Introduction
Acute myocardial infarction (MI) is a major cause of
death worldwide (1). Despite the
availability of primary percutaneous coronary intervention or
thrombolytic therapy as the most effective treatment for myocardial
blood reconstruction in patients who are suffering from MI
(2), myocardial
ischemia/reperfusion (I/R) injury remains the major cause of heart
failure worldwide (3). This type
of injury activates apoptosis and autophagy, which results in cell
death (4). Therefore, an
increasing number of studies have investigated the roles of
apoptosis and autophagy during I/R injury in myocytes (5–7).
Apoptosis is an active energy-dependent mode of cell
death, which is regulated by programmed cellular signaling
pathways. Previous studies have demonstrated that I/R-induced
myocyte injury results in apoptosis and the suppression of
cardiomyocyte apoptosis provides significant cardioprotection
against I/R injury (8–10). By contrast, autophagy is a natural
biological process, which removes unnecessary proteins and damaged
organelles and maintains cellular homeostasis (11). The upregulation of autophagy during
mild ischemia is associated with the preservation of the
mitochondrial membrane potential and cellular membrane integrity,
accompanied by a significant delay in the onset of apoptosis and
necrosis (12). However, excessive
autophagy damages essential proteins and organelles, leading to
collapse of all cellular functions (13). Evidence supporting this concept by
Matsui et al demonstrated that excessive autophagy may be
detrimental to the heart during reperfusion (14). Therefore, the identification of
treatments with anti-apoptotic and anti-autophagic effects is
important for protection of the heart from I/R injury. The
investigation of traditional medicines as a potential treatment
option in I/R injury has received interest due to their wide range
of pharmacological effects (15–16).
Curcumin, a natural polyphenolic compound present in
the rhizomes of Curcuma longa, has potential
anti-inflammatory, antioxidant, anti-carcinogenic and
cardiovascular protective effects (17–19).
Previous studies have demonstrated that curcumin attenuates I/R
injury in various organs in vivo and in vitro,
including the intestines, retina, liver and renal system, through
anti-oxidative stress and anti-apoptotic mechanisms (20–23).
In addition, Gonzalez-Salazar et al (24) demonstrated that curcumin mitigated
myocardial I/R injury through the attenuation of oxidative stress
and mitochondrial dysfunction. However, the beneficial effects of
curcumin on the extent of apoptosis and autophagy in myocardial
I/R, and their potential mechanisms, remain to be elucidated.
The present study examined the protective effect of
curcumin on hypoxia/reoxygenation (H/R)-induced H9c2 myocytes,
focusing on the regulation of apoptosis and autophagy. The
potential mechanism by which curcumin mediates these processes was
also investigated.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS) and 10,000 U/ml penicillin/streptomycin were
obtained from Gibco Life Technologies (Carlsbad, CA, USA). Dimethyl
sulfoxide (DMSO) and curcumin were obtained from Sigma-Aldrich (St.
Louis, MO, USA). Rabbit monoclonal antibody against GAPDH was
produced by Abcam (Cambridge, UK). Rabbit monoclonal anti-LC3B
antibody was provided by Zymed Life Technologies (San Francisco,
CA, USA). Rabbit monoclonal anti-beclin-1, Bcl-2/adenovirus E1B 19
kDa interacting protein 3 (BNIP3) and silent information regulation
1 (SIRT1) antibodies were purchased from the Cell Signaling
Technology, Inc. (Danvers, MA, USA). Goat anti-rabbit secondary
antibody (cat. no. A-21109) was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). A cellular adenosine triphosphate
(ATP) kit was obtained from Beyotime Institute of Biotechnology
(Shanghai, China). All other chemicals for western blot analysis
were of the highest commercial purity grade available.
Cell culture and treatment
The H9c2 rat myocardium-derived cardiac myoblast
cell line was purchased from the American Type Culture Collection
(Rockville, MD, USA). The cells were cultured in DMEM containing
4,500 mg/l glucose and supplemented with 10% (v/v) FBS, 10 mM HEPES
(Sigma-Aldrich) and 1% penicillin/streptomycin at 37°C in a 5%
CO2 incubator for 24 h. Prior to pretreatment with
curcumin, the cell media were replaced with FBS-free media for 4 h
for cell cycle synchronization until the cell density conformed
with the experimental standard of achieving a unified cell state.
To identify the cytotoxicity of curcumin on H9c2 myocytes, the
cells (1×106/ml) were pretreated with curcumin (1, 5,
10, 20, 40 and 80 μM) for 1 h under normoxic conditions with
an atmosphere of 21% O2, 5% CO2 and 74%
N2 in FBS- and glucose-free DMEM. To simulate
starvation, the cells were cultured in FBS- and glucose-free DMEM,
with the exception of the normal control group cells which were
cultured in complete DMEM. To simulate I/R injury, the
glucose-deprived cells were cultured under hypoxic conditions with
an atmosphere of 1% O2, 5% CO2 and 94%
N2 for 1 h and then cultured under normoxic conditions
for 3 h. The cells in the normal control and starvation groups were
exposed to normoxic conditions for 4 h.
Cell viability detection by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
An MTT assay was performed to assess the cell
viability following treatment with curcumin (1, 5, 10, 20, 40 and
80 μM). Subsequent to the previously described treatments,
the cells were incubated with 0.5 mg/ml MTT (Roche Diagnostics,
Basel, Switzerland) in RPMI-1640 medium (Life Technologies,
Carlsbad, CA, USA) for a further 4 h. The blue formazan crystals of
the viable cells were dissolved in 150 μl DMSO and the
absorbance was measured at a wavelength of 570 nm using a
spectrophotometer (DNM-9602; Perlong, Beijing, China).
