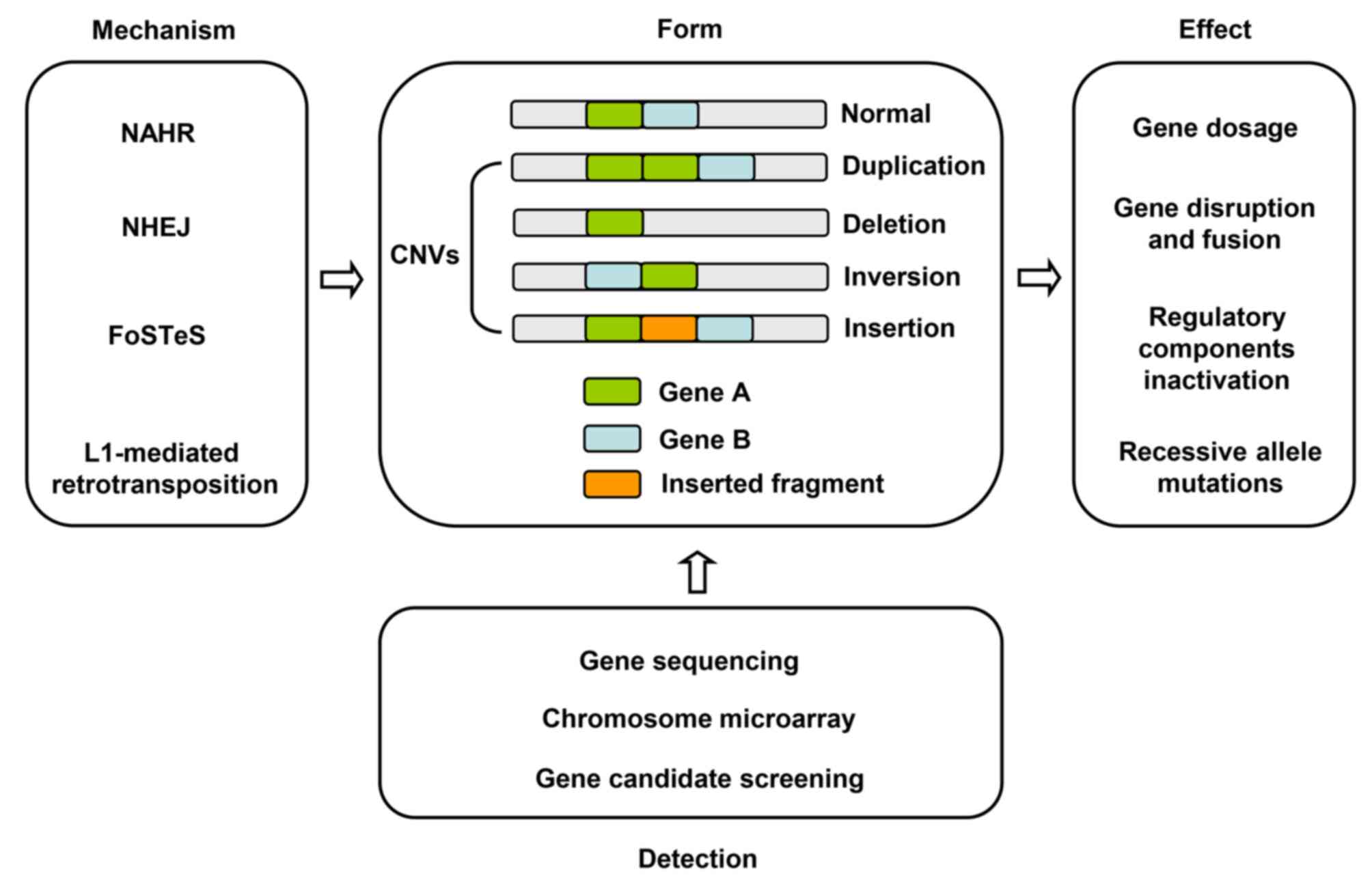
CNVs are widely distributed across the human genome
and refer to the copy number change by deletion, insertion,
inversion, translocation, duplication, and other types of
chromosomal rearrangements (14).
CNVs are generally DNA segments of >1 kb in size, with a mean
fragment length of ~2.9 kb (15,16).
CNVs are mainly generated by non-allele homologous recombination
(NAHR), non-homologous end joining (NHEJ), a DNA replication-based
mechanism such as fork stalling and template switching (FoSTeS),
and long interspersed nuclear element-1 (L1) retrotransposition
(17). NAHR is the principal
mechanism for the formation of CNVs (18). DNA is damaged and repaired via a DNA
double-stranded break repair mechanism to produce NHEJ following
exposure to ionizing radiation or oxidative stress (19,20).
In 2007, Lee et al (21)
proposed a FoSTeS model to explain the complex CNV rearrangements,
which may explain the process of producing more complex and longer
CNVs. Retrotransposon is primarily produced by spontaneous
transposon L1s (22).
CNVs play a prominent role in tumorigenesis in
various cancer types, including breast cancer, ovarian cancer,
hepatocellular carcinoma, colorectal cancer, and bladder carcinoma
(23–27). Amplified genomic regions are often
detected to harbor oncogenes, while tumor suppressor genes are
often located in the deleted regions. The molecular mechanisms of
pathogenesis primarily include gene dosage, gene disruption and
fusion, position effect, and recessive allele mutations (18). The gene dosage effect is
characterized by changes in gene expression levels along with the
CNVs. Gene breakups and breakpoint fusions are caused by
interruptions directly by CNVs, resulting in loss of function or
gain of function mutations. The position effect leading to
rearrangement regulates distal and proximal gene expression, and
affects the translation of adjacent genes (28,29).
In recent years, with the rapid development of
sequencing technologies, the methods of detecting CNVs have made
notable progress. Common detection methods for CNVs can be
primarily divided into two categories: i) Genome-wide detection of
unknown CNVs and ii) specific detection of known CNVs. Microarray
and sequencing are commonly used to detect genome-unknown CNVs.
Currently, the mainstream approaches for analyzing
CNVs are gene sequencing-based methods. Sequencing techniques
comprise Sanger DNA sequencing, next-generation sequencing (NGS),
and third-generation sequencing (TGS), of which NGS and TGS are
commonly used. Whole genome sequencing (WGS), whole exome
sequencing (WES), and targeted sequencing (TS) are frequently
utilized for CNV detection by NGS or TGS. Each kind of sequencing
may be applied to different aspects. WGS is a high-resolution and
reliable approach to detect CNVs with greater coverage and
increased accuracy (30,31). Compared to WGS, WES needs less time
and is cheaper, but its application is limited. Since exomes
account for 1–1.5% of the human genome, WES is a better choice when
only detecting coding region copy number changes. TS focuses on
select DNA segments that can be used to identify somatic changes
(32). In recent decades, NGS has
revealed aberrant genes and signaling pathways, and has certain
advantages regarding throughput capacity and price. TGS can be used
to screen out single molecular genes even without PCR sequencing,
and can generate longer and more accurate reads with faster speed
(33,34). It can read >15 kb on average,
which provides the possibility of obtaining longer sequences and
avoids the need for fragment rejoining (35). By TGS, CNV fragment length, and
specific sites can be detected more precisely. However, it has some
drawbacks such as low throughput, and high cost and error rate.
