Introduction
Renal cell carcinoma (RCC), the prevailing kidney
malignancy in adults, has been shown to be resistant to
chemotherapy and radiation therapy (1). Though the use of targeted agents, such
as sunitinib, axitinib, temsirolimus, pazopanib and cabozatinib has
been reported to notably prolong survival in patients with advanced
RCC, the responses induced by these drugs are only transient
(2,3). The recent introduction of
immunotherapy utilizing immune checkpoint inhibitors, including
nivolumab, pembrolizumab, ipilimumab and avelumab, holds promise
for providing significant antitumor activity, as well as enduring
responses, in patients with advanced RCC. However, the complete
response rates induced by these drugs are limited, ranging from
4–10% (4,5). Another related concern is the
occurrence of immune-related adverse events, as these can have
effects on nearly all organs with varying frequency and severity,
including hypophysitis, thyroiditis, hepatitis, interstitial
pneumonitis, colitis and interstitial nephritis (6). Consequently, a pressing need for the
development of innovative and effective therapeutic approaches for
metastatic RCC has become evident.
Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL), a TNF superfamily member, is
promising as an effective anticancer agent due to its capacity to
selectively induce apoptosis in various tumor cells and has been
revealed to be relatively non-toxic towards normal cells (7). TRAIL triggers apoptosis by binding to
the death receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5). Activation
of these receptors results in a signal transduction cascade that
initiates both extrinsic and intrinsic apoptotic pathways (8). TRAIL also binds to two other receptors
that lack functional cytoplasmic death domains, TRAIL-R3 (DcR1) and
TRAIL-R4 (DcR2), as well as osteoprotegerin, a secreted TNF
receptor homolog. These receptors have been considered to
potentially inhibit TRAIL-induced apoptosis. Therefore, use of a
specific activator of TRAIL-R1 or TRAIL-R2 would be favorable to
exclude potential interference from competition with DcRs. Previous
studies have reported that monoclonal antibodies (mAbs) targeting
human TRAIL-R1 or TRAIL-R2 from mice or rabbits exhibit antitumor
activities in vitro and in vivo (9,10).
These agonistic antibodies have functions similar to TRAIL in
triggering apoptotic pathways mediated by TRAIL activation. In
addition, several studies have reported that lexatumumab, a fully
human agonistic mAb specific for TRAIL-R2, induces apoptotic cell
death in some tumor cells (11–13).
It was previously reported by the authors that low concentrations
of doxorubicin enhance lexatumumab-mediated apoptosis and
cytotoxicity by inducing TRAIL-R2 expression in various human solid
cancer cells, including RCC, prostate, bladder and lung cancer
cells (14).
Celastrol, a plant triterpene, exhibits a diverse
range of pharmacological properties, including anti-inflammatory,
antioxidative and antitumor effects (15,16).
Previous studies have demonstrated that celastrol has
broad-spectrum anticancer activities, including against prostate
cancer, glioblastoma, lung cancer, colon cancer, ovarian cancer
cells, melanoma, osteosarcoma and RCC (16–23).
However, studies have demonstrated that periods of celastrol
injection cause obvious weight loss in mice, indicating that its
toxicity poses a threat to normal cells and tissues (24,25).
Nevertheless, celastrol enhances TRAIL-induced apoptosis in some
tumor cells via the death receptor pathway, indicating a potential
strategy to effectively reduce associated side effects (25–27).
In the present study, the enhancement of
TRAIL-R2-mediated apoptosis and cytotoxicity in RCC cells by
celastrol was examined. The molecular mechanisms that are possibly
related to enhanced cytotoxicity were also explored.
Materials and methods
Reagents
Celastrol was obtained from Sigma-Aldrich; Merck
KGaA, dissolved in dimethyl sulfoxide (DMSO), and subsequently
diluted in culture medium. The concentration of DMSO utilized
during treatment was <0.1%. Lexatumumab, highly specific for
binding to TRAIL-R2, was generously supplied by Human Genome
Sciences (28). The concentration
of lexatumumab used was 1–100 ng/ml because it did not have
significant cytotoxic effects in a variety of cancer cells at 100
ng/ml in previous studies (14,29,30).
RCC cell lines and primary RCC
cells
Two human RCC cell lines were used: ACHN (cat. no.
CRL-1611) and Caki-1 (cat. no. HTB-46) were obtained from the
American Type Culture Collection. Primary RCC cells were separated
from the surgical specimens of six patients with untreated RCC as
previously described (31).
Pathologic stage and grade were consistent with the 2004 worlds
health organization (WHO) criteria (https://www.patologi.com/WHO%20kidney%20testis.pdf):
T3N0M1 grade 2 in patient 1 (77 years, female), T2N0M0 grade 2 in
patient 2 (72 years, male), T3N1M0 grade 2 in patient 3 (68 years,
female), T2N0M0 grade 1 in patient 4 (70 years, male), T2N0M0 grade
1 in patient 5 (63 years, male), and T2N0M0 grade 2 in patient 6
(70 years, male). All cells were cultured in RPMI-1640 medium (cat.
no. 11875119) supplemented with 10% FBS (cat. no. 10099141) and 1%
penicillin and streptomycin (cat. no. 15140122) (all from Gibco;
Thermo Fisher Scientific, Inc.), and then maintained at 37°C in 5%
CO2.
Ethical approval for use of human tissue was granted
by Hyogo College of Medicine (approval no. 202306; Hyogo, Japan).
All patients provided individual written informed consent for use
of their sampled tissues.
Cytotoxicity assays
Cytotoxicity was determined based on colorimetric
assays of cell viability using Cell Counting Kit-8 (CCK-8) (cat.
no. 343-07623; Dojindo Laboratories, Inc.) (32). Briefly, a 100-µl suspension
containing 0.5×104 cells was added to a 96-well flat
bottom microtiter plate. Following 24 h of incubation at 37°C, 100
µl of lexatumumab or celastrol, alone or in combination, or medium
alone (control) was added to the plates in triplicate. Each plate
was then incubated at 37°C for an additional 3–24 h. Next, 10 µl of
CCK-8 solution was added and incubated for 3 h. Absorbance (A) was
quantified using a SPECTRAmax PLUS384 (Molecular Devices, LLC) at
450 nm as the reference, and cell viability was determined based on
the percentage of control cells using the following formula:
percent cell viability=(A of treated wells/A of
control wells) ×100.
The coefficient of drug interaction (CDI) was
determined to assess the interactions between celastrol and
lexatumumab. CDI was calculated as AB/(AxB), where AB is the
A of the mixture of the two active agents/A of the
control, and A and B are the A of the single active
agent/A of the control. Based on the CDI, the interactions
were categorized as synergistic (CDI <1), additive (CDI=1), or
antagonistic (CDI >1) (33). CDI
<0.7 was considered to indicate a significant level of synergism
between the drugs.
Cell viability was assessed using a trypan blue dye
exclusion test. Initially, cells were seeded in six-well plates at
3×105 cells/well and cultured at 37°C for 24 h.
Subsequently, they were treated in duplicate with lexatumumab
and/or celastrol for 6 h. Following treatment, cells were harvested
and viable cells counted by staining with a 0.5% trypan blue dye
solution (Sigma-Aldrich; KGaA) under an optical microscope
(magnification, ×10) (Olympus Corporation).
Apoptosis assays
Apoptosis was assessed using two distinct
methodologies. First, following incubation with 100 ng/ml of
lexatumumab and/or 1 µM celastrol for 6 h, floating and adherent
cells were harvested. The obtained cells were washed twice with
cold PBS and resuspended in 1X binding buffer at a concentration of
1×106 cells/ml. Subsequently, the cells were stained
with 5 µl of FITC Annexin V and 5 µl of propidium iodide (PI) (FITC
annexin V Apoptosis Detection Kit; cat. no. 556547; BD
Biosciences), and then analyzed by an LSRFortessa™ X-20 instrument
(BD Biosciences) equipped with the BD FACSDiva and FlowJo (v10.7.1;
FlowJo LLC) software packages. Second, DNA fragmentation was
quantified using an enzyme-linked immunosorbent assay (ELISA) Kit
for cell death detection (cat. no. 11544675001; Roche Life Science
Products; Merck KGaA) according to the manufacturer's instructions.
