Introduction
Acute myeloid leukemia (AML) is a clonal disorder
arising from uncontrolled proliferation of hematopoietic progenitor
or stem cells (1). Genetic and
epigenetic aberrations, including promoter hypermethylation and
covalent histone modification, have been implicated in the
pathogenesis of leukemia (2),
leading to enhanced proliferation and self-renewal, evasion of
apoptosis and differentiation arrest of leukemia stem cells.
Frizzled 9 is a of protein encoded by the FZD9 gene
in humans. Members of the 'frizzled' gene family encodes seven
transmembrane domain proteins that are receptors for Wnt signaling
proteins. Wnt proteins secrete glycoprotein to regulate early
B-cell growth and survival (3).
The Wnt/FZD signaling pathway has important roles in development
(4), tumorigenesis (5) and lymphoid maturation (6,7). The
FZD9 gene is located within a region of chromosome 7, which is
commonly deleted in Williams Beuren syndrome (8–10).
FZD9 is expressed predominantly in the muscle, kidney, bones, eyes,
testis and brain, and has an important role in the maintenance of
stem cell populations in the blood, skin and gut (11–14).
FZD9-deficient mice show a reduction in the number of pro/pre-B
cells at developmental stages (15). In addition, aberrant DNA
methylation has been identified to occur during the transformation
of myelodysplastic syndrome (MDS) to AML (16). In addition, non-small cell lung
cancer cell lines showed a decrease of Wnt7a expression (17,18),
while restoration of Wnt7a and FZD9 expression inhibited cell
proliferation and anchorage-independent growth, thus promoting
cellular differentiation and reversing the transformed
phenotype.
Aberrant Wnt signaling has been studied in a number
of leukemia types, including lymphoid and myeloid lineages, as this
pathway has an important role in the renewal of normal
hematopoietic stem cells and its dysregulation may lead to leukemia
(19). Transcriptional repression
by DNA promoter hypermethylation has been shown to affect Wnt
antagonists in several human malignancies, including leukemia. The
loss of function of Wnt antagonists due to promoter
hypermethylation contributes to the activation of the Wnt pathway
in AML and may be involved in its pathogenesis, in addition to
representing a possible prognostic factor (20,21).
DNA methylation in the promoter region leads to
epigenetic gene inactivation by transcriptional silencing (22–24).
Aberrant DNA methylation of candidate tumor suppressor genes (TSGs)
has been found in various tumor types and may represent an
alternative pathway of TSG inactivation (25–27).
The present study assessed aberrant DNA methylation of the promoter
region of the FZD9 gene in hematological malignancies and examined
the association between DNA methylation of the FZD9 gene and its
gene expression.
Materials and methods
Samples and DNA/RNA preparation
Total DNA was isolated from bone marrow mononuclear
cells (BMMCs) (28) of 78
patients, including 51 patients with AML (excluding acute
promyelocytic leukemia), 18 patients with B-cell acute lymphocytic
leukemia (B-ALL), 9 patients with chronic myeloid leukemia (CML),
at the time of initial diagnosis or relapse stage, who were seen at
The First Affiliated Hospital of Harbin Medical University,
(Nangang, China) from 2011 to 2014, and grouped according to
criteria of the French-American-British classification (29). DNA was also isolated from
peripheral blood mononuclear cells (PBMCs) (30) of six healthy volunteers. The
patients and volunteers provided informed consent to participate in
this study. In addition, 10 hematological cell lines were examined.
U937, JURKAT, K562, SUP-1, THP-1, MOLT4, HL60 and NB4 cells were
provided by the Blood and Lymphatic Tumor Research Center of Nagoya
University (Nagoya, Japan). NALM6 and RPMI8226 were provided by the
Internal Hematology Laboratory of China Medical University
(Shenyang, China). Cells were maintained in RPMI 1640 culture
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.) and 100 U/ml penicillin/streptomycin, and were incubated in a
5% CO2-humidified incubator at 37°C. Total RNA was
extracted using the Aqua-SPIN RNA Isolation Mini kit (Watson,
Shanghai, China) according to the manufacturer's instructions.
