Introduction
Renal cell carcinoma (RCC) is a renal malignancy
originating from the epithelium of the renal tubules of nephrons,
which accounts for 2–3% of adult malignancies and 80–90% of adult
renal malignancies globally (1,2). RCC
is often asymptomatic until its advanced stages, with ~30% of
patients diagnosed at the metastatic stage (3). Despite being the third most common
urogenital malignancy after prostate and bladder cancer, RCC has a
high mortality rate (4), and its
incidence and mortality rates are increasing worldwide
annually.
Clear cell RCC (ccRCC) is the predominant
histological subtype of RCC, accounting for ~75% of all RCC cases
(2). The current treatment for
localized RCC is partial or radical nephrectomy. However, advanced
RCC is resistant to chemotherapy and radiotherapy, making treatment
challenging (5,6). Recently, molecular targeted drugs and
immune checkpoint inhibitors (ICIs) have emerged as significant
therapeutic options in urological oncology. Randomized controlled
trials have demonstrated the efficacy and safety of combination
immunotherapy, particularly with ICIs and molecular targeted drugs
(tyrosine kinase and multikinase inhibitors), as a first-line
treatment for advanced RCC (7).
Although some patients with RCC (5–15%) achieve complete remission,
30–60% do not respond to treatment, and no predictive markers
currently exist to determine which patients will respond.
Consequently, a number of patients experience disease progression
and eventually succumb to the cancer.
Genomic studies have revealed that >90% of human
genes are actively transcribed, but only 2% encode proteins. This
highlights the prevalence of non-coding RNAs (ncRNAs) in the genome
(8,9). ncRNAs are categorized based on size
into small ncRNAs and long ncRNAs (lncRNAs), with lncRNAs being
>200 nucleotides in length. Although small ncRNAs, such as
microRNAs (miRNAs/miRs), have been extensively studied, and their
roles in gene regulation and cellular processes in various cancer
types are well-documented (9),
lncRNAs have more recently garnered attention for their significant
roles in normal development and disease, including cancer (10). lncRNAs are involved in various
biological processes, such as epigenetic regulation, nuclear
import, cell cycle control, nuclear and cytoplasmic trafficking,
imprinting, cell differentiation, alternative splicing, RNA decay,
and transcription and translation (11,12).
Consequently, lncRNAs have emerged as serving a critical role in
cancer research, with studies indicating that certain lncRNAs can
function as oncogenes, tumor suppressors or both, depending on the
context (13–15).
lncRNAs are crucial for regulating immune cell
activation and the tumor microenvironment, potentially enhancing
the efficacy of immunotherapy by modulating the expression and
function of immune checkpoint molecules (16,17).
For example, AGAP2-AS1 can polarize M0 macrophages to M2, promoting
RCC cell proliferation, invasion and migration (18). Additionally, some lncRNAs regulate
immune checkpoint expression in tumor immunity and can predict
response to ICIs (19).
According to the competing endogenous RNA (ceRNA)
hypothesis, lncRNAs bind to miRNAs and compete with mRNAs that
share similar miRNA response elements, thereby influencing the
expression of miRNA target genes. Various ceRNAs have been
identified, demonstrating their ability to sequester miRNAs or
block miRNA-mRNA interactions, ultimately leading to the enhanced
stability and expression of mRNAs (17).
Several lncRNAs (GAS5, MEG3/GTL2, HIF-1α-AS1, H19,
KCQN1OT1, MALAT1 and HOTAIR) have been implicated in renal cancer
(20–27). However, numerous lncRNAs remain
uncharacterized. In the present study, the lncRNA expression in RNA
extracted from the surgical specimens of patients who responded and
did not respond to ICI therapy was initially analyzed. The lncRNA
leucine-rich repeat containing 75 A-antisense RNA1 (LRRC75A-AS1)
has been proposed as a potential prognostic marker in RCC treatment
based on its expression patterns in patients with differing
responses to ICI therapy.
It has previously been reported that LRRC75A-AS1 is
located on chromosome 17 and predominantly acts in the cytoplasm
(28). Subcellular fractionation
data have indicated that cytoplasmic lncRNAs primarily function by
suppressing miRNAs. Notably, previous studies have only examined
the role of LRRC75A-AS1 in colorectal cancer, cervical cancer,
triple-negative breast cancer and neuroblastoma (29–32);
however, its mechanisms and association with ICI responses remain
unclear, including in RCC.
The present study aimed to investigate whether the
high expression of LRRC75A-AS1 in RCC is associated with
responsiveness to ICI therapy or RCC progression, thereby affecting
prognosis. The role of LRRC75A-AS1 as a novel biomarker was
examined and functional analyses were conducted using immortalized
RCC cell lines. To the best of our knowledge, the present study is
the first to suggest that LRRC75A-AS1 may serve as a novel
biomarker for RCC, influencing cancer invasion and metastasis.
Materials and methods
Clinical samples
The present study included 212 patients who
underwent partial or radical nephrectomy at Yamaguchi University
Hospital (Ube, Japan) between October 2005 and December 2022. Tumor
tissue samples were collected from all patients, while adjacent
normal tissue samples were collected from a subset of patients
(n=25) for further analysis. All patients were pathologically
diagnosed with ccRCC. Blood samples were collected preoperatively
and 7 days postoperatively for the analysis of clinical data, such
as neutrophil-to-lymphocyte ratio (NLR). NLR was calculated by
dividing the absolute neutrophil count by the absolute lymphocyte
count. Among these patients, 10 underwent ICI therapy, with renal
cancer tissue samples from five effective and five ineffective
patients available for analysis. The detailed patient
characteristics are presented in Table
I.
 | Table I.Characteristics of 212 patients who
underwent partial or radical nephrectomy. |
Table I.
