Introduction
Age-related macular degeneration (AMD) is the
leading cause of blindness in the elderly population, as it results
in a loss of vision due to damage to the retina. Based on clinical
findings, AMD may be divided into 'dry' and 'wet' AMD (1,2).
Drusen are often used to diagnose AMD, they are also associated
with the geographic atrophy of the retinal pigment epithelial (RPE)
cells in dry-AMD and increase the risk of developing the exudative
form of AMD (wet-AMD) (3,4). Amyloid-β (Aβ) is an important
constituent of drusen and is primarily associated with
neurodegenerative processes in the brain during Alzheimer's disease
(AD) (5,6). Previous studies indicated that Aβ is
also an important contributor to the progression of AMD (3,7).
The Aβ is a peptide that is ubiquitously and
normally expressed in humans in two forms of 39- and 43-amino acids
in length (termed Aβ40 and Aβ42, respectively) (8,9).
Aβ42 is associated with AD plaques that are composed of a multitude
of highly aggregated Aβ fibrils, and represent abnormal
pathological lesions (10). Aβ40,
the more common and less harmful form, is present in drusen and
activates the inflammatory response in RPE cells aiding in AMD
progression (11). Bruban et
al (12) reported that the
oligomeric form of Aβ1-42 (OAβ1-42), is toxic for RPE cells in
vitro and in vivo. Furthermore, subsequent studies
demonstrated that Aβ upregulated the inflammasome-associated
factors interleukin-1β (IL-1β), IL-18 and caspase-1 (11,13),
and cytokines IL-6, IL-8 and tumor necrosis factor-α (TNF-α)
(14,15). Additionally, angiogenic factors
have also been associated with Aβ in AD. Ets-1, an angiogenic
transcription factor has been identified to co-localize with
vascular endothelial growth factor (VEGF) and soluble Aβ in the
microvasculature (16). In
addition, astrocytes have been shown to be involved with Aβ-induced
angiogenesis in rat hippocampal cells (17). Therefore, Aβ may be responsible for
triggering the angiogenic and inflammatory responses involved in
the pathogenesis of AMD (14–18).
Toll-like receptor 4 (TLR4) is involved in microbial
and autoimmune pathogenesis, as well as chronic inflammatory
conditions (19). The TLR4 gene is
located on the 9q32-33 chromosome region that harbors a potential
AMD susceptibility locus (20,21).
TLR4 was also identified to be involved in the phagocytosis of the
outer photoreceptor segments in RPE cells (22). Impairing phagocytosis in the
photoreceptor layer, causes accumulation of lipofuscin fluorophore,
which may lead to AMD. Aβ may induce upregulation of TLR4 and
nuclear factor-κB (NF-κB) expression in microglia as reported by
Zhou et al (23). However,
the link between the TLR4 signaling pathway and Aβ in RPE cells
remains poorly understood.
The current study aimed to determine whether Aβ
triggered upregulation of TLR4 and NF-κB expression and whether Aβ
activates the release of cytokines and growth factors via the TLR4
and NF-κB signaling pathways in RPE, thus aiding the progression of
AMD. The results of the present study provide strong evidence in
support of the involvement of Aβ-induced local inflammatory
response in RPE cells in the pathogenesis of AMD.
Materials and methods
RPE cell culture and cell treatment
The human ARPE19 cell line [CRL-2302; American Type
Culture Collection (ATCC), Manassas, VA, USA] is a non-transformed
human RPE cell line that has numerous differentiated properties
typical of RPE cells in vivo. ARPE-19 cells were cultured in
Dulbecco's modified Eagle's medium: F12 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal calf serum (Gibco; Thermo Fisher Scientific,
Inc.). At 80% confluence, the ARPE-19 cultures were treated with 1
mM concentrations of the Aβ peptides (Bachem Distribution Services
GmbH, Weil am Rhein, Germany) for 30 min, 1, 3, 6, 12 or 24 h and
their respective inactive reverse peptides (Bachem Distribution
Services GmbH), which served as controls. All of the cells were
used between passage 3 and 7. For the TLR4 inhibitor (COBRA; Novus
Biologicals, LLC, San Diego, CA, USA) treatment group, cells were
pre-treated with the TLR4 inhibitor, 20 µM COBRA, 4 h prior
to being exposed to the Aβ peptides. COBRA interferes with
interaction between TIRAP/Mal and the TIR domain of TLR2 or
TLR4.
Procedure of Aβ oligomerization
Aβ1-42 (H-1368), Aβ42-1 (H-3976, inactive reverse
control peptide of Aβ1-42), Aβ1-40 (H-1194) and Aβ40-1 (H-2972,
inactive reverse control peptide of Aβ1-40), were used in the
experiments and supplied by Bachem Holding AG (Bubendorf,
Switzerland). The preparation of the oligomerization Aβ1-42 has
been previously described by Bruban et al (12) (technical notes on the
solubilization and oligomerization of the Aβ peptides supplied by
Bachem Holding AG). Non-oligomerized Aβ1-40 and Aβ1-42 (Bachem
Distribution Services GmbH) were incubated at 37°C for 5 days and
stored at −20°C until they were used. Each aliquot was used only
once.
Gene expression of cytokines in ARPE-19
cells using the QuantiGenePlex 6.0 Reagent system
Target-specific RNA molecules of ARPE-19 cells
[TLR4: NM_003266; myeloid differentiation factor 88 (MyD88):
NM_002468; NF-κB: NM_003998; IL-6:NM_000600; IL-8:NM_000584; IL-33:
NM_033439; VEGF: NM_003376; basic fibroblast growth factor (bFGF):
NM_002006; angiopoietin 2 (Ang2): NM_001147] were detected using
the QuantiGenePlex 6.0 Reagent System according to the
manufacturer's protocol (Affymetrix, Inc., Fremont, CA, USA). RNA
extracted from cell lysates was captured by fluorescent
microspheres (Affymetrix, Inc.). Signals of cascade amplification
were detected with the Luminex 100 xMAP system and Bio-Plex
software (version 5.0; Bio-Rad Laboratories, Hercules, CA, USA).