Briefly, after treatment with 100 ng/ml of lexatumumab and/or 1 µM
of celastrol for 6 h, the cells were resuspended in 500 µl of
incubation buffer at a concentration of 1×105 cells/ml
at room temperature. After 30 min, the lysate was centrifuged at
20,000 × g for 10 min at 4°C and the resulting supernatant
prediluted 1:10 with incubation buffer. Using a pipette, 100 µl of
coating solution was added to each well of MP modules and incubated
overnight at 4°C, after which the incubation buffer was removed and
then 200 µl of incubation buffer were added into each well of the
MP modules via pipette and incubated for 30 min at room
temperature. After removing the solution, the cells were rinsed
three times with washing solution and 100 µl of sample solution
added to each well using a pipette and incubated for 90 min at room
temperature. Subsequently, the solution was removed and the wells
rinsed. A total of 100 µl of conjugate solution (dilution of 10 µl
anti-DNA-POD solution with 90 µl incubation buffer) was added to
each well using a pipette and incubated for 90 min at room
temperature. The solution was then removed and the wells rinsed
three times before adding 100 µl of substrate solution to each well
using a pipette. The module was placed on a plate shaker and
incubated with shaking at 250 × g for 20 min. Finally, the A value
of each well was determined using a SPECTRAmax PLUS384 (Molecular
Devices, LLC) at 405 nm.
Western blotting
Cells were initially plated in 9-cm plates and held
for 24 h. Subsequently, lexatumumab and/or celastrol treatment was
performed for 6 h at 37°C. Following treatment, the cells were
lysed for 20 min on ice using lysis buffer (cat. no. 89900; Thermo
Fisher Scientific, Inc.) and the protein concentrations determined
using a Bradford Assay Kit (Bio-Rad Laboratories, Inc.). Next, 30
µg of protein was loaded into each lane of 10% SDS-PAGE gel, and
then transferred onto a PVDF membrane after separation. After
blocking non-specific binding sites with 5% skim milk in TBS
containing 0.1% Tween-20 for 2 h at room temperature, the membranes
were incubated overnight at 4°C with the following primary
antibodies: TRAIL-R2 (cat. no. sc-166624), Bid (cat. no. sc-6538),
Bcl-2 (cat. no. sc-7382), Bax (cat. no. sc-20067), AIF (cat. no.
sc-13116), and caspase-3 (cat. no. sc-7272) obtained from Santa
Cruz Biotechnology, Inc. at a 1:100 dilution; FLIP (cat. no.
OPA1-01011; http://www.antibodydirectory.com/moreinfos.php?Item=155921)
obtained from Thermo Fisher Scientific, Inc. at 1:1,000 dilution;
caspase-8 (cat. no. M032-3) and caspase-9 (cat. no. M054-3)
purchased from MBL International Co. at a 1:1,000 dilution; and
B-actin mouse polyclonal (cat. no. E4D9Z), FADD (cat. no. 2782) and
caspase-6 (cat. no. 9762) obtained from Cell Signaling Technology,
Inc. at a 1:2,000 dilution. Subsequently, the membranes were washed
three times with TBST buffer for 30 min at room temperature, and
then incubated with horseradish peroxidase (HRP)-conjugated
anti-mouse IgG secondary antibody (cat. no. 330; MBL International
Co.) at a 1:2,000 dilution and HRP-conjugated goat anti-rabbit IgG
secondary antibody (sc-2004; Santa Cruz Biotechnology) at a 1:1,000
dilution for 1.5 h at room temperature. Finally, the membranes were
washed three times with TBST buffer for 30 min at room temperature.
Signals were detected using a chemiluminescence ECL kit (GE
Healthcare) and an ImageQuant LAS 4010 system (GE Healthcare).
Caspase activity and inhibition
assays
To assess the activities of caspase-8, −9, −6, and
−3, quantitative colorimetric assays were performed with
caspase-specific kits (BioVision, Inc.) (34). Briefly, cells were treated with 100
ng/ml lexatumumab and/or 1 µM celastrol in a cell culture incubator
at 37°C for 3–24 h. Following treatment, cells were homogenized in
200 µl of cell lysis buffer, and then incubated on ice for 10 min.
Next, the lysate was centrifuged at 10,000 × g for 1 min at 4°C and
the supernatant collected to determine the protein concentrations.
For the assay procedure, 50 µl of cell lysate with 100 µg of total
protein, 50 µl of 2X reaction buffer, and 5 µl of a 4 mM
Asp-Glu-Val-Asp-pNA (cat. no. K113-100),
Val-Glu-lle-Asp-pNA (cat. no. K119-100),
lle-Glu-Thr-Asp-pNA (cat. no. K115-100), or
Leu-Glu-His-Asp-pNA (cat. no. K106-100) substrate (all from
BioVision, Inc.) were added to each well of a 96-well plate and
incubated at 37°C overnight. The A value of each well was
determined using a SPECTRAmax PLUS384 (Molecular Devices, LLC) at
405 nm.
Caspase inhibition assays were performed with the
caspase-8 inhibitor Z-LETD-FMK (cat. no. C8734), caspase-9
inhibitor Z-LEHD-FMK (cat. no. C1355), caspase-6 inhibitor
Z-VEID-FMK (cat. no. C1730), caspase-3 inhibitor Z-DQMD-FMK (cat.
no. C0480) (all from Sigma-Aldrich; Merck KGaA), or the general
caspase inhibitor Z-VAD-FMK (cat. no. HY-16658Bp; MedChemExpress),
or the human recombinant DR5:Fc chimeric protein (cat. no.
ALX-522-005-C050; Enzo Life Sciences, Inc.). Cells were pre-treated
with the respective caspase inhibitor (50 µM) or DR5:Fc chimeric
protein (1 µg/ml) for 1 h, and then exposed to 1 µM celastrol and
100 ng/ml lexatumumab for 6 h. Cell viability was assessed using a
CCK-8 kit.
Statistical analysis
Each experiment was performed at least three times,
and the results are presented as the mean ± SD. The data were
analyzed using one-way analysis of variance (ANOVA). In addition,
homogeneity of variance was tested, and post hoc multiple
comparisons tests were performed. Data with equal variances were
compared using Tukey's post hoc multiple comparisons test and data
with unequal variances were compared with Dunnett's T3 test.
Two-way ANOVA test was performed for the drug combination assays.
GraphPad Prism V8 for MacOS (Dotmatics) was used for all of the
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
Celastrol enhances lexatumumab-induced
cytotoxicity in RCC cells
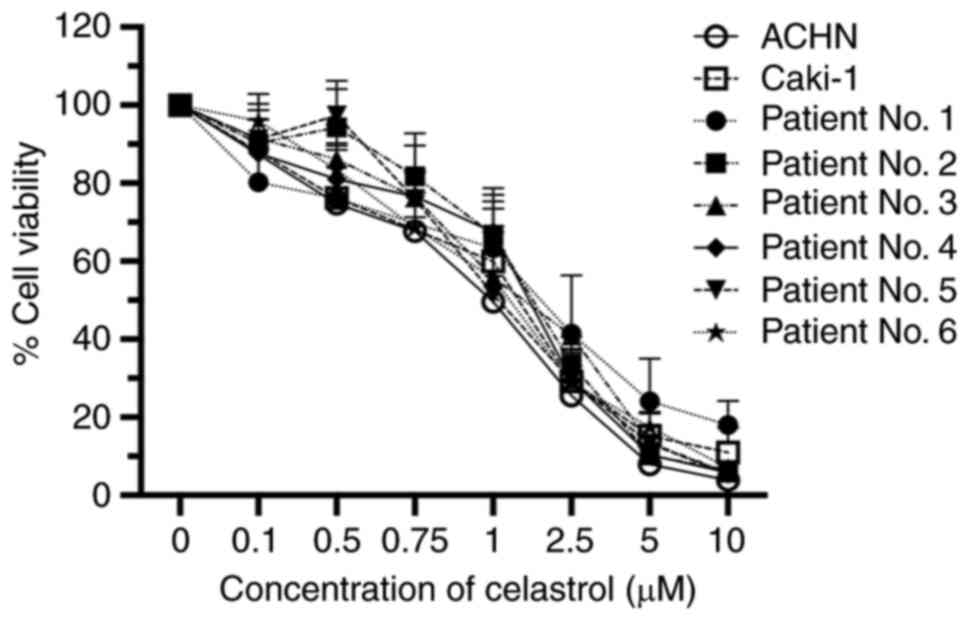
First, the effects of different concentrations of
celastrol on cell viability were examined using the human ACHN RCC
cell line. After a 24 h treatment period, celastrol suppressed cell
proliferation in a dose-dependent manner with a 50% inhibitory
concentration (IC50) of 1.099 µM. Similar cytotoxic
effects were noted in examinations of another RCC cell line,
Caki-1, as well as primary RCC cells obtained from 6 patients
(Fig. 1).