Chromosome microarrays primarily consist of
comparative genomic hybridization and single nucleotide
polymorphisms. Candidate-gene screening is generally utilized to
identify the CNVs of specific sites. High-throughput gene
sequencing, chromosome microarray, and candidate-gene screening can
be used for further detection (Fig.
1).
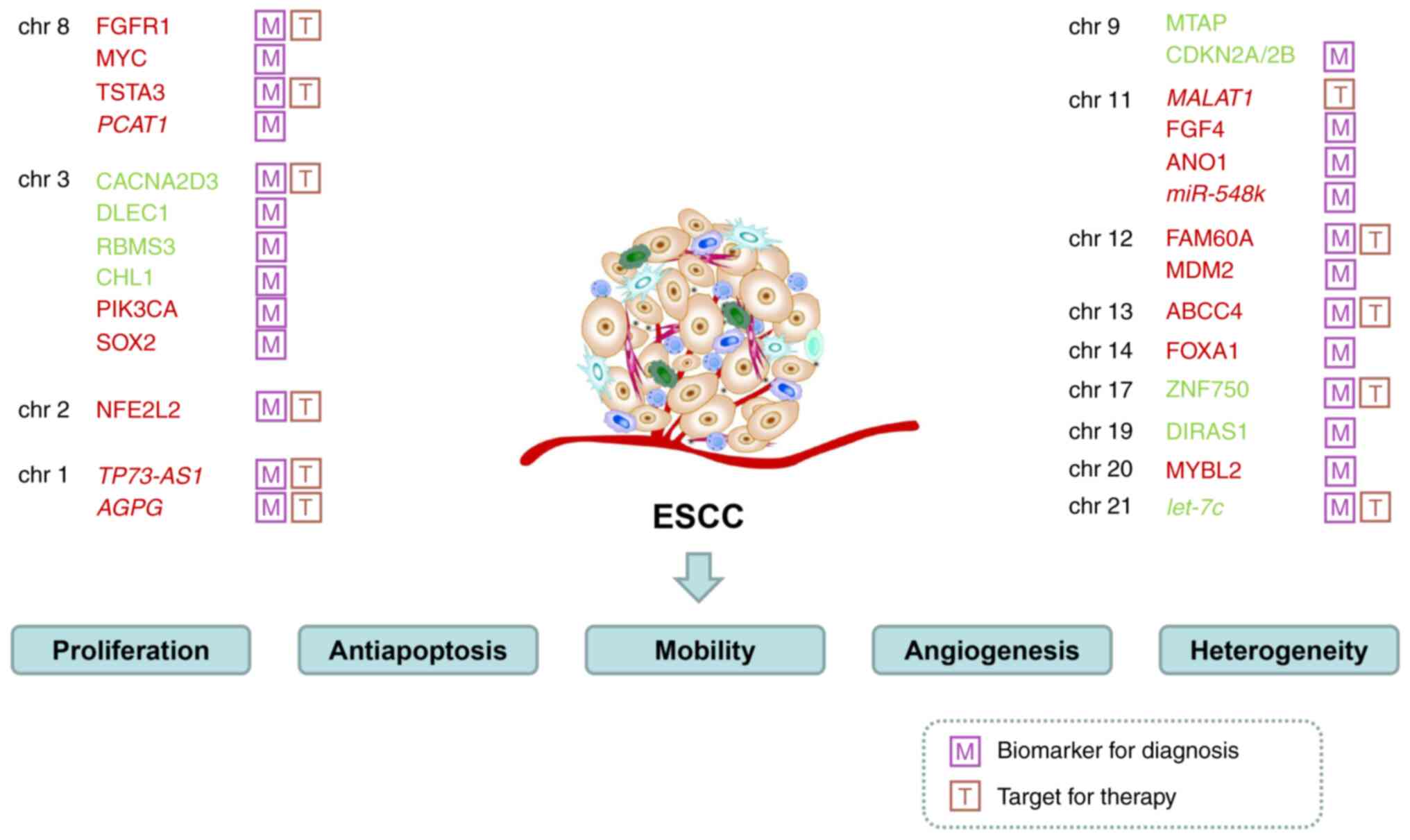
Previous studies on 1p36.32 have demonstrated that
CNVs in long non-coding RNA (lncRNA) regions play critical roles in
cancer progression. Importantly, certain lncRNAs may serve as
prognostic biomarkers for clinical diagnosis. For example, lncRNA
TP73-AS1 is located in 1p36.32 and is upregulated in ESCC,
promoting proliferation and suppressing apoptosis. TP73-AS1
enhances the sensitivity of ESCC to cisplatin and 5-fluorouracil.
In clinical samples, TP73-AS1 is associated with TNM staging
and tumor localization of ESCC, but not with sex, age, lymphatic
metastasis, or differentiation. Increased copy numbers of
TP73-AS1 were found in early-stage ESCC, whereas decreased
expression of TP73-AS1 was found in advanced TNM stages
(41).
The 3q26 segment has been found to be amplified in
various tumors such as cervical, ovarian, lung, and head and neck
cancer (55). The 3q26 region is
notably highly amplified in ESCC, and harbors oncogenes such as
PIK3CA, PRKCI, MDS1-EVI1, and SOX2 (56–58).
As an oncogene, PIK3CA encodes the catalytic subunit
p110-kinase of PI3K, which is highly expressed in esophageal cancer
and is involved in the PI3K signaling pathway to promote the
development of ESCC (57,59). Gene amplification results in
overexpression of PRKCI. Immunohistochemical analysis using 180
ESCC samples showed that overexpression of PRKCI was correlated
with lymph node metastasis, while upregulated expression of PRKCI
was correlated with poor prognosis in ESCC (56). SRY-box transcription factor 2 (SOX2)
has been evaluated in several studies. For instance, Gen et
al (60,61) reported the role of SOX2 as a
protooncogene in ESCC. The change in copy number of SOX2 was
positively correlated with SOX2 expression levels (62). SOX2 promotes ESCC and leads to a
poorer prognosis in ESCC. High expression of SOX2 is related to
differentiation in ESCC (60). By
regulating Slug, SOX2 activates the HIF-1α/STAT3 signaling pathway
and promotes metastasis in ESCC (63). These aforementioned studies suggest
that 3q26 is a vital region for the tumorigenesis of ESCC.