Characteristics of 212 patients who
underwent partial or radical nephrectomy.
| Variable | Value |
|---|
| Sex, n (%) |
|
|
Male | 139 (65.6) |
|
Female | 73 (34.4) |
| Age, years |
|
|
Median | 67 |
|
Range | 28-92 |
| Pathological T
stage, n (%) |
|
| 1a | 124 (58.5) |
| 1b | 31 (14.6) |
| 2a | 10 (4.7) |
| 3a | 41 (19.3) |
| 3b | 5 (2.4) |
| 4 | 1 (0.5) |
| Fuhrman grade |
|
| G
1/2 | 184 (86.8) |
| G
3/4 | 28 (13.2) |
| Follow-up duration,
months |
|
|
Median | 37.1 |
|
Range | 0.6–194.6 |
| Progression |
|
| No | 178 (84.0) |
|
Yes | 34 (16.0) |
| Overall
survival |
|
| No | 193 (91.0) |
|
Yes | 19 (9.0) |
lncRNA expression profiling using
microarray
To identify lncRNAs that may predict the efficacy of
ICI therapy in patients with ccRCC, a Human V5.0 LncRNA Array
Service (cat. no. AS-S-LNC-H; ArrayStar Inc.) was performed on
formalin-fixed paraffin-embedded (FFPE) samples (4 µm; fixed in 10%
neutral-buffered formalin at room temperature for 48 h) from
patients who continued ICI therapy for 2 years (n=2) and those who
experienced disease progression within 6 months after ICI therapy
(n=2). After nephrectomy, samples of renal cancer tissues were
collected from all 212 patients, while adjacent normal renal
tissues were obtained from a subset of 25 patients. One part was
stored at −80°C in RNAlater to stabilize and protect RNA through
immediate RNase inactivation, while the other part was processed
into FFPE blocks. The FFPE sections were stained with hematoxylin
and eosin using the Tissue-Tek Prisma Plus system (Sakura Finetek
Japan). Hematoxylin staining was performed at room temperature for
8 min, followed by eosin staining for 6 min, according to the
standard protocol. The sections were then microdissected to
identify cancerous and normal tissues. An optical microscope (Nikon
Corporation) was used for the observation. RNA was extracted from
each of these microdissected tissues using the Maxwell®
RSC simplyRNA Tissue Kit (cat. no. AS1340; Promega Corporation).
The total RNA concentration was measured using NanoDrop One (Thermo
Fisher Scientific, Inc.). Total RNA purity was confirmed by
measuring the RNA integrity number equivalent. Using 100 ng total
RNA, the samples were processed for hybridization onto the
Arraystar Human LncRNA Expression Array V5.0 platform. This
platform covers 39,317 well-annotated lncRNAs. The extracted RNA
was labeled with Cy3 using the Arraystar RNA Labeling Kit
(Arraystar Inc), and then hybridized onto the lncRNA microarray
slides according to the manufacturer's protocol. After
hybridization, the slides were washed and scanned using the Agilent
G2565CA Microarray Scanner System (Agilent Technologies, Inc.). The
raw signal intensities were extracted using Agilent Feature
Extraction software (v11.0.1.1; Agilent Technologies, Inc.) and
data analysis was performed using GeneSpring GX software (v12.1;
Agilent Technologies, Inc.). The raw data were normalized using the
quantile normalization method, and differentially expressed lncRNAs
were identified by comparing the two patient groups. Statistical
significance was determined using an unpaired Student's t-test, and
lncRNAs with a fold change >1.8 and a P<0.5 were considered
significantly differentially expressed.
Cell culture
Renal cancer cell lines [769-P (ATCC no. CRL-1,933),
786-O (ATCC no. CRL-1,932), ACHN (ATCC no. CRL-1,611) and A498
(ATCC no. HTB-44)] were purchased from American Type Culture
Collection. The 769-P, 786-O, ACHN and A498 cell lines were
cultured in Roswell Park Memorial Institute 1640 medium (Gibco;
Thermo Fisher Scientific, Inc.), supplemented with 10% fetal bovine
serum (cat. no. 172012; Nichirei Biosciences Inc.), and penicillin
G (100 U/ml) and streptomycin sulfate (0.1 mg/ml) (cat. no. A5955;
Sigma-Aldrich; Merck KGaA), and were maintained in a humidified
incubator at 37°C with 5% CO2.
Total RNA extraction from tissues and
cells
Total RNA was extracted from human renal cancer
tissues and adjacent non-cancerous normal renal tissues using the
miRNeasy FFPE Kit (Qiagen GmbH) after pathological examination and
the FFPE method. Additionally, RNA (miRNA and total RNA) was
extracted from frozen tissues and RCC cell lines using the
PureLink® RNA Mini Kit (Invitrogen; Thermo Fisher
Scientific Inc.) according to the manufacturer's protocol.
cDNA synthesis
cDNA was synthesized from the extracted RNA by
reverse transcription (RT) using the PrimeScript RT Reagent Kit
(Takara Biotechnology Co., Ltd.), following the manufacturer's
instructions, and was subsequently used for quantitative
(q)PCR.
Knockdown of LRRC75A-AS1 in RCC
cells
The 769-P and 786-O cells were transfected with
LRRC75A-AS1 small interfering (si)RNA (si-LRRC75A-AS1; cat. no.
n547418) or a negative control siRNA [si-negative control (si-NC);
cat. no. 4390843] (both from Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. Briefly, the cells were
grown in six-well plates at a density of 0.25–1×106
cells/well and transfected individually with si-LRRC75A-AS1 at a
concentration of 50 pmol/well. The transfection was performed at
37°C and incubated for 72 h. The effect of si-LRRC75A-AS1 knockdown
was examined by reverse transcription (RT)-qPCR using RNA extracted
48 h after transfection. Cell viability was assessed 24, 48, and 72
h after transfection, and invasion 48 h after transfection.
Transfection was performed using Lipofectamine™ RNAiMAX
Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions.
Cell viability and cell invasion
assays
Cell viability was assessed using the MTS assay
(CellTiter 96 AQueous One Solution Cell Proliferation Assay;
Promega Corporation) according to the manufacturer's protocol.
Measurements were taken at 24, 48 and 72 h after plating by
determining the optical density (OD) at 490 nm. The OD measurements
were conducted in triplicate.
Cell invasion was assessed using the CytoSelect
24-well Cell Invasion Assay Kit (Cell Biolabs Inc.) on a 24-well
Transwell plate (pore size, 8 µm). The chambers were coated with
Matrigel at room temperature for 1 h. The transfected cells were
transferred to the upper chamber in triplicate at a cell density of
0.5–1.0×106 cells/ml; a total of 300 µl cell suspension
was added to each well, resulting in 1.5–3.0×105
cells/well. After 48 h of incubation at 37°C (5% CO2),
the cells that migrated through the membrane were stained.
Extraction was performed using the Extraction Solution from the
kit, and the results were expressed as the number of invaded cells.