Reverse transcription-polymerase chain
reaction (RT-PCR)
First-strand cDNA was synthesized from 1 µg
total RNA using random hexamers as primers and Moloney murine
leukemia virus-H-reverse transcriptase (Gibco; Thermo Fisher
Scientific, Inc.). PCR was then performed using first-strand cDNA
as a template. The PCR thermocycling program was as follows:
Initial denaturation for 5 min at 95°C, followed by 35 cycles of
denaturation for 30 sec at 95°C, annealing for 30 sec at 56°C and
extension for 30 sec at 72°C, followed by a final extension for 10
min at 72°C. The primers FZD9 forward (5′-TCAAGGTCAGGCAAGTGAGCA-3′)
and FZD9 reverse (5′-AGCTTCCAGAGGAACGCAACA-3′) were used to
generate 249-bp products. The PCR products were separated on 2%
agarose gels and visualized by ethidium bromide staining (Shanghai
Tuo Yang Biotechnology Co., Ltd., Shanghai, China). As the control,
GAPDH cDNA was amplified by RT-PCR (25 cycles) in separate tubes by
using the primers GAPDH1 (5′-CCATGGAGAAGGCTGGGG-3′) and GAPDH2
(5′-CAAAGTTGTCATGGATGACC-3′) to generate 225-bp products.
Culture with 5-aza-2-deoxycytidine
On day two after seeding, U937 and K562 cells
(2×105/ml) were incubated with 5-aza-2′-deoxycytidine
(0, 5 or 10 µM; Sigma-Aldrich, St. Louis, MO, USA) for 24 h.
The cells were then washed with phosphate-buffered saline and
incubated for another 72 h. Finally, the cells were harvested on
day five to assess mRNA expression and DNA methylation of the FZD9
gene.
Bisulfite sequencing
A total of 200 ng genomic DNA extract from PBMCs of
healthy volunteers, cell lines and clinical samples were modified
by sodium bisulfite using a DNA modification kit (Methylamp™ DNA
Modification kit; Epigentek, NewYork, NY, USA). Modified DNA (25
ng) was amplified by PCR using the primers FZD9 forward
(5′-GTTTTTTTTTTAGAGTAAAATGAGG-3′) and FZD9 reverse
(5′-ACCRAAACTACCAACCCC-3′) to obtain the promoter sequence of the
FZD9 gene. The methylation status of the FZD9 gene was assessed at
501-246 bp upstream of the transcription initiation site. PCR
products were separated on 1.5% low-melting agarose gels, excised
and then digested with β-agarase (New England Biolabs, Beverly, MA,
USA). The digestion products were sub-cloned into the pMD18-T
vector as previously described (31), and a minimum of 8 clones from each
product were subjected to cycle sequencing (Applied Biosystems,
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and analyzed
using the ABI 310 sequencer (Applied Biosystems).
Methylation-specific PCR (MSP)
The modified DNA was selectively amplified by PCR
with the primers FZD9 forward and FZD9 reverse under the conditions
described above, followed by MSP analysis with the use of primer
sets specific for unmethylated (U) DNA (MSP-U forward,
5′-GATTTAGTTTGAGAGTGTGGGTATG-3′ and reverse,
5′-CAAAACTCCAAAAAAACACA-3′) and methylated (M) DNA (MSP-M forward,
5′-ATTTAGTTTGAGAGTGTGGGTACG-3′ and reverse,
5′-GAAACTCCGAAAAAACACGC-3′). The thermocycling conditions were as
follows: Initial denaturation for 5 min at 95°C, followed by 35
cycles of denaturation for 30 sec at 95°C, annealing for 30 sec at
58°C/62°C and extension for 40 sec at 72°C, followed by a final
extension for 10 min at 72°C. Each PCR was 'hot-started' at 95°C,
and the products were separated on 2% agarose gels and then
visualized by ethidium bromide staining as described above.