ATP determination
The intracellular levels of ATP were determined
using an ATP bioluminescent assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer’s instructions.
Annexin V/propidium iodide (PI) double
staining and flow cytometry
The apoptotic rate was measured by flow cytometry,
which was performed using primary mouse anti-human monoclonal
antibodies and fluorescein isothiocyanate (FITC)/PI double
staining-annexin V (BD Biosciences, Franklin Lakes, NJ, USA)
according to the manufacturer’s instructions. The cells were
incubated with 5 μl annexin V-FITC and PI at room
temperature for 15 min in the dark and apoptosis was detected by
flow cytometry (BD accuri C6; BD Biosciences). The mean fluorescent
intensity of the annexin V/PI double staining in the myocytes was
analyzed using a BD fluorescent activated cell sorter (FACS)
calibur (BD Biosciences).
Protein isolation and western blot
analysis
Protein isolation and western blot analyses were
performed, as previously described (25), with the exception that the
membranes were probed with either rabbit primary Bcl-2-associated X
protein (Bax), Bcl-2, LC3B-II/I, beclin-1, BNIP3 or SIRT1
antibodies diluted 1:1,000 in Tris-buffered saline containing
Tween-20 (TBST; Solarbio, Beijing, China) for 2 h or rabbit
anti-GAPDH diluted 1:5,000 in TBST for 1 h. The membranes were then
incubated with goat anti-rabbit secondary antibody labeled with
far-red-fluorescent AlexaFluor 680 dye (1:1,000 in TBST; Abcam,
Cambridge, UK). All signals were detected using the Odyssey CLx