Quantification was performed by measuring the OD at 560 nm using a
plate reader, according to the manufacturer's instructions.
qPCR
qPCR was performed in triplicate using the Applied
Biosystems StepOnePlus and TaqMan Universal PCR Master Mix (both
from Applied Biosystems; Thermo Fisher Scientific, Inc.), according
to the manufacturer's protocols. TaqMan probes and primers were
also purchased from Applied Biosystems; Thermo Fisher Scientific,
Inc. Human β-actin (Assay ID: Hs01060665_g1) and human RNU48 (Assay
ID: 001006) were used as endogenous controls. RNU48 was used as a
control for miRNA, while human β-actin was used as a control for
LRRC75A-AS1 and ADAMTS5. The expression levels of lncRNA
LRRC75A-AS1 (Assay ID: Hs00415106_m1), hsa-miR-370-5p (Assay ID:
462392_mat) and ADAMTS5 (Assay ID: Hs01095518_m1) were determined
using StepOnePlus software (version 2.1; Applied Biosystems; Thermo
Fisher Scientific, Inc.) and the 2−ΔΔCq method (33). The following thermocycling
conditions were used: For lncRNA and ADAMTS5, initial denaturation
at 95°C for 20 sec, followed by 40 cycles at 95°C for 1 sec and
60°C for 20 sec; for miRNA, initial denaturation at 95°C for 20
sec, followed by 40 cycles at 95°C for 3 sec and 60°C for 30
sec.
Bioinformatics analysis
To identify miRNAs with strong binding affinity to
LRRC75A-AS1, the miRNA target scanner miRanda (http://www.microrna.org/) was used (34). The Ensembl Canonical cDNA sequence
(https://rapid.ensembl.org/index.html)
for LRRC75A-AS1 (ENST05220043563.1; 3,310 bp) and mature miRNA
sequences for Homo sapiens from miRBase (35) were used as input for miRanda. The
mature miRNA sequences for Homo sapiens used as input of
miRanda were extracted from the mature miRNA sequences file
downloaded from the miRBase (https://www.mirbase.org/download/). Pairing score ≥160
and energy score ≤-20 were used as cutoff thresholds for miRanda
analysis. Target genes for miRNAs were searched through
TargetScanHuman (https://www.targetscan.org/vert_80/) and miRDB
(https://mirdb.org/mirdb/index.html).
Statistical analysis
Continuous variables were compared using the
unpaired Student's t-test, the paired Student's t-test for matched
samples, or the Mann-Whitney U test. Survival analysis was
performed using the Kaplan-Meier method, and comparisons were made
using the log-rank test. A Cox proportional hazards regression
model was employed in both univariate and multivariate analyses to
identify the risk factors for recurrence and progression.
Statistical significance for functional in vitro analysis
was determined using an unpaired Student's t-test for comparing two
groups, or one-way analysis of variance (ANOVA) followed by the
Tukey post hoc test for more than two groups. Statistical analyses
were performed using JMP software (Pro.16; SAS Institute, Inc.).
All numerical data are presented as the mean ± standard deviation.
P-values were two-sided, and P<0.05 was considered to indicate a
statistically significant difference.
Results
lncRNA microarray data analysis of
RCC
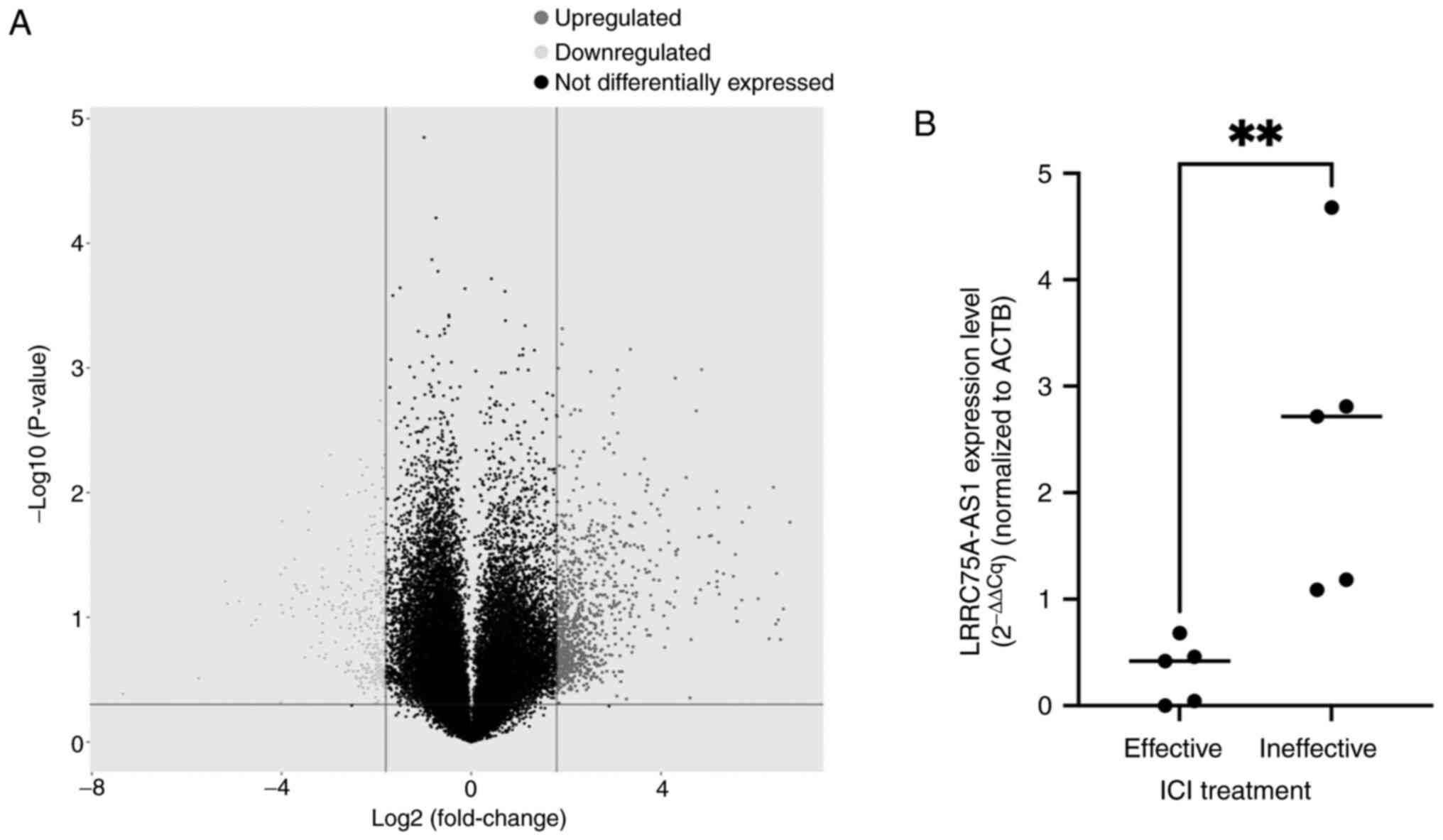
The lncRNA microarray analysis identified several
lncRNAs that were highly expressed in patients who were
unresponsive to ICI therapy (Fig.