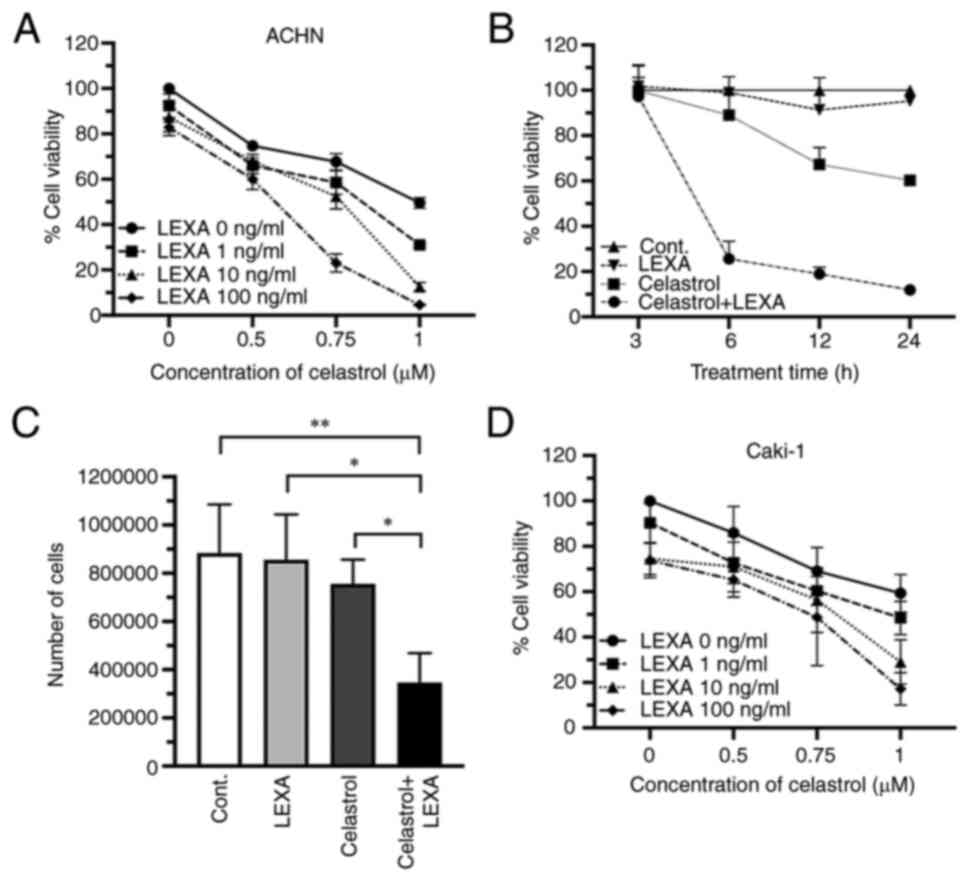
Next, the enhancement of lexatumumab-induced
cytotoxicity in RCC cells by celastrol was assessed. A significant
potentiation of cytotoxicity was achieved in ACHN cells 24 h after
treatment with a combination of lexatumumab and celastrol (Fig. 2A). Such an enhanced cytotoxic effect
was also observed when the lexatumumab and celastrol treatment
duration was shortened from 24 to 6 h, though there was no effect
when shortened further to 3 h (Fig.
2B). Treatment with the combination of lexatumumab and
celastrol led to a significant decrease in the number of ACHN cells
in trypan blue dye exclusion tests, whereas treatment with either
alone led to only a slight decrease (Fig. 2C). A similar enhanced cytotoxic
effect of lexatumumab and celastrol was observed in Caki-1 cells
(Fig. 2D).
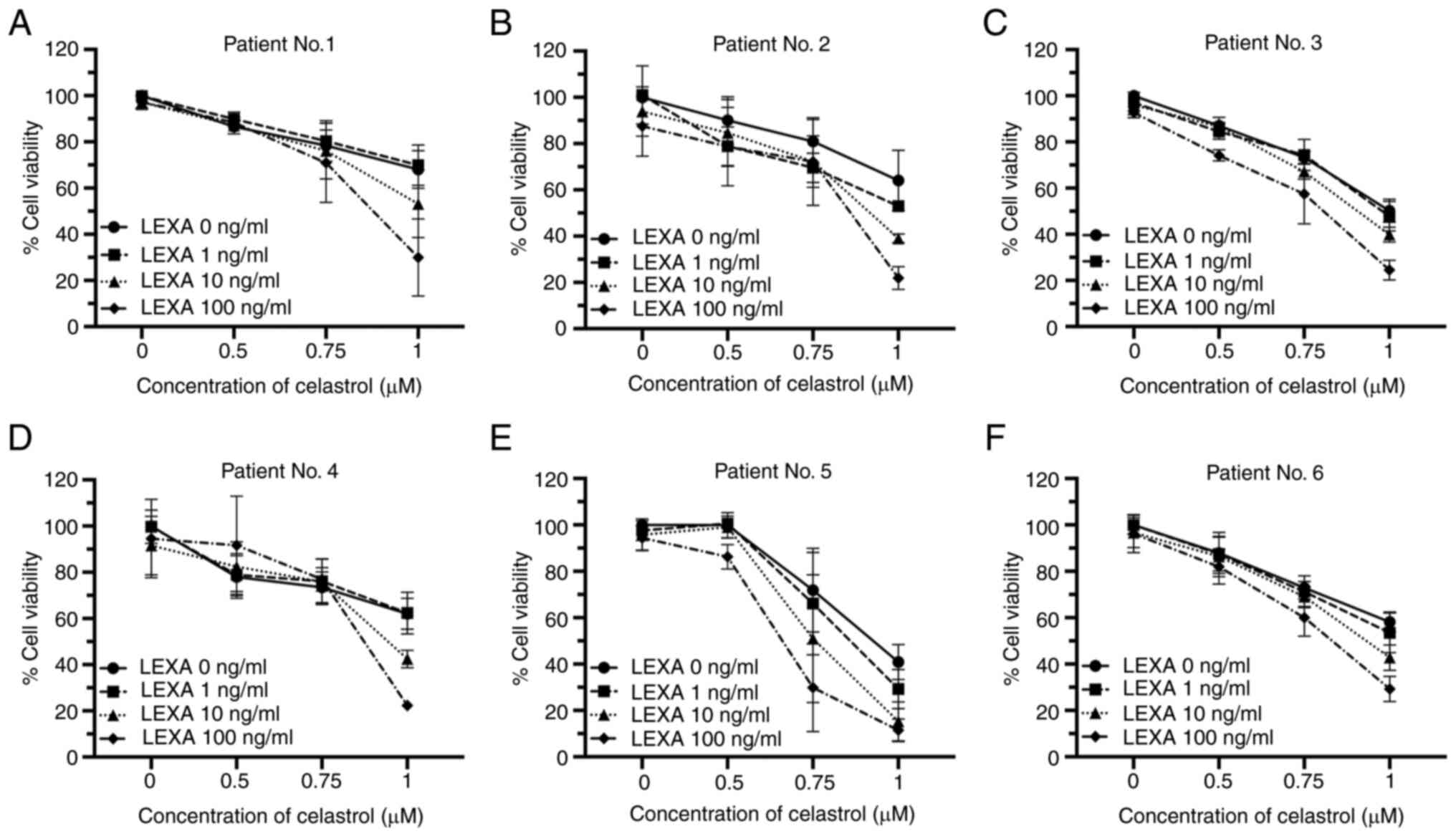
The cytotoxic effects of lexatumumab and celastrol
were also evaluated in primary RCC cells obtained from 6 patients.