The 11q13.2–11q13.4 region is frequently amplified
in ESCC and other various tumors, such as HNSCC and breast cancer
(78,79). Amplification of 11q13.3 indicates
poor outcomes in patients with ESCC (10). The 11q13.2–11q13.4 segment contains
‘FGFs-FADD-SHANK2’. Moreover, the MYEOV, CCND1, CPT1A, CTTN,
PPFIA, FADD, TMEM16, and CTTS genes are also located in
this region. FGFs include FGF3, FGF4, and FGF19.
Upregulated expression of SHANK2 contributes to a poor prognosis of
ESCC (38). FGF4 gene
amplification is associated with clinical stages and can be used to
predict 5-year survival and recurrence rates. Serving as an
independent prognostic factor, FGF4 upregulation is indicative of a
shorter OS and disease-free survival (80). The ANO1 gene, also known as
TMEM16A or DOG1, encoding the
Ca2+-activated Cl− channel protein, is an
oncogene that can facilitate cell proliferation, invasion, and
migration. Upregulated ANO1 expression is associated with a poor
prognosis, and is significantly associated with lymphatic
metastasis and advanced TNM stages. Immunohistochemical experiments
demonstrated that ANO1 was upregulated in tumor specimens from
patients with ESCC (37). Previous
in vivo and in vitro studies demonstrated that the
downregulation of ANO1 via the TGF-β signaling pathway could
significantly inhibit the carcinogenesis of ESCC (81). ANO1 may thus be used as a biomarker
for ESCC (82).
miR-548k has been identified as an oncogene that is
present in the 11q13.3–13.4 amplicon, which induces malignant
phenotypes of ESCC (6). In
addition, miR-548k notably facilitated lymphatic metastasis and
regulated the microenvironment through increased secretion of
VEGFC. miR-548k and VEGFC may thus have potential as biomarkers for
the early diagnosis of their clinical features in serum detection,
and may thus serve as novel targets for the prognostic prediction
of ESCC (83).
FAM60A is also known as SIN3-HDAC Complex Associated
Factor. Gains of copy numbers lead to the upregulation of FAM60A in
ESCC. Thus, as a driver gene of ESCC, it may be a clinical target.
FAM60A is upregulated in tumor tissues of ESCC, and is closely
associated with tumor size, lymph node metastasis, and TNM staging.
Knockdown of the FAM60A gene can repress FAM60A expression,
arrest cell cycle events at the G2/M phase, reduce the
proportion of G1 phase cells, inhibit cell
proliferation, and promote apoptosis. FAM60A contributes to
metastasis of ESCC, and is predictive of a poor outcome (84).
Research on CNVs aims to identify candidate genes
and provide clues for the development of effective ESCC clinical
treatment. To date, copy number variant genes that have been used
in clinical targeted therapy of ESCC include EGFR and VEGFR. Mapped
on chromosome 7p11.2, amplified EGFR belongs to the ErbB family of
RTKs, and can transfer the downstream signal to the
RAS-RAF-MEK-ERK-MAPK and PI3K-AKT-mTOR pathways (100). With an amplification rate of
24.3%, excessive activation of EGFR leads to cell proliferation,
invasion, metastasis and poor prognosis in ESCC (6,101).
As a well-known valid target for various tumors, EGFR is considered
to have potential in ESCC treatment. Thus far, several drugs have
been applied to block EGFR signaling such as the monoclonal
antibodies cetuximab and nimotuzumab, and the small molecular
inhibitor gefitinib. Combination of cetuximab with other adjuvant
therapies such as chemotherapeutic drugs and radiotherapy has been
shown to exert a remarkable effect on reducing ESCC progression
(102). However, the adverse
effects need to be taken into consideration. Nimotuzumab exhibits
low toxicity, and may thus be a promising treatment strategy for
advanced or metastatic ESCC (103). In addition to EGFR, VEGFR also
performs well as an effective therapeutic target. VEGFR binds to
VEGF to form the VEGF/VEGFR complex and trigger the PI3K/AKT and
MAPK/ERK signaling pathways (104). Apatinib and Endor, two
anticarcinogens independently developed by Chinese scientists that
target the VEGF/VEGFR axis, have been evaluated in clinical trials
and may become novel drugs for patients with ESCC (105–107).
Another target, HER-2, has been used for various
tumors, particularly breast cancer (108). HER-2 may have potential for the
treatment of esophageal cancer as well. In a clinical phase III
trial, patients with advanced esophageal adenocarcinoma received
combined treatment of the HER-2 specific inhibitor lapatinib and
CapeOx; OS reached 12.2 months compared with that of the untreated
group (10.5 months), showing a distinct curative effect in
esophageal adenocarcinoma (109).
At present, clinical evaluation of lapatinib is still required in
ESCC. In addition to the utilization of copy number variant genes
for targeted therapy, they may also be used for chemoradiotherapy.
The MYC locus is highly amplified with a rate of 43% and is
expressed throughout the entire process of chemoradiotherapy, which
may facilitate an increase in resistance (4). Furthermore, a focal copy-number
increase in MYC is markedly associated with a reduced survival rate
(110). With an increased
understanding of ESCC, additional targets are expected to be
identified in the future.
CNVs play crucial roles in ESCC occurrence and
development by altering numerous protein-coding genes. Future
studies should focus on identifying the emerging functions of
non-coding RNAs [including miRNAs, lncRNAs, and circular RNAs
(circRNAs)] in copy number aberrations, since protein-coding
sequences constitute only 2% of the total genome sequence, while
the remaining 98% corresponds to non-coding sequences. However, the
function of non-coding RNA has not yet been fully elucidated with
regard to CNVs.
The expression of lncRNAs is more specific,
particularly in cells, tissues, and during the developmental
stages, and in diseased tissues (111,112). Compared with linear RNA, circRNAs
are more stable, abundant, and better conserved. Thus, they may
serve as a potential target. For instance, lower expression of
deleted miRNA let-7c is correlated with a poorer prognosis
and reaction to chemotherapy, and it may be used to predict
clinical effects. In vitro, let-7c suppresses the IL6/STAT3
signaling pathway after cisplatin exposure. In short, let-7c
is a therapeutic target for ESCC and is deserved of further
research and exploitation for the management of cancer (37,113).
