Introduction
Renal cell carcinoma (RCC), which accounts for ~3%
of all malignancies, is one of the most lethal urologic
malignancies (1) and 20–30% of all
patients are diagnosed with metastatic disease (2). Systemic therapeutic strategies for
advanced RCC include surgical management, chemotherapy,
radiotherapy, immunotherapy and molecular targeted therapy
(3–5). Following nephrectomy, 20% of patients
will suffer a relapse and develop metastatic (m)RCC (6). Cytotoxic chemotherapy has
consistently failed to benefit patients (7) and RCC has been identified as being
intrinsically radioresistant (8).
Molecular targeted therapy may prolong the life of patients,
although they often acquire resistance over time (9,10).
In addition, adverse side-effects are often associated with the
treatment, including rashes, diarrhea, edema and weight gain
(11).
Since the prognosis is poor for patients with
advanced RCC or mRCC, there is an urgent demand for further
prognostic improvements. As RCC is an immunogenic tumour, it is a
putative target for immunotherapeutic intervention strategies
(12). Interferon (IFN)-α is an
immunotherapeutic agent generated predominantly by monocytes and
macrophages, which elicit beneficial effects on human health in a
variety of ways. Previous studies revealed that IFN-α modulates the
immune response (13), induces
apoptosis (14) and directly
inhibits the proliferation (15,16)
and differentiation of tumour cells (17). As a type I IFN, IFN-α has been used
clinically. In addition, IFN-α was recommended as a first-line
treatment for clear-cell mRCC in systemic therapy; however, the
therapeutic effects of IFN-α monotherapy are limited in duration
(18).
The cancer immunoediting theory, which hypothesizes
that malignancy results from the imbalance between
immunosurveillance and tumour immune escape (19), has reinvigorated much research
effort in the field of cancer immunology. Previous studies have
revealed that myeloid-derived suppressor cells (MDSCs) are one of
the key drivers of tumourmediated immune evasion. MDSCs promote
tumour growth via different mechanisms (20,21),
and consequently, MDSCs exert a clear prognostic importance in
multiple solid tumour types. Newly acquired data support the
suitability of circulating MDSCs as a predictive marker for cancer
immunotherapy (22).
Lycium barbarum
(Goji berry) has been used in China for 2,000 years.
L. barbarum polysaccharides (LBP), derived from the
water-soluble portion of extract from L. barbarum, are
important bioactive compounds. Previous studies have suggested that
LBP may exert a role in a variety of biological processes,
including immunomodulation, as an anticancer agent, in enhancing
metabolism and in the amelioration of physical fatigue (23,24).
Furthermore, novel data revealed that LBP may activate T-cells
(25), increase macrophage
phagocytosis (26), stimulate the
phenotypic maturation of dendritic cells (DCs) with marked
immunogenicity (27) and arrest
the cell cycle of tumour cells (28). Additionally, a
polysaccharide-protein complex markedly suppressed the growth of
transplantable sarcomas and enhanced the action of macrophages
(26); however, the precise
effects of LBP on RCC cells and the underlying molecular mechanisms
remain to be elucidated.
In the present study the effects and the mechanism
of LBP in combination with IFN-α2b on the murine RCC Renca cell
line in vitro and on renal tumour xenografts in vivo
were analyzed to provide a basis for the clinical use of LBP and
recombinant human IFN-α2b in patients with RCC.
Materials and methods
Murine RCC cell line and cell
culture
The murine RCC cell line, Renca, was purchased from
Shanghai Cell Bank (Shanghai Xin Yu Biotech Co., Ltd, Shanghai,
China). The cells were grown in RPMI-1640 media (Gibco Life
Technologies, Carlsbad, CA, USA), including 10% fetal bovine serum
(FBS; HyClone, GE Healthcare Life Sciences, Logan, UT, USA), 100
U/ml penicillin and 100 µg/ml streptomycin (Gibco Life
Technologies) at 37°C in a humidity-controlled incubator with 5%
CO2.
Cell viability assay
The Renca cells (3.0×103 cells/well) were
seeded into 96-well plates, cultured for 24 h and stimulated with
either fresh RPMI-1640 culture medium, containing either 10% FBS
(control), IFN-α2b (1,000, 2,000, 4,000 or 8,000 IU/ml; Furen
Pharmaceutical Group Co., Ltd., Beijing, China) or LBP (50, 100,
200 or 400 µg/ml; 70% purity, Xian Plant Bio-Engineering
Co., Ltd., Xian, China) for 24, 48 and 72 h. A total of 20
µl 0.5 mg/ml MTT solution (Ameresco, Inc., Framingham, MA,
USA) was added to each well, incubated for 4 h and subsequently the
culture medium was replaced with 150 µl/well dimethyl
sulfoxide (Sigma-Aldrich, St. Louis, MO, USA). The cells were
agitated for 10 min to dissolve the purple crystals. The absorbance
(A) at 570 nm was analyzed using a microplate reader (680; Bio-Rad
Laboratories, Hercules, CA, USA). The cell viability rate (%) was
quantified as follows: (Atreated)/(Acontrol)
× 100%.
Subsequently, the Renca cells (3.0×103
cells/well) were seeded into 96-well plates and cultured for 24 h
in fresh RPMI-1640 culture medium, containing 10% FBS (control),
IFN-α2b (4,000 IU/ml), LBP (200 mg/ml) or IFN-α2b (4,000 IU/ml) in
combination with LBP (200 mg/ml). The cells were cultured for 24,
48 or 72 h, and the remaining stages were performed, as described
above. Each experiment was repeated three times with four wells for
each concentration.
Apoptosis assay
The Renca cells (2×105/well) were seeded
into 6-well plates and following overnight attachment, were treated
with either 10% FBS (control), IFN-α2b (4,000 IU/ml), LBP (200
µg/ml) or IFN-α2b (4,000 IU/ml) in combination with LBP (200
µg/ml), for 48 h. The cells were collected by centrifugation
at 1,000 rpm for 5 min at 37°C. The collected cells were washed
with ice-cold phosphate buffered saline (PBS) and 1X binding buffer
(BD Biosciences, Franklin Lakes, NJ, USA). Subsequently, a 100
µl suspension solution containing 5 µl annexin
V-fluorescein isothiocyanate (FITC; BD Biosciences) and 5 µl
propidium iodide (PI; Sigma-Aldrich) were incubated at room
temperature in the dark for 15 min. Subsequently, 500 µl 1X
binding buffer was added and the samples were measured using a
FACSVantage SE flow cytometer (BD Biosciences). CellQuest software
(BD Biosciences) was used to analyze the flow cytometry data.
Cell cycle analysis
Renca cells stimulated, as described above for the
apoptosis assay for 48 h, were treated with trypsin, centrifuged at
800 rpm for 5 min at 37°C and fixed with pre-chilled 75% ethanol
for >18 h at 4°C. Following treatment with 1% ribonuclease A
(Sigma-Aldrich) for 30 min at 37°C, the cells were stained with 50
µg/ml PI for 30 min at 4°C. A BD FACSVantage SE flow
cytometer was used to examine the cell cycle distribution at 490
nm. The proportion of cells in each cycle were analyzed using
multicycle DNA content and CellQuest cell analysis software. Each
experiment was performed in triplicate.
Western blot analysis
Following seeding the Renca cells (1×106)
into a culture flask, the cells were treated, as described for the
apoptosis assay, for 48 h. The cells were subsequently lysed in
radioimmunoprecipitation assay buffer (Beijing ComWin Biotech Co.,
Ltd., Beijing, China), containing protease inhibitor,
phenylmethylsulfonyl fluoride, and incubated for 30 min on ice. The
lysates were ultracentrifuged at 13,800 × g for 15 min at 4°C and
the supernatant was collected. The protein concentration was
measured using the bicinchoninic acid method (Beyotime Institute of
Biotechnology, Haimen, China). The protein lysates (30 µg)
were separated by SDS-polyacrylamide gel electrophoresis (Beyotime
Institute of Biotechnology) and were transferred onto
polyvinylidene difluoride membranes (Amersham Biosciences, Beijing,
China) using a wet transfer apparatus. Non-fat milk (5%) was used
to block the membranes for 1 h at room temperature, then the
membranes were incubated with primary antibodies overnight at 4°C.