1A). However, the present study focused on lncRNA LRRC75A-AS1
due to the limited existing literature on this particular
lncRNA.
LRRC75A-AS1 expression in ccRCC
tissues between ICI effective and ineffective groups
RT-qPCR was performed to determine whether
LRRC75A-AS1 expression was upregulated in human ccRCC tissues.
Although the sample size was small, the expression of LRRC75A-AS1
was significantly higher in ccRCC tissues from the ICI ineffective
group (n=5) compared with that in the effective group (n=5)
(P<0.01; Fig. 1B).
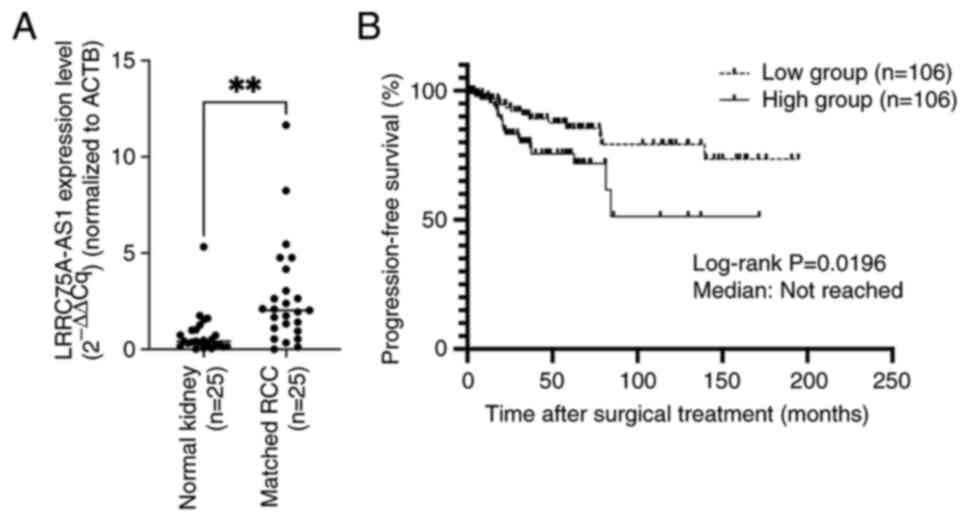
Relationship between LRRC75A-AS1
expression levels and the prognosis of patients with RCC
The present study compared LRRC75A-AS1 expression in
25 matched normal renal tissues and renal cancer tissues (all
ccRCC) by RT-qPCR. LRRC75A-AS1 expression was significantly higher
in all renal cancer tissues compared with that in the matched
normal renal tissues (P=0.0016; Fig.
2A). Subsequently, the expression levels of LRRC75A-AS1 were
assessed in 212 ccRCC samples, and the samples were divided into
two groups based on the median expression of LRRC75A-AS1. The high
LRRC75A-AS1 group exhibited significantly shorter progression-free
survival (PFS) compared with the low LRRC75A-AS1 group (log-rank
P=0.0196) (Fig. 2B). However, no
significant difference was found in the overall survival rate
between the groups (P=0.08; data not shown). Subsequently, the
present study analyzed the association between LRRC75A-AS1
expression and various clinicopathological parameters (Table II). In addition, prognostic factors
for PFS, including sex, age, body mass index,
neutrophil-to-lymphocyte ratio, stage, Fuhrman grade, sarcomatoid
features and LRRC75A-AS1 expression levels were assessed in
patients with ccRCC (Table II).
Elevated LRRC75A-AS1 expression emerged as a significant
independent risk factor for PFS in the multivariate analysis,
similar to other clinical prognostic factors (hazard ratio=2.88;
P=0.006).
 | Table II.Univariate and multivariate analyses
of predictive factors of progression-free survival. |
Table II.
Univariate and multivariate analyses
of predictive factors of progression-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex, male vs.
female | 0.69 | 0.74–2.85 | 0.278 |
|
|
|
| Age, ≥65 vs. <65
years | 0.95 | 0.48–1.88 | 0.888 |
|
|
|
| BMI, ≥25 vs. <25
kg/m2 | 0.79 | 0.39–1.63 | 0.526 |
|
|
|
| NLR, ≥3 vs.
<3 | 1.03 | 0.51–2.10 | 0.888 |
|
|
|
| Stage, ≥II vs.
I | 6.49 | 3.16–13.35 |
<0.0001a | 3.48 | 1.34–8.99 | 0.01a |
| Metastasis, ≥1 vs.
0 | 2.59 | 0.79–8.52 | 0.117 |
|
|
|
| Fuhrman grade, ≥3
vs. <3 | 4.38 | 2.12–9.06 |
<0.0001a | 3.2 | 1.37–7.46 | 0.007a |
| Sarcomatoid, yes
vs. no | 4.68 | 2.12–9.07 | 0.0003a | 3.77 | 1.60–8.89 | 0.003a |
| LRRC75A-AS1, high
vs. low | 2.26 | 2.03–10.78 | 0.023a | 2.88 | 1.35–6.14 | 0.006a |
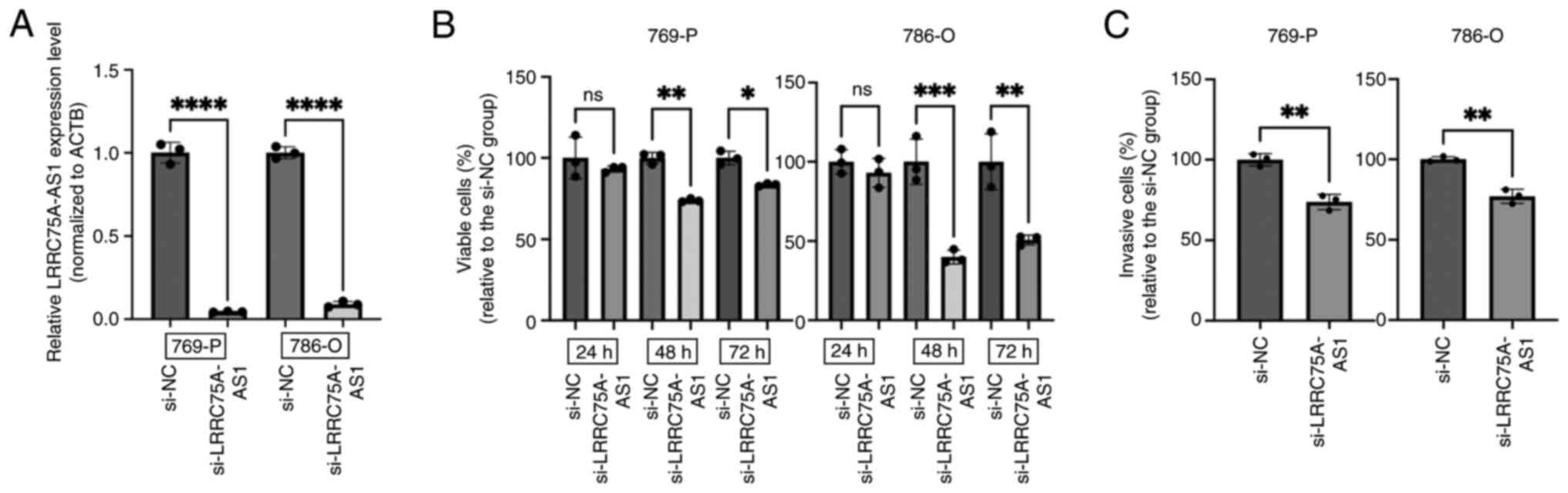
Effect of LRRC75A-AS1 knockdown on
cell viability and invasion in vitro
The present study analyzed and compared the
expression levels of LRRC75A-AS1 in four RCC lines (769-P, 786-O,
ACHN and A498) using RT-qPCR. Among these, 769-P and 786-O cells
exhibited the highest expression of LRRC75A-AS1 and were selected
for further experiments (Fig. S1).