Amplified lncRNA PCAT1 acts as an oncogene and sponges
miR-326 as a competing endogenous RNA. PCAT1 was notably
detected in exosomes secreted by ESCC cells, highlighting its
potential as a non-invasive biomarker for patients with cancer
(38,114). Overexpression of the lncRNA
MALAT1 may result from frequent amplification of 11q13.1 in
ESCC (115). MALAT1
inhibits the effect of radiotherapy partly due to enhanced Cks1
expression. These results demonstrate that MALAT1-targeted therapy
may be feasible for improving the effectiveness of radiotherapy
(116). Though promising,
non-coding RNA-based therapies remain in an early stage of
development and need further validation in confirmatory clinical
trials. Numerous studies have been conducted to investigate ESCC;
however, ESCC still lacks efficient marker genes for diagnosis and
subtyping, unlike HER2 in breast cancer, and EGFR and
ROS in lung cancer.
Tumors are comprised of not only cancer cells but
also various stromal cells, which form the microenvironment the
tumor is situated in, and this contributes to cell proliferation,
drug resistance, invasion, migration, and the poor outcomes of
clinical treatment (117,118). In addition, intra-tumor
heterogeneity may lead to differences in the therapeutic efficacy
when treating malignant tumors (119). However, the heterogeneity and
tumor components of ESCC have not been clearly elucidated yet.
Although admixed samples of diverse cell types have been analyzed
by second-generation or TGS, heterogeneity was not noticeably
detected (120). Single-cell
sequencing offers the possibility of exploring genuine cellular and
molecular components during tumorigenesis (121). Tumor cells showed higher CNV
levels compared with normal epidermal cells in ESCC analyzed at the
single-cell level, which exhibited remarkable heterogeneity
(122). However, further research
is needed to explore the microenvironment of ESCC and to identify
more effective targets for tumor therapy.
In conclusion, this review comprehensively
summarized the CNV events and CNV-affected genes in ESCC, which
contributed to a deeper understanding of the molecular mechanisms
underlying the occurrence, development, recurrence, and metastasis
of ESCC. Improving our understanding of CNVs in ESCC will be
valuable for identifying biomarkers for early diagnosis and
molecular typing, as well as prognostic markers, and novel
therapeutic targets for the management of ESCC.
Not applicable.
This study was supported by the National Natural Science
Foundation of China (grant no. 81972613 and 82072746), the
Fundamental Research Program of Shanxi Province (grant no.
202203021222253), the Science and Technology Innovation Team of
Shanxi Province (grant no. 202204051001024) and the Science and
Technology Achievements Transformation Project of Shanxi Province
(grant no. 202204021301062).
Not applicable.
JR prepared the manuscript. LZ conceived the
subject of review. PK, YW, and DG reviewed and edited the
manuscript. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smyth EC, Lagergren J, Fitzgerald RC,
Lordick F, Shah MA, Lagergren P and Cunningham D: Oesophageal
cancer. Nat Rev Dis Primers. 3:170482017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang J, Tan W, Ling Z, Xi R, Shao M, Chen
M, Luo Y, Zhao Y, Liu Y, Huang X, et al: Genomic analysis of
oesophageal squamous-cell carcinoma identifies alcohol
drinking-related mutation signature and genomic alterations. Nat
Commun. 8:152902017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen XX, Zhong Q, Liu Y, Yan SM, Chen ZH,
Jin SZ, Xia TL, Li RY, Zhou AJ, Su Z, et al: Genomic comparison of
esophageal squamous cell carcinoma and its precursor lesions by
multi-region whole-exome sequencing. Nat Commun. 8:5242017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in oesophageal squamous cell cancer. Nature. 509:91–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Zhou Y, Cheng C, Cui H, Cheng L,
Kong P, Wang J, Li Y, Chen W, Song B, et al: Genomic analyses
reveal mutational signatures and frequently altered genes in
esophageal squamous cell carcinoma. Am J Hum Genet. 96:597–611.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng C, Zhou Y, Li H, Xiong T, Li S, Bi
Y, Kong P, Wang F, Cui H, Li Y, et al: Whole-genome sequencing
reveals diverse models of structural variations in esophageal
squamous cell carcinoma. Am J Hum Genet. 98:256–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng C, Cui H, Zhang L, Jia Z, Song B,
Wang F, Li Y, Liu J, Kong P, Shi R, et al: Genomic analyses reveal
FAM84B and the NOTCH pathway are associated with the progression of
esophageal squamous cell carcinoma. Gigascience. 5:12016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui Y, Chen H, Xi R, Cui H, Zhao Y, Xu E,
Yan T, Lu X, Huang F, Kong P, et al: Whole-genome sequencing of 508
patients identifies key molecular features associated with poor
prognosis in esophageal squamous cell carcinoma. Cell Res.
30:902–913. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lian Y, Niu X, Cai H, Yang X, Ma H, Ma S,
Zhang Y and Chen Y: Clinicopathological significance of c-MYC in
esophageal squamous cell carcinoma. Tumour Biol.
39:10104283177158042017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan T, Cui H, Zhou Y, Yang B, Kong P,
Zhang Y, Liu Y, Wang B, Cheng Y, Li J, et al: Multi-region
sequencing unveils novel actionable targets and spatial
heterogeneity in esophageal squamous cell carcinoma. Nat Commun.
10:16702019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin DC, Wang MR and Koeffler HP: Targeting
genetic lesions in esophageal cancer. Cell Cycle. 13:2013–2014.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Almal SH and Padh H: Implications of gene
copy-number variation in health and diseases. J Hum Genet. 57:6–13.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conrad DF, Pinto D, Redon R, Feuk L,
Gokcumen O, Zhang Y, Aerts J, Andrews TD, Barnes C, Campbell P, et
al: Origins and functional impact of copy number variation in the
human genome. Nature. 464:704–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Redon R, Ishikawa S, Fitch KR, Feuk L,
Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen W, et
al: Global variation in copy number in the human genome. Nature.
444:444–454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang F, Gu W, Hurles ME and Lupski JR:
Copy number variation in human health, disease, and evolution. Annu
Rev Genomics Hum Genet. 10:451–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lupski JR and Stankiewicz P: Genomic
disorders: Molecular mechanisms for rearrangements and conveyed
phenotypes. PLoS Genet. 1:e492005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lieber MR, Ma Y, Pannicke U and Schwarz K:
Mechanism and regulation of human non-homologous DNA end-joining.