The antibodies were as follows: Rabbit monoclonal anti-Bcl-2
(1:1,000; cat. no. sc-492), anti-Bax (1:1,000; cat. no. sc-526),
anti-cyclin D1 (1:1,000; cat. no. sc-718) and anti-c-Myc (1:1,000;
cat. no. sc-764; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), and rabbit polyclonal anti-glyceralde-hyde-3-phosphate
dehydrogenase (GAPDH; cat. no. sc-25778; 1:5,000). Subsequently,
the membranes were incubated with a 1:2,000 dilution of horseradish
peroxidaselabelled secondary antibody (cat. no. CW0103; Beijing
ComWin Biotech Co., Ltd.) for 1 h. The protein bands were analyzed
using enhanced chemiluminescence (Beijing ComWin Biotech Co., Ltd.)
and an Odyssey Two-Color Infrared Imaging system (LI-COR
Bioscience, Inc., Lincoln, NE, USA). The blots were re-probed and
incubated with anti-GAPDH as a loading control for protein
normalization.
Mouse xenograft model treatment
Animal experiments were performed on the basis of
the institutional animal care and use committee guidelines at
Chongqing Medical University (Chongqing, China). The study was
approved by the ethics committee of the First Affiliated Hospital
of Chongqing Medical University. A total of 28 male BALB/c mice,
aged 4 weeks, were obtained from the Experimental Animal Center of
Chongqing Medical University (Chongqing, China) and were fed on a
standard sterile laboratory diet for >3 days prior to
experiments. The mice were housed 5 per cage in a temperature,
humidity, and light/dark cycles-controlled room (temperature:
22±2°C, humidity: 60%, 12 h light/dark cycles) with ad
libitum access to food and water. The weight of the mice was
15±0.36 g. Renca cells (2×106) mixed 1:1 with Matrigel
(BD Biosciences) in 100 µl PBS were injected subcutaneously
into each mouse. When the tumour size reached 40–50 mm3,
28 mice were selected and randomly assigned to either the control,
IFN-α2b, LBP or IFN-α2b in combination with LBP groups. IFN-α2b was
dissolved in sterile PBS prior to injection and LBP were diluted in
sterile PBS for oral administration. IFN-α2b (200 IU/g) was
administered by intraperitoneal injection twice weekly for 15 days
and LBP were administered by gavage at dosages of 20 µg/g
once daily for 15 days. The control and the IFN-α2b alone groups
were administered 100 µl sterile PBS by gavage, and the
control and the LBP alone groups were administered 100 µl
sterile PBS by intraperitoneal injection. The tumour sizes were
measured using callipers twice weekly and counted as follows: π/6 ×
large diameter × (small diameter)2. The body weights
were measured twice weekly. On the day following the completion of
the 15 day treatment, the animals were sacrificed via cervical
dislocation and the tumors were carefully resected, weighed,
measured and stored at −80°C.
Detection of the CD11b+
Gr-1+ cells from murine bone marrow using flow cytometry
and confocal laser scanning microscopy
The femurs and tibias were obtained immediately
after the four groups of male BALB/c mice were sacrificed, from
which isolated bone marrow cells were labelled using the FITC
anti-mouse Ly-6G/Ly-6C and PE anti-mouse CD11b antibodies (Tianjin
Sungene Biotech Co., Ltd., Tianjin, China). The cells were
subsequently incubated for 30 min at 4°C in the dark. Following two
washes, the cells were analyzed using the BD FACSAriaTMII Special
Order system (BD Biosciences) and a Leica TCS SP2 laser scanning
confocal microscope (Leica, Mannheim, Germany).
Statistical analysis
Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The data are expressed as
the mean ± standard deviation. Multiple comparisons in the
interblock were analyzed using a one-way analysis of variance test,
and individual comparisons were analyzed using Fisher's least
significance difference post-hoc test between the control and
treatment groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of cell proliferation
following treatment with LBP and IFN-α2b in the Renca cells
To investigate the role of LBP and IFN-α2b on the
proliferation of the Renca cells, the cells treated with LBP and
IFN-α2b were assessed using an MTT assay. To confirm the mode of
action of IFN-α2b or LBP alone, and their use in combination, in
Renca cells, the absorption was detected at various concentrations
and durations. The cell viability rate was subsequently calculated
using the above-mentioned equation.
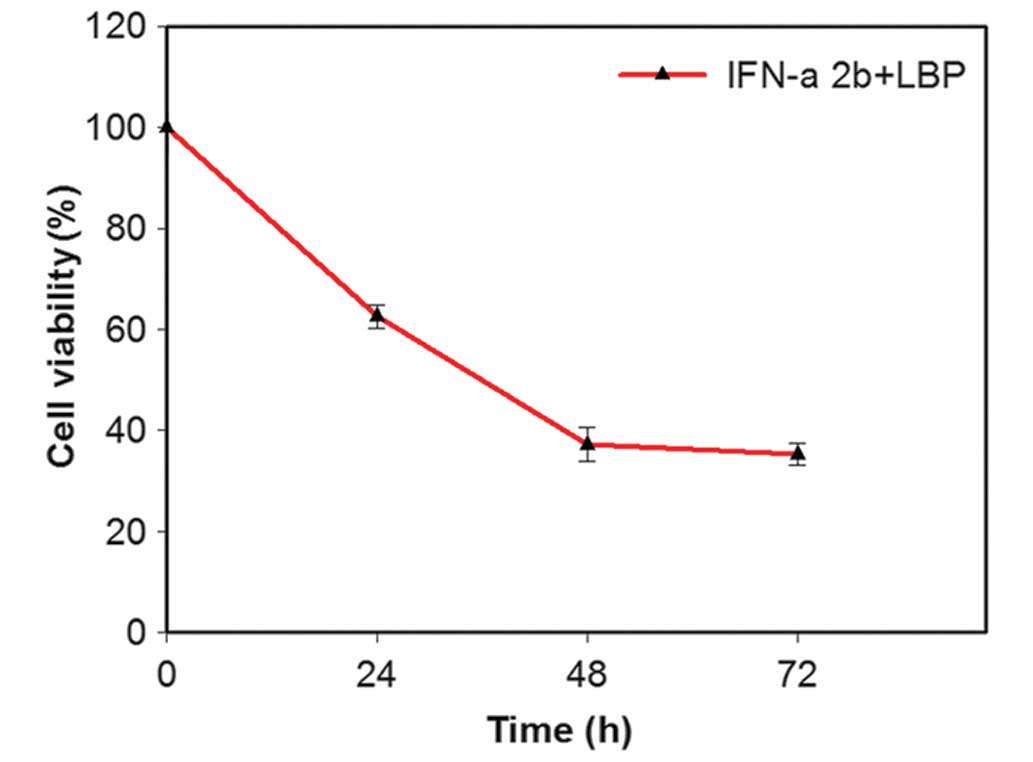
The dose-effect curve demonstrated an effect 48 h
following the addition of IFN-α2b at a concentration of 4,000 IU/ml
and of LBP at a concentration of 200 µg/ml, whereby the
viability of the Renca cells was significantly inhibited. Therefore
concentrations of 4,000 IU/ml IFN-α2b and 200 µg/ml LBP were
selected for subsequent experiments. IFN-α2b (4,000 IU/ml) in
combination with LBP (200 µg/ml) significantly inhibited the
cell proliferation in a time-dependent manner
(**P<0.01; Fig. 1).
The viability of the cells was reduced to 37.0% following this
treatment for 48 h. The Renca cells were treated with different
concentrations of IFN-α2b alone (1,000, 2,000, 4,000 and 8,000
IU/ml) for 48 h, and the cell viability was 79±1.40, 61±1.30,
40±1.30 and 50±1.24%, respectively. The Renca cells were treated
with different concentrations of LBP alone (50, 100, 200 and 400
µg/ml) for 48 h, and the cell viability was 72±1.51,
60±1.21, 41±1.23 and 50±1.11%, respectively.