In 769-P and 786-O cells, LRRC75A-AS1 expression was significantly
decreased after si-LRRC75A-AS1 transfection (P<0.0001; Fig. 3A). The MTS assay results revealed
that the knockdown of LRRC75A-AS1 significantly suppressed cell
proliferation in both cell lines 48 h post-transfection, compared
with that in the si-NC group (P<0.01 and P<0.001,
respectively; Fig. 3B). In the
invasion assay, LRRC75A-AS1 knockdown significantly reduced the
invasive capacity of 769-P and 786-O cells compared with that in
the si-NC group (P<0.01; Fig.
3C). These results indicated that the knockdown of LRRC75A-AS1
expression significantly inhibited the proliferation and invasion
of RCC cell lines in vitro.
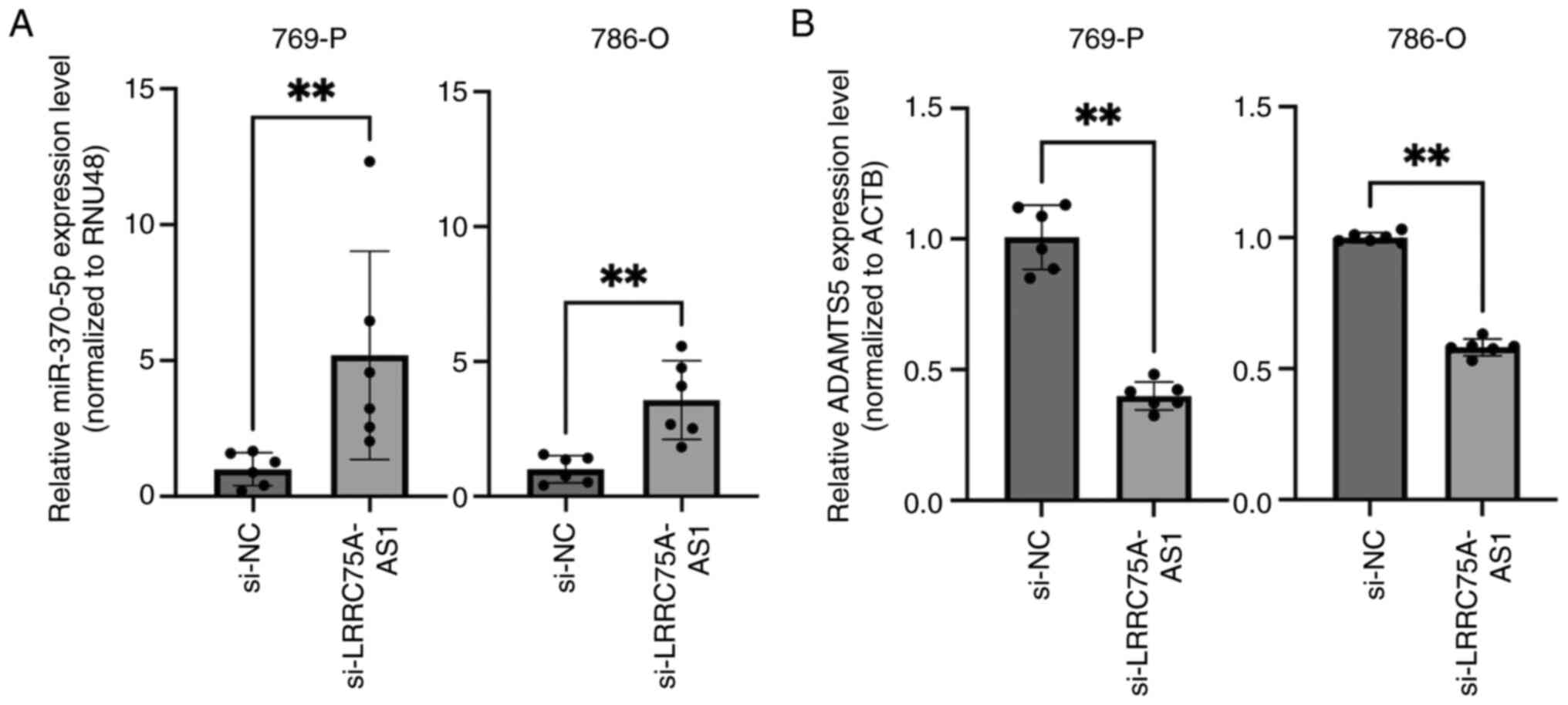
Interaction among LRRC75A-AS1,
miR-370-5p and ADAMTS5 in RCC
The present study investigated the potential
relationship between LRRC75A-AS1 and miRNAs. Cytoplasmic lncRNAs
act as miRNA sponges; therefore, it was hypothesized that
LRRC75A-AS1 may function as a miRNA regulator. A total of 66 miRNAs
identified by bioinformatics analysis as having potential binding
sites on LRRC75A-AS1 were selected (Table SI). Among these, miR-370-5p
functions as a tumor suppressor in renal cancer (36). Therefore, the present study focused
on miR-370-5p and investigated its interaction with LRRC75A-AS1 in
a renal cancer cell line. LRRC75A-AS1 knockdown significantly
increased the expression of miR-370-5p in the 769-P and 786-O cell
lines (P<0.01; Fig. 4A). These
results suggested a reciprocal interaction between LRRC75A-AS1 and
miR-370-5p. To identify the potential target genes of miR-370-5p,
public databases such as miRDB and TargetScanHuman were utilized.