The geometric means of the two housekeeping genes, peptidylprolyl
isomerase B (NM_011149) and hypoxanthine phosphoribosyltransferase
1 (NM_013556) for the ARPE19 cells were used for normalizations.
Fold-changes were the relative ratios between the normalized values
of the four infected groups and the values of the untreated group.
ARPE19 cells from three different cell samples were combined for
one detection, and the experiments were repeated three times.
Multiplex cytokine assay
Procarta cytokine profiling kit (Affymetrix, Inc.)
was used to simultaneously detect IL-6, IL-8, VEGF, and bFGF in the
cell culture supernatants, according to manufacturer's protocol.
Antibody beads (50 µl) were added to each well of the filter
plate and washed with wash buffer. Then, 50 µl cell culture
supernatant was added to each well, incubated for 1 h at room
temperature, and washed with wash buffer. Subsequently, 25
µl per well of the detection antibody was added and the
filter plate was shaken at 30 × g for 30 min at room temperature.
Following the addition of Streptavidin-phycoerythrin, the signals
were detected using a Luminex 200 instrument (Bio-Rad
Laboratories).
Enzyme-linked immunosorbent assay
(ELISA)
The levels of IL-33 and Ang2 were determined using
Human IL-33 Quantikine ELISA kit (R&D Systems, Inc.,
Minneapolis, MN, USA) and Human Ang2 Quantikine ELISA kit (R&D
Systems, Inc.,) according to the manufacturer's instructions. The
100-µl cell culture supernatant was added to the wells
(1×105 cells/well), which was pre-coated with IL-33 and
Ang2 antibodies. The absorbance was measured at 450 nm by
microplate reader, model 450 (Bio-Rad Laboratories). All
experiments were performed at least three times.
Western blot analysis
RPE cells were harvested and lysed in
radioimmunoprecipitation assay buffer (1% Nonidet P-40, 0.5% sodium
deoxycholate and 0.1% sodium dodecyl sulfate in phosphate-buffered
saline; Affymetrix, Inc.) and centrifuged at 25,000 × g for 15 min
at 4°C. NuPAGE Bis-Tris gels (10%; Invitrogen) were used according
to the manufacturer's protocol. The membranes were blocked with 10%
fat-free milk and incubated with primary antibodies overnight at
4°C. The anti-TLR4 antibody, purchased from Abcam (ab22048;
Cambridge, MA, USA) was used at a 1:1,000 dilution. The following
primary antibodies were purchased from Cell Signaling Technology,
Inc. (Beverly, MA, USA) and were used at a 1:1,000 dilution: MyD88
(cat. no. 4283), phosphorylation-NF-κB (cat. no. 3033), and β-actin
(cat. no. 4970). The anti-rabbit (cat. no. 7074) and anti-mouse
(cat. no. 7076) horseradish peroxidase-conjugated secondary
antibodies (dilution, 1:2,000 for the two; Cell Signaling
Technology, Inc.) were applied for 1 h at room temperature. Labeled
proteins were detected using an enhanced chemiluminescence western
blotting system (Pierce Biotechnology, Inc., Rockford, IL, USA).
Image J analysis software (version 1.49; National Institutes of
Health, Bethesda, MD, USA) was used to quantify the optical density
of each band. Relative changes in protein expression were
calculated in relation to normal group and expressed as a
fold-change. Each experiment was repeated at least three times.
Tube-formation assay
Human umbilical vein endothelial cells (HUVECs;
CRL-1730, ATCC) were used in the current study for in vitro
evaluation of angiogenesis as previously described (24). All RPE cells were exposed to Aβ;