Combination treatment with LBP and
IFN-α2b promotes the apoptosis of Renca cells
To determine the effect of LBP and IFN-α2b on
apoptosis in Renca cells, the percentage of cells undergoing
apoptosis was confirmed and quantified using an annexin V-FITC/PI
assay. The percentages featured in the lower right and upper right
quadrant of the histograms represent the early and late apoptotic
cells, respectively. Following treatment of the cells, as described
above for 48 h, the total percentage of apoptotic cells was 23.26,
34.39 and 56.37% in the IFN-α2b, LBP and IFN-α2b in combination
with LBP groups, respectively, compared with the control group
(5.43%; *P<0.05 and **P<0.01; Figs. 2A–E). The results demonstrated the
anticancer effect of IFN-α2b in combination with LBP in the Renca
cells.
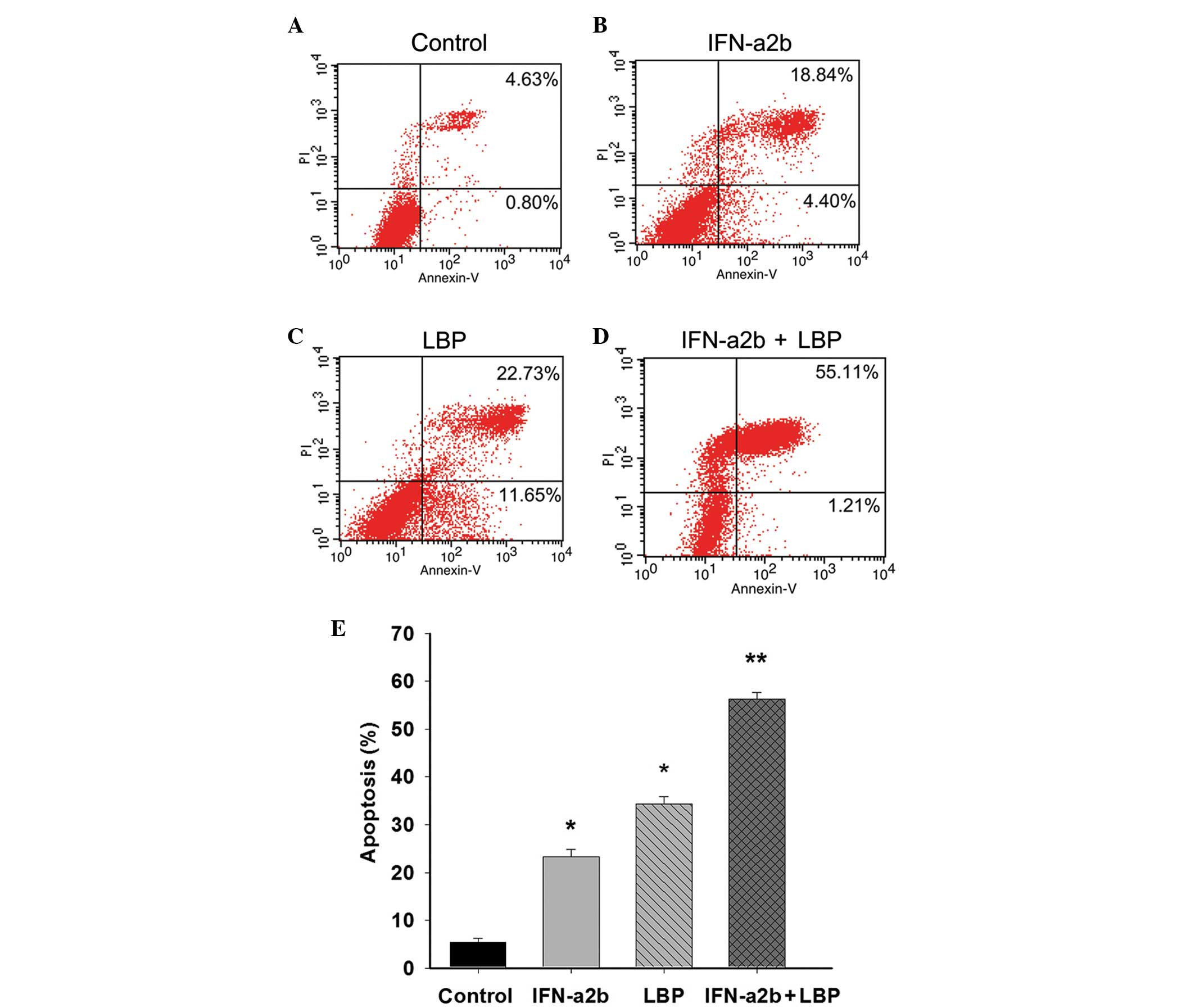 | Figure 2IFN-α2b, in combination with LBP,
promotes apoptosis in Renca cells. The Renca cells were (A)
non-treated or treated with (B) IFN-α2b (4,000 IU/ml), (C) LBP (200
µg/ml) or (D) IFN-α2b plus LBP for 48 h prior to staining
with fluorescein isothiocyanate-annexin V and PI. The percentage of
surviving cells was indicated in the lower left panel of the
quadrant. The percentages indicated in the lower right and upper
right quadrant of the histograms represent the early and late
apoptotic cells, respectively. (E) The percentages of cells
undergoing apoptosis induced by IFN-α2b (4,000 IU/ml), LBP (200
µg/ml) and IFN-α2b plus LBP were quantified. The data are
expressed as the mean ± standard deviation (*P<0.05,
vs. control; **P<0.01, vs. the IFN-α2b or LBP groups
alone). IFN-α2b, interferon-α2b; LBP, L. barbarum
polysaccharides; PI, propidium iodide. |
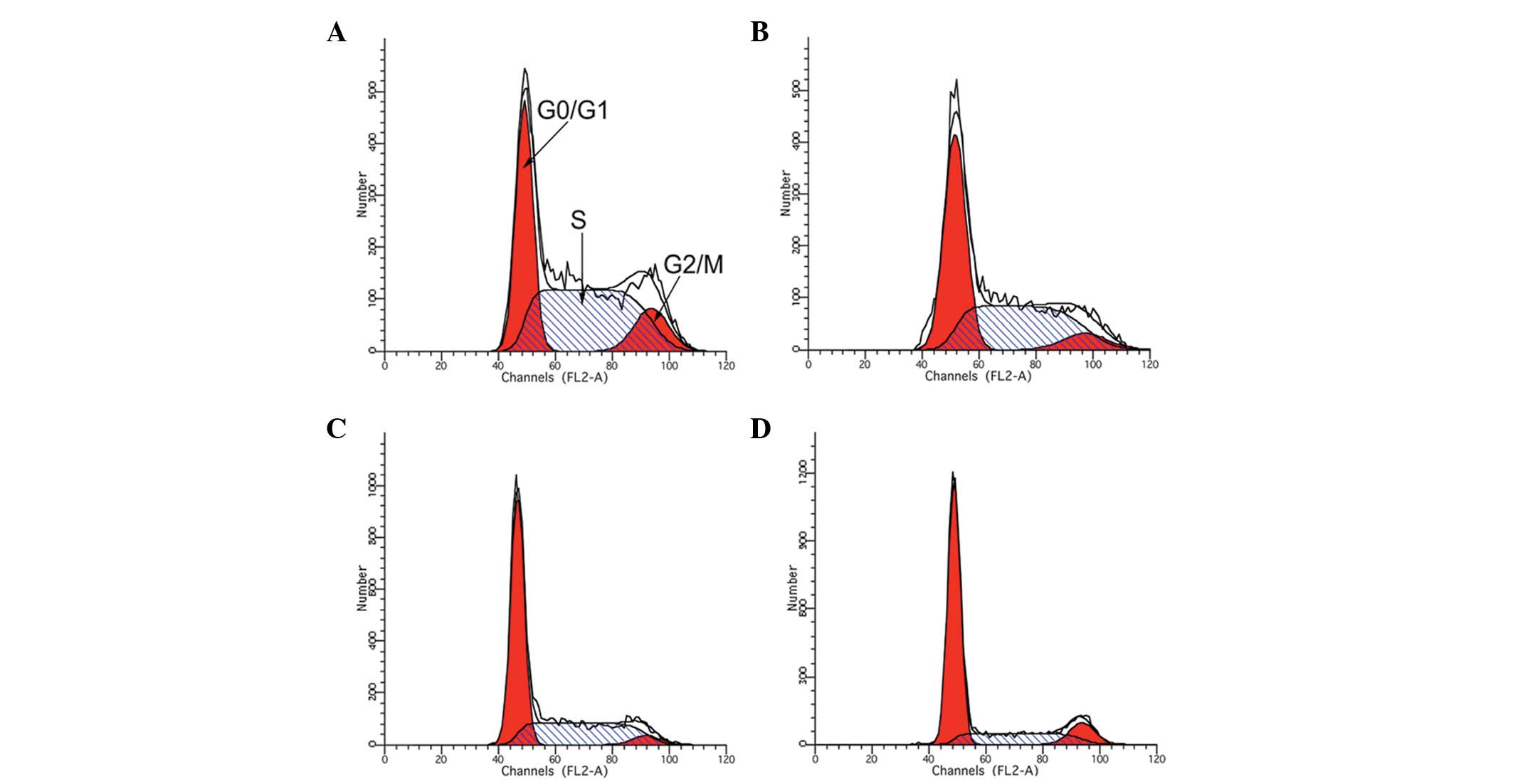
Treatment with LBP and IFN-α2b arrests
the Renca cell cycle
Treatment with IFN-α2b and LBP inhibited Renca cell
growth, which was always associated with cell cycle arrest.