Statistics and analysis
Each experiment was repeated 3 times. X-test,
one-way analysis of variance and logistic analysis were conducted
using the SPSS software, version 16.0 (SPSS, Inc., Chicago, IL,
USA). P=0.05 was set as the test standard and P<0.05 was
considered to indicate a statistically significant difference.
Results
FZD9 expression is lost in a proportion
of leukemia cell lines
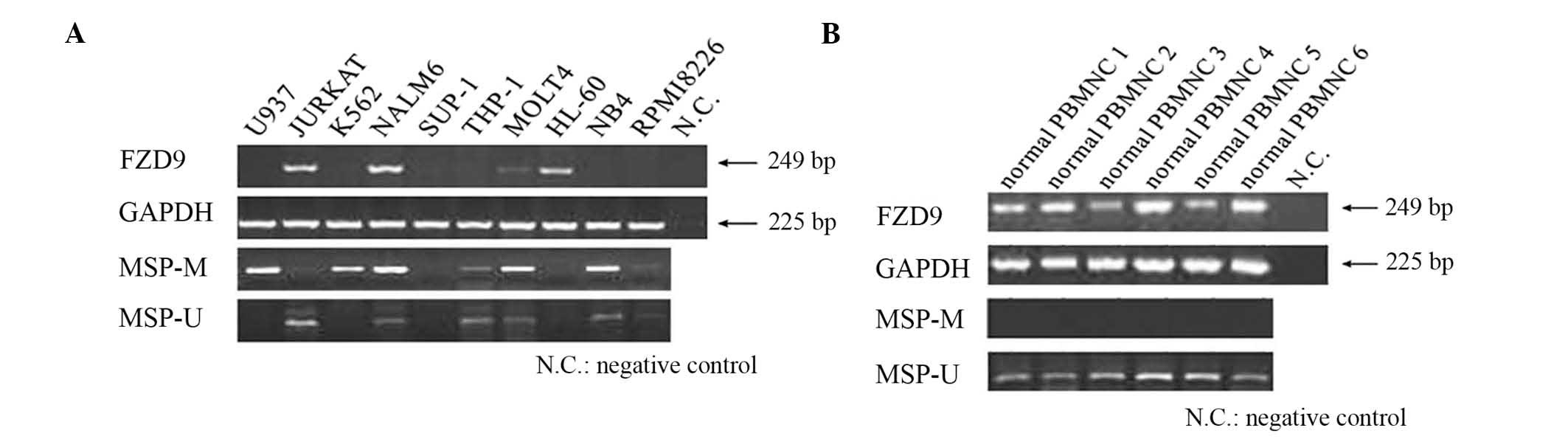
The expression of the FZD9 gene in 10 leukemic cell
lines and 6 normal PBMC samples was evaluated by RT-PCR. The
results showed that the FZD9 gene was not expressed in six of the
cell lines (U937, K562, SUP-1, THP-1, NB4 and RPMI8226) (Fig. 1). Although these cell lines were
derived from diverse hematological malignancies, the loss of FZD9
gene expression tended to be more frequent in myeloid leukemia cell
lines. However, the FZD9 gene was expressed in all normal PBMNC
samples.