Infrared Imaging system (Li-Cor Biosciences, Lincoln, NE, USA).
Densitometric analysis was performed using the Quantity One system
(170–9600; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Groups of three or more were compared using one-way
analysis of variance (ANOVA) with Student-Newman-Keuls and Dunnet
methods as post-hoc analysis if the result of the ANOVA was
significant. The data are expressed as the mean ± standard
deviation and were analyzed using SPSS 18.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed at least three times.
Results
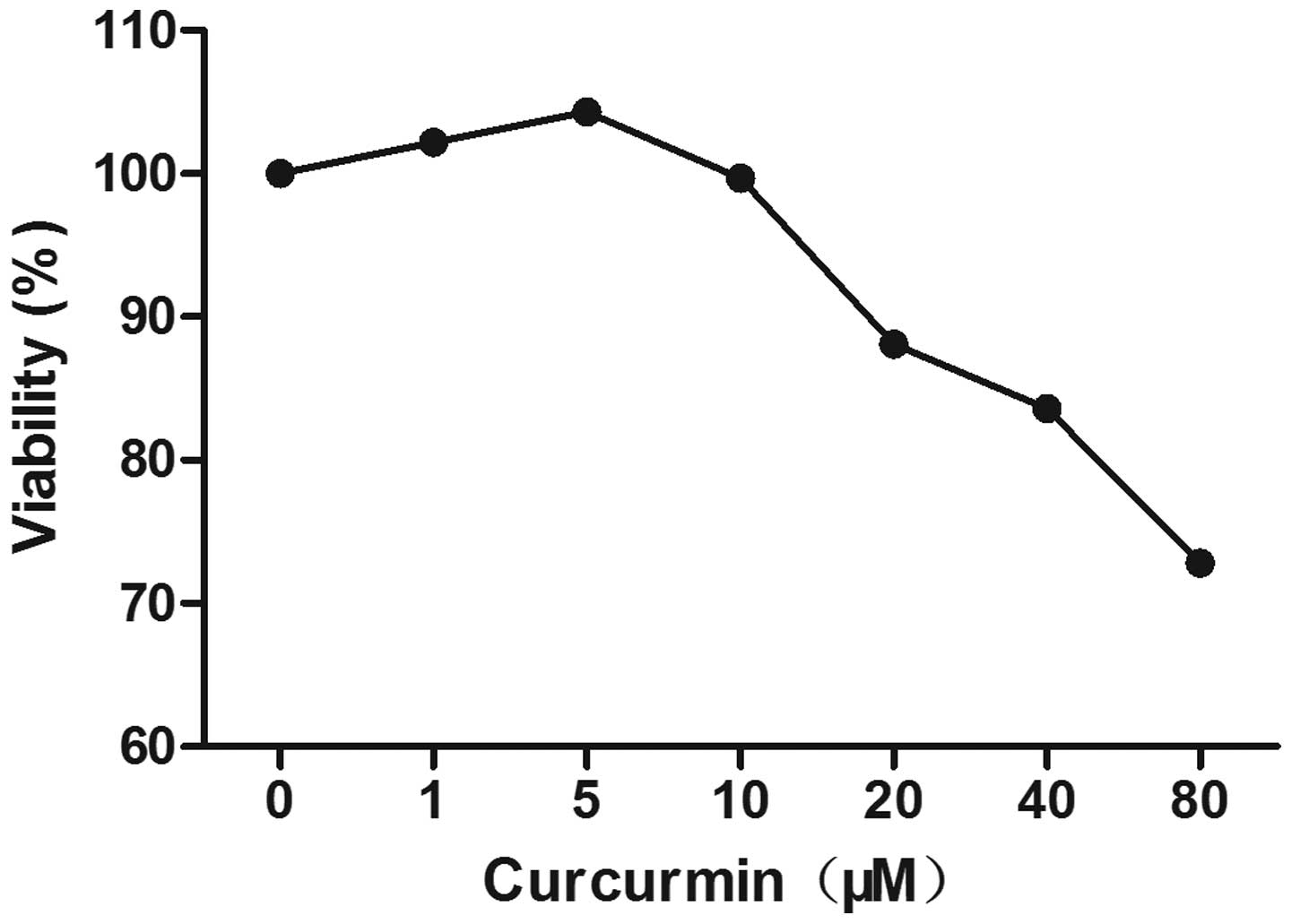
Effect of curcumin on cell viability
An MTT assay was used to assess the effect of
curcumin on the viability of the H9c2 myocytes. As shown in
Fig. 1 the concentrations of
curcumin, ranging between 1 and 20 μM, had no significant
effect on cell viability. Therefore, a curcumin concentration
<20 μM was considered non-cytotoxic and a concentration
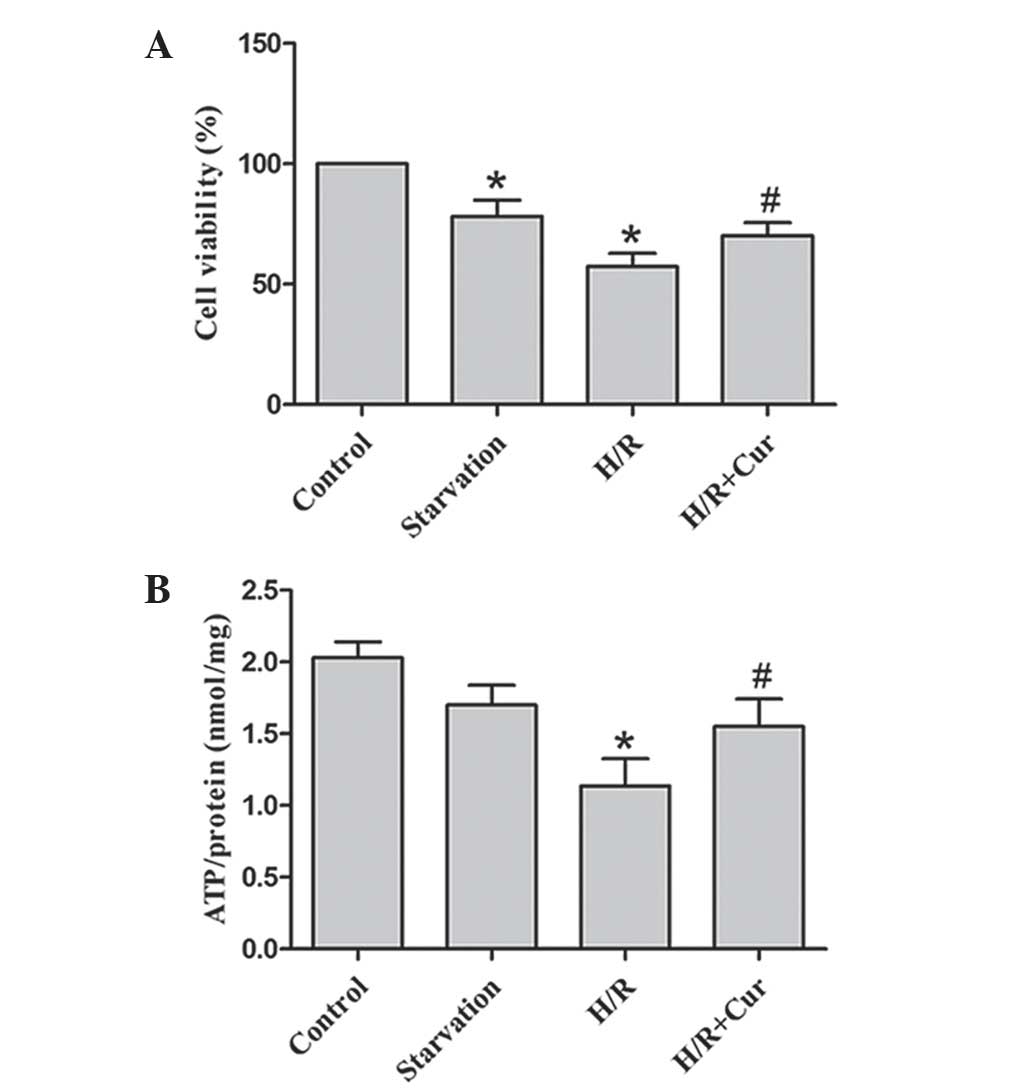
of 10 μM was used in the subsequent experiments. As shown in
Fig. 2A, the viability of the H9c2
cells was significantly reduced in the starvation and H/R treatment
groups. Treatment with 10 μM curcumin increased the
viability of the H9c2 cells during the H/R period compared with the
control group.
Curcumin abrogates the depletion of
cellular ATP in H9c2 cells exposed to H/R
The starvation and H/R treatments resulted in the
depletion of cellular ATP contents in the H9c2 myocytes. Treatment
with curcumin significantly reversed the depletion of cellular ATP,
which improved cell survival (Fig.
2B).