Nat Rev Mol Cell Biol. 4:712–720. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lieber MR: The mechanism of human
nonhomologous DNA end joining. J Biol Chem. 283:1–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JA, Carvalho CMB and Lupski JR: A DNA
replication mechanism for generating nonrecurrent rearrangements
associated with genomic disorders. Cell. 131:1235–1247. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kazazian HH Jr and Moran JV: The impact of
L1 retrotransposons on the human genome. Nat Genet. 19:19–24. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang S, Lu Z, Unruh AK, Ivan C, Baggerly
KA, Calin GA, Li Z, Bast RC Jr and Le XF: Clinically relevant
microRNAs in ovarian cancer. Mol Cancer Res. 13:393–401. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zucman-Rossi J, Villanueva A, Nault JC and
Llovet JM: Genetic landscape and biomarkers of hepatocellular
carcinoma. Gastroenterology. 149:1226–1239.e4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Liang L, Fang JY and Xu J: Somatic
gene copy number alterations in colorectal cancer: New quest for
cancer drivers and biomarkers. Oncogene. 35:2011–2019. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi W, Ochoa A, McConkey DJ, Aine M,
Höglund M, Kim WY, Real FX, Kiltie AE, Milsom I, Dyrskjøt L and
Lerner SP: Genetic alterations in the molecular subtypes of bladder
cancer: Illustration in the cancer genome atlas dataset. Eur Urol.
72:354–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berger AC, Korkut A, Kanchi RS, Hegde AM,
Lenoir W, Liu W, Liu Y, Fan H, Shen H, Ravikumar V, et al: A
comprehensive pan-cancer molecular study of gynecologic and breast
cancers. Cancer Cell. 33:690–705.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kleinjan DA and van Heyningen V:
Long-range control of gene expression: Emerging mechanisms and
disruption in disease. Am J Hum Genet. 76:8–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Girirajan S, Campbell CD and Eichler EE:
Human copy number variation and complex genetic disease. Annu Rev
Genet. 45:203–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meyerson M, Gabriel S and Getz G: Advances
in understanding cancer genomes through second-generation
sequencing. Nat Rev Genet. 11:685–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alkan C, Coe BP and Eichler EE: Genome
structural variation discovery and genotyping. Nat Rev Genet.
12:363–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mamanova L, Coffey AJ, Scott CE, Kozarewa
I, Turner EH, Kumar A, Howard E, Shendure J and Turner DJ:
Target-enrichment strategies for next-generation sequencing. Nat
Methods. 7:111–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ari Å and Arikan M: Next-generation
sequencing: Advantages, disadvantages, and future. In: Plant omics:
Trends and applications. Springer; Berlin: pp. 109–135. 2016
|
|
34
|
Ogawa A, Celikkol-Aydin S, Gaylarde C,
Baptista-Neto JA and Beech I: Microbiomes of biofilms on decorative
siliceous stone: Drawbacks and advantages of next generation
sequencing. Curr Microbiol. 74:848–853. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Berná L, Rodriguez M, Chiribao ML,
Parodi-Talice A, Pita S, Rijo G, Alvarez-Valin F and Robello C:
Expanding an expanded genome: Long-read sequencing of Trypanosoma
cruzi. Microb Genom. 4:e0001772018.PubMed/NCBI
|
|
36
|
Lin DC, Hao JJ, Nagata Y, Xu L, Shang L,
Meng X, Sato Y, Okuno Y, Varela AM, Ding LW, et al: Genomic and
molecular characterization of esophageal squamous cell carcinoma.
Nat Genet. 46:467–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi ZZ, Shang L, Jiang YY, Hao JJ, Zhang
Y, Zhang TT, Lin DC, Liu SG, Wang BS, Gong T, et al: Consistent and
differential genetic aberrations between esophageal dysplasia and
squamous cell carcinoma detected by array comparative genomic
hybridization. Clin Cancer Res. 19:5867–5878. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qin HD, Liao XY, Chen YB, Huang SY, Xue
WQ, Li FF, Ge XS, Liu DQ, Cai Q, Long J, et al: Genomic
characterization of esophageal squamous cell carcinoma reveals
critical genes underlying tumorigenesis and poor prognosis. Am J
Hum Genet. 98:709–727. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin DC, Wang MR and Koeffler HP: Genomic
and epigenomic aberrations in esophageal squamous cell carcinoma
and implications for patients. Gastroenterology. 154:374–389. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang R, Dai Q, Yang R, Duan Y, Zhao Q,
Haybaeck J and Yang Z: A review: PI3K/AKT/mTOR signaling pathway
and its regulated eukaryotic translation initiation factors may be
a potential therapeutic target in esophageal squamous cell
carcinoma. Front Oncol. 12:8179162022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zang W, Wang T, Wang Y, Chen X, Du Y, Sun
Q, Li M, Dong Z and Zhao G: Knockdown of long non-coding RNA
TP73-AS1 inhibits cell proliferation and induces apoptosis in
esophageal squamous cell carcinoma. Oncotarget. 7:19960–19974.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu J, Liu ZX, Wu QN, Lu YX, Wong CW, Miao
L, Wang Y, Wang Z, Jin Y, He MM, et al: Long noncoding RNA AGPG
regulates PFKFB3-mediated tumor glycolytic reprogramming. Nat
Commun. 11:15072020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu X, Zhang M, Ying S, Zhang C, Lin R,
Zheng J, Zhang G, Tian D, Guo Y, Du C, et al: Genetic alterations
in esophageal tissues from squamous dysplasia to carcinoma.
Gastroenterology. 153:166–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma S, Paiboonrungruan C, Yan T, Williams
KP, Major MB and Chen XL: Targeted therapy of esophageal squamous
cell carcinoma: The NRF2 signaling pathway as target. Ann N Y Acad
Sci. 1434:164–172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shen H, Yang Y, Xia S, Rao B, Zhang J and
Wang J: Blockage of Nrf2 suppresses the migration and invasion of
esophageal squamous cell carcinoma cells in hypoxic
microenvironment. Dis Esophagus. 27:685–692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kawasaki Y, Okumura H, Uchikado Y, Kita Y,
Sasaki K, Owaki T, Ishigami S and Natsugoe S: Nrf2 is useful for
predicting the effect of chemoradiation therapy on esophageal
squamous cell carcinoma. Ann Surg Oncol. 21:2347–2352. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shibata T, Kokubu A, Saito S,
Narisawa-Saito M, Sasaki H, Aoyagi K, Yoshimatsu Y, Tachimori Y,
Kushima R, Kiyono T and Yamamoto M: NRF2 mutation confers malignant
potential and resistance to chemoradiation therapy in advanced
esophageal squamous cancer. Neoplasia. 13:864–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bollong MJ, Yun H, Sherwood L, Woods AK,
Lairson LL and Schultz PG: A small molecule inhibits deregulated
NRF2 transcriptional activity in cancer. ACS Chem Biol.