FZD9 gene expression in leukemia cells is
restored by 5-aza-2′-deoxycytidine
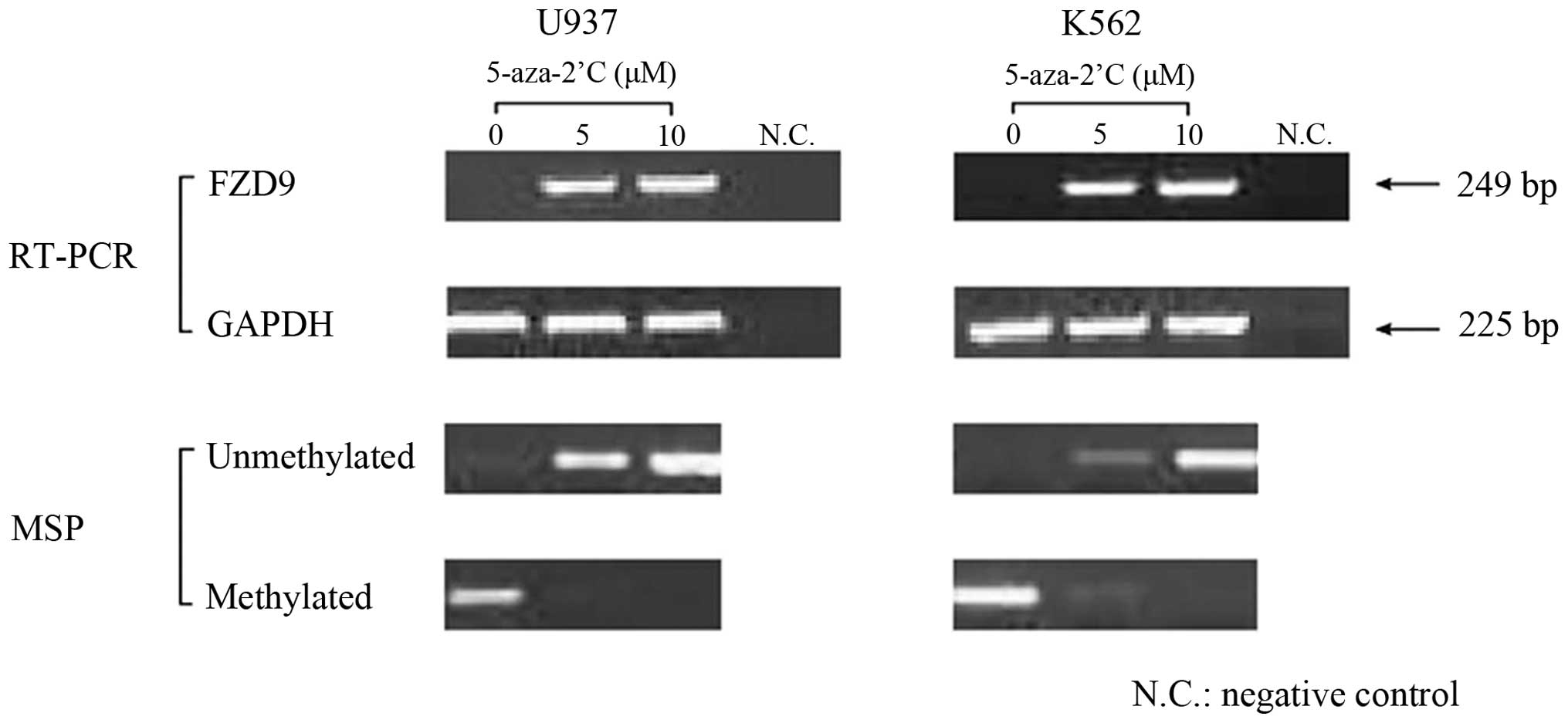
The mechanism of the observed downregulation of the
expression of the FZD9 gene was examined. The U937 and K562 cell
lines, which did not express FZD9, were exposed to
5-aza-2′-deoxycytidine (0, 5 or 10 µM). The results showed
that the expression of the FZD9 gene was restored in these cells
following treatment with 5-aza-2′-deoxycytidine (Fig. 2).