Based on its high TargetScores and relevance to cancer,
particularly as an unfavorable prognostic marker of RCC, ADAMTS5
was selected for further investigation. The expression levels of
ADAMTS5 were compared between si-NC- and si-LRRC75A-AS1-transfected
769-P and 786-O cell lines. The results demonstrated a significant
decrease in ADAMTS5 expression in the si-LRRC75A-AS1 group
(P<0.01; Fig. 4B). These
findings suggested that LRRC75A-AS1 may regulate ADAMTS5 expression
through a sponge effect on miR-370-5p, thereby contributing to RCC
progression. Specifically, LRRC75A-AS1 acted as a molecular sponge
for miR-370-5p, preventing miR-370-5p from binding to ADAMTS5 mRNA.
This inhibition could lead to increased ADAMTS5 expression, which
is implicated in RCC progression and metastasis. The present
results demonstrated that LRRC75A-AS1, through its sponging effect
on miR-370-5p, may serve a critical role in the regulation of these
target genes, contributing to the pathogenesis of RCC.
Discussion
The present study investigated the oncogenic role of
lncRNA LRRC75A-AS1 in RCC and its potential as a prognostic
biomarker. Based on the lncRNA microarray data, the study focused
on LRRC75A-AS1, which, to the best of our knowledge, had not
previously been investigated in RCC. Using tissue samples obtained
post-surgery, it was demonstrated that high LRRC75A-AS1 expression
in RCC was associated with a poor prognosis. Specifically, high
LRRC75A-AS1 expression was significantly associated with PFS and
served as an independent predictor, similar to clinical prognostic
factors such as Fuhrman grade, stage (37,38),
and sarcomatoid differentiation. Additionally, the expression
levels of LRRC75A-AS1 were examined in four RCC cell lines. Using
specific siRNAs to knock down LRRC75A-AS1 expression in 769-P and
786-O cell lines, a reduction in cell proliferation and invasion
was observed. These results suggested that LRRC75A-AS1 may function
as an oncogene, contributing to RCC development.
Despite their limited protein-coding abilities,
lncRNAs regulate the stemness of cancer stem cells (CSCs) and
mediate chemoresistance (39). A
previous review discussed the critical roles of various lncRNAs in
maintaining CSC properties and mediating resistance to chemotherapy
(40). Notable lncRNAs, such as
HOTAIR, MALAT1 and H19, were highlighted for their involvement in
these processes. These lncRNAs contribute to CSC maintenance and
drug resistance by interacting with key signaling pathways, such as
Wnt/β-catenin, Notch and Hedgehog. In RCC, lncRNAs have also been
implicated in sunitinib resistance. For example, lncRNA IGFL2-AS1
can induce sunitinib resistance through extracellular vesicles
(41). Furthermore, LRRC75A-AS1,
the central focus of the present study, may be associated with
these processes, particularly CSC maintenance and chemoresistance,
through pathways such as Wnt/β-catenin, Notch and Hedgehog.
Continued research in this area is essential to address the
challenges associated with RCC treatment.
The present study also examined the potential
interactions between LRRC75A-AS1 and other ncRNAs, such as miRNAs.
lncRNAs can influence post-transcriptional regulation by competing
for shared miRNA response elements and acting as natural miRNA
sponges, thereby reducing the binding of endogenous miRNAs to their
target genes (42). Cytoplasmic
lncRNAs and miRNAs have been implicated in post-transcriptional
regulation across various types of cancer, including RCC, through
their sponge effects. Although the mechanisms by which LRRC75A-AS1
operates in RCC remain unexplored, LRRC75A-AS1 has been identified
as a ceRNA in triple-negative breast cancer. Specifically,
LRRC75A-AS1 has been shown to regulate miR-30a-5p through a sponge
effect, resulting in increased BAALC expression, and contributing
to cancer proliferation and invasion (31). The present study identified the
miRNAs that may bind to LRRC75A-AS1. Among these, miR-370-5p
emerged as a significant miRNA in RCC and is located in the
cytoplasm of renal cancer cells (36). Various studies have shown that
miR-370-5p may act as a tumor suppressor in various types of
cancer, including RCC, and lung (43), colorectal (44) and nasopharyngeal (45) cancer. For example, low miR-370-5p
expression levels have been observed in RCC cells; by contrast, the
artificial upregulation of miR-370-5p expression has been shown to
mitigate the pro-tumor effects of circCOL5A1 by suppressing tumor
malignancy and glycolysis (36).
In the present study, a significant association was
observed between miR-370-5p and LRRC75A-AS1 expression, suggesting
a potential regulatory interaction. This interaction supports the
ceRNA hypothesis indicating that LRRC75A-AS1 acts as a sponge for
miR-370-5p, thereby regulating its target mRNAs involved in RCC
progression. Specifically, the present cellular experiments
revealed that the siRNA-mediated knockdown of LRRC75A-AS1 led to
increased miR-370-5p expression, consistent with its role as a
tumor suppressor. These results suggested that LRRC75A-AS1 may
suppress miR-370-5p expression, thereby contributing to RCC
progression. Understanding these interactions could provide new
insights into the molecular mechanisms driving RCC and facilitate
the identification of novel therapeutic targets. Using the miRDB
and TargetScanHuman databases, the target genes of miR-370-5p were
predicted and ADAMTS5 was identified as a candidate mRNA. ADAMTS5
is involved in extracellular matrix remodeling, and promotes tumor
invasion and metastasis in various cancer types (46). Notably, high ADAMTS5 expression in
hepatocellular carcinoma has been associated with a poor prognosis
(47). These findings suggested
that ADAMTS5 may be regulated by miR-370-5p in RCC, contributing to
tumor malignancy. The sponging effect of LRRC75A-AS1 on miR-370-5p
could result in increased ADAMTS5 expression, thereby facilitating
RCC progression. Understanding this interaction could provide new
insights into the molecular mechanisms driving RCC and present
innovative therapeutic targets.