however, two treatment groups were also established, one where the
cells were exposed to 20 µM COBRA for 4 h and another where
they were not. Subsequently, 200 µl Matrigel (BD
Biosciences, San Diego, CA, USA) was added to a 24-well pre-cooled
plate and then allowed to polymerize at 37°C for 30 min.
Trypsin-harvested HUVEC cells (1×105 cells/well)
suspended in 300 µl of the fresh assay medium were seeded
onto Matrigel and incubated for 8 h. The networks from five
randomly selected fields were photographed (Eclipse 50i; Nikon
Corporation, Tokyo, Japan). The length of the capillary-like
structures, in two-dimensional microscope images, was measured
using Image J software (version 1.49). The experiments were
repeated three times.
Statistical analysis
Data analysis was performed using the following
statistical software programs: Prism version 5.0 (GraphPad
Software, Inc., San Diego, CA, USA) and SPSS version 16.0 (SPSS,
Inc., Chicago, IL, USA). All data are presented as the mean ±
standard error. Data sets were examined by one-way analysis of
variance followed by a post hoc Dunnett's test. For group
comparisons, generalized linear mixed models were used as the
present data was measured and obtained at various time-points.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Different Aβ agents upregulate mRNA
levels of cytokines in ARPE19 cells
In the current study, different Aβ agents were used
to stimulate ARPE19 cells. The mRNA expression levels of all
cytokines was shown in Fig. 1. In
this assay, TLR4, MyD88 and NF-κB were upregulated at 3, 6 and 12
h, respectively. Following a 24 h incubation, TLR4, MyD88 and NF-κB
increased to 6.0±0.37, 4.3±0.44 and 5.4±0.32-fold, respectively, in
the OAβ1-42 group, which was significantly higher than in the other
groups (Fig. 1A–C)
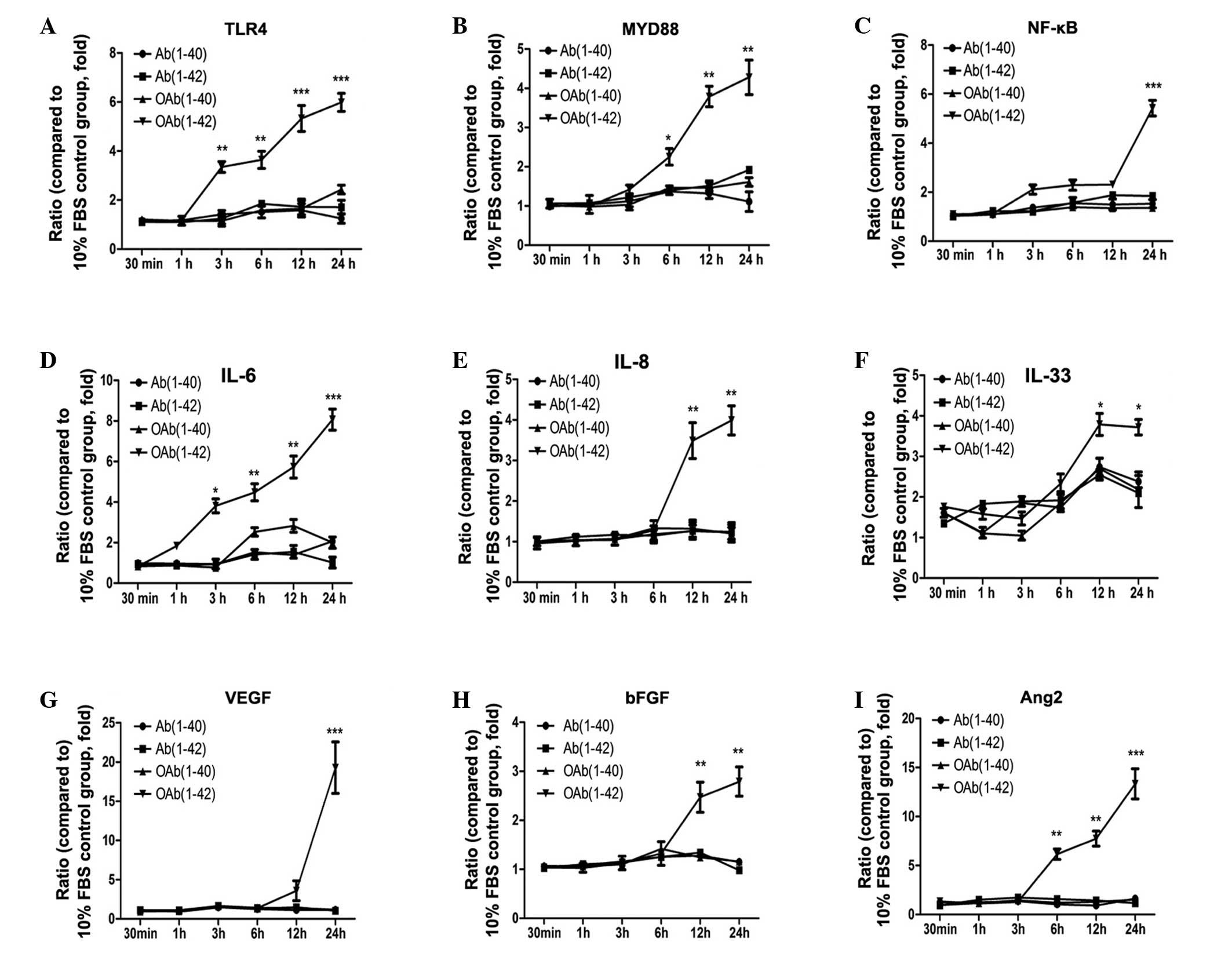 | Figure 1mRNA expression levels of cytokines
in RPE cells. QuantiGene assay was used to determine the mRNA
levels in RPE cells treated with or without 1 µM OAβ1-42.
Fold-changes were the relative ratios of (A) TLR4, (B) MyD88, (C)
NF-κB, (D) IL-6, (E) IL-8, (F) IL-33, (G) VEGF, (H) bFGF and (I)
Ang2 between the normalized values of the four infected groups and
the values of the untreated group. Values are expressed as the mean
± standard error, n=3, *P<0.05,
**P<0.01 and ***P<0.001. FSB, fetal
bovine serum; OAβ1-42, oligomeric form of Aβ; TLR4, toll-like
receptor 4; MyD88, myeloid differentiation factor 88; NF-κB,
nuclear factor-κB; IL-6,-8,-33, interleukin-6,-8,-33; VEGF,
vascular endothelial growth factor; bFGF, basic fibroblast growth
factor; Ang2, angiopoierin-2; RPE, retinal pigment epithelial. |
As presented in Fig.
1D–F, inflammatory factors were upregulated from 3 and 12 h.
IL-6, IL-8, IL-33 increased to 8.1±0.52, 4.0±0.36 and
3.7±0.19-fold, respectively, in the OAβ1-42 group respectively,
which was significantly higher than other groups. The mRNA
expression of angiogenic cytokines was upregulated from 6 h. VEGF
expression was increased to 19.3±3.28 in the OAβ1-42 group, which
was significantly higher compared with the other groups after 24 h
(Fig. 1G). The mRNA expression
level of bFGF increased by 3.0±0.30-fold at 24 h in the OAβ1-42
group (P<0.01; Fig. 1H). Ang2
steadily increased from 6 h onwards (13.3±3.0-fold; Fig. 1I). The expression of TLR2 and TLR3
mRNA was also determined; however, there was no significant
difference compared with the control group (data not shown).