The FZD9 gene promoter region is
partially methylated in leukemia cell lines with loss of FZD9
expression
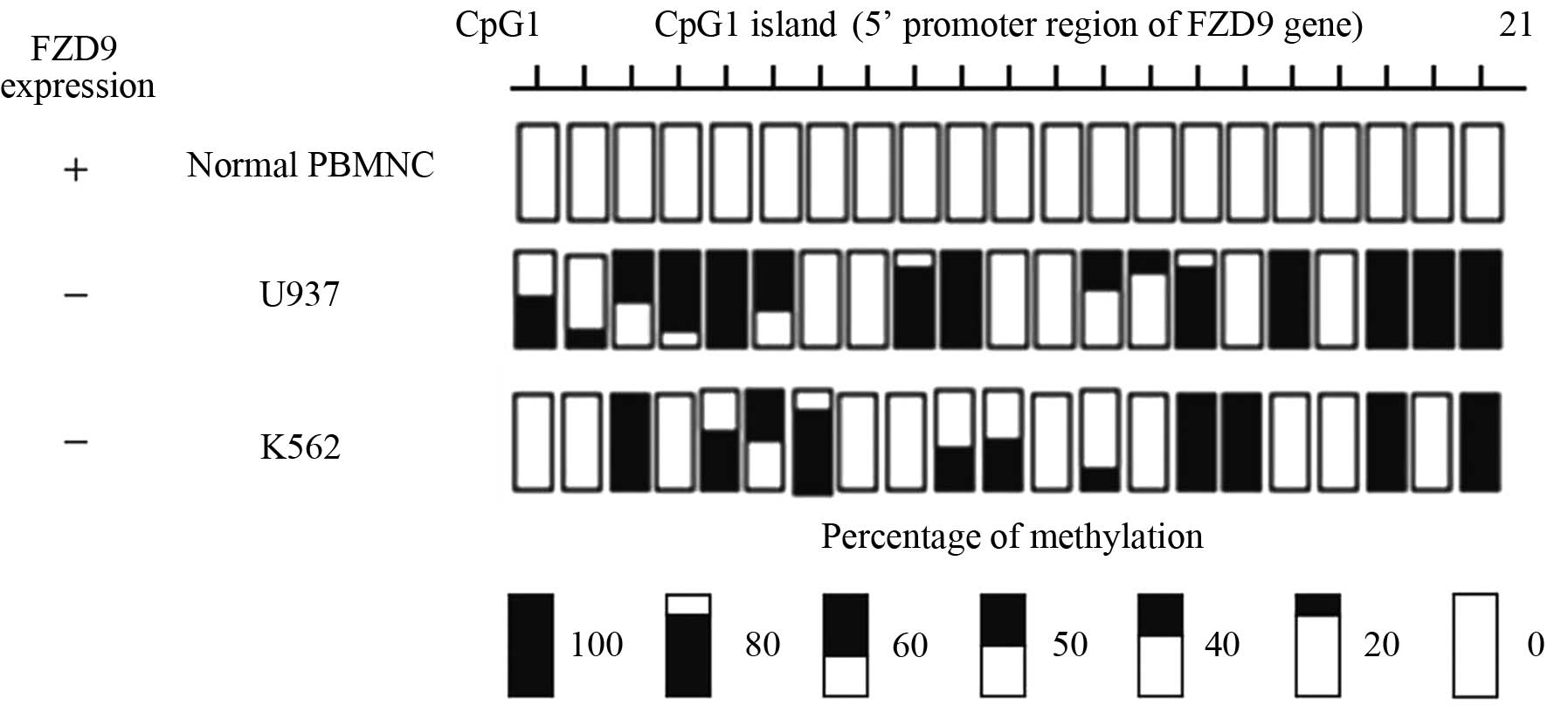
The association between DNA methylation and FZD9
gene expression was further examined by bisulfite genomic
sequencing of 21 CpG sites at the promoter region of the FZD9 gene.
PBMCs from a healthy volunteer and the U937 and K562 cell lines,
which did not express the FZD9 gene, were analyzed (Fig. 1). The normal PBMCs expressed the
FZD9 gene and the 21 CpG sites of the promoter region were
completely unmethylated. By contrast, the U937 and K562 cell lines
did not express the FZD9 gene and their promoter regions were
partially methylated (Fig. 3).