10:2193–2198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Singh A, Venkannagari S, Oh KH, Zhang YQ,
Rohde JM, Liu L, Nimmagadda S, Sudini K, Brimacombe KR, Gajghate S,
et al: Small molecule inhibitor of NRF2 selectively intervenes
therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem
Biol. 11:3214–3225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Zhu CL, Nie CJ, Li JC, Zeng TT, Zhou
J, Chen J, Chen K, Fu L, Liu H, et al: Investigation of tumor
suppressing function of CACNA2D3 in esophageal squamous cell
carcinoma. PLoS One. 8:e600272013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nie C, Qin X, Li X, Tian B, Zhao Y, Jin Y,
Li Y, Wang Q, Zeng D, Hong A and Chen X: CACNA2D3 enhances the
chemosensitivity of esophageal squamous cell carcinoma to cisplatin
via inducing Ca2+-mediated apoptosis and suppressing
PI3K/Akt pathways. Front Oncol. 9:1852019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li L, Xu J, Qiu G, Ying J, Du Z, Xiang T,
Wong KY, Srivastava G, Zhu XF, Mok TS, et al: Epigenomic
characterization of a p53-regulated 3p22.2 tumor suppressor that
inhibits STAT3 phosphorylation via protein docking and is
frequently methylated in esophageal and other carcinomas.
Theranostics. 8:61–77. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Y, Chen L, Nie CJ, Zeng TT, Liu H, Mao
X, Qin Y, Zhu YH, Fu L and Guan XY: Downregulation of RBMS3 is
associated with poor prognosis in esophageal squamous cell
carcinoma. Cancer Res. 71:6106–6115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tang H, Jiang L, Zhu C, Liu R, Wu Y, Yan
Q, Liu M, Jia Y, Chen J, Qin Y, et al: Loss of cell adhesion
molecule L1 like promotes tumor growth and metastasis in esophageal
squamous cell carcinoma. Oncogene. 38:3119–3133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sugita M, Tanaka N, Davidson S, Sekiya S,
Varella-Garcia M, West J, Drabkin HA and Gemmill RM: Molecular
definition of a small amplification domain within 3q26 in tumors of
cervix, ovary, and lung. Cancer Genet Cytogenet. 117:9–18. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang YL, Chu JY, Luo ML, Wu YP, Zhang Y,
Feng YB, Shi ZZ, Xu X, Han YL, Cai Y, et al: Amplification of
PRKCI, located in 3q26, is associated with lymph node metastasis in
esophageal squamous cell carcinoma. Genes Chromosomes Cancer.
47:127–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu Y, Liu X, Hu L, Tao H, Guan X, Zhang K,
Bai Y and Yang K: Copy number loss of variation_91720 in PIK3CA
predicts risk of esophageal squamous cell carcinoma. Int J Clin Exp
Pathol. 8:14479–14485. 2015.PubMed/NCBI
|
|
58
|
Wang P, Shan L, Xue L, Zheng B and Lu N:
Genome wide copy number analyses of superficial esophageal squamous
cell carcinoma with and without metastasis. Oncotarget.
8:5069–5080. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li B, Cheung PY, Wang X, Tsao SW, Ling MT,
Wong YC and Cheung AL: Id-1 activation of PI3K/Akt/NFkappaB
signaling pathway and its significance in promoting survival of
esophageal cancer cells. Carcinogenesis. 28:2313–2320. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gen Y, Yasui K, Zen Y, Zen K, Dohi O, Endo
M, Tsuji K, Wakabayashi N, Itoh Y, Naito Y, et al: SOX2 identified
as a target gene for the amplification at 3q26 that is frequently
detected in esophageal squamous cell carcinoma. Cancer Genet
Cytogenet. 202:82–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gen Y, Yasui K, Nishikawa T and Yoshikawa
T: SOX2 promotes tumor growth of esophageal squamous cell carcinoma
through the AKT/mammalian target of rapamycin complex 1 signaling
pathway. Cancer Sci. 104:810–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang X, Ge X, Wang H, Huang J, Song Q, Xu
C, Jiang Z, Su J, Wang H, Tan L, et al: SOX2 amplification and
chromosome 3 gain significantly impact prognosis in esophageal
squamous cell carcinoma. Ann Transl Med. 9:3212021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gao H, Teng C, Huang W, Peng J and Wang C:
SOX2 promotes the epithelial to mesenchymal transition of
esophageal squamous cells by modulating slug expression through the
activation of STAT3/HIF-α Signaling. Int J Mol Sci. 16:21643–21657.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen B, Liu S, Gan L, Wang J, Hu B, Xu H,
Tong R, Yang H, Cristina I, Xue J, et al: FGFR1 signaling
potentiates tumor growth and predicts poor prognosis in esophageal
squamous cell carcinoma patients. Cancer Biol Ther. 19:76–86. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Guagnano V, Kauffmann A, Wöhrle S, Stamm
C, Ito M, Barys L, Pornon A, Yao Y, Li F, Zhang Y, et al: FGFR
genetic alterations predict for sensitivity to NVP-BGJ398, a
selective pan-FGFR inhibitor. Cancer Discov. 2:1118–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
von Loga K, Kohlhaussen J, Burkhardt L,
Simon R, Steurer S, Burdak-Rothkamm S, Jacobsen F, Sauter G and
Krech T: FGFR1 amplification is often homogeneous and strongly
linked to the squamous cell carcinoma subtype in esophageal
carcinoma. PLoS One. 10:e01418672015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Luo H, Quan J, Xiao H, Luo J, Zhang Q, Pi
G, Ye Y, He R, Liu Y, Su X, et al: FGFR inhibitor AZD4547 can
enhance sensitivity of esophageal squamous cell carcinoma cells
with epithelial-mesenchymal transition to gefitinib. Oncol Rep.
39:2270–2278. 2018.PubMed/NCBI
|
|
68
|
Huang J, Jiang D, Zhu T, Wang Y, Wang H,
Wang Q, Tan L, Zhu H, Yao J and Hou Y: Prognostic significance of
c-MYC amplification in esophageal squamous cell carcinoma. Ann
Thorac Surg. 107:436–443. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang HF, Wu C, Alshareef A, Gupta N, Zhao
Q, Xu XE, Jiao JW, Li EM, Xu LY and Lai R: The PI3K/AKT/c-MYC axis
promotes the acquisition of cancer stem-like features in esophageal
squamous cell carcinoma. Stem Cells. 34:2040–2051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li W, Zhang L, Guo B, Deng J, Wu S, Li F,
Wang Y, Lu J and Zhou Y: Exosomal FMR1-AS1 facilitates maintaining
cancer stem-like cell dynamic equilibrium via TLR7/NFκB/c-Myc
signaling in female esophageal carcinoma. Mol Cancer. 18:222019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang Y, Cheng J, Xie D, Ding X, Hou H,
Chen X, Er P, Zhang F, Zhao L, Yuan Z, et al: NS1-binding protein
radiosensitizes esophageal squamous cell carcinoma by
transcriptionally suppressing c-Myc. Cancer Commun (Lond).