OAβ1-42 is involved in the activation of
the TLR4 signaling pathway in ARPE19 cells
Western blot analysis detected the protein
expression of TLR4, MyD88 and phosphorylation-NF-κB in ARPE19 cells
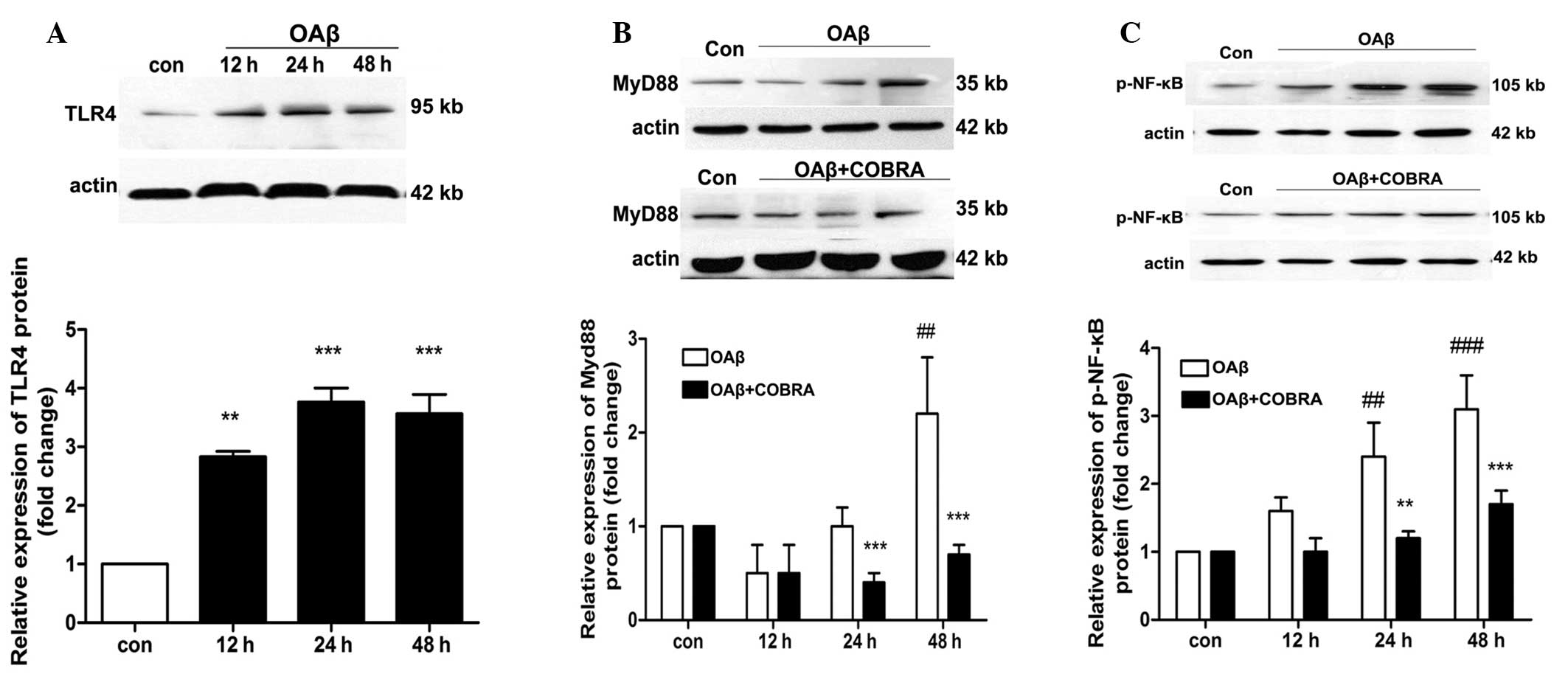
treated with OAβ1-42 (Fig. 2).
Fig. 2 indicates that the protein
expression of TLR4 increased at 12 h and reached peak at 24 h. The
expression of TLR4 was upregulated 3.7-fold compared with the
control group (Fig. 2A).
Similarly, the expression levels of MyD88 and p-NF-κB were also
increased in RPE cells treated with OAβ1-42 (P<0.01 and
P<0.001 vs. OAβ + COBRA; Fig. 2B
and C).
Western blot analysis was also used to determine the
effect of COBRA on the protein expression levels of MyD88 and
p-NF-κB. As presented in Fig. 2B and
C the protein levels of MyD88 and NF-κB were significantly
reduced in ARPE19 cells following COBRA treatment when compared
with the OAβ1-42 group at 24 and 48 h.
COBRA downregulates the expression of
inflammatory and angiogenic factors in ARPE19 cells induced by
OAβ1-42
The protein expression level of inflammatory factors
increased from 6 h of treatment in the two groups. IL-8 was
upregulated by 9.17±0.43-fold at 12 h in the OAβ1-42 group
(P<0.001 vs. control). IL-6 and IL-33 increased by 5.1±0.75-fold
and 3.3±0.14-fold at 24 h, respectively, in the OAβ1-42 group
(P<0.05 vs. control; Fig. 3A and
C).
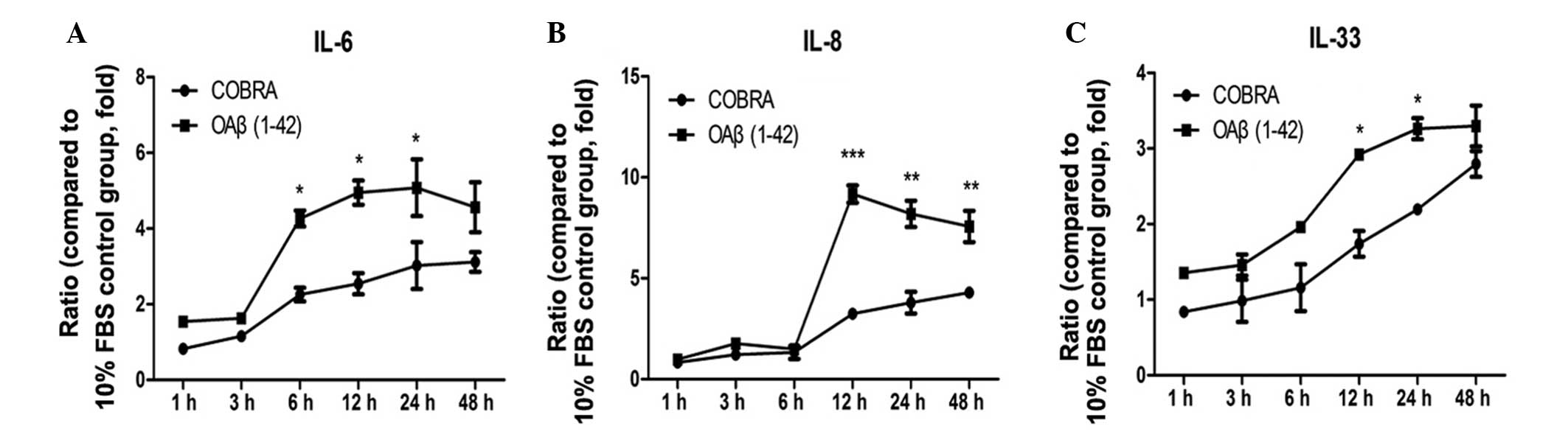 | Figure 3Effects of COBRA on the expression of