Methylation of FZD9 gene promoter region
detected by MSP
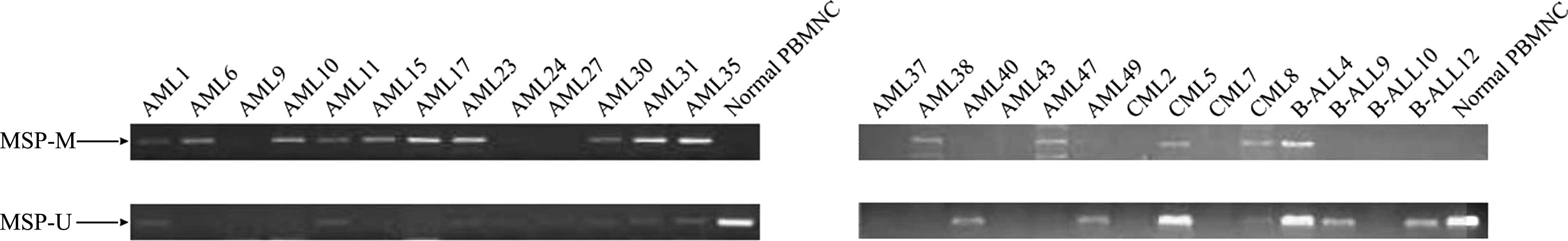
The DNA methylation in the promoter region of the
FZD9 gene was further screened using the MSP method. FZD9 gene
expression in 10 leukemic cell lines was examined using MSP
analysis. In analogy with the results of the bisulfite sequencing
analysis, MSP analysis revealed that the DNA in the promoter
regions of the FZD9 gene was methylated in the U937 and K562 cell
lines. Furthermore, the NALM6, MOLT4, THP-1, NB4 and RPMI8226 cell
lines showed partial methylation in their FZD9 promoter regions.
However, the FZD9 promoter region of Jurkat cells was not
methylated, while neither methylation nor unmethylation was
detected in SUP-1 and HL-60 cell lines. In addition, analysis of
the normal PBMNCs by MSP did not show any methylated bands
(Fig. 1B). Analysis of patient
samples showed that the methylation rate of the FZD9 gene promoter
was 52.9, 33.3 and 5.6% in AML samples, CML samples and ALL
samples, respectively (Fig. 4).
The frequencies of FZD9 gene methylation in various hematological
malignancies are shown in Table I.
These data suggested that aberrant DNA methylation of the FZD9 gene
was present in certain types hematological malignancies, and that
the frequency of this methylation was higher in AML compared with
that in CML or B-ALL.
 | Table IFrequency of FZD9 gene methylation in
various hematological malignancies. |
Table I
Frequency of FZD9 gene methylation in
various hematological malignancies.
| Malignancy | Methylation rate of
FZD9 gene | Frequency (%) |
|---|
| AML (except M3) | 27/51 | 52.9 |
| AML-M1 | 2/6 | 33.3 |
| AML-M2 | 13/22 | 59.0 |
| AML-M2 (ETO
positive) | 5/6 | 83.3 |
| AML-M4 | 2/5 | 40.0 |
| AML-M5 | 5/10 | 50.0 |
| AML-M6 | 0/2 | 0.0 |
| CML | 3/9 | 33.3 |
| B-ALL | 1/18 | 5.6 |
Discussion
In addition to point mutation or gene deletion,
transcriptional repression by the hypermethylation of promoter
sequences may represent an alternative mechanism of TSG
inactivation in cancers (32). The
FZD9 gene has been indicated to be a TSG, which has, however,
remained to be directly evidenced. FZD9 expression has been shown
to be decreased in chronic lymphocytic leukemia (21), myelodysplastic syndromes and
non-small cell lung cancer (17,18),
indicating its TSG role in hematogenic malignancies.