Following treatment of the Renca cells with either IFN-α2b, LBP or
IFN-α2b in combination with LBP for 48 h, flow cytometry was
performed. The results revealed that the combined treatment of the
cells with IFN-α2b and LBP resulted in a more marked accumulation
in the G0/G1 phase compared with that of the control (P<0.01;
Table I and Fig. 3).
 | Table IRenca cell cycle phases stimulated by
IFN-α2b or LBP alone or in combination for 48 h. |
Table I
Renca cell cycle phases stimulated by
IFN-α2b or LBP alone or in combination for 48 h.
| Group | G0/G1 (%) | S (%) | G2/M (%) |
|---|
| Control |
35.4430±0.9710a |
54.3030±2.1500a | 12.693±0.774 |
| IFN-α2b |
47.6160±0.3610a |
46.0430±0.7670a | 6.336±0.80 |
| LBP |
57.0900±0.2950a |
37.780±0.6238a | 4.32±0.353 |
| IFN-α2b + LBP |
67.8160±0.5080a |
21.1060±0.4636a | 14.37±5.637 |
Effect of LBP and IFN-α2b on the
expression of Renca cell-associated proteins
Cyclin D1, one of the critical cell cycle
regulators, promotes the G1/S-phase transition and induces cell
proliferation. c-Myc also influences cell cycle regulation, as the
overexpression of c-Myc stimulates cell cycle progression, whereas
c-Myc downregulation exerts the opposite effect. Bcl-2 protein
inhibits target cell apoptosis, whereas the protein Bax enhances
target cell apoptosis. Following the treatment of the Renca cells
with either IFN-α2b, LBP or IFN-α2b in combination with LBP for 48
h, the expression levels of the above proteins were measured by
western blot analysis (Fig. 4A).
The results revealed that cyclin D1, c-Myc and Bcl-2 were
downregulated, whereas Bax was upregulated, in all treatment groups
compared with the control group, and more significant changes were
identified in the IFN-α2b in combination with LBP group (Figs. 4B–E).
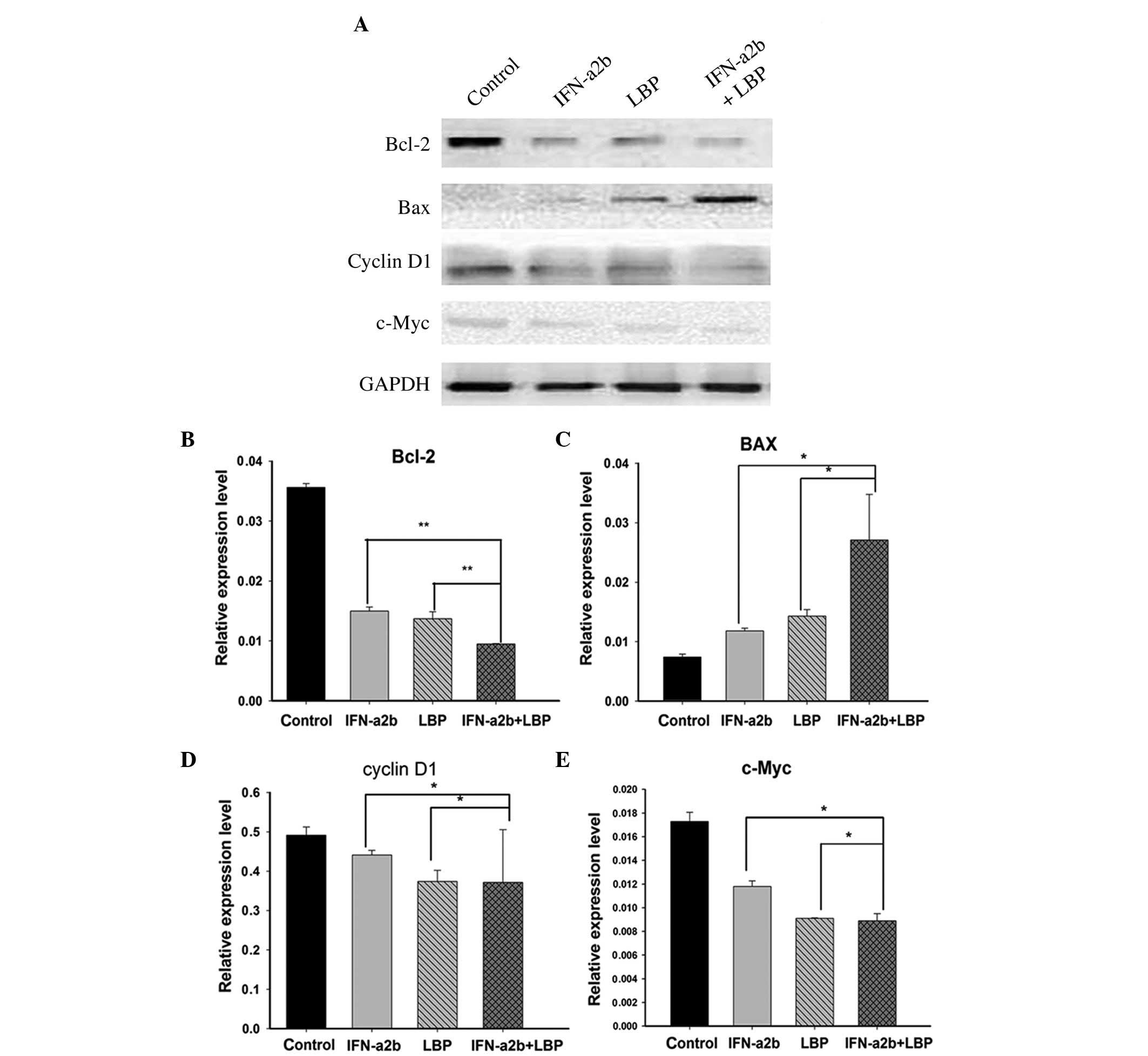 | Figure 4Effect of IFN-α2b and LBP on protein
expression levels. (A) The effects of IFN-α2b (4,000I U/ml) and LBP
(200 µg/ml) on the expression levels of cell
cycle-associated proteins (cyclin D1 and c-Myc) and cell
apoptosis-associated proteins (Bcl-2 and Bax) were investigated by
western blot analysis. The data are representative of three
independent experiments. (B–E) The intensity of the bands was