the inflammatory cytokines (A) IL-6, (B) IL-8 and (C) IL-33 in
retinal pigment epithelial cells exposed to OAβ1-42. Three
independent experiments were performed and data are expressed as
the mean ± standard error. *P<0.05,
**P<0.01 and ***P<0.01 vs. COBRA group.
FBS, fetal bovine serum; OAβ1-42, oligomeric form of Aβ;
IL-6,-8,-33, interleukin-6,-8,-33; COBRA, TLR4 inhibitor. |
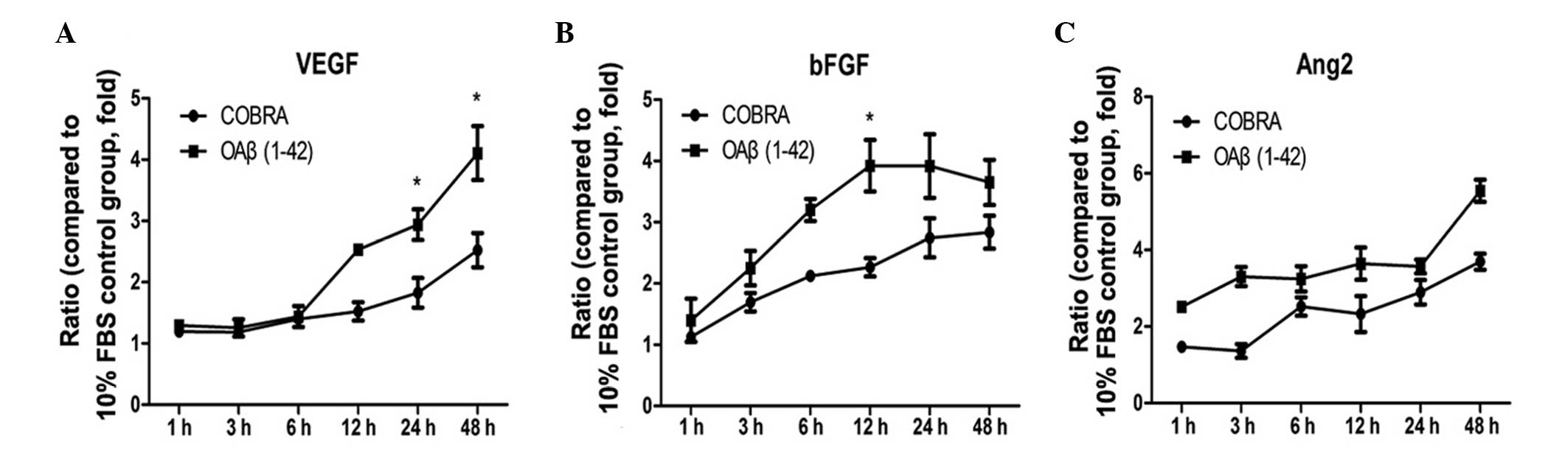
Furthermore, changes in the protein concentration of
inflammatory and angiogenic factors in cells treated with COBRA and
OAβ1-42 were detected (Figs. 3 and
4). IL-6 and IL-8 expression was
significantly reduced at 12 h following COBRA treatment (P<0.05
and P<0.001 vs. the COBRA group, respectively). However, IL-33
expression in the COBRA group was at a similar level as the OAβ1-42
group at 48 h (Fig. 3C). VEGF and
Ang2 expression was increased by 4.1±0.44-fold and 5.5±0.29-fold at
4 h, while bFGF, increased by 3.9±0.52-fold at 24 h (P<0.05 vs.
control; Fig. 4). The levels of
VEGF and bFGF were reduced in the COBRA group vs. the OAβ1-42 group
(P<0.05; Fig. 4A and B). No
significant differences were identified between the OAβ1-42 group
and the COBRA treatment group for Ang2 (Fig. 4C).
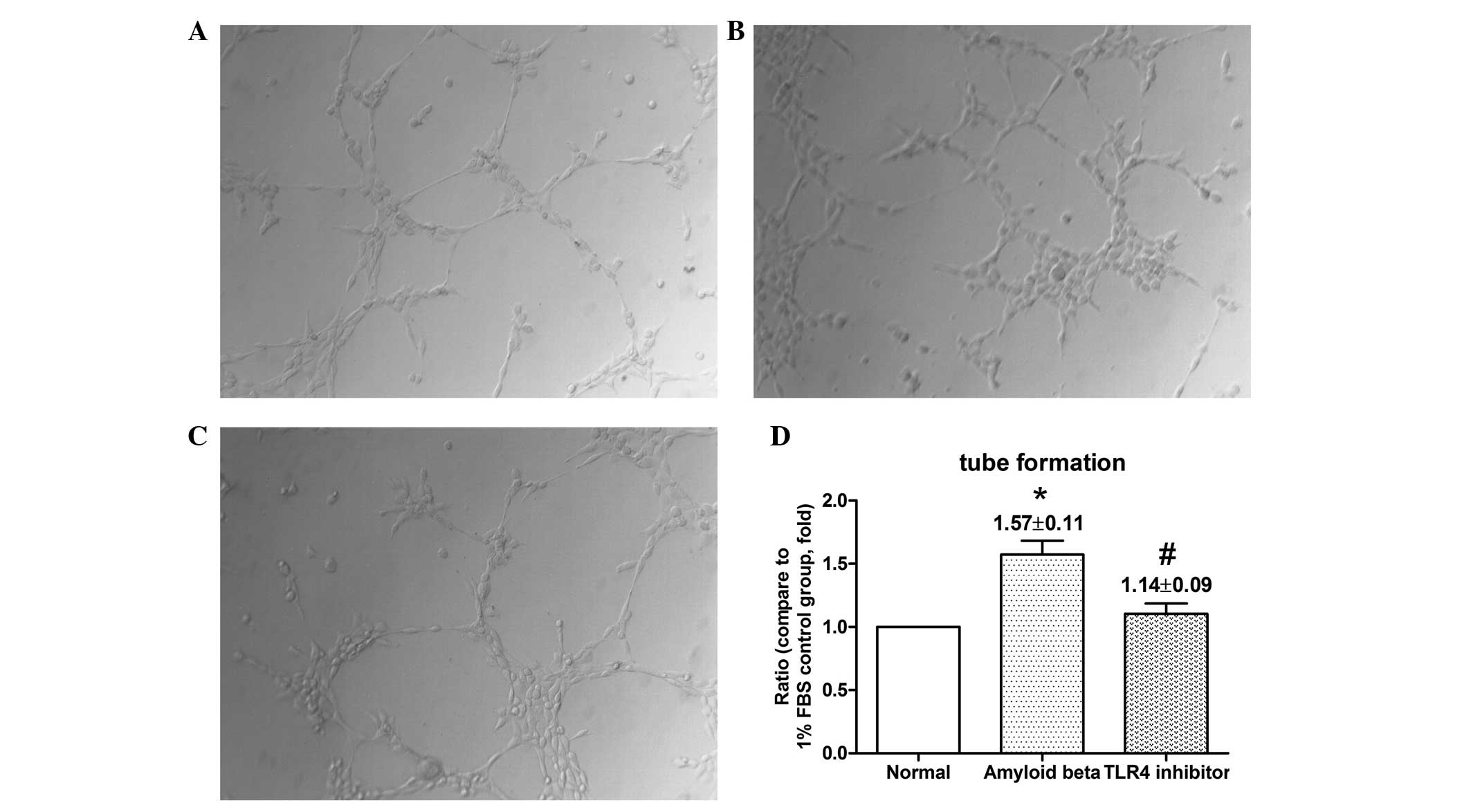
COBRA reduces capillary-like structure
formation
The Matrigel tube formation assay is a quantifiable
method of testing the angiogenic properties of compounds on
vascular endothelial cells in vitro, as it determines the
ability of endothelial cells to form capillary-like structures.
Cells were cultured on Matrigel-coated wells for 8 h. HUVECs
cultured in the cell supernatant of RPE cells, which were exposed
to COBRA, exhibited an impaired capacity to form a regular network
(due to impaired tube formation ability; Fig. 5C and D). The length of the
angiogenic network indicated a significant difference between the
COBRA and OAβ1-42 treatment groups (Fig. 5D).
Discussion
Accumulation of drusen in the extracellular
compartment between the choroid and the RPE is an early event in
the course of AMD (3,4). Aβ is an important constituent of
drusen and has been associated with the pathogenesis of AMD
(3,4), which has been verified by AMD mice
models (25) and RPE cells
(7). A previous study also
indicated that Aβ may induce inflammation and barrier disruption in
RPE cells in vivo (11).
Additionally, it has been demonstrated that chronic inflammation
has a prominent role in the pathogenesis of AMD (26). However, the underlying molecular
mechanism is largely unknown. To investigate the dysfunction of
Aβ-stimulated RPE cells, the current in vitro study was
conducted, focusing on angiogenic and inflammatory factors. The
present study also demonstrated that OAβ1-42 activates the TLR4,
MyD88 and NF-κB signaling pathways, and induced the expression of
inflammatory and angiogenic factors in ARPE19 cells. The secretion