The results of the present study suggested that the
methylation status of the FZD9 gene in leukemia cell lines was
reversed after treatment with the demethylating agent
5-aza-2′-deoxycytidine. While methylated bands in the K562 cell
line remained after exposure to 5 µM 5-aza-2′-deoxycytidine,
they disappeared in U937 cells, along with a restoration of FZD9
expression. These results suggested that DNA methylation of the
FZD9 gene or genes that transcriptionally regulate FZD9 may
represent a mechanism of the inactivation of the FZD9 gene in
leukemia.
However, it is likely that silencing of FZD9 is not
exclusively mediated via DNA methylation in the promoter region.
Jiang et al (16) assessed
the contribution of aberrant DNA methylation to TSG silencing
during disease progression by DNA methylation microarray and
high-density single-nucleotide polymorphism array karyotyping
methods, revealing that FZD9 gene methylation was common in early
MDS, and that the methylation ratio increased with the progression
of the disease. Thus, despite the absence of genetic mutations,
FZD9 is likely to be a TSG, which is silenced during the genesis
and progression of cancers by epigenetic modifications.
MSP is a simple and sensitive method to detect DNA
methylation and requires only small specimens containing limited
amounts of DNA (33). In the
present study, analysis of the SUP-1 and HL-60 cell lines showed
that neither methylated nor unmethylated FDZ9 was present,
indicating that FZD9 expression may be deactivated by mechanisms
other than DNA methylation of its gene promoter region, such as
deregulation of transcription upstream of the FZD9 gene, histone
deacetylation or genetic mutations. Analysis of clinical specimens
revealed that the promoter region of FZD9 was 52.9% methylated in
BMMCs from patients with AML, 33.3% methylated in BMMCs from
patients with CML, but only 5.6% methylated in BMMCs from patients
with B-ALL, suggesting that aberrant FZD9 gene methylation occurred
more frequently in primary or relapse AML compared with other types
of leukemia. In patients with B-ALL, FZD9 expression may be
silenced through other mechanisms, such as gene mutations. FZD9
gene methylation was frequent in patients with AML, which was
AML1/ETO fusion gene positive. Whether the methylation status of
the FZD9 promoter in AML with translocation (8,21) is
indicative of chromosomal translocation, patient prognosis or drug
resistance requires further study. An understanding of the
association between methylation of the FZD9 gene promoter and the
clinical outcome of primary or relapse AML may aid in the
elucidation of the clinical significance of such epigenetic
alterations. As only limited clinical data of the subjects were
available, these associations could not be examined in the present
study.
According to a previous study, FZD9 knockout mice
showed no obvious features of Williams Beuren syndrome, but
revealed that the FZD9 gene had an important role in lymphoid
development and maturation (15).
FZD9 knockout mice showed thymic atrophy, pronounced splenomegaly
and lymphadenopathy with accumulation of plasma cells in lymph
nodes. These results showed that the FZD9 gene was associated with
abnormal B-cell development. However, the underlying mechanisms of
neoplastic transformations in humans may differ from those in
mice.
In conclusion, the present study indicated that the
methylation profile of the FZD9 gene corresponded to that of a
candidate tumor-suppressor gene in AML, and that demethylation of
FZD9 may represent a novel therapeutic strategy.
Acknowledgments
This work was funded by the Specialized Research
Fund for the Doctoral Program of Higher Education (no.
20132307110022).
References
|
1
|
Löwenberg B, Downing JR and Burnett A:
Acute myeloid leukemia. N Engl J Med. 341:1051–1062. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
3
|
Kaucká M, Plevová K, Pavlová S, Janovská
P, Mishra A, Verner J, Procházková J, Krejcí P, Kotasková J, Ovesná
P, et al: The planar cell polarity pathway drives pathogenesis of
chronic lymphocytic leukemia by the regulation of B-lymphocyte
migration. Cancer Res. 73:1491–1501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huelsken J, Vogel R, Brinkmann V, Erdmann
B, Birchmeier C and Birchmeier W: Requirement for beta-catenin in
anterior-posterior axis formation in mice. J Cell Biol.