In each case, significant enhancement of cytotoxic effects and
synergy was identified, regardless of the sensitivity of RCC cells
to celastrol or lexatumumab when each was used alone (Table I and Fig. 3A-F).
 | Table I.The CDI. |
Table I.
The CDI.
|
| Lexatumumab (100
ng/ml) |
|---|
|
|
|
|---|
| Renal cell
carcinoma cells | Celastrol 0.5
µM | Celastrol 1 µM |
|---|
| ACHN | 0.96825323 | 0.112518579 |
| Caki-1 | 0.76507638 | 0.391270559 |
| Patient No. 1 | 1.05027777 | 0.443204229 |
| Patient No. 2 | 0.995838373 | 0.394370361 |
| Patient No. 3 | 0.921209366 | 0.537397991 |
| Patient No. 4 | 1.249032099 | 0.394213415 |
| Patient No. 5 | 0.916766395 | 0.287956402 |
| Patient No. 6 | 0.974511663 | 0.522562291 |
Taken together, these findings clearly demonstrated
that treatment with lexatumumab and celastrol in combination
enhances cytotoxicity towards RCC cell lines and primary RCC
cells.
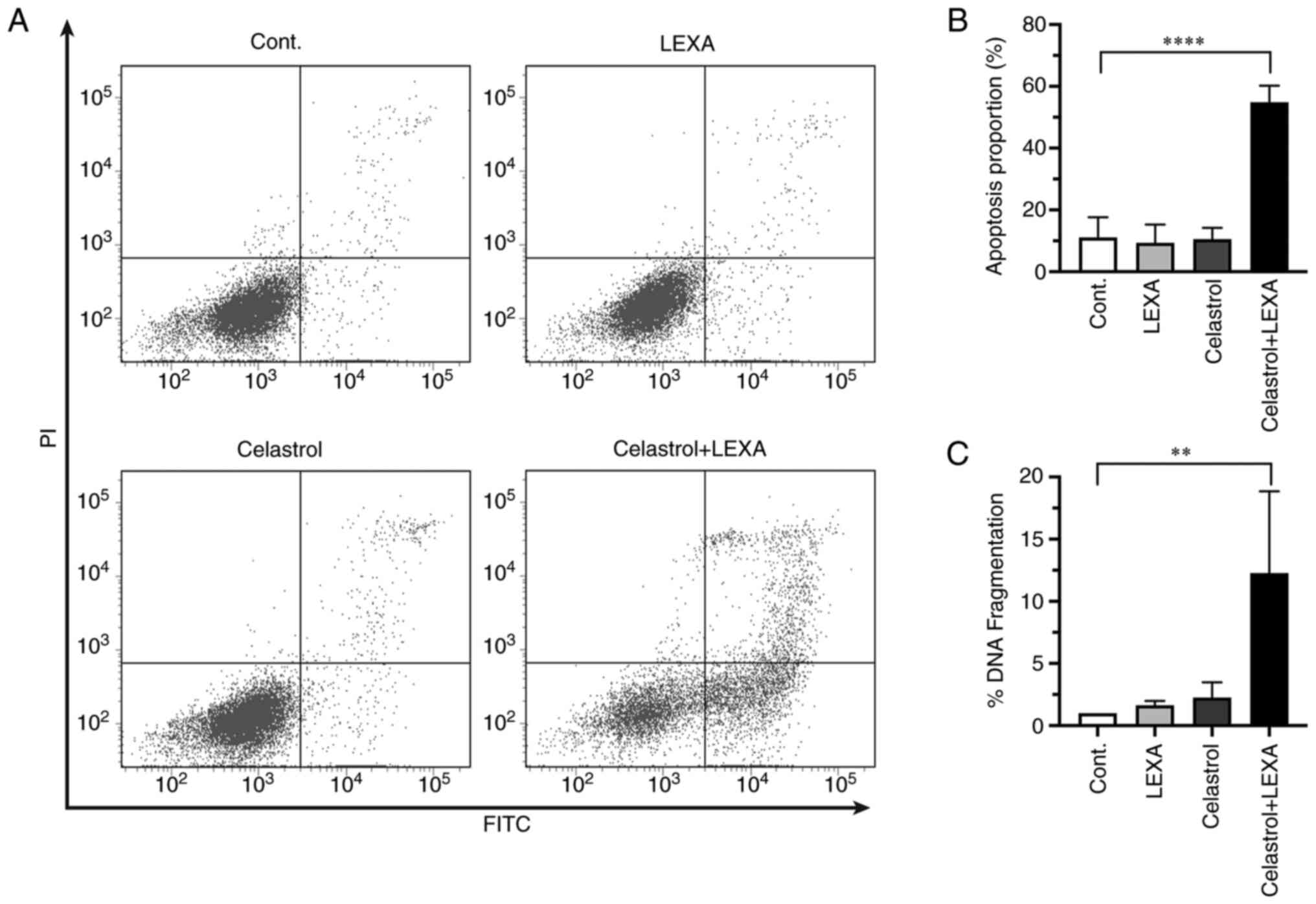
Induction of apoptosis
RCC cells were analyzed to improve evaluation of
whether the observed enhancement of cytotoxicity was mediated by
apoptosis. Enhanced apoptosis of cells treated with lexatumumab in
combination with celastrol was detected by both flow cytometry
(Fig. 4A and B), and a quantitative
apoptosis-specific ELISA kit (Fig.
4C). Thus, the enhanced cytotoxicity of lexatumumab and
celastrol was related to their ability to trigger apoptotic cell
death.