38:332018.PubMed/NCBI
|
|
72
|
Yang J, Kong P, Yang J, Jia Z, Hu X, Wang
Z, Cui H, Bi Y, Qian Y, Li H, et al: High TSTA3 expression as a
candidate biomarker for poor prognosis of patients with ESCC.
Technol Cancer Res Treat. 17:5330338187814052018. View Article : Google Scholar
|
|
73
|
Zhang L, Gao Y, Zhang X, Guo M, Yang J,
Cui H, Kong P, Niu X, Bi Y, Xu J, et al: TSTA3 facilitates
esophageal squamous cell carcinoma progression through regulating
fucosylation of LAMP2 and ERBB2. Theranostics. 10:11339–11358.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lin X, Yan C, Gao Y, Du J, Zhu X, Yu F,
Huang T, Dai J, Ma H, Jiang Y, et al: Genetic variants at 9p21.3
are associated with risk of esophageal squamous cell carcinoma in a
Chinese population. Cancer Sci. 108:250–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shen TY, Mei LL, Qiu YT and Shi ZZ:
Identification of candidate target genes of genomic aberrations in
esophageal squamous cell carcinoma. Oncol Lett. 12:2956–2961. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang Q, Bai J, Abliz A, Liu Y, Gong K, Li
J, Shi W, Pan Y, Liu F, Lai S, et al: An old story retold: Loss of
G1 control defines a distinct genomic subtype of esophageal
squamous cell carcinoma. Genomics Proteomics Bioinformatics.
13:258–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Su D, Zhang D, Jin J, Ying L, Han M, Chen
K, Li B, Wu J, Xie Z, Zhang F, et al: Identification of predictors
of drug sensitivity using patient-derived models of esophageal
squamous cell carcinoma. Nat Commun. 10:50762019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Clark ES, Brown B, Whigham AS,
Kochaishvili A, Yarbrough WG and Weaver AM: Aggressiveness of HNSCC
tumors depends on expression levels of cortactin, a gene in the
11q13 amplicon. Oncogene. 28:431–444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kwek SS, Roy R, Zhou H, Climent J,
Martinez-Climent JA, Fridlyand J and Albertson DG: Co-amplified
genes at 8p12 and 11q13 in breast tumors cooperate with two major
pathways in oncogenesis. Oncogene. 28:1892–1903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Huang J, Song Q, Wang H, Wang H, Xu C,
Wang X, Jiang Z, Wang Y, Xu Y, Su J, et al: Poor prognostic impact
of FGF4 amplification in patients with esophageal squamous cell
carcinoma. Hum Pathol. 80:210–218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yu Y, Cao J, Wu W, Zhu Q, Tang Y, Zhu C,
Dai J, Li Z, Wang J, Xue L, et al: Genome-wide copy number
variation analysis identified ANO1 as a novel oncogene and
prognostic biomarker in esophageal squamous cell cancer.
Carcinogenesis. 40:1198–1208. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shang L, Hao JJ, Zhao XK, He JZ, Shi ZZ,
Liu HJ, Wu LF, Jiang YY, Shi F, Yang H, et al: ANO1 protein as a
potential biomarker for esophageal cancer prognosis and
precancerous lesion development prediction. Oncotarget.
7:24374–24382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang W, Hong R, Li L, Wang Y, Du P, Ou Y,
Zhao Z, Liu X, Xiao W, Dong D, et al: The chromosome 11q13.3
amplification associated lymph node metastasis is driven by
miR-548k through modulating tumor microenvironment. Mol Cancer.
17:1252018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Dong G, Mao Q, Yu D, Zhang Y, Qiu M, Dong
G, Chen Q, Xia W, Wang J, Xu L and Jiang F: Integrative analysis of
copy number and transcriptional expression profiles in esophageal
cancer to identify a novel driver gene for therapy. Sci Rep.
7:420602017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sawada R, Maehara R, Oshikiri T, Nakamura
T, Itoh T, Kodama Y, Kakeji Y and Zen Y: MDM2 copy number increase:
A poor prognostic, molecular event in esophageal squamous cell
carcinoma. Hum Pathol. 89:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xiao FK, Guo S, Yang F, Zhao LS and Wang
LD: MDM2 and its functional polymorphism SNP309 contribute to the
development of esophageal carcinoma. J Gene Med. 21:e30862019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
He T, Guo J, Song H, Zhu H, Di X, Min H,
Wang Y, Chen G, Dai W, Ma J, et al: Nutlin-3, an antagonist of
MDM2, enhances the radiosensitivity of esophageal squamous cancer
with wild-type p53. Pathol Oncol Res. 24:75–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Okamoto H, Fujishima F, Kamei T, Nakamura
Y, Ozawa Y, Miyata G, Nakano T, Katsura K, Abe S, Taniyama Y, et
al: Murine double minute 2 predicts response of advanced esophageal
squamous cell carcinoma to definitive chemoradiotherapy. BMC
Cancer. 15:2082015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sun Y, Shi N, Lu H, Zhang J, Ma Y, Qiao Y,
Mao Y, Jia K, Han L, Liu F, et al: ABCC4 copy number variation is
associated with susceptibility to esophageal squamous cell
carcinoma. Carcinogenesis. 35:1941–1950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yasui K, Imoto I, Fukuda Y, Pimkhaokham A,
Yang ZQ, Naruto T, Shimada Y, Nakamura Y and Inazawa J:
Identification of target genes within an amplicon at 14q12-q13 in
esophageal squamous cell carcinoma. Genes Chromosomes Cancer.
32:112–118. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sano M, Aoyagi K, Takahashi H, Kawamura T,
Mabuchi T, Igaki H, Tachimori Y, Kato H, Ochiai A, Honda H, et al:
Forkhead box A1 transcriptional pathway in KRT7-expressing
esophageal squamous cell carcinomas with extensive lymph node
metastasis. Int J Oncol. 36:321–330. 2010.PubMed/NCBI
|
|
92
|
Xu Y, Wang W, Li L, Liu J, Wu X, Yu J,
Wang H, Cui W and Zhang R: FOXA1 and CK7 expression in esophageal
squamous cell carcinoma and its prognostic significance. Neoplasma.
65:469–476. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bi Y, Guo S, Xu X, Kong P, Cui H, Yan T,
Ma Y, Cheng Y, Chen Y, Liu X, et al: Decreased ZNF750 promotes
angiogenesis in a paracrine manner via activating
DANCR/miR-4707-3p/FOXC2 axis in esophageal squamous cell carcinoma.