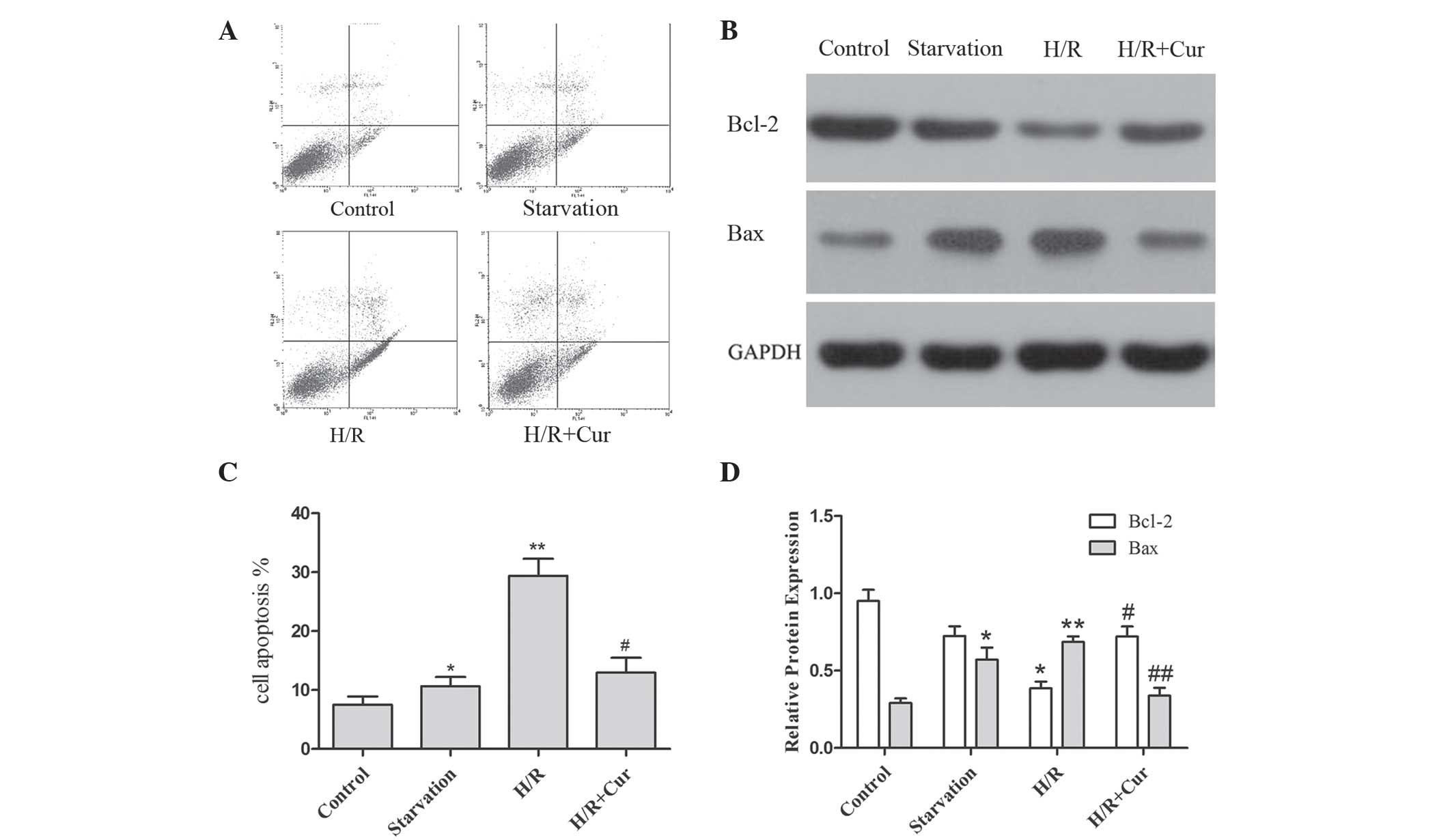
Curcumin inhibits apoptosis in the H9c2
myocytes
The present study aimed to determine whether
apoptosis was involved in the effect of curcumin on cell viability.
Annexin V/PI double staining was used to assess the apoptotic rate
in the H9c2 cells. The results demonstrated that the expression of
annexin V was significantly increased in the starvation (glucose
deprivation; GD) and H/R groups compared with the normal group.
However, an apparent reduction in the expression of annexin V was
observed in the H/R-induced H9c2 myocytes, which were pretreated
with curcumin (Fig. 3A).
The Bcl-2 protein family is important in the
regulation of apoptosis. The anti-apoptotic protein, Bcl-2, is
mainly located in the mitochondria, protecting these organelles by
preventing the movement of pro-apoptotic proteins, including Bax,
Bcl-2-antagonist/killer 1 and Bcl-2 homology 3 (BH3)
interacting-domain, to the mitochondria (26). Therefore, the expression levels of
these proteins were assessed to determine the initial apoptotic
events caused by curcumin. As shown in Fig. 3B, H/R activated the expression of
Bax and downregulated the expression of Bcl-2 in the H9c2 myocytes.
Treatment with curcumin markedly reversed these effects on the
expression levels of Bcl-2 and Bax in the cells exposed to H/R.
Curcumin inhibits autophagy in the H9c2
myocytes
In mammalian cells, LC3B-II is produced from LC3B-I
and is modified into a membrane-bound form to prompt its
localization to autophagosomes. Thus, LC3B-II is considered to be
an autophagosomal marker (27).
The present study investigated the expression of LC3B-II using
western blot analysis. As shown in Fig. 4A and B, the ratio of LC3B-II/LC3B-I
was increased in response to H/R and reduced by curcumin in the
H9c2 myocytes.