Synergistic cytotoxicity of
lexatumumab and celastrol is TRAIL-R2-dependent
Western blot analysis was performed to determine
whether expression of TRAIL-R2 was related to the sensitization of
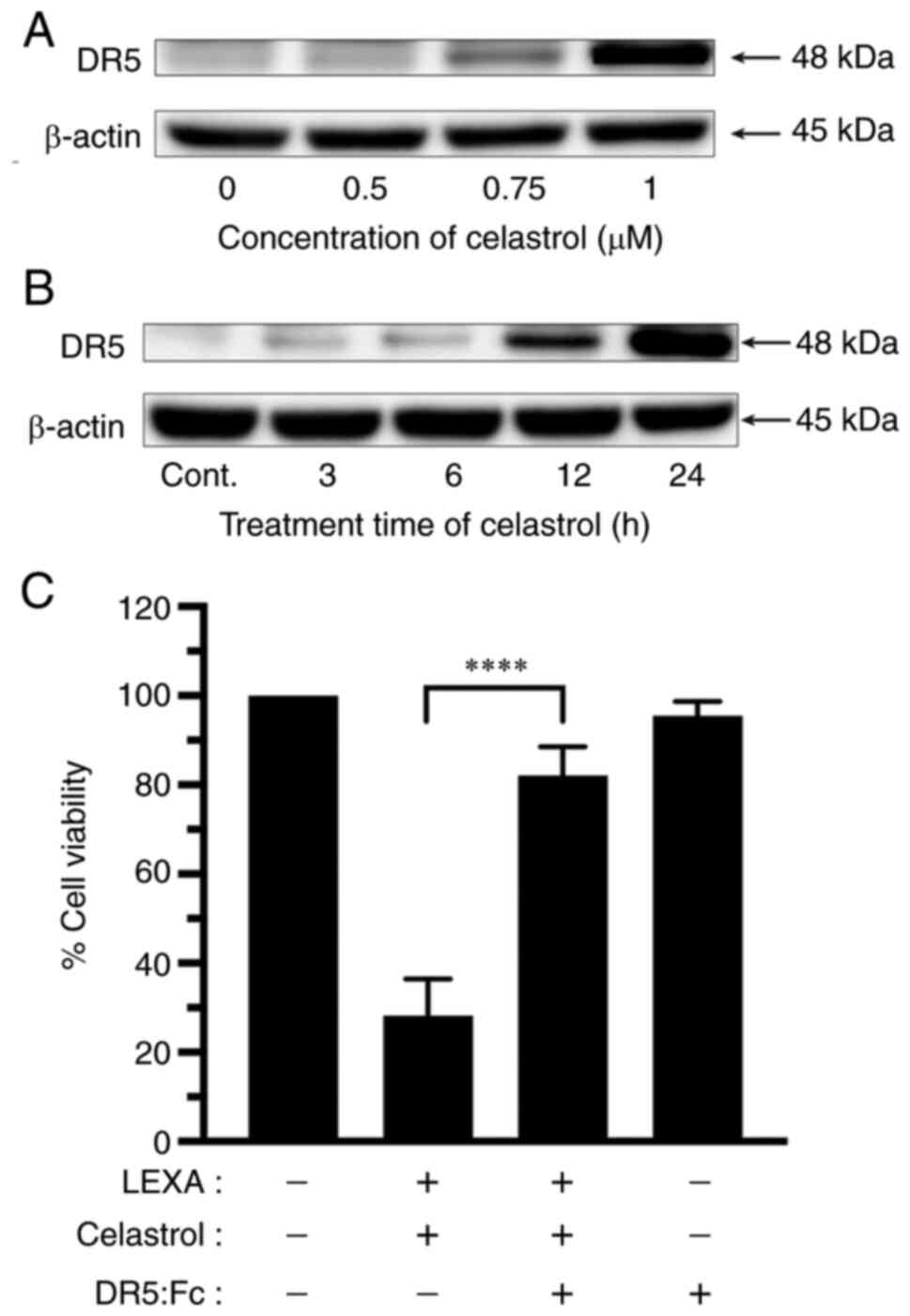
RCC cells to lexatumumab-induced apoptosis by celastrol. Celastrol
remarkably increased TRAIL-R2 expression in RCC cells in both a
dose- and time-dependent manner (Fig.
5A and B).
To further assess the underlying molecular mechanism
of enhanced cytotoxicity induced by the combination of lexatumumab
and celastrol, the authors examined the effects of a human
recombinant DR5:Fc chimeric protein (cat. no. ALX-522-005-C050;
Enzo Life Sciences, Inc.) known to have a dominant role negating
the cytotoxicity of TRAIL-R2. As demonstrated in Fig. 5C, celastrol-mediated enhancement of
lexatumumab-induced cytotoxicity was significantly inhibited when
the DR5:Fc chimeric protein was present.
These results indicated a TRAIL-R2-dependency of the
synergistic cytotoxicity and apoptosis that occurs with the
combination of lexatumumab and celastrol.
Effects of lexatumumab and celastrol
combination on expression of apoptotic compounds
The regulation of compounds related to
TRAIL-R2-mediated apoptosis were examined by the combination of
lexatumumab and celastrol in RCC cells using western blotting.
Expression of FADD, FLIP, Bid, Bax, Bcl-2 and AIF was not affected
when RCC cells were treated with celastrol and/or lexatumumab for 6
h. However, the combination treatment remarkably induced activation
of cleaved caspase-8, −9, −6, and −3 (Fig. 6A).
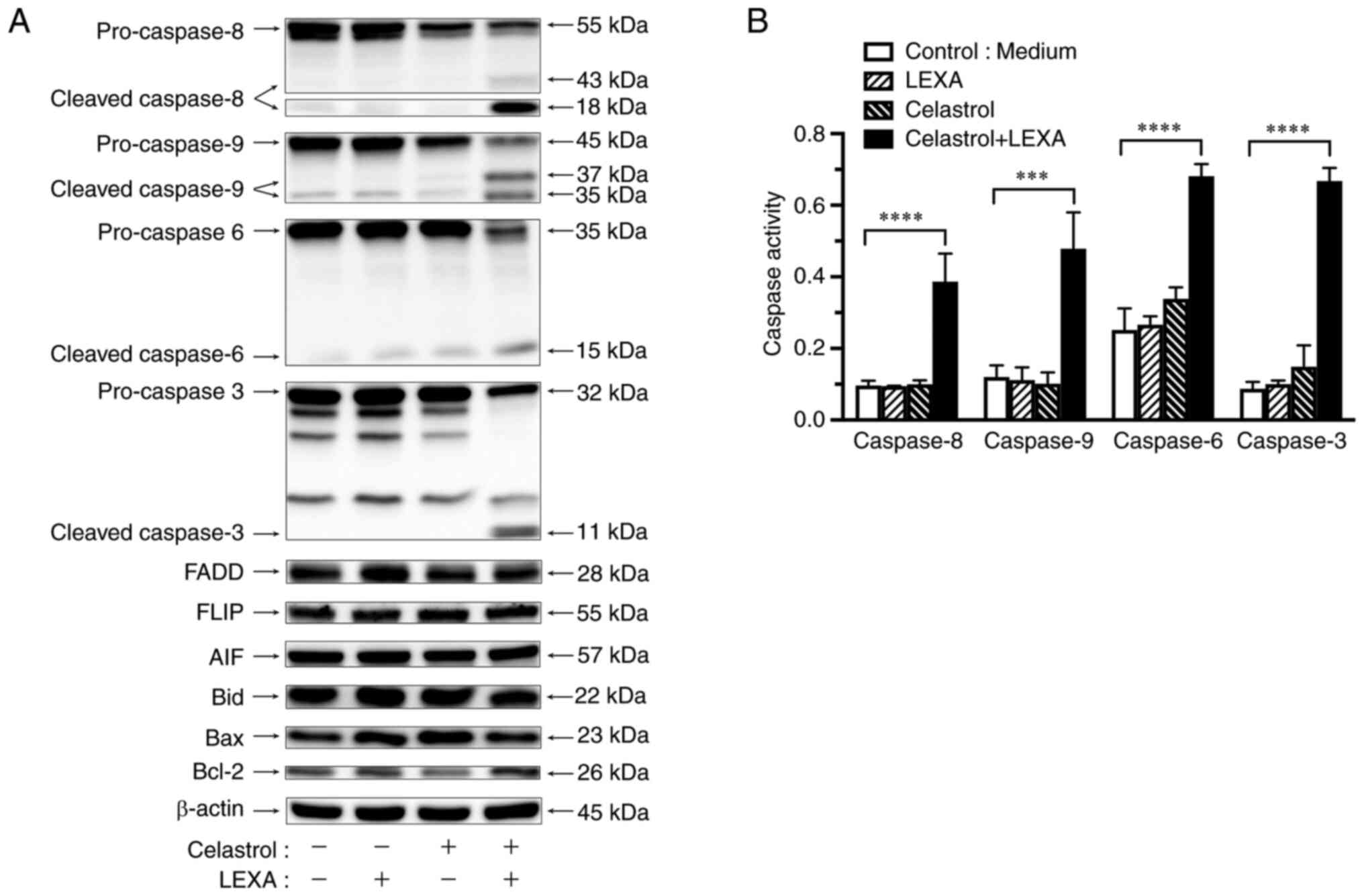 | Figure 6.Activation of caspases by lexatumumab
and celastrol. ACHN cells were treated with 100 ng/ml lexatumumab
alone, 1 µM celastrol alone, or the combination for 6 h. (A)
Expression of caspase-8, −9, −6, and −3, AIF, Bid, Bax and Bcl-2
was determined by western blot analysis. β-actin was used as
a loading control. (B) Activities of caspase-8, −9, −6 and −3 were
determined by colorimetric assay. Values are shown as the mean ± SD
of three individual experiments. ***P<0.001 and ****P<0.0001
vs. untreated control. LEXA, lexatumumab; Cont., control. |
Activation of caspase cascade by
lexatumumab and celastrol
Minor activation of caspase-6 and −3 in RCC cells
was observed following lexatumumab treatment, but with no
detectable activation of caspase-8 or −9. Exposure to celastrol
alone did not activate caspase-8 or −9, whereas activation of
caspase-6 and −3 was slightly induced, though their levels were
significantly lower than in treatment with the combination of
lexatumumab and celastrol. The combination induced remarkable
activation of caspase-8, −9, −6, and −3 (Fig. 6B).