Cell Death Dis. 11:2962020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kong P, Xu E, Bi Y, Xu X, Liu X, Song B,
Zhang L, Cheng C, Yan T, Qian Y, et al: Novel ESCC-related gene
ZNF750 as potential prognostic biomarker and inhibits
epithelial-mesenchymal transition through directly depressing SNAI1
promoter in ESCC. Theranostics. 10:1798–1813. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Du Plessis L, Dietzsch E, Van Gele M, Van
Roy N, Van Helden P, Parker MI, Mugwanya DK, De Groot M, Marx MP,
Kotze MJ and Speleman F: Mapping of novel regions of DNA gain and
loss by comparative genomic hybridization in esophageal carcinoma
in the black and colored populations of South Africa. Cancer Res.
59:1877–1883. 1999.PubMed/NCBI
|
|
96
|
Gorringe KL, Ramakrishna M, Williams LH,
Sridhar A, Boyle SE, Bearfoot JL, Li J, Anglesio MS and Campbell
IG: Are there any more ovarian tumor suppressor genes? A new
perspective using ultra high-resolution copy number and loss of
heterozygosity analysis. Genes Chromosomes Cancer. 48:931–942.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Girard L, Zöchbauer-Müller S, Virmani AK,
Gazdar AF and Minna JD: Genome-wide allelotyping of lung cancer
identifies new regions of allelic loss, differences between small
cell lung cancer and non-small cell lung cancer, and loci
clustering. Cancer Res. 60:4894–4906. 2000.PubMed/NCBI
|
|
98
|
Zhu YH, Fu L, Chen L, Qin YR, Liu H, Xie
F, Zeng T, Dong SS, Li J, Li Y, et al: Downregulation of the novel
tumor suppressor DIRAS1 predicts poor prognosis in esophageal
squamous cell carcinoma. Cancer Res. 73:2298–2309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Qin H, Li Y, Zhang H, Wang F, He H, Bai X
and Li S: Prognostic implications and oncogenic roles of MYBL2
protein expression in esophageal squamous-cell carcinoma. Onco
Targets Ther. 12:1917–1927. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chong CR and Jänne PA: The quest to
overcome resistance to EGFR-targeted therapies in cancer. Nat Med.
19:1389–1400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ciardiello F and Tortora G: EGFR
antagonists in cancer treatment. N Engl J Med. 358:1160–1174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ruhstaller T, Thuss-Patience P, Hayoz S,
Schacher S, Knorrenschild JR, Schnider A, Plasswilm L, Budach W,
Eisterer W, Hawle H, et al: Neoadjuvant chemotherapy followed by
chemoradiation and surgery with and without cetuximab in patients
with resectable esophageal cancer: A randomized, open-label, phase
III trial (SAKK 75/08). Ann Oncol. 29:1386–1393. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Han X, Lu N, Pan Y and Xu J: Nimotuzumab
combined with chemotherapy is a promising treatment for locally
advanced and metastatic esophageal cancer. Med Sci Monit.
23:412–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling-in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li J and Wang L: Efficacy and safety of
apatinib treatment for advanced esophageal squamous cell carcinoma.
Onco Targets Ther. 10:3965–3969. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang B, Qi L, Wang X, Xu J, Liu Y, Mu L,
Wang X, Bai L and Huang J: Phase II clinical trial using
camrelizumab combined with apatinib and chemotherapy as the
first-line treatment of advanced esophageal squamous cell
carcinoma. Cancer Commun (Lond). 40:711–720. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Xu M, Huang H, Xiong Y, Peng B, Zhou Z,
Wang D and Yang X: Combined chemotherapy plus endostar with
sequential stereotactic radiotherapy as salvage treatment for
recurrent esophageal cancer with severe dyspnea: A case report and
review of the literature. Oncol Lett. 8:291–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu
JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, et al:
Lapatinib in combination with capecitabine plus oxaliplatin in
human epidermal growth factor receptor 2-positive advanced or
metastatic gastric, esophageal, or gastroesophageal adenocarcinoma:
TRIO-013/LOGiC-a randomized phase III trial. J Clin Oncol.
34:443–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hirata H, Niida A, Kakiuchi N, Uchi R,
Sugimachi K, Masuda T, Saito T, Kageyama SI, Motomura Y, Ito S, et
al: The evolving genomic landscape of esophageal squamous cell
carcinoma under chemoradiotherapy. Cancer Res. 81:4926–4938. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Flynn RA and Chang HY: Long noncoding RNAs
in cell-fate programming and reprogramming. Cell Stem Cell.
14:752–761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sugimura K, Miyata H, Tanaka K, Hamano R,
Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori
M and Doki Y: Let-7 expression is a significant determinant of
response to chemotherapy through the regulation of IL-6/STAT3
pathway in esophageal squamous cell carcinoma. Clin Cancer Res.
18:5144–5153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Huang L, Wang Y, Chen J, Wang Y, Zhao Y,
Wang Y, Ma Y, Chen X, Liu W, Li Z, et al: Long noncoding RNA PCAT1,
a novel serum-based biomarker, enhances cell growth by sponging
miR-326 in oesophageal squamous cell carcinoma. Cell Death Dis.
10:5132019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y
and Yang K: Up-regulation of long noncoding RNA MALAT1 contributes
to proliferation and metastasis in esophageal squamous cell
carcinoma. J Exp Clin Cancer Res. 34:72015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li Z, Zhou Y, Tu B, Bu Y, Liu A and Kong
J: Long noncoding RNA MALAT1 affects the efficacy of radiotherapy
for esophageal squamous cell carcinoma by regulating Cks1
expression. J Oral Pathol Med. 46:583–590. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Azizi E, Carr AJ, Plitas G, Cornish AE,
Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M,
et al: Single-cell map of diverse immune phenotypes in the breast
tumor microenvironment. Cell. 174:1293–1308.e36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gao S, Yan L, Wang R, Li J, Yong J, Zhou
X, Wei Y, Wu X, Wang X, Fan X, et al: Tracing the temporal-spatial
transcriptome landscapes of the human fetal digestive tract using
single-cell RNA-sequencing. Nat Cell Biol. 20:721–734. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Xiao Z, Dai Z and Locasale JW: Metabolic
landscape of the tumor microenvironment at single cell resolution.
Nat Commun. 10:37632019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zong C, Lu S, Chapman AR and Xie XS:
Genome-wide detection of single-nucleotide and copy-number
variations of a single human cell. Science. 338:1622–1626. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Vitak SA, Torkenczy KA, Rosenkrantz JL,
Fields AJ, Christiansen L, Wong MH, Carbone L, Steemers FJ and Adey
A: Sequencing thousands of single-cell genomes with combinatorial
indexing. Nat Methods. 14:302–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Chen Z, Zhao M, Liang J, Hu Z, Huang Y, Li
M, Pang Y, Lu T, Sui Q, Zhan C, et al: Dissecting the single-cell
transcriptome network underlying esophagus non-malignant tissues
and esophageal squamous cell carcinoma. EBioMedicine.
69:1034592021. View Article : Google Scholar : PubMed/NCBI
|