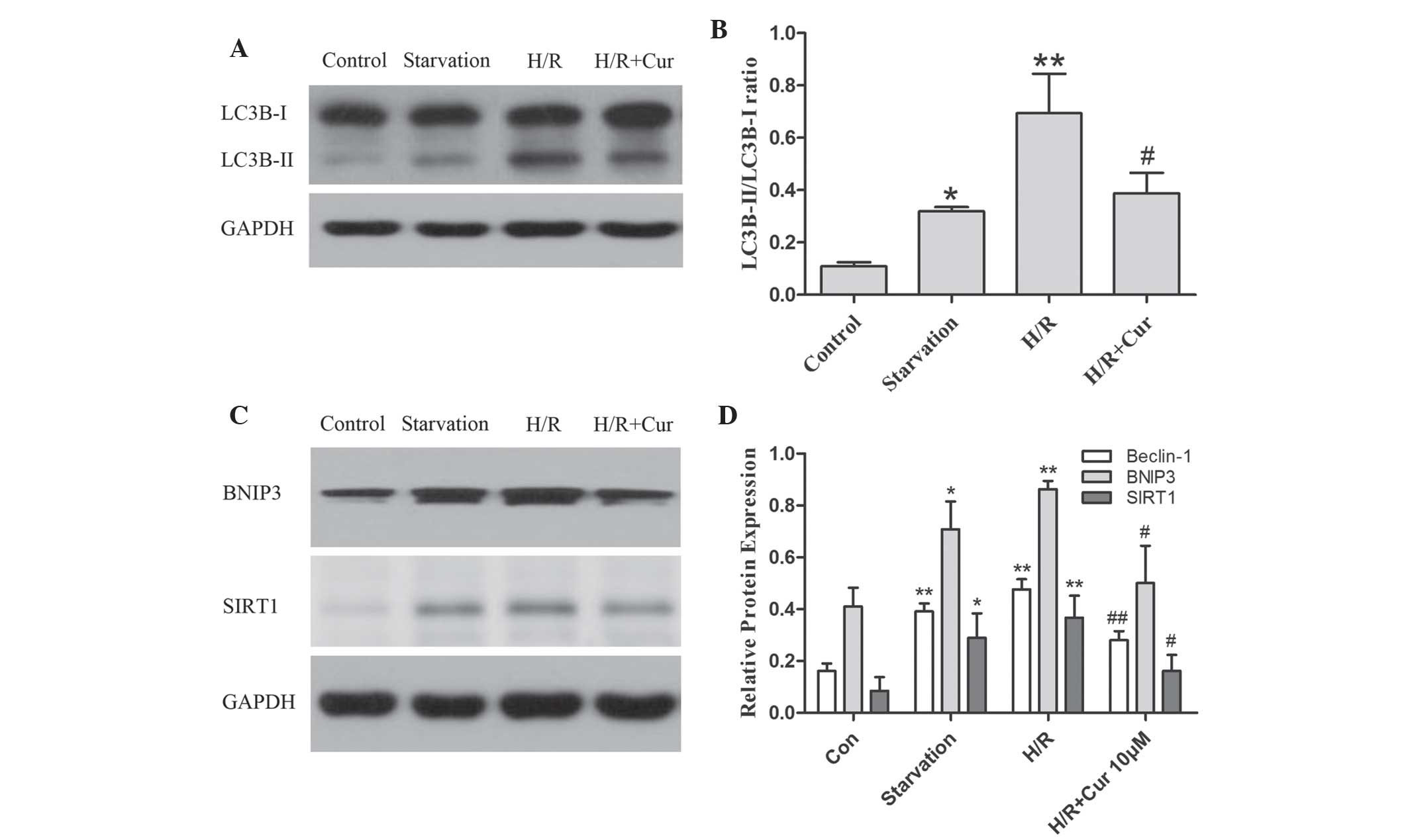 | Figure 4Curcumin downregulates the ratio of
LC3B-II/LC3B-I, a marker of autophagy and autophagy-associated
proteins, including beclin-1, BNIP3 and SIRT1 in H9c2 myocytes
during H/R. The cells were subjected to glucose deprivation, with
the exception of the control group, and were cultured under hypoxic
conditions for 1 h and then reoxygenated for 3 h, with or without
curcumin. The cell lysates from the H9c2 cells were subjected to
(A) western blot analysis. and (B) densitometric measurements of
LC3B-II/LC3B-I. (C) Representative western blot analysis and (D)
densitometric measurements of beclin-1, BINP3, and SIRT1. The band
densities were measured using the Quantity One 1D analysis software
program. Data are expressed as the mean ± standard deviation and
were obtained from three independent experiments
(*P<0.05 and **P<0.01, compared with
the control group; #P<0.05 and
##P<0.01, compared with the H/R group). H/R,
hypoxia/reoxygenation; Cur, curcumin; BNIP3, adenovirus E1B 19 kDa
interacting protein 3 SIRT1, silent information regulation 1. |
Autophagy is a complex biological process involving
several distinct regulatory modules. Therefore, the present study
further examined autophagy-associated proteins, including beclin-1,
SIRT1 and BNIP3, to confirm whether they were involved in the
effects of curcumin on the level of autophagy in myocardial H/R
injury. The results demonstrated that the expression levels of
beclin-1, SIRT1 and BNIP3 were markedly upregulated in the GD and
H/R groups and were significantly downregulated by treatment with
curcumin (Fig. 4C and D). These
results suggested that curcumin affected the level of autophagy by
downregulating the expression levels of beclin-1, SIRT1 and BNIP3
in the H9c2 myocytes during H/R injury.
Discussion
In present study, curcumin significantly increased
the viability of the H9c2 myocytes subjected to H/R by inhibiting
the levels of apoptosis and autophagy. Upregulation of the
expression of Bcl-2 and downregulation of the expression of Bax
were involved in the inhibitory effect of curcumin on the apoptosis
of myocytes during H/R. Additionally, suppression of the expression
levels of beclin-1, SIRT1 and BNIP3 were identified as necessary
molecular mechanism by which curcumin mitigated autophagy activity
during myocardial H/R injury.