A limitation of the present study was the small
number of patients treated with ICIs, which limits the
generalizability of the findings. Further studies are warranted to
fully elucidate the role of LRRC75A-AS1 in ICI therapy. The limited
sample size hinders the ability to draw definitive conclusions
regarding the interaction between LRRC75A-AS1 expression and ICI
treatment response. Future studies with a larger cohort of patients
treated with ICIs are essential to validate these findings and to
explore the potential of LRRC75A-AS1 as a predictive biomarker for
ICI therapy response.
Another limitation is the lack of in vivo
experiments, such as those utilizing an orthotopic animal model
with siRNA- or short hairpin RNA-induced LRRC75A-AS1 knockdown.
Such studies would aid in elucidating the molecular mechanisms
involved in RCC invasion and metastasis. Additionally, siRNA was
used in cell lines to investigate the effect of LRRC75A-AS1 on cell
proliferation and invasion in vitro, obtaining significant
results; however, overexpression experiments have not yet been
conducted. Furthermore, the direct interaction between LRRC75A-AS1
and miR-370-5p has not been experimentally validated, which is
another limitation of the present study. Similarly, the direct
interaction between miR-370-5p and ADAMTS5 has not been
experimentally validated, such as via a luciferase reporter assay.
Future studies should include in vitro and in vivo
experiments to confirm the regulatory interactions and functional
effects of LRRC75A-AS1 and its target genes.
In conclusion, LRRC75A-AS1 may function as an
oncogene in RCC, promoting tumorigenesis and progression. The
significant upregulation of LRRC75A-AS1 in ccRCC tissues and its
association with poor prognosis suggests its potential as a
biomarker for RCC. Furthermore, the regulatory interaction between
LRRC75A-AS1 and miR-370-5p, which may influence the expression of
ADAMTS5, underscores their roles in RCC progression. Specifically,
the increased expression of ADAMTS5 facilitated by the
LRRC75A-AS1-mediated sponging of miR-370-5p highlights a potential
pathway contributing to tumor invasion and metastasis.
Understanding this interaction and its impact on ADAMTS5 expression
could provide new insights into the molecular mechanisms underlying
RCC development and identify novel therapeutic targets. Continued
research into the molecular mechanisms of LRRC75A-AS1, including
its effect on ADAMTS5 and its clinical applications, could
significantly improve RCC management and patient outcomes.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by a Grant-in-Aid for Scientific
Research Foundation (C) from the Japan Society for the Promotion of
Science (grant no. KAKENHI-PROJECT- 21K09399).
Availability of data and materials
The microarray data generated in the present study
may be found in the Gene Expression Omnibus under accession number
GSE276355 or at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE276355.
The other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
HH and KS conceived and designed the study. All
authors provided guidance on the work. TT and HH performed the
experiments. TT, YH and HH prepared the figures and drafted the
original manuscript. YH, NF, KK, TH, YA, HH and KS contributed to
the analysis or interpretation of data. TH and YA were responsible
for the software and formal analysis. HH and KS reviewed and
revised the manuscript. HH and KS confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Graduate School of Medicine at Yamaguchi University
(Institutional Review Board No. 2022-051; Ube, Japan), and written
informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ccRCC
|
clear cell renal cell carcinoma
|
|
ceRNA
|
competing endogenous RNA
|
|
ICI
|
immune checkpoint inhibitor
|
|
lncRNA
|
long non-coding RNA
|
|
miRNA
|
microRNA
|
|
ncRNA
|
non-coding RNA
|
|
PFS
|
progression-free survival
|
|
RCC
|
renal cell carcinoma
|
|
si-NC
|
small interfering RNA-negative
control
|
|
CSCs
|
cancer stem cells
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bahadoram S, Davoodi M, Hassanzadeh S,
Bahadoram M, Barahman M and Mafakher L: Renal cell carcinoma: An
overview of the epidemiology, diagnosis, and treatment. G Ital
Nefrol. 3:392022.
|
|
3
|
Mori K, Mostafaei H, Miura N, Karakiewicz
PI, Luzzago S, Schmidinger M, Bruchbacher A, Pradere B, Egawa S and
Shariat SF: Systemic therapy for metastatic renal cell carcinoma in
the first-line setting: A systematic review and network
meta-analysis. Cancer Immunol Immunother. 70:265–273. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wein A, Kavoussi L, Partin A and Peters C:
Campbell-Walsh urology: 4-Volume Set. (11th Ed.). Fac Bookshelf;
Elsevier, Philadelphia, PA: 2016
|
|
5
|
Liu X, Hao Y, Yu W, Yang X, Luo X, Zhao J,
Li J, Hu X and Li L: Long non-coding RNA emergence during renal
cell carcinoma tumorigenesis. Cell Physiol Biochem. 47:735–746.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gilbert N: Surgical treatment of pulmonary
metastases in metastatic renal cell carcinoma. Aktuelle Urol.
51:271–274. 2020.(In German). PubMed/NCBI
|
|
7
|
Bosma NA, Warkentin MT, Gan CL, Karim S,
Heng DYC, Brenner DR and Lee-Ying RM: Efficacy and safety of
first-line systemic therapy for metastatic renal cell carcinoma: A
systematic review and network meta-analysis. Eur Urol Open Sci.
37:14–26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martens-Uzunova ES, Böttcher R, Croce CM,
Jenster G, Visakorpi T and Calin GA: Long noncoding RNA in
prostate, bladder, and kidney cancer. Eur Urol. 65:1140–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao N, Li Y, Li J, Gao Z, Yang Z, Li Y,
Liu H and Fan T: Long non-coding RNAs: The regulatory mechanisms,
research strategies, and future directions in cancers. Front Oncol.
10:5988172020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou S, Wang J and Zhang Z: An emerging
understanding of long noncoding RNAs in kidney cancer. J Cancer Res
Clin Oncol. 140:1989–1995. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Braga EA, Fridman MV, Filippova EA,
Loginov VI, Pronina IV, Burdennyy AM, Karpukhin AV, Dmitriev AA and
Morozov SG: LncRNAs in the regulation of genes and signaling
pathways through miRNA-mediated and other mechanisms in clear cell
renal cell carcinoma. Int J Mol Sci. 22:111932021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pi YN, Qi WC, Xia BR, Lou G and Jin WL:
Long non-coding RNAs in the tumor immune microenvironment:
Biological properties and therapeutic potential. Front Immunol.