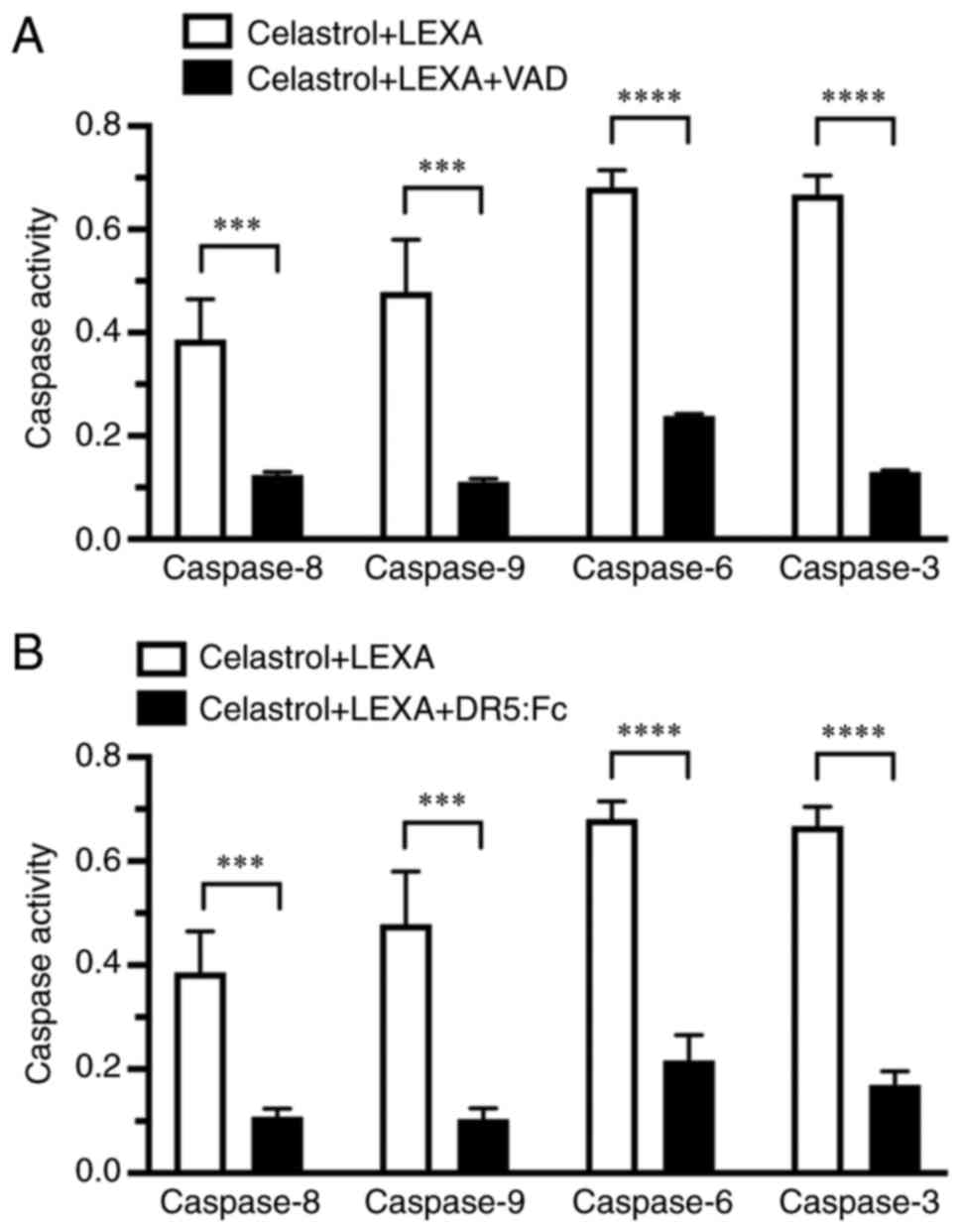
Suppressive effects of VAD and DR5:Fc
chimeric protein toward caspase activation
The activities of caspase-8, −9, −6 and −3 in cells
treated with lexatumumab in combination with celastrol in the
absence or presence of the general caspase inhibitor or DR5:Fc
chimeric protein were also examined. Caspase-8, −9, −6 and −3
activities in the cells, which were elevated by use of the drug
combination, were significantly suppressed by both Z-VAD-FMK
(Fig. 7A) and DR5:Fc chimeric
protein (Fig. 7B).
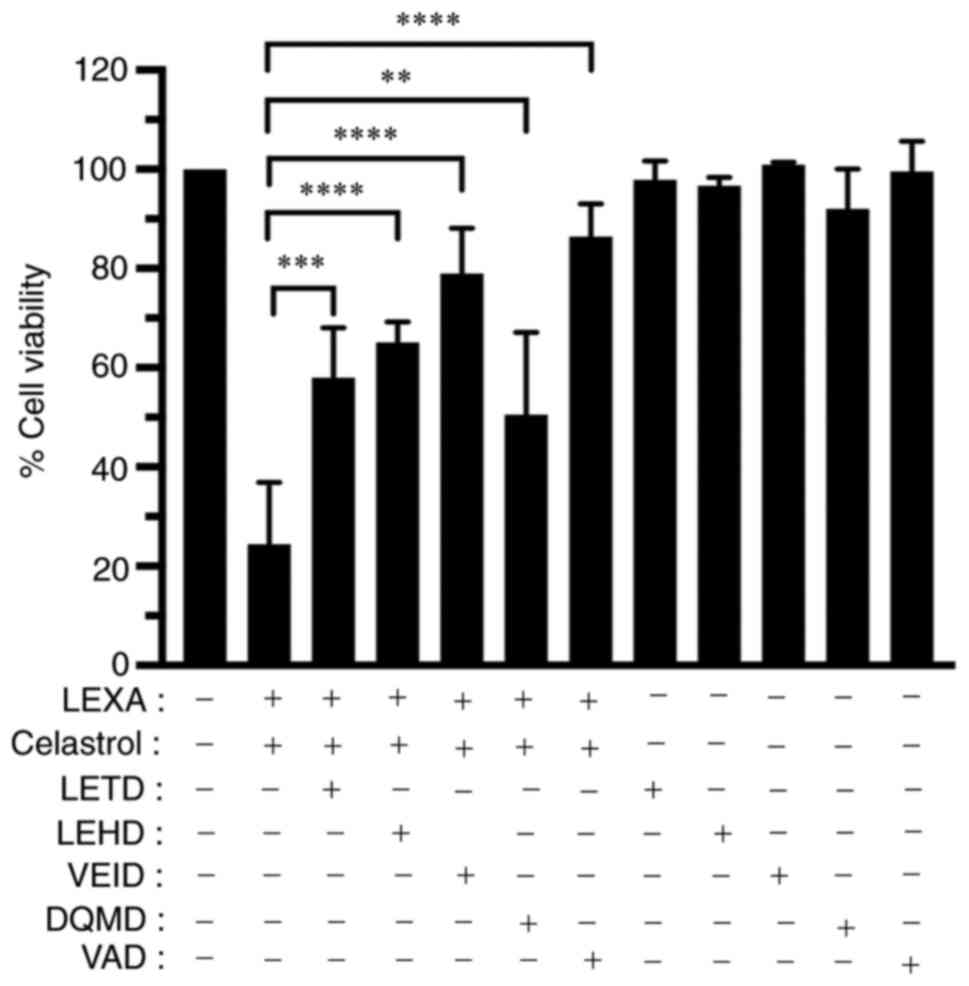
Caspase inhibitors inhibit synergistic
cytotoxic effect of lexatumumab and celastrol
To confirm mediation of the synergistic cytotoxic
effect of lexatumumab and celastrol through caspase activation, the
effects of specific inhibitors of caspase-8, −9, −6 and −3, as well
as a general caspase inhibitor, on cell death induced by
lexatumumab and celastrol, were examined. The findings showed that
cytotoxicity was significantly inhibited by the specific inhibitors
of caspase-8, −9, −6, and −3, as well as the general caspase
inhibitor (Fig. 8).