Apoptosis is a well-characterized, specific
morphological aspect of cell death, accompanied with nuclear
pyknosis (chromatin condensation) and karyorhexis (nuclear
fragmentation) (28) and is linked
with I/R-induced myocardial injury. The Bcl-2 family protein, Bax,
is a pro-apoptotic protein, which forms complexes with other
proteins in the mitochondrial outer membrane, promoting the release
of pro-apoptotic molecules, including cytochrome C, and activates
apop-tosis (29). Bcl-2 is an
anti-apoptotic molecule that reduces myocardial apoptosis during
reperfusion (30). Bcl-2 inhibits
the action of Bax through direct or indirect mechanisms and reduces
cell death (31,32). The present study demonstrated that
the expression of Bcl-2 was upregulated and the expression of Bax
was downregulated in the H9c2 myocytes treated with curcumin,
promoting cell survival during H/R. Therefore, curcumin inhibited
apoptosis by affecting the expression levels of Bax and Bcl-2.
Autophagy has a dual role in cell survival and cell
death during heart ischemia and reperfusion (33). Induction of autophagy maintains the
homeostasis of cellular ATP and promotes cell survival in the
ischemic heart (34). However,
excessive autophagy is detrimental and can lead to myocyte death
during the reperfusion phase (14). The present study established a
model of H/R damage in H9c2 myocytes to investigate the effect of
curcumin on the level of autophagy. The ratio of LCB3-II/LC3B-I
increased significantly and was associated with a reduction in cell
viability in the myocyte H/R period, suggesting that autophagy
during the reperfusion phase may be detrimental for myocyte
survival. Notably, curcumin treatment significantly downregulated
the ratio of LC3B-II/LC3B-I and improved the survival rate of the
H9c2 myocytes. These results indicated that curcumin promoted cell
survival through inhibiting the excessive activation of autophagy
by H/R.
Several molecular mechanisms, including beclin-1,
BNIP3 and SIRT1, respond to the regulation of autophagy (35–37).
Therefore, the present study examined the changes in the levels of
these autophagy-associated proteins in myocytes during H/R.
Beclin-1, a BH3 domain-only protein (38), is necessary for activating
autophagy during reperfusion and exacerbates cardiomyocyte death
(39). Downregulation of the
expression of beclin-1 using RNA interference in myocytes reduces
the level of I/R-induced autophagy, accompanied by an increased
cell survival (40). These results
indicated that inhibiting the expression of beclin-1 is beneficial
for the survival of myocytes. It has been previously demonstrated
that the upregulation of Bcl-2 is important in suppressing
autophagy (26,41,42).
Bcl-2 binds to beclin-1 via the BH3 domain to form a Bcl-2:beclin-1
complex. This complex prevents beclin-1 from assembling the
pre-autophagosomal structure, thereby inhibiting autophagy
(43). Notably, a previous study
revealed that suppressing the expression of Bcl-2 using small
interfering RNA in breast cancer cells did not activate apoptosis
as expected, but induced autophagic cell death accompanied by an
upregulation in the expression of beclin-1 (44). Due to the association between Bcl-2
and beclin-1 and the importance of the Bcl-2/beclin-1 complex for
autophagy, the present study examined the expression levels of
these proteins in myocytes during H/R. The results demonstrated
that the expression of Bcl-2 was downregulated and the expression
of beclin-1 was upregulated, accompanied by an upregulation in
autophagy in the myocytes during H/R. However, these changes in the
expression levels of Bcl-2 and beclin-1 and the ratio of
LC3B-II/LC3B-I were markedly reversed by curcumin treatment.
Therefore, it was suggested that curcumin may upregulate the
quantity of the Bcl-2/beclin-1 complex and downregulate the
expression of beclin-1, thereby inhibiting autophagy in the H9c2
myocytes during H/R.
BNIP3, another member of the BH3-contaning Bcl-2
family, is responsible for excessive autophagy and the
over-expression of BNIP3 significantly induces autophagy (45). Bellot et al (46) demonstrated that hypoxia-induced
BNIP3 induces autophagy by disrupting the Bcl-2/beclin-1 complex.
Additionally, overexpression of BNIP3 promoted apoptotic cell death
(47,48). SIRT1, a nicotinamide adenine
dinucleotide-dependent deacetylase, regulates critical metabolic
and physiological processes, including autophagy (37). SIRT1 induces autophagy by forming a
molecular complex with several autophagy-associated genes and
directly deacetylating these components (49). The overexpression of SIRT1 is
sufficient to induce an autophagic flux (50), and SIRT1-/- mouse embryonic
fibroblasts fail to fully activate autophagy under conditions of
starvation (49). The upregulation
of BNIP3 and SIRT1 stimulates autophagy or apoptosis in different
cell types (51,52). In the present study the expression
levels of BNIP3 and SIRT1 were significantly upregulated during H/R
in myocytes and curcumin treatment significantly down-regulated the
expression levels of BNIP3 and SIRT1. This was accompanied by
downregulation in the levels of apoptosis and autophagy in the H9c2
myocytes during H/R. Therefore, curcumin inhibited cell
apoptosis/excessive autophagy and promoted cell survival by
downregulating BNIP3 and SIRT1. Therefore, the results suggested
that curcumin assisted in reducing the levels of apoptosis and
autophagy, improving cell viability, upregulating the expression of
Bcl-2 and downregulating the expression levels of Bax and beclin-1.