normalized against the internal standard, GAPDH, and the results
are shown for (B) Bcl-2, (C) BAX, (D) cyclin D1 and (E) c-Myc as a
ratio against the control. The data are expressed as the mean ±
standard deviation (*P<0.05, **P<0.01,
vs. the IFN-α2b alone and LBP alone groups). GAPDH,
glyceraldehyde-3-phosphate dehydrogenase, IFN-α2b, interferon-α2b;
LBP, L. barbarium polysaccharides. |
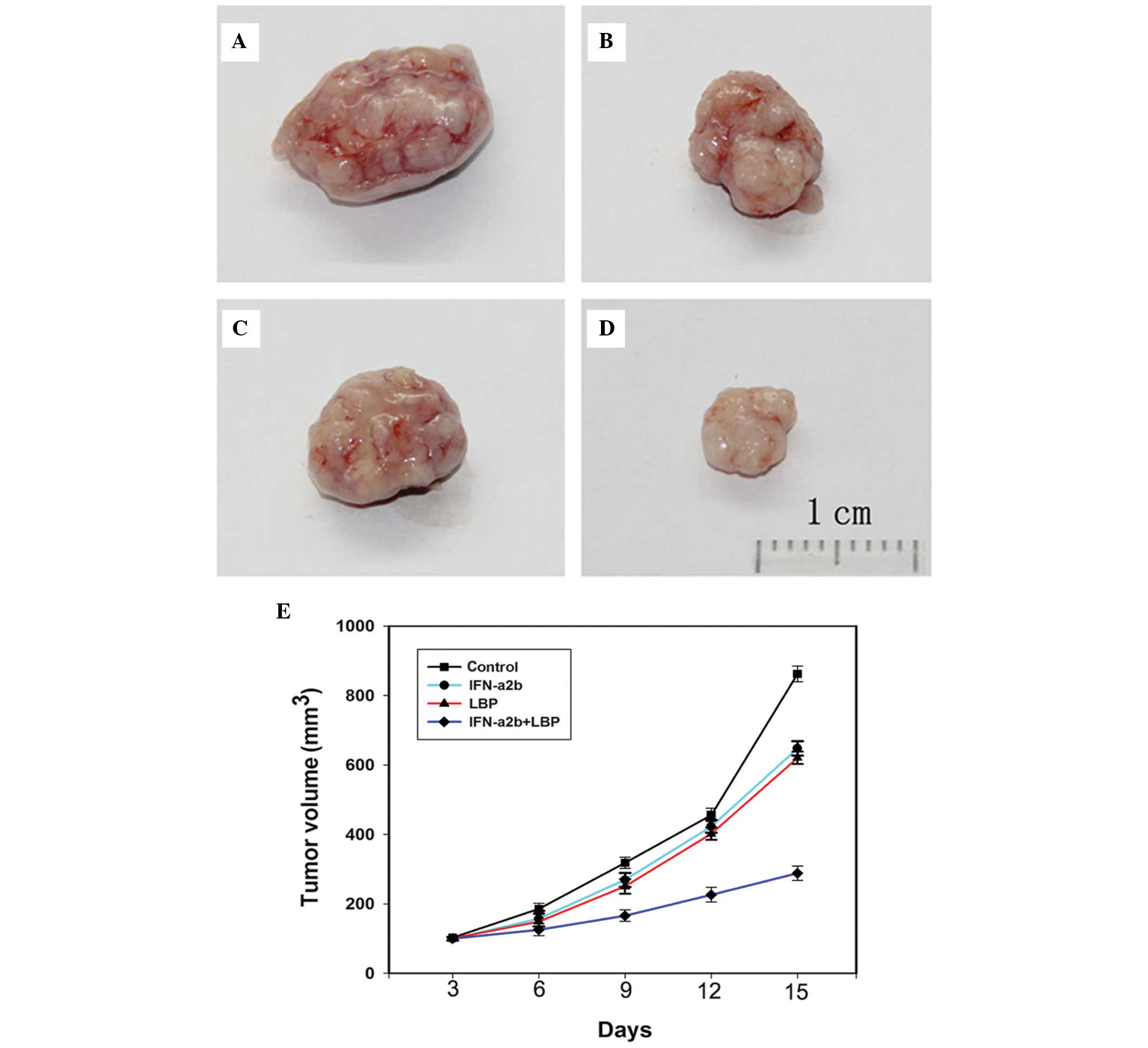
Combined treatment with LBP and IFN-α2b
inhibits tumour growth in a Renca xenograft model
To further determine whether IFN-α2b in combination
with LBP inhibits tumour growth in vivo, BALB/c mice bearing
Renca tumour xenografts were treated with either IFN-α2b, LBP or
IFN-α2b in combination with LBP for 15 days. The doses of drugs
used were determined according to a previous study (29). Compared with the control BALB/c
mice (Fig. 5A), those treated with
IFN-α2b (Fig. 5B) and LBP
(Fig. 5C) exhibited a
significantly smaller tumour volume. In addition, compared with the
groups receiving monotherapy, the tumour volumes in the combination
group xenografts (Fig. 5D) were
markedly smaller. In conclusion, the data indicated that the
antitumor efficacy of LBP combined with IFN-α2b is greater compared
with LBP and IFN-α2b alone (Fig.
5E).
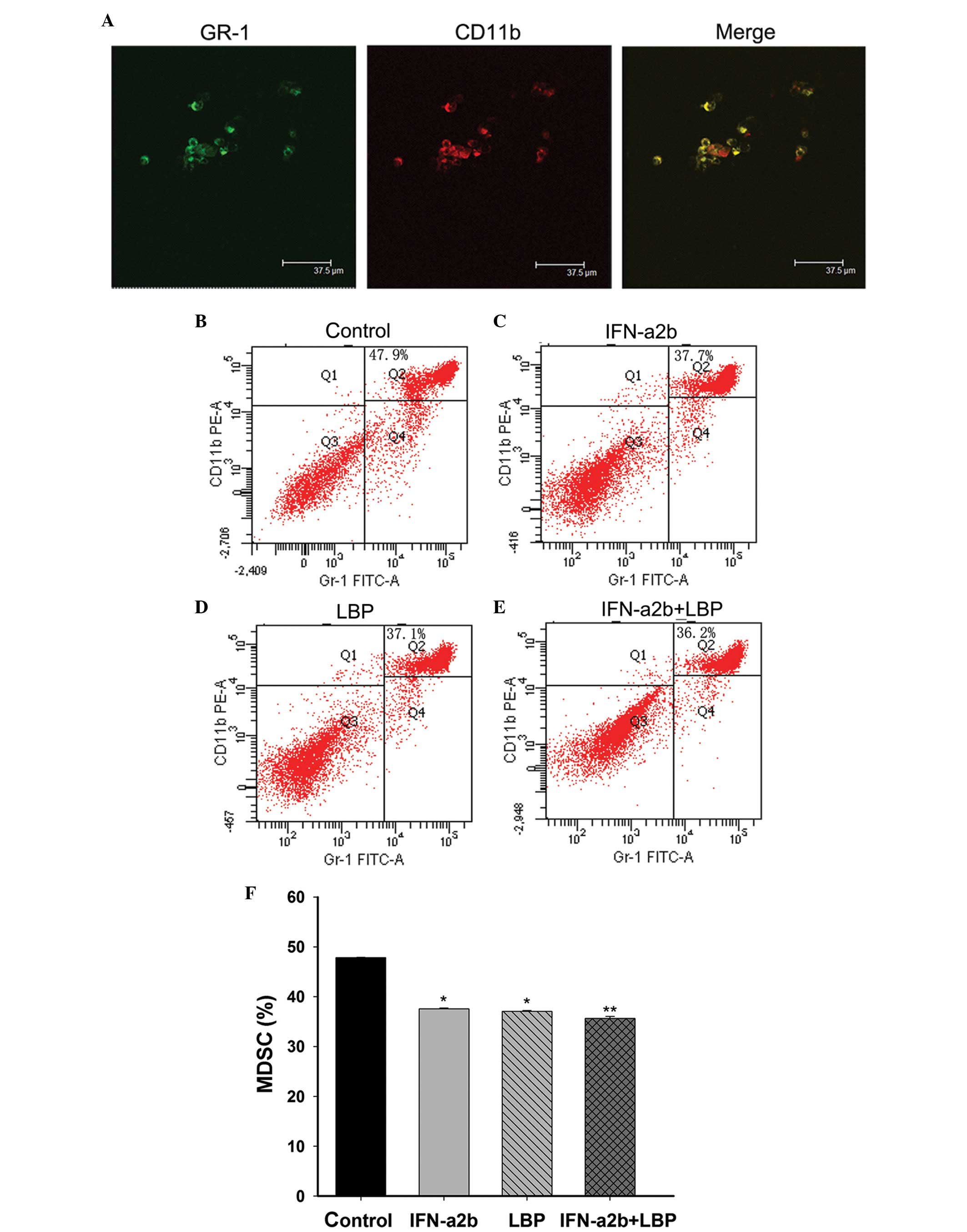
Effect of combination treatmetn with LBP
and IFN-α2b on the CD11b+ Gr-1+ cells in the
bone marrow of tumour-bearing mice and the morphology of the
CD11b+ Gr-1+ cells
MDSCs, which facilitate tumour growth, are one of
the crucial negative immune regulators. MDSCs were defined as
CD11b+Gr-1+ cells in the mouse. The
morphology of the MDSCs was examined using confocal laser scanning
microscopy (Fig. 6A). To determine
the mechanism of IFN-α2b and LBP on Renca cells in vivo, the
proportion of MDSCs in the bone marrow cells from BALB/c mice
bearing Renca tumour xenografts were gated. The tumour-bearing mice
were treated with either IFN-α2b, LBP or IFN-α2b in combination
with LBP for 15 days. The results revealed that the percentages of
CD11b+Gr-1+ bone marrow cells were clearly
reduced in the treatment groups compared with the control group
(Fig. 6B; *P<0.05).