of cytokines triggered by exposure to OAβ1-42 was significantly
reduced when COBRA was applied as a treatment. The capillary-like
structures that form as a response to OAβ1-42 were also reduced
following COBRA treatment.
The current study verified that OAβ1-42 was the
pathological form of Aβ and was responsible for the changes in the
mRNA expression levels of cytokines rather than the other forms of
Aβ. Additionally, mRNA levels were upregulated, particularly in the
OAβ1-42 treatment groups (Fig. 1).
The present study also determined mRNA expression levels of other
TLRs; however, TLR2 and TLR3 were not increased following OAβ1-42
stimulation (data not shown). Therefore, TLR4 was selected as a
target to investigate the pathological function of OAβ1-42 on RPE
cells.
Numerous studies have demonstrated the diverse
response to TLR4 activation including, the intracellular expression
of adaptor protein MyD88, the phosphorylation of IκBα and NF-κB,
and elevated mRNA expression of TNF-α, IL-6 and IL-8, along with
monocyte chemoattractant protein-1 (MCP-1) (27–29).
Qi et al (27) indicated
that retinal ischemia-reperfusion injury may trigger the TLR4
signaling pathway through MyD88, TNF receptor-associated factor 6
(TRAF6) and NF-κB, leading to the release of mature IL-1β and
IL-18. Smith et al (28)
revealed that infection of macrophages enhanced TNF-α, IL-6 and
IL-8 gene expression and protein production in response to the TLR4
ligand (lipopolysaccharide) stimulation. These findings suggest
that TLR4-signaling activation, triggered by damage- and
pathogen-associated molecular patterns, regulated the release of
inflammatory factors in response to various injuries. The current
results were consistent with previous studies, and found that
OAβ1-42 triggered TLR4 signaling pathway through MyD88 and NF-κB in
ARPE19 cells. Additionally, COBRA was able to attenuate the
expression of MyD88 and p-NF-κB in OAβ1-42-treated RPE cells. These
results suggest the potential function of the TLR4 signaling
pathway in the pathological mechanisms of AMD.
The present study indicates that mRNA and protein
levels of VEGF, bFGF and Ang2 were upregulated, in the
OAβ1-42-treated groups. VEGFA (also termed VEGF165) is considered
to be a major angiogenic factor to date (30). bFGF is also important in vascular
generation and fibrosis of endothelial cells, RPE cells and
membrane formation (31,32). Angiopoietin-2 is also a member of
the angiogenic factor family, it facilitates VEGF-induced
neovascularization and initiates neovascularization (33). The current results are consistent
with those of Yoshida et al (7), as stimulation of human RPE cells with
Aβ resulted in the altered expression of angiogenic genes,
specifically VEGF. The current study provides further evidence that
Aβ accumulation affects the balance of angiogenic factors in RPE
cells, which may be a key contributor to the development of 'wet'
AMD (7).