Additionally, curcumin inhibited the expression of BNIP3 and SIRT1
in the H9c2 myocytes subjected to H/R. Therefore, these findings
demonstrated the protective effect of curcumin against myocardial
H/R injury by reducing the levels of apoptosis and autophagy.
Curcumin may offer a potential therapeutic approach for preventing
myocardial I/R injury.
Acknowledgments
The authors would like to thank Dr Zuoquan Xie for
their technical assistance. This study was supported by grants from
the National Natural Science Foundation of China (no. 81102837),
the Natural Science Foundation of Zhejiang Province (no.
LY13H280004) and the Key Construction Academic Subject (Traditional
Chinese Medicine) of Zhejiang Province (no. 2012-XK-A28).
References
|
1
|
Keeley EC, Boura JA and Grines CL: Primary
angioplasty versus intravenous thrombolytic therapy for acute
myocardial infarction: a quantitative review of 23 randomised
trials. Lancet. 361:13–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ong SB and Gustafsson AB: New roles for
mitochondria in cell death in the reperfused myocardium. Cardiovasc
Res. 94:190–196. 2012. View Article : Google Scholar :
|
|
5
|
Zeng M, Wei X, Wu Z, et al: NF-κB-mediated
induction of autophagy in cardiac ischemia/reperfusion injury.
Biochem Biophys Res Commun. 436:180–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner C, Tillack D, Simonis G, Strasser
RH and Weinbrenner C: Ischemic post-conditioning reduces infarct
size of the in vivo rat heart: role of PI3-K, mTOR, GSK-3beta, and
apoptosis. Mol Cell Biochem. 339:135–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma X, Liu H, Foyil SR, Godar RJ,
Weinheimer CJ and Diwan A: Autophagy is impaired in cardiac
ischemia-reperfusion injury. Autophagy. 8:1394–1396. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin Y, Guan Y, Duan J, et al:
Cardioprotective effect of Danshensu against myocardial
ischemia/reperfusion injury and inhibits apoptosis of H9c2
cardiomyocytes via Akt and ERK1/2 phosphorylation. Eur J Pharmacol.
699:219–226. 2013. View Article : Google Scholar
|
|
9
|
Hadi NR, Yusif FG, Yousif M and Jaen KK:
Both castration and goserelin acetate ameliorate myocardial
ischemia reperfusion injury and apoptosis in male rats. ISRN
Pharmacol. 2014:2069512014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hadi NR, Al-Amran F, Yousif M and Zamil
ST: Antiapoptotic effect of simvastatin ameliorates myocardial
ischemia/reperfusion injury. ISRN Pharmacol. 2013:8150942013.
View Article : Google Scholar
|
|
11
|
Levine B and Klionsky DJ: Development by
self-digestion: molecular mechanisms and biological functions of
autophagy. Dev cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loos B, Genade S, Ellis B, et al: At the
core of survival: autophagy delays the onset of both apoptotic and
necrotic cell death in a model of ischemic cell injury. Exp Cell
Res. 317:1437–1453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levine B and Yuan J: Autophagy in cell
death: An innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsui Y, Takagi H, Qu X, et al: Distinct
roles of autophagy in the heart during ischemia and reperfusion:
roles of AMP-activated protein kinase and Beclin 1 in mediating
autophagy. Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang M, Kim JH, Cho C, et al: Effect of
Acori graminei Rhizoma on contractile dysfunction of ischemic and
reperfused rat heart. Biol Pharm Bull. 29:483–488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang M, Kim JH, Cho C, et al:
Anti-ischemic effect of Aurantii Fructus on contractile dysfunction
of ischemic and reperfused rat heart. J Ethnopharmacol.
111:584–591. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abe Y, Hashimoto S and Horie T: Curcumin
inhibition of inflammatory cytokine production by human peripheral
blood monocytes and alveolar macrophages. Pharmacol Res. 39:41–47.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhandapani KM, Mahesh VB and Brann DW:
Curcumin suppresses growth and chemoresistance of human
glioblastoma cells via AP-1 and NF-kappaB transcription factors. J
Neurochem. 102:522–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kapakos G, Youreva V and Srivastava AK:
Cardiovascular protection by curcumin: molecular aspects. Indian J
Biochem Biophys. 49:306–315. 2012.PubMed/NCBI
|
|
20
|
Yucel AF, Kanter M, Pergel A, Erboga M and
Guzel A: The role of curcumin on intestinal oxidative stress, cell
proliferation and apoptosis after ischemia/reperfusion injury in
rats. J Mol Histol. 42:579–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen SQ, Zhang Y, Xiang JJ and Xiong CL:
Protective effect of curcumin against liver warm
ischemia/reperfusion injury in rat model is associated with
regulation of heat shock protein and antioxidant enzymes. World J
Gastroenterol. 13:1953–1961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Awad AS and El-Sharif AA: Curcumin
immune-mediated and anti-apoptotic mechanisms protect against renal
ischemia/reperfusion and distant organ induced injuries. Int
Immunopharmacol. 11:992–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Li C, Guo H, Kern TS, Huang K and
Zheng L: Curcumin inhibits neuronal and vascular degeneration in
retina after ischemia and reperfusion injury. PloS One.
6:e231942011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gonzalez-Salazar A, Molina-Jijon E, Correa
F, et al: Curcumin protects from cardiac reperfusion damage by
attenuation of oxidant stress and mitochondrial dysfunction.