Discussion
From a clinical perspective, combination treatment
with lexatumumab and celastrol is promising. The present study
indicated that celastrol at a low concentration (0.5–1 µM) in
combination with lexatumumab exerts a synergistic effect not only
on human RCC cell lines, but also primary RCC cells derived from
patients. It was also revealed that TRAIL-R2-dependent induction of
apoptosis and caspase cascade activation, indicate synergistic
cytotoxicity of celastrol and lexatumumab. Interestingly, the
growth of RCC cells was significantly inhibited at 24 h by
treatment with 1 µM celastrol plus 100 ng/ml lexatumumab. A similar
result was achieved when the treatment duration was shortened to 6
h. These findings suggested that the synergistic cytotoxicity of
lexatumumab and celastrol is due to the induction of apoptosis via
upregulation of TRAIL-R2 expression and activation of the caspase
cascade.
Cell surface expression of TRAIL-R1 or TRAIL-R2
plays a crucial role in mediating TRAIL-induced apoptosis, though
the sensitivity of tumor cells expressing these death receptors to
TRAIL is not always consistent due to intracellular mechanisms
(35). Furthermore, the efficacy of
TRAIL correlated with levels of TRAIL-R1 and/or TRAIL-R2 on the
surface of leukemia cells (36). On
the other hand, previous studies have found no connection between
TRAIL receptor expression and the synergy of TRAIL and
chemotherapeutic drugs in examinations of certain cell lines
(37,38). The results of the present study
demonstrated that celastrol significantly upregulated TRAIL-R2
expression in RCC cells. The synergistic cytotoxicity of
lexatumumab and celastrol was also significantly inhibited by
DR5:Fc chimeric protein, which had a dominant negative function
against TRAIL-R2. The findings of the present study suggested that
lexatumumab and celastrol induce cytotoxicity and apoptosis in RCC
cells synergistically through upregulation of TRAIL-R2.
Caspases are essential protease mediators of
apoptosis known to be triggered by various stimuli, including TRAIL
(39,40), but it is difficult to examine
isolated TRAIL-mediated signal transduction because some receptors
complicate this transduction. Using lexatumumab, a specific
TRAIL-R2 mAb, the authors were able to specifically assess caspase
involvement in TRAIL-R2-mediated apoptosis. The findings of the
present study demonstrated that lexatumumab and celastrol in
combination significantly activate initiative caspases, such as
caspase-8 and −9, and effective caspases, including caspase-6 and
−3. The elevated activities of caspase-8, −9, −6 and −3 in cells
exposed to the drug combination were significantly suppressed by
the DR5:Fc chimeric protein and general caspase inhibitor
Z-VAD-FMK, whereas the synergistic cytotoxicity of lexatumumab and
celastrol was inhibited considerably by specific inhibitors of
caspase-8, −9, −6 and −3, as well as a general caspase inhibitor.
Taken together, these findings indicated that the caspase cascade
comprised molecules downstream of death receptors plays a crucial
role in the synergistic cytotoxicity of lexatumumab and celastrol
in RCC cells.
A previous study demonstrated that celastrol
enhances TRAIL-mediated apoptosis through upregulation of TRAIL-R2
and the activation of caspase-8 and −3 in human glioblastoma cells,
whereas celastrol has no effect on TRAIL-mediated apoptosis in
normal human astroglial cells (27). Another study found that simultaneous
administration of TRAIL and celastrol had an anticancer effect
against human colorectal tumors in nude mice (26). The results of the present study
indicated that lexatumumab and celastrol have a synergistic effect
not only on human RCC cell lines, but also primary RCC cells. Thus,
treatment of RCC with the combination of celastrol and lexatumumab
is promising for potential clinical application.
The toxicity of celastrol exposure to normal kidney
cells is a concern. Consequently, a method to alleviate the
toxicity of celastrol is needed. Some studies have demonstrated
that low concentrations of celastrol enhance TRAIL-induced
apoptosis in some tumor cells via the death receptor pathway,
indicating a potential strategy to effectively reduce associated
side effects (25–27). In the present study, the authors
focused on how to reduce the cytotoxicity of celastrol through
combination with lexatumumab in RCC cells. Further studies will be
needed to clarify the cytotoxic effect of celastrol in combination
with lexatumumab on normal kidney cells.
In conclusion, the results obtained in the present
study clearly indicated that administration of lexatumumab with
celastrol has cytotoxic effects on both human RCC cell lines and
primary RCC cells, particularly at a low concentration of celastrol
(0.5–1 µM). This synergistic cytotoxicity of lexatumumab and
celastrol is due to induction of apoptosis by the upregulation of
TRAIL-R2 expression and activation of the caspase cascade. Thus,
the use of celastrol in combination with lexatumumab may be a novel
therapeutic strategy for treatment of RCC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in the current published article.
Authors' contributions
YB, YKi and YKa performed the cell proliferation and
western blot assays, and statistical analysis of the data obtained
in all of the experiments. TK and AK performed the trypan blue
staining analysis. SY and XW planned, analyzed and interpreted all
of the experiments and validity of the data, and drafted the
manuscript. All authors read and approved the final manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work and data are
appropriately investigated and resolved. YB and XW confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Ethical approval for the use of human tissue was
granted by Hyogo College of Medicine (approval no. 202306; Hyogo,
Japan). All patients provided individual written informed consent
for the use of their sampled tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albiges L, Oudard S, Negrier S, Caty A,
Gravis G, Joly F, Duclos B, Geoffrois L, Rolland F, Guillot A, et
al: Complete remission with tyrosine kinase inhibitors in renal
cell carcinoma. J Clin Oncol. 30:482–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Powles T, Burotto M, Escudier
B, Bourlon MT, Shah AY, Suárez C, Hamzaj A, Porta C, Hocking CM, et
al: Nivolumab plus cabozantinib versus sunitinib in first-line
treatment for advanced renal cell carcinoma (CheckMate 9ER):
Long-term follow-up results from an open-label, randomised, phase 3
trial. Lancet Oncol. 23:888–898. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ, Rini BI, McDermott DF, Arén
Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B,
Beuselinck B, Amin A, et al: Nivolumab plus ipilimumab versus
sunitinib in first-line treatment for advanced renal cell
carcinoma: Extended follow-up of efficacy and safety results from a
randomised, controlled, phase 3 trial. Lancet Oncol. 20:1370–1385.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Powles T, Plimack ER, Soulières D, Waddell
T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko
I, et al: Pembrolizumab plus axitinib versus sunitinib monotherapy
as first-line treatment of advanced renal cell carcinoma
(KEYNOTE-426): Extended follow-up from a randomised, open-label,
phase 3 trial. Lancet Oncol. 21:1563–1573. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chuntharapai A, Dodge K, Grimmer K,
Schroeder K, Marsters SA, Koeppen H, Ashkenazi A and Kim KJ:
Isotype-dependent inhibition of tumor growth in vivo by monoclonal
antibodies to death receptor 4. J Immunol. 166:4891–4898. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ichikawa K, Liu W, Zhao L, Wang Z, Liu D,
Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberly RP and Zhou T:
Tumoricidal activity of a novel anti-human DR5 monoclonal antibody
without hepatocyte cytotoxicity. Nat Med. 7:954–960. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng Y, Wu XX, Fiscella M, Shimada O,
Humphreys R, Albert V and Kakehi Y: Monoclonal antibody to tumor
necrosis factor-related apoptosis-inducing ligand receptor 2
(TRAIL-R2) induces apoptosis in primary renal cell carcinoma cells
in vitro and inhibits tumor growth in vivo. Int J Oncol.