Cytokines are key drivers of inflammation and their
imbalances are often implicated in diseases. Cytokines have gained
attention due to their importance in AMD pathophysiology (34). Additionally, IL-6, IL-8 and IL-33
were upregulated in the cell group treated with OAβ1-42. IL-6 is a
powerful cytokine that mediates the inflammatory response in a
variety of disease states and is implicated in the progression of
AMD (34). A previous clinical
study determined that the aqueous humor from patients with AMD
contains higher concentrations of IL-6 and IL-8 (35). IL-8 is a notable cytokine as its
expression may be promoted by IL-1β via direct and indirect
mechanisms in RPE cells. Gene microarrays indicated that the mRNA
levels of IL-1β and IL-8 were significantly expressed, which is
consistent with the present findings (36). In the current study, the
concentration of IL-8 increased by 9.17-fold in OAβ1-42-treated RPE
cells when compared with the control group. Therefore, IL-8 may
account for the observed accumulation of inflammatory cells in the
regions of drusen formation in patients with AMD. IL-33 is also
important in human inflammatory diseases, and its high level of
expression was detected in the inflammatory status of various
tissues (37). However, in the
present study, IL-33 increased slightly in the COBRA treatment
group and it almost recovered to a similar level as the OAβ1-42
only group following 48 h. These results provide support for the
hypothesis that OAβ1-42 triggers inflammatory responses in RPE
cells and the production of inflammatory factors aiding in the
development of AMD. However, inflammatory factors are only one
aspect of the progression of AMD.
The present study concluded that COBRA regulates the
expression of IL-6, IL-8, IL-33, VEGF, bFGF and Ang2 through
suppressing MyD88 and p-NF-κB activation. However, with prolonged
exposure, the levels of IL-6, IL-8, IL-33, VEGF and bFGF in the
COBRA treatment group approached a similar level as the OAβ1-42
group. Therefore, the release of cytokines in RPE cells induced by
Aβ is a complex mechanism, which involves numerous signaling
pathways. Previous studies demonstrated that Aβ-induced RPE barrier
disruption and expression of inflammation factors was associated
with NF-κB, ERK1/2 and p38 MAPK signaling (14,15).
The TLR4 signaling pathway is a possible mechanism that is involved
with stimulation of inflammatory and angiogenic factors in RPE
cells induced by Aβ. This supports the hypothesis that Aβ promotes
local inflammation and angiogenic factors near drusen sites and
within the surrounding RPE layer that may facilitate the
pathogenesis of AMD.
Acknowledgments
The present study was supported by the Beijing Nova
Program (grant no. Z131102000413004).
References
|
1
|
Coleman HR, Chan CC, Ferris FL III and
Chew EY: Age-related macular degeneration. Lancet. 372:1835–1845.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jager RD, Mieler WF and Miller JW:
Age-related macular degeneration. N Engl J Med. 358:2606–2617.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bird AC, Bressler NM, Bressler SB,
Chisholm IH, Coscas G, Davis MD, de Jong PT, Klaver CC, Klein BE,
Klein R, et al The International ARM Epidemiological Study Group:
An international classification and grading system for age-related
maculopathy and age-related macular degeneration. Surv Ophthalmol.
39:367–374. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bressler NM, Silva JC, Bressler SB, Fine
SL and Green WR: Clinicopathologic correlation of drusen and
retinal pigment epithelial abnormalities in age-related macular
degeneration. Retina. 14:130–142. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferreira ST, Vieira MN and De Felice FG:
Soluble protein oligomers as emerging toxins in Alzheimer's and
other amyloid diseases. IUBMB Life. 59:332–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson LV, Leitner WP, Rivest AJ, Staples
MK, Radeke MJ and Anderson DH: The Alzheimer's A beta-peptide is
deposited at sites of complement activation in pathologic deposits
associated with aging and age-related macular degeneration. Proc
Natl Acad Sci USA. 99:11830–11835. 2002. View Article : Google Scholar
|
|
7
|
Yoshida T, Ohno-Matsui K, Ichinose S, Sato
T, Iwata N, Saido TC, Hisatomi T, Mochizuki M and Morita I: The
potential role of amyloid beta in the pathogenesis of age-related
macular degeneration. J Clin Invest. 115:2793–2800. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teplow DB, Lazo ND, Bitan G, Bernstein S,
Wyttenbach T, Bowers MT, Baumketner A, Shea JE, Urbanc B, Cruz L,
et al: Elucidating amyloid beta-protein folding and assembly: A
multidisciplinary approach. Acc Chem Res. 39:635–645. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoyer W, Grönwall C, Jonsson A, Ståhl S
and Härd T: Stabilization of a beta-hairpin in monomeric
Alzheimer's amyloid-beta peptide inhibits amyloid formation. Proc
Natl Acad Sci USA. 105:5099–5104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gouras GK, Olsson TT and Hansson O:
β-Amyloid peptides and amyloid plaques in Alzheimer's disease.
Neurotherapeutics. 12:3–11. 2015. View Article : Google Scholar :
|
|
11
|
Liu RT, Gao J, Cao S, Sandhu N, Cui JZ,
Chou CL, Fang E and Matsubara JA: Inflammatory mediators induced by
amyloid-beta in the retina and RPE in vivo: Implications for
inflammasome activation in age-related macular degeneration. Invest
Ophthalmol Vis Sci. 54:2225–2237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruban J, Glotin AL, Dinet V, Chalour N,
Sennlaub F, Jonet L, An N, Faussat AM and Mascarelli F:
Amyloid-beta(1–42) alters structure and function of retinal
pigmented epithelial cells. Aging Cell. 8:162–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu RT, Wang A, To E, Gao J, Cao S, Cui JZ
and Matsubara JA: Vinpocetine inhibits amyloid-beta induced
activation of NF-κB, NLRP3 inflammasome and cytokine production in
retinal pigment epithelial cells. Exp Eye Res. 127:49–58. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao L, Liu C, Wang F and Wang H: SIRT1
negatively regulates amyloid-beta-induced inflammation via the
NF-κB pathway. Braz J Med Biol Res. 46:659–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu XC, Liu XF, Jian CX, Li CJ and He SZ:
IL-33 is induced by amyloid-β stimulation and regulates
inflammatory cytokine production in retinal pigment epithelium
cells. Inflammation. 35:776–784. 2012. View Article : Google Scholar
|
|
16
|
Jantaratnotai N, Ling A, Cheng J, Schwab
C, McGeer PL and McLarnon JG: Upregulation and expression patterns
of the angiogenic transcription factor ets-1 in Alzheimer's disease
brain. J Alzheimers Dis. 37:367–377. 2013.PubMed/NCBI
|
|
17
|
Fioravanzo L, Venturini M, Di Liddo R,
Marchi F, Grandi C, Parnigotto PP and Folin M: Involvement of rat
hippocampal astrocytes in β-amyloid-induced angiogenesis and
neuroinflammation. Curr Alzheimer Res. 7:591–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kijlstra A, La Heij E and Hendrikse F:
Immunological factors in the pathogenesis and treatment of
age-related macular degeneration. Ocul Immunol Inflamm. 13:3–11.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bryant CE, Spring DR, Gangloff M and Gay
NJ: The molecular basis of the host response to lipopolysaccharide.