Cardiovasc Toxicol. 11:357–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Z, Wang C, Wei L, et al: Resveratrol
inhibits EMMPRIN expression via P38 and ERK1/2 pathways in
PMA-induced THP-1 cells. Biochem Biophys Res Commun. 374:517–521.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levine B, Sinha S and Kroemer G: Bcl-2
family members: Dual regulators of apoptosis and autophagy.
Autophagy. 4:600–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kroemer G, Galluzzi L, Vandenabeele P, et
al: Classification of cell death: Recommendations of the
nomenclature committee on cell death. Cell Death Differ. 16:3–11.
2009. View Article : Google Scholar :
|
|
29
|
Wagener FA, Dekker D, Berden JH, et al:
The role of reactive oxygen species in apoptosis of the diabetic
kidney. Apoptosis. 14:1451–1458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian Y, Zhang W, Xia D, et al:
Postconditioning inhibits myocardial apoptosis during prolonged
reperfusion via a JAK2-STAT3-Bcl-2 pathway. J Biomed Sci.
18:532011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fletcher JI, Meusburger S, Hawkins CJ, et
al: Apoptosis is triggered when prosurvival Bcl-2 proteins cannot
restrain Bax. Proc Natl Acad Sci USA. 105:18081–18087. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim H, Rafiuddin-Shah M, Tu HC, et al:
Hierarchical regulation of mitochondrion-dependent apoptosis by
BCL-2 subfamilies. Nat Cell Biol. 8:1348–1358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takagi H, Matsui Y and Sadoshima J: The
role of autophagy in mediating cell survival and death during
ischemia and reperfusion in the heart. Antioxid Redox Signal.
9:1373–1381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takagi H, Matsui Y, Hirotani S, et al:
AMPK mediates autophagy during myocardial ischemia in vivo.
Autophagy. 3:405–407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu LL, Cheng Y and Liu B: Beclin-1:
autophagic regulator and therapeutic target in cancer. Int J
Biochem Cell Biol. 45:921–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J and Ney PA: Role of BNIP3 and NIX
in cell death, autophagy, and mitophagy. Cell Death Differ.
16:939–946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shin BH, Lim Y, Oh HJ, et al:
Pharmacological activation of Sirt1 ameliorates
polyglutamine-induced toxicity through the regulation of autophagy.
PloS One. 8:e649532013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sinha S and Levine B: The autophagy
effector Beclin 1: A novel BH3-only protein. Oncogene. 27:S137–148.
2008. View Article : Google Scholar
|
|
39
|
Przyklenk K, Dong Y, Undyala VV and
Whittaker P: Autophagy as a therapeutic target for
ischaemia/reperfusion injury? Concepts, controversies, and
challenges. Cardiovasc Res. 94:197–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Valentim L, Laurence KM, Townsend PA, et
al: Urocortin inhibits Beclin1-mediated autophagic cell death in
cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol
Cell Cardiol. 40:846–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marquez RT and Xu L: Bcl-2:Beclin 1
complex: Multiple, mechanisms regulating autophagy/apoptosis toggle
switch. Am J Cancer Res. 2:214–221. 2012.PubMed/NCBI
|
|
42
|
Pattingre S, Tassa A, Qu X, et al: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liang XH, Kleeman LK, Jiang HH, et al:
Protection against fatal Sindbis virus encephalitis by beclin, a
novel Bcl-2-interacting protein. J Virol. 72:8586–8596.
1998.PubMed/NCBI
|
|
44
|
Akar U, Chaves-Reyez A, Barria M, et al:
Silencing of Bcl-2 expression by small interfering RNA induces
autophagic cell death in MCF-7 breast cancer cells. Autophagy.
4:669–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hamacher-Brady A, Brady NR, Logue SE, et
al: Response to myocardial ischemia/reperfusion injury involves
Bnip3 and autophagy. Cell Death Differ. 14:146–157. 2007.
View Article : Google Scholar
|
|
46
|
Bellot G, Garcia-Medina R, Gounon P, et
al: Hypoxia-induced autophagy is mediated through hypoxia-inducible
factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol
Cell Biol. 29:2570–2581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yasuda M, Theodorakis P, Subramanian T and
Chinnadurai G: Adenovirus E1B-19K/BCL-2 interacting protein BNIP3
contains a BH3 domain and a mitochondrial targeting sequence. J
Biol Chem. 273:12415–12421. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen G, Ray R, Dubik D, et al: The E1B
19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein
that activates apoptosis. J Exp Med. 186:1975–1983. 1997.
View Article : Google Scholar
|
|
49
|
Hariharan N, Maejima Y, Nakae J, Paik J,
Depinho RA and Sadoshima J: Deacetylation of FoxO by Sirt1 plays an
essential role in mediating starvation-induced autophagy in cardiac
myocytes. Circ res. 107:1470–1482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee IH, Cao L, Mostoslavsky R, et al: A
role for the NAD-dependent deacetylase Sirt1 in the regulation of
autophagy. Proc Natl Acad Sci USA. 105:3374–3379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ou X, Lee MR, Huang X, Messina-Graham S
and Broxmeyer HE: SIRT1 positively regulates autophagy and
mitochondria function in embryonic stem cells under oxidative
stress. Stem Cells. 32:1183–1194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee Y, Lee HY, Hanna RA and Gustafsson AB:
Mitochondrial autophagy by Bnip3 involves Drp1-mediated
mitochondrial fission and recruitment of Parkin in cardiac
myocytes. Am J Physiol Heart Circ Physiol. 301:H1924–1931. 2011.
View Article : Google Scholar : PubMed/NCBI
|