12:6970832021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu P, Feng D, Wang J, Wang YD, Xie G,
Zhang B, Li XH, Zeng JW and Feng JF: LncRNA AGAP2 antisense RNA 1
stabilized by insulin-like growth factor 2 mRNA binding protein 3
promotes macrophage M2 polarization in clear cell renal cell
carcinoma through regulation of the microRNA-9-5p/THBS2/PI3K-Akt
pathway. Cancer Cell Int. 23:3302023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao Y, Wang X, Dong L, Qu C, Lu Q, Wang P,
Xin M, Zheng W, Liu C and Ning S: Identifying immune
checkpoint-related lncRNA biomarkers for immunotherapy response and
prognosis in cancers. Sci Data. 10:6632023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiao HP, Gao WS, Huo JX and Yang ZS: Long
non-coding RNA GAS5 functions as a tumor suppressor in renal cell
carcinoma. Asian Pac J Cancer Prev. 14:1077–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawakami T, Chano T, Minami K, Okabe H,
Okada Y and Okamoto K: Imprinted DLK1 is a putative tumor
suppressor gene and inactivated by epimutation at the region
upstream of GTL2 in human renal cell carcinoma. Hum Mol Genet.
15:821–830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bertozzi D, Iurlaro R, Sordet O, Marinello
J, Zaffaroni N and Capranico G: Characterization of novel antisense
HIF-1α transcripts in human cancers. Cell Cycle. 10:3189–3197.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frevel MA, Sowerby SJ, Petersen GB and
Reeve AE: Methylation sequencing analysis refines the region of H19
epimutation in Wilms tumor. J Biol Chem. 274:29331–29340. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiesa N, De Crescenzo A, Mishra K, Perone
L, Carella M, Palumbo O, Mussa A, Sparago A, Cerrato F, Russo S, et
al: The KCNQ1OT1 imprinting control region and non-coding RNA: New
properties derived from the study of Beckwith-Wiedemann syndrome
and Silver-Russell syndrome cases. Hum Mol Genet. 21:10–25. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davis IJ, Hsi BL, Arroyo JD, Vargas SO,
Yeh YA, Motyckova G, Valencia P, Perez-Atayde AR, Argani P, Ladanyi
M, et al: Cloning of an Alpha-TFEB fusion in renal tumors harboring
the t(6;11)(p21;q13) chromosome translocation. Proc Natl Acad Sci
USA. 100:6051–6056. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuiper RP, Schepens M, Thijssen J, van
Asseldonk M, van den Berg E, Bridge J, Schuuring E, Schoenmakers EF
and van Kessel AG: Upregulation of the transcription factor TFEB in
t(6;11)(p21;q13)-positive renal cell carcinomas due to promoter
substitution. Hum Mol Genet. 12:1661–1669. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiyomaru T, Fukuhara S, Saini S, Majid S,
Deng G, Shahryari V, Chang I, Tanaka Y, Enokida H, Nakagawa M, et
al: Long non-coding RNA HOTAIR is targeted and regulated by miR-141
in human cancer cells. J Biol Chem. 289:12550–12565. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeong G, Kwon DH, Shin S, Choe N, Ryu J,
Lim YH, Kim J, Park WJ, Kook H and Kim YK: Long noncoding RNAs in
vascular smooth muscle cells regulate vascular calcification. Sci
Rep. 9:58482019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Lan J, Ye Z, Duan S, Hu Y, Zou Y
and Zhou J: Long noncoding RNA LRRC75A-AS1 inhibits cell
proliferation and migration in colorectal carcinoma. Exp Biol Med
(Maywood). 244:1137–1143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han L, Li Z, Jiang Y, Jiang Z and Tang L:
SNHG29 regulates miR-223-3p/CTNND1 axis to promote glioblastoma
progression via Wnt/β-catenin signaling pathway. Cancer Cell Int.
19:3452019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Wu D, Jia H and Zhang Z: Long
non-coding RNA LRRC75A-AS1 facilitates triple negative breast
cancer cell proliferation and invasion via functioning as a ceRNA
to modulate BAALC. Cell Death Dis. 11:6432020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Wang H, Zhang R, Li D and Gao MQ:
LRRC75A antisense lncRNA1 knockout attenuates inflammatory
responses of bovine mammary epithelial cells. Int J Biol Sci.
16:251–263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Enright AJ, John B, Gaul U, Tuschl T,
Sander C and Marks DS: MicroRNA targets in Drosophila. Genome Biol.
5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kozomara A, Birgaoanu M and
Griffiths-Jones S: miRBase: From microRNA sequences to function.
Nucleic Acids Res. 47:D155–D162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie Q, Qin F, Luo L, Deng S, Zeng K, Wu Y,
Liao D, Luo L and Wang K: hsa_circ_0003596, as a novel oncogene,
regulates the malignant behavior of renal cell carcinoma by
modulating glycolysis. Eur J Med Res. 28:3152023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang T, Alvarez A, Hu B and Cheng SY:
Noncoding RNAs in cancer and cancer stem cells. Chin J Cancer.
32:582–593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fang Y and Fullwood MJ: Roles, functions,
and mechanisms of long non-coding RNAs in cancer. Genomics
Proteomics Bioinformatics. 14:42–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pan Y, Lu X, Shu G, Cen J, Lu J, Zhou M,
Huang K, Dong J, Li J, Lin H, et al: Extracellular vesicle-mediated
transfer of LncRNA IGFL2-AS1 confers sunitinib resistance in renal
cell carcinoma. Cancer Res. 83:103–116. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dykes IM and Emanueli C: Transcriptional
and post-transcriptional gene regulation by long non-coding RNA.
Genomics Proteomics Bioinformatics. 15:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li C, Ge Q, Liu J, Zhang Q, Wang C, Cui K
and Chen Z: Effects of miR-1236-3p and miR-370-5p on activation of
p21 in various tumors and its inhibition on the growth of lung
cancer cells. Tumor Biol. 39:10104283177108242017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Li L, Lu KX, Yu LB, Meng J and
Liu CY: LncRNA SNHG3 is responsible for the deterioration of
colorectal carcinoma through regulating the miR-370-5p/EZH1 axis.
Eur Rev Med Pharmacol Sci. 25:6131–6137. 2021.PubMed/NCBI
|
|
45
|
Zhou Z, Xu F and Zhang T: Circular RNA
COL1A1 promotes Warburg effect and tumor growth in nasopharyngeal
carcinoma. Discov Oncol. 15:1202024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mochizuki S and Okada Y: ADAMs in cancer
cell proliferation and progression. Cancer Sci. 98:621–628. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu Z, Xu J, Wu X, Lin S, Li L, Ye W and
Huang Z: In silico identification of contradictory role of ADAMTS5
in hepatocellular carcinoma. Technol Cancer Res Treat.
20:15330338209868262021. View Article : Google Scholar : PubMed/NCBI
|