28:421–430. 2006.PubMed/NCBI
|
|
12
|
Shimada O, Wu X, Jin X, Nouh MA, Fiscella
M, Albert V, Matsuda T and Kakehi Y: Human agonistic antibody to
tumor necrosis factor-related apoptosis-inducing ligand receptor 2
induces cytotoxicity and apoptosis in prostate cancer and bladder
cancer cells. Urology. 69:395–401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szliszka E, Mazur B, Zydowicz G, Czuba ZP
and Król W: TRAIL-induced apoptosis and expression of death
receptor TRAIL-R1 and TRAIL-R2 in bladder cancer cells. Folia
Histochem Cytobiol. 47:579–585. 2009.PubMed/NCBI
|
|
14
|
Wu XX, Jin XH, Zeng Y, El Hamed AM and
Kakehi Y: Low concentrations of doxorubicin sensitizes human solid
cancer cells to tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL)-receptor (R) 2-mediated apoptosis by inducing
TRAIL-R2 expression. Cancer Sci. 98:1969–1976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Allison AC, Cacabelos R, Lombardi VR,
Alvarez XA and Vigo C: Celastrol, a potent antioxidant and
anti-inflammatory drug, as a possible treatment for Alzheimer's
disease. Prog Neuropsychopharmacol Biol Psychiatry. 25:1341–1357.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang H, Chen D, Cui QC, Yuan X and Dou QP:
Celastrol, a triterpene extracted from the Chinese ‘Thunder of God
Vine,’ is a potent proteasome inhibitor and suppresses human
prostate cancer growth in nude mice. Cancer Res. 66:4758–4765.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Liu X, Zhao P, Zhao H, Gao W and
Wang L: Celastrol suppresses glioma vasculogenic mimicry formation
and angiogenesis by blocking the PI3K/Akt/mTOR signaling pathway.
Front Pharmacol. 11:252020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen G, Zhu X, Li J, Zhang Y, Wang X,
Zhang R, Qin X, Chen X, Wang J, Liao W, et al: Celastrol inhibits
lung cancer growth by triggering histone acetylation and acting
synergically with HDAC inhibitors. Pharmacol Res. 185:1064872022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ni H, Han Y and Jin X: Celastrol inhibits
colon cancer cell proliferation by downregulating miR-21 and
PI3K/AKT/GSK-3β pathway. Int J Clin Exp Pathol. 12:808–816.
2019.PubMed/NCBI
|
|
20
|
Wang X, Liu Q, Wu S, Xu N, Li H and Feng
A: Identifying the effect of celastrol against ovarian cancer with
network pharmacology and in vitro experiments. Front Pharmacol.
13:7394782022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JH, Won YS, Park KH, Lee MK, Tachibana
H, Yamada K and Seo KI: Celastrol inhibits growth and induces
apoptotic cell death in melanoma cells via the activation
ROS-dependent mitochondrial pathway and the suppression of PI3K/AKT
signaling. Apoptosis. 17:1275–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li HY, Zhang J, Sun LL, Li BH, Gao HL, Xie
T, Zhang N and Ye ZM: Celastrol induces apoptosis and autophagy via
the ROS/JNK signaling pathway in human osteosarcoma cells: An in
vitro and in vivo study. Cell Death Dis. 6:e16042015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang CJ, Zhu N, Long J, Wu HT, Wang YX,
Liu BY, Liao DF and Qin L: Celastrol induces lipophagy via the
LXRα/ABCA1 pathway in clear cell renal cell carcinoma. Acta
Pharmacol Sin. 42:1472–1485. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu H, Zhao H, Ding C, Jiang D, Zhao Z, Li
Y, Ding X, Gao J, Zhou H, Luo C, et al: Celastrol suppresses
colorectal cancer via covalent targeting peroxiredoxin 1. Signal
Transduct Target Ther. 8:512023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu H, Ding WJ, Wu R, Weng QJ, Lou JS, Jin
RJ, Lu W, Yang B and He QJ: Synergistic anti-cancer activity by the
combination of TRAIL/APO-2L and celastrol. Cancer Invest. 28:23–32.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cha Z, Cheng J, Xiang H, Qin J, He Y, Peng
Z, Jia J and Yu H: Celastrol enhances TRAIL-induced apoptosis in
human glioblastoma via the death receptor pathway. Cancer Chemother
Pharmacol. 84:719–728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen SR, Dai Y, Zhao J, Lin L and Wang Y
and Wang Y: A mechanistic overview of triptolide and celastrol,
natural products from Tripterygium wilfordii Hook F. Front
Pharmacol. 9:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pukac L, Kanakaraj P, Humphreys R,
Alderson R, Bloom M, Sung C, Riccobene T, Johnson R, Fiscella M,
Mahoney A, et al: HGS-ETR1, a fully human TRAIL-receptor 1
monoclonal antibody, induces cell death in multiple tumour types in
vitro and in vivo. Br J Cancer. 92:1430–1441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luster TA, Carrell JA, McCormick K, Sun D
and Humphreys R: Mapatumumab and lexatumumab induce apoptosis in
TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when
treated in combination with bortezomib. Mol Cancer Ther. 8:292–302.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marini P, Denzinger S, Schiller D, Kauder
S, Welz S, Humphreys R, Daniel PT, Jendrossek V, Budach W and Belka
C: Combined treatment of colorectal tumours with agonistic TRAIL
receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy:
Enhanced effects in vitro and dose-dependent growth delay in vivo.
Oncogene. 25:5145–5154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizutani Y, Bonavida B, Fukumoto M and
Yoshida O: Enhanced susceptibility of c-myc antisense
oligonucleotide-treated human renal cell carcinoma cells to lysis
by peripheral blood lymphocytes. J Immunother Emphasis Tumor
Immunol. 17:78–87. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bao Y, Wu X, Jin X, Kanematsu A, Nojima M,
Kakehi Y and Yamamoto S: Apigenin inhibits renal cell carcinoma
cell proliferation through G2/M phase cell cycle arrest. Oncol Rep.
47:602022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu SP, Sun GP, Shen YX, Peng WR, Wang H
and Wei W: Synergistic effect of combining paeonol and cisplatin on
apoptotic induction of human hepatoma cell lines. Acta Pharmacol
Sin. 28:869–878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu XX, Kakehi Y, Mizutani Y, Lu J, Terachi
T and Ogawa O: Activation of caspase-3 in renal cell carcinoma
cells by anthracyclines or 5-fluorouracil. Int J Oncol. 19:19–24.
2001.PubMed/NCBI
|
|
35
|
Amantana A, London CA, Iversen PL and Devi
GR: X-linked inhibitor of apoptosis protein inhibition induces
apoptosis and enhances chemotherapy sensitivity in human prostate
cancer cells. Mol Cancer Ther. 3:699–707. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Q, Hilsenbeck S and Gazitt Y: Arsenic
trioxide-induced apoptosis in myeloma cells: p53-dependent G1 or
G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and
synergy with APO2/TRAIL. Blood. 101:4078–4087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galligan L, Longley DB, McEwan M, Wilson
TR, McLaughlin K and Johnston PG: Chemotherapy and TRAIL-mediated
colon cancer cell death: The roles of p53, TRAIL receptors, and
c-FLIP. Mol Cancer Ther. 4:2026–2036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lacour S, Hammann A, Wotawa A, Corcos L,
Solary E and Dimanche-Boitrel MT: Anticancer agents sensitize tumor
cells to tumor necrosis factor-related apoptosis-inducing
ligand-mediated caspase-8 activation and apoptosis. Cancer Res.
61:1645–1651. 2001.PubMed/NCBI
|
|
39
|
Derosier LC, Vickers SM, Zinn KR, Huang Z,
Wang W, Grizzle WE, Sellers J, Stockard CR Jr, Zhou T, Oliver PG,
et al: TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce
apoptosis and inhibit radiologically validated orthotopic
pancreatic tumor growth. Mol Cancer Ther. 6:3198–3207. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
O'Kane HF, Watson CJ, Johnston SR, Petak
I, Watson RW and Williamson KE: Targeting death receptors in
bladder, prostate and renal cancer. J Urol. 175:432–438. 2006.
View Article : Google Scholar : PubMed/NCBI
|