Compared with IFN-α2b alone (Fig.
6C) and LBP alone (Fig. 6D)
groups, the reduction was more pronounced in the tumour-bearing
mice treated with IFN-α2b plus LBP (Fig. 6E; **P<0.01). No
significant reduction was observed between the IFN-α2b and LBP
alone groups.
Discussion
In the present study, the results revealed that the
synergistic immunotherapeutic effects of LBP in combination with
IFN-α2b inhibited the proliferation of mouse RCC Renca cells in
vitro and in vivo.
RCC is considered to be an immunogenic tumour, for
which immunotherapy is one of the systemic therapies. As a
multifunctional cytokine, IFN-α intervenes in tumour cell
proliferation and apoptosis, and has been used in the treatment of
various malignancies. A previous study determined that cell growth
was inhibited by treatment with recombinant human IFN-α1b in human
nasopharyngeal carcinoma (15).
IFN-α markedly inhibits the growth of the pheochromocytoma PC12
line and increases apoptosis (16). Booy et al (30) identified that IFN-α significantly
reduces cell growth in eight human pancreatic cancer cell lines.
Since the short duration of therapeutic effects and
immunotherapeutic efficacy of IFN-α are limited, novel therapeutic
strategies for advanced RCC are required.
LBP perform numerous roles, including in
immunomodulation and anticancer functions. Previous studies
identified that LBP inhibit the proliferation of human QGY7703
hepatoma cells (29), and human
MGC-803 and SGC-7901 gastric cancer cells (28). Since IFN-α and LBP operate
according to different mechanisms of action in tumour cells, their
combined use may potentially offer novel treatment options for RCC;
however, the effect and mechanism of LBP and IFN-α2b in combination
therapy remains to be elucidated.
To investigate the combined effects of LBP and
IFN-α2b in RCC cell lines, the effects of IFN-α2b and LBP on cell
viability were initially measured. An MTT assay revealed that there
was a marked decrease in cell viability in the Renca cells treated
with IFN-α2b or LBP alone; however, cell viability was markedly
diminished by co-treatment with LBP and IFN-α2b for 48 h. From
these observations, co-treatment with IFN-α2b and LBP appears to be
more effective against Renca cell proliferation in vitro
compared with treatment with IFN-α2b or LBP alone.
Data have suggested that the antitumor effects of
immunotherapeutic drugs are associated with the cell cycle. A
previous study demonstrated that T-cells were arrested in the
G1-phase by recombinant human IFN-α1b in human nasopharyngeal
carcinoma (15). Maeda et
al (14) also reported that
IFN-α induces cell cycle arrest in the G0/G1 phase in the HuH7
hepatocellular carcinoma cell line.
To elucidate the inhibitory mechanisms of LBP and
IFN-α2b in Renca cells, flow cytometric analysis was used to detect
the changes in the cell-cycle distribution. The results revealed
that the number of Renca cells in the G0/G1 phase were increased
following drug treatment. In particular, the ratio of G0/G1 was
increased more markedly compared with IFN-α2b or LBP alone. These
results were consistent with previous reports using IFN-α treatment
in RCC (31), human MGC-803
gastric cancer (28) and human
SW480 and Caco-2 colon cancer (32) cells. However, DNA synthesis in
human QGY7703 hepatoma cells was affected and arrested in the S
phase by LBP action (29). The
mechanism of LBP in cancer cells is complicated and differences may
result from comparing across varying types of cells.
With the exception of arresting the cell cycle, the
effect of certain immunotherapeutic agents against cancer has been
largely associated with the induction of apoptosis. Maeda et
al (14) reported that IFN-α
induces apoptosis in the human HuH7 hepatocellular carcinoma cell
line through an IFN-α type-2 receptor-dependent signalling pathway.
Markowitz et al (33)
reported that the combined use of bortezomib and IFN-α
synergistically induced apoptosis in human melanoma cells in
melanoma tumour-bearing mice. Additionally, another previous study
revealed that LBP suppresses the proliferation of HeLa cells
(34) and human QGY7703 hepatoma
cells by inducing apoptosis (29).
In particular, apoptosis was induced to a greater extent by
co-treatment with IFN-α2b or LBP compared with treatment with
IFN-α2b or LBP alone. These results suggested that co-treatment
with IFN-α2b and LBP suppressed Renca cell proliferation by
activating apoptosis.
Emerging evidence has revealed that the cell cycle
regulatory- and apoptosis-associated proteins are implicated in the
anticancer activity of certain immunotherapeutic agents. To confirm
the mechanism of inhibition of proliferation at the protein level,
western blot analysis was performed to detect the expression of
cyclin D1, c-Myc, Bcl-2 and Bax. In the G0/G1 phase, cyclin D1,
which is considered to be one of the predominant cell-cycle
regulators, promoted the G1-S phase transition (35). c-Myc is associated with cell cycle
regulation, as the expression levels of c-Myc are tightly
correlated with cell proliferation. Overexpression of c-Myc
stimulates cell cycle progression, whereas Myc downregulation
exerts the opposite effect. A previous study suggested that IFN-α
inhibits the expression of c-Myc (36). In the present study, the protein
expression levels of cyclin D1 and c-Myc were markedly
downregulated in Renca cells following treatment with LBP and
IFN-α2b, which was as expected, given the known characteristics of
the cell-cycle distribution. These results suggested that the
combination of LBP and IFN-α2b suppressed Renca cell proliferation
to decrease the expression levels of cyclin D1 and c-Myc.
As a proto-oncogene, the Bcl-2 gene inhibits
apoptosis. Overexpression of Bcl-2 enhances the resistance of
cancer cells to the majority of cytotoxins, whereas downregulation
of Bcl-2 expression has been demonstrated to improve
chemo-sensitivity in clinical studies with various carcinomas. As a
proapoptotic gene, the Bax gene exerts the opposite effects.
Zitzmann et al (37)
reported that type I IFN-α mediates apoptosis by changing the ratio
of antiapoptotic Bcl-2 and Bcl-xL to the proapoptotic molecules Bak
and Bax in neuroendocrine tumour cells. A previous study indicated
that tumour necrosis factor-α-induced apoptosis was further
enhanced by IFN-α by lowering the protein expression of c-Myc in
HL-60 cells (38). The expression
levels of the Bcl-2 gene were reduced, and the expression of the
c-Myc gene was inhibited, by IFN-α in the bone marrow mononuclear
cells from chronic granulocytic patients (39).
A previous study revealed that LBP mediates the
expression of Bcl-2 and Bax, while inducing apoptosis in human
prostate cancer cells (40), and
that it also suppresses the expression of the antiapoptotic Bcl-2
gene product in human HL-60 cells (41). Gómez-Benito et al (42) also reported that IFN-α induced
myeloma cell apoptosis via the mitochondrial route. Zhu and Zhang
(34) reported that the inhibitory
effect of LBP on the proliferation of HeLa cells was caused by
inducing apoptosis through the mitochondrial pathway.
In the present study, the expression of Bcl-2 was
reduced and the expression of Bax was higher in the co-treatment
group compared with the control or single drug treatment groups,
which may be due to the downregulation of the expression of Bcl-2
at the protein level by the combined use of LBP and IFN-α2b via the
mitochondrial pathway.
Subsequently, the combined effect of LBP and IFN-α2b
on xenograft tumours was examined, and it was observed that the
tumour volume and weight were markedly reduced by co-treatment with
LBP and IFN-α2b compared with the control or monotherapy groups. No
apparent loss of body weight was observed in the mice treated with
the drugs. From these results, it appears that combination therapy
with LBP and IFN-α2b will be effective against Renca cell
proliferation in vivo.
To further investigate the mechanism of this effect,
the population of MDSCs in isolated bone marrow cells was measured
using flow cytometry and confocal laser scanning microscopy. MDSCs
are a heterogeneous population, comprising progenitor and immature
myeloid cells, which are at different stages of maturation and
continually differentiate into different types of mature immune
cells, including macrophages, DCs and granulocytes. In the mouse,
these cells are usually described as
CD11b+GR-1+ cells (21). MDSCs inhibit the innate and
adaptive immune responses, and immunosuppression is the predominant
function of MDSCs. Immune evasion is a hallmark of cancer, and
MDSCs are one of the key drivers of tumour-mediated immune evasion.
Preclinical data in immunosuppressed murine models suggested that
MDSCs are closely associated with tumour progression and the
metastatic process, independent of their immunosuppressive
properties. The MDSC population promotes tumour growth (20).
Previous studies have revealed that levels of MDSCs
are increased in Sokal high-risk chronic myeloid leukemia (43). IFN-α enhanced antitumor efficiency
by modulating of the suppressor of cytokine signaling 1 function of
CD4 and CD8 T-cells in the context of melanoma (13). Zoglmeier et al (44) reported that recombinant IFN-α
diminished the suppressive abilities of MDSCs in tumour-bearing
mice. Additionally, a previous study reported that LBP induce
phenotypic changes and the maturation of DCs, and enhance host
immunity with marked immunogenicity (45). The antitumor mechanism of LBP in
H22-bearing mice may be exerted through increasing the numbers of
CD4+ and CD8+ T-cells, restoring the function
of the immune system (39).
In the present study, combination treatment with LBP
and IFN-α2b markedly reduced the ratio of MDSCs in vivo.
This indicated that treatment with the combination may decrease the
numbers of MDSCs, increase the numbers of macrophages, DCs,
granulocytes and CD4 and CD8 T-cells, relieve immunosuppression,
and restore innate and adaptive immunity to inhibit the process of
tumour growth. However, certain limitations to the present study
exist, including the use of only one type of RCC cell line and the
in vivo data were generated only from Renca xenograft
tumours. Therefore, the investigation of other RCC cell lines is
required to further evaluate the clinical potential of the combined
use of LBP and IFN-α2b. Additionally, further investigation of the
immune cells in the blood and spleen of mice is required.
In conclusion, LBP and IFN-α2b may inhibit the
progression of Renca xenografts via the following mechanism: The
cells were arrested in the G0/G1 phase, cell proliferation was
inhibited, apoptosis was induced, MDSCs were diminished,
immunosuppression was relieved, and the innate and adaptive
immunity pathways were restored.
Acknowledgments
This study was supported by the National Science
Foundation Research Grant of China (no. 81272572) and the
Foundation of Chongqing Municipal Health Bureau (no. 2012-2-042).
The authors would like to thank the Experimental Animal Center and
Institute of Life Sciences of Chongqing Medical University
(Chongqing, China) for providing a facility to perform the animal
experiments.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev.
34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karam JA, Rini BI, Varella L, Garcia JA,
Dreicer R, Choueiri TK, Jonasch E, Matin SF, Campbell SC, Wood CG,
et al: Metastasectomy after targeted therapy in patients with
advanced renal cell carcinoma. J Urol. 185:439–444. 2011.
View Article : Google Scholar
|
|
4
|
Paly JJ, Hallemeier CL, Biggs PJ,
Niemierko A, Roeder F, Martínez-Mong R, Whitson J, Calvo FA,
Fastner G, Sedlmayer F, et al: Outcomes in a multi-institutional
cohort of patients treated with intraoperative radiation therapy
for advanced or recurrent renal cell carcinoma. Int J Radiat Oncol
Biol Phys. 88:618–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richey SL, Tamboli P, Ng CS, Lin E, Lim
ZD, Araujo JC, Jonasch E, Sharma P, Pagliaro LC and Tannir NM:
Phase II trial of pemetrexed plus gemcitabine in patients with
locally advanced and metastatic nonclear cell renal cell carcinoma.
Am J Clin Oncol. 36:450–454. 2013. View Article : Google Scholar
|
|
6
|
Athar U and Gentile TC: Treatment options
for metastatic renal cell carcinoma: A review. Can J Urol.
15:3954–3966. 2008.PubMed/NCBI
|
|
7
|
Milowsky MI and Nanus DM: Advanced renal
cell carcinoma. Curr Treat Options Oncol. 2:437–445. 2001.
View Article : Google Scholar
|
|
8
|
Blanco AI, Teh BS and Amato RJ: Role of
radiation therapy in the management of renal cell cancer. Cancers
(Basel). 3:4010–4023. 2011. View Article : Google Scholar
|
|
9
|
Rini BI and Atkins MB: Resistance to
targeted therapy in renal-cell carcinoma. Lancet Oncol.
10:992–1000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katakami N, Atagi S, Goto K, Hida T, Horai
T, Inoue A, Ichinose Y, Koboyashi K, Takeda K, Kiura K, et al:
LUX-Lung 4: a phase II trial of afatinib in patients with advanced
non-small-cell lung cancer who progressed during prior treatment
with erlotinib, gefitinib, or both. J Clin Oncol. 31:3335–3341.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smolle E, Taucher V, Petru E and Haybaeck
J: Targeted treatment of ovarian cancer–the multiple - kinase -
inhibitor sorafenib as a potential option. Anticancer Res.
34:1519–1530. 2014.PubMed/NCBI
|
|
12
|
Yang JC and Childs R: Immunotherapy for
renal cell cancer. J Clin Oncol. 24:5576–5583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guenterberg KD, Lesinski GB, Mundy-Bosse
BL, Karpa VI, Jaime-Ramirez AC, Wei L and Carson WE III: Enhanced
anti-tumor activity of interferon-alpha in SOCS1-deficient mice is
mediated by CD4+ and CD8+ T-cells. Cancer
Immunol Immunother. 60:1281–1288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maeda S, Wada H, Naito Y, Nagano H,
Simmons S, Kagawa Y, Naito A, Kikuta J, Ishii T, Tomimaru Y, et al:
Interferon-α acts on the S/G2/M phases to induce apoptosis in the
G1 phase of an IFNAR2-expressing hepatocellular carcinoma cell
line. J Biol Chem. 289:23786–23795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Lu J, He ML, i Z, Zhang B, Zhou LH,
Li Q, Li G, Wang L, Tian WD, et al: Antitumor effects of
interferon-alpha on cell growth and metastasis in human
nasopharyngeal carcinoma. Curr Cancer Drug Targets. 12:561–570.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motylewska E, Lawnicka H,
Kowalewicz-Kulbat M, Sicinska P, Niedziela A, Melen-Mucha G and
Stepien H: Interferon alpha and rapamycin inhibit the growth of
pheochromocytoma PC12 line in vitro. Endokrynol Pol. 64:368–374.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolf B, Schwarzer A, Côté AL, Hampton TH,
Schwaab T, Huarte E, Tomlinson CR, Gui J, Fisher JL, Fadul CE, et
al: Gene expression profile of peripheral blood lymphocytes from
renal cell carcinoma patients treated with IL-2, interferon-α and
dendritic cell vaccine. PloS One. 7:e502212012. View Article : Google Scholar
|
|
18
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A and Bex A:
EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol.
67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dunn GP, Old LJ and Schreiber RD: The
three Es of cancer immunoediting. Annu Rev Immunol. 22:329–360.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marigo I, Dolcetti L, Serafini P,
Zanovello P and Bronte V: Tumor-induced tolerance and immune
suppression by myeloid derived suppressor cells. Immunol Rev.
222:162–179. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diaz-Montero M, Finke J and Montero AJ:
Myeloid derived suppressor cells in cancer: Therapeutic,
predictive, and prognostic implications. Semin Oncol. 41:174–184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao R, Li Q and Xiao B: Effect of Lycium
barbarum polysac-charide on the improvement of insulin resistance
in NIDDM rats. Yakugaku Zasshi. 125:981–988. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XM: Protective effect of Lycium
barbarum polysaccharides on streptozotocin-induced oxidative stress
in rats. Int J Biol Macromol. 40:461–465. 2007. View Article : Google Scholar
|
|
25
|
Chen Z, Kwong Huat Tan B and Chan SH:
Activation of T lymphocytes by polysaccharide-protein complex from
Lycium barbarum L. Int Immunopharmacol. 8:1663–1671. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gan L, Hua Zhang S, Liang Yang X and Bi Xu
H: Immunomodulation and antitumor activity by a
polysaccharide-protein complex from Lycium barbarum. Int
Immunopharmacol. 4:563–569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Z, Lu J, Srinivasan N, Tan BK and
Chan SH: Polysaccharide-protein complex from Lycium barbarum L. is
a novel stimulus of dendritic cell immunogenicity. J Immunol.
182:3503–3509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miao Y, Xiao B, Jiang Z, Guo Y, Mao F,
Zhao J, Huang X and Guo J: Growth inhibition and cell-cycle arrest
of human gastric cancer cells by Lycium barbarum polysaccharide.
Med Oncol. 27:785–790. 2010. View Article : Google Scholar
|
|
29
|
Zhang M, Chen H, Huang J, Li Z, Zhu C and
Zhang S: Effect of Lycium barbarum polysaccharide on human hepatoma
QGY7703 cells: Inhibition of proliferation and induction of
apoptosis. Life Sci. 76:2115–2124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Booy S, van Eijck CH, Dogan F, van
Koetsveld PM and Hofland LJ: Influence of type–I Interferon
receptor expression level on the response to type-I Interferons in
human pancreatic cancer cells. J Cell Mol Med. 18:492–502. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shang D, Yang P, Liu Y, Song J, Zhang F
and Tian Y: Interferon-α induces G1 cell-cycle arrest in renal cell
carcinoma cells via activation of Jak-Stat signaling. Cancer
Invest. 29:347–352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao F, Xiao B, Jiang Z, Zhao J, Huang X
and Guo J: Anticancer effect of Lycium barbarum polysaccharides on
colon cancer cells involves G0/G1 phase arrest. Med Oncol.
28:121–126. 2011. View Article : Google Scholar
|
|
33
|
Markowitz J, Luedke EA, Grignol VP, Hade
EM, Paul BK, Mundy-Bosse BL, Brooks TR, Dao TV, Kondalasula SV,
Lesinski GB, et al: A phase I trial of bortezomib and
interferon-alpha-2b in metastatic melanoma. J Immunother. 37:55–62.
2014. View Article : Google Scholar :
|
|
34
|
Zhu CP and Zhang SH: Lycium barbarum
polysaccharide inhibits the proliferation of HeLa cells by inducing
apoptosis. J Sci Food Agric. 93:149–156. 2013. View Article : Google Scholar
|
|
35
|
McIntosh GG, Anderson JJ, Milton I,
Steward M, Parr AH, Thomas MD, Henry JA, Angus B, Lennard TW and
Horne CH: Determination of the prognostic value of cyclin D1
overexpression in breast cancer. Oncogene. 11:885–891.
1995.PubMed/NCBI
|
|
36
|
Chen H, Tang L, Peng X, Luo Z, Luo S and
Tan W: Effects of IFN-alpha combined with IL-6 on cell growth and
related genes expression and apoptosis of bone marrow cells from
CGL patients. Zhonghua Xue Ye Xue Za Zhi. 21:341–344. 2000.In
Chinese.
|
|
37
|
Zitzmann K, Brand S, De Toni EN, Baehs S,
Göke B, Meinecke J, Spöttl G, Meyer HH and Auernhammer CJ: SOCS1
silencing enhances antitumor activity of type I IFNs by regulating
apoptosis in neuroendocrine tumor cells. Cancer Res. 67:5025–5032.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakashima A, Kumakura S, Mishima S,
Ishikura H and Kobayashi S: IFN-alpha enhances TNF-alpha-induced
apoptosis through downregulation of c-Myc protein expression in
HL-60 cells. J Exp Clin Cancer Res. 24:447–456. 2005.PubMed/NCBI
|
|
39
|
He YL, Ying Y, Xu YL, Su JF, Luo H and
Wang HF: Effects of Lycium barbarum polysaccharide on tumor
microenvironment T-lymphocyte subsets and dendritic cells in
H22-bearing mice. Chin Integr Med. 3:374–377. 2005.In Chinese.
View Article : Google Scholar
|
|
40
|
Luo Q, Li Z, Yan J, Zhu F, Xu RJ and Cai
YZ: Lycium barbarum polysaccharides induce apoptosis in human
prostate cancer cells and inhibits prostate cancer growth in a
xenograft mouse model of human prostate cancer. J Med Food.
12:695–703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gan L, Wang J and Zhang S: Inhibition the
growth of human leukemia cells by Lycium barbarum polysaccharide. J
Hygiene Res. 30:333–335. 2001.In Chinese.
|
|
42
|
Gómez-Benito M, Balsas P, Carvajal-Vergara
X, Pandiella A, Anel A, Marzo I and Naval J: Mechanism of apoptosis
induced by IFN-alpha in human myeloma cells: Role of Jak1 and Bim
and potentiation by rapamycin. Cell Signal. 19:844–854. 2007.
View Article : Google Scholar
|
|
43
|
Christiansson L, Söderlund S, Svensson E,
Mustjoki S, Bengtsson M, Simonsson B, Olsson-Strömberg U and Loskog
AS: Increased level of myeloid-derived suppressor cells, programmed
death receptor ligand 1/programmed death receptor 1, and soluble
CD25 in Sokal high risk chronic myeloid leukemia. PLoS One.
8:e558182013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zoglmeier C, Bauer H, Nörenberg D,
Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S and
Bourquin C: CpG blocks immunosuppression by myeloid-derived
suppressor cells in tumor-bearing mice. Clin Cancer Res.
17:1765–1775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu J, Zhao LH and Chen Z: Stimulation by
Lycium bararum polysaccharides of the maturation of dendritic cells
in murine bone marrow. Zhejiang da xue xue bao. Journal of Zhejiang
University. Med Sci. 35:648–652. 2006.In Chinese.
|