148:567–578. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI
|
|
6
|
Yu S, Zhou X, Steinke FC, Liu C, Chen SC,
Zagorodna O, Jing X, Yokota Y, Meyerholz DK, Mullighan CG, et al:
The TCF-1 and LEF-1 transcription factors have cooperative and
opposing roles in T cell development and malignancy. Immunity.
37:813–826. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y, Banerjee D, Huelsken J, Birchmeier W
and Sen JM: Deletion of beta-catenin impairs T cell development.
Nat Immunol. 4:1177–1182. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fusco C, Micale L, Augello B, Teresa
Pellico M, Menghini D, Alfieri P, Cristina Digilio M, Mandriani B,
Carella M, Palumbo O, et al: Smaller and larger deletions of the
Williams Beuren syndrome region implicate genes involved in mild
facial phenotype, epilepsy and autistic traits. Eur J Hum Genet.
22:64–70. 2014. View Article : Google Scholar
|
|
9
|
Laurito S, Branham T, Herrero G, Marsa S,
Garro F and Roqué M: Detection of a Williams Beuren syndrome case
by MLPA. Medicina (B Aires). 73:47–50. 2013.In Spanish.
|
|
10
|
Van Hagen JM, Eussen HJ, Van Schooten R,
van Der Geest JN, Lagers-van Haselen GC, Wouters CH, De Zeeuw CI
and Gille JJ: Comparing two diagnostic laboratory tests for
Williams syndrome: Fluorescent in situ hybridization versus
multiplex ligation-dependent probe amplification. Genet Test.
11:321–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kolben T, Peröbner I, Fernsebner K,
Lechner F, Geissler C, Ruiz-Heinrich L, Capovilla S, Jochum M and
Neth P: Dissecting the impact of Frizzled receptors in
Wnt/β-catenin signaling of human mesenchymal stem cells. Biol Chem.
393:1433–1447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reya T, Duncan AW, Ailles L, Domen J,
Scherer DC, Willert K, Hintz L, Nusse R and Weissman IL: A role for
Wnt signalling in self-renewal of haematopoietic stem cells.
Nature. 423:409–414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huelsken J, Vogel R, Erdmann B, Cotsarelis
G and Birchmeier W: beta-Catenin controls hair follicle
morphogenesis and stem cell differentiation in the skin. Cell.
105:533–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ranheim EA, Kwan HC, Reya T, Wang YK,
Weissman IL and Francke U: Frizzled 9 knock-out mice have abnormal
B-cell development. Blood. 105:2487–2494. 2005. View Article : Google Scholar
|
|
16
|
Jiang Y, Dunbar A, Gondek LP, Mohan S,
Rataul M, O'Keefe C, Sekeres M, Saunthararajah Y and Maciejewski
JP: Aberrant DNA methylation is a dominant mechanism in MDS
progression to AML. Blood. 113:1315–1325. 2009. View Article : Google Scholar :
|
|
17
|
Tennis M, Van Scoyk M, Heasley LE,
Vandervest K, Weiser-Evans M, Freeman S, Keith RL, Simpson P,
Nemenoff RA and Winn RA: Prostacyclin Inhibits non-small cell lung
cancer growth by a frizzled 9-dependent pathway that is blocked by
secreted frizzled-related protein 1. Neoplasia. 12:244–253. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Winn RA, Van Scoyk M, Hammond M, Rodriguez
K, Crossno JT Jr, Heasley LE and Nemenoff RA: Antitumorigenic
effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is
mediated through ERK-5-dependent activation of peroxisome
proliferator-activated receptor gamma. J Biol Chem.
281:26943–26950. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khan NI and Bendall LJ: Role of WNT
signaling in normal and malignant hematopoiesis. Histol
Histopathol. 21:761–774. 2006.PubMed/NCBI
|
|
20
|
Román-Gómez J, Cordeu L, Agirre X,
Jiménez-Velasco A, San José-Eneriz E, Garate L, Calasanz MJ,