Nat Rev Microbiol. 8:8–14. 2010.
|
|
20
|
Seddon JM, Santangelo SL, Book K, Chong S
and Cote J: A genomewide scan for age-related macular degeneration
provides evidence for linkage to several chromosomal regions. Am J
Hum Genet. 73:780–790. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abecasis GR, Yashar BM, Zhao Y, Ghiasvand
NM, Zareparsi S, Branham KE, Reddick AC, Trager EH, Yoshida S,
Bahling J, et al: Age-related macular degeneration: A
high-resolution genome scan for susceptibility loci in a population
enriched for late-stage disease. Am J Hum Genet. 74:482–494. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kindzelskii AL, Elner VM, Elner SG, Yang
D, Hughes BA and Petty HR: Toll-like receptor 4 (TLR4) of retinal
pigment epithelial cells participates in transmembrane signaling in
response to photoreceptor outer segments. J Gen Physiol.
124:139–149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou X, Yuan L, Zhao X, Hou C, Ma W, Yu H
and Xiao R: Genistein antagonizes inflammatory damage induced by
β-amyloid peptide in microglia through TLR4 and NF-κB. Nutrition.
30:90–95. 2014. View Article : Google Scholar
|
|
24
|
Mohr T and Desser L: Plant proteolytic
enzyme papain abrogates angiogenic activation of human umbilical
vein endothelial cells (HUVEC) in vitro. BMC Complement Altern Med.
13:2312013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malek G, Johnson LV, Mace BE, Saloupis P,
Schmechel DE, Rickman DW, Toth CA, Sullivan PM and Bowes Rickman C:
Apolipoprotein E allele-dependent pathogenesis: A model for
age-related retinal degeneration. Proc Natl Acad Sci USA.
102:11900–11905. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buschini E, Piras A, Nuzzi R and Vercelli
A: Age related macular degeneration and drusen: Neuroinflammation
in the retina. Prog Neurobiol. 95:14–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi Y, Zhao M, Bai Y, Huang L, Yu W, Bian
Z, Zhao M and Li X: Retinal ischemia/reperfusion injury is mediated
by Toll-like receptor 4 activation of NLRP3 inflammasomes. Invest
Ophthalmol Vis Sci. 55:5466–5475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith PD, Shimamura M, Musgrove LC, Dennis
EA, Bimczok D, Novak L, Ballestas M, Fenton A, Dandekar S, Britt WJ
and Smythies LE: Cytomegalovirus enhances macrophage TLR expression
and MyD88-mediated signal transduction to potentiate inducible
inflammatory responses. J Immunol. 193:5604–5612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park MY and Mun ST: Carnosic acid inhibits
TLR4-MyD88 signaling pathway in LPS-stimulated 3T3-L1 adipocytes.
Nutr Res Pract. 8:516–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Novack GD: Pharmacotherapy for the
treatment of choroidal neovascularization due to age-related
macular degeneration. Annu Rev Pharmacol Toxicol. 48:61–78. 2008.
View Article : Google Scholar
|
|
31
|
Stahl A, Paschek L, Martin G, Feltgen N,
Hansen LL and Agostini HT: Combinatory inhibition of VEGF and FGF2
is superior to solitary VEGF inhibition in an in vitro model of
RPE-induced angiogenesis. Graefes Arch Clin Exp Ophthalmol.
247:767–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nagineni CN, Samuel W, Nagineni S,
Pardhasaradhi K, Wiggert B, Detrick B and Hooks JJ: Transforming
growth factor-beta induces expression of vascular endothelial
growth factor in human retinal pigment epithelial cells:
Involvement of mitogen-activated protein kinases. J Cell Physiol.
197:453–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Asahara T, Chen D, Takahashi T, Fujikawa
K, Kearney M, Magner M, Yancopoulos GD and Isner JM: Tie2 receptor
ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced
postnatal neovascularization. Circ Res. 83:233–240. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seddon JM, George S, Rosner B and Rifai N:
Progression of age-related macular degeneration: Prospective
assessment of C-reactive protein, interleukin 6, and other
cardiovascular biomarkers. Arch Ophthalmol. 123:774–782. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jonas JB, Tao Y, Neumaier M and Findeisen
P: Cytokine concentration in aqueous humour of eyes with exudative
age-related macular degeneration. Acta Ophthalmol. 90:e381–e388.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kurji KH, Cui JZ, Lin T, Harriman D,
Prasad SS, Kojic L and Matsubara JA: Microarray analysis identifies
changes in inflammatory gene expression in response to amyloid-beta
stimulation of cultured human retinal pigment epithelial cells.
Invest Ophthalmol Vis Sci. 51:1151–1163. 2010. View Article : Google Scholar
|
|
37
|
Préfontaine D, Nadigel J, Chouiali F,
Audusseau S, Semlali A, Chakir J, Martin JG and Hamid Q: Increased
IL-33 expression by epithelial cells in bronchial asthma. J Allergy
Clin Immunol. 125:752–754. 2010. View Article : Google Scholar : PubMed/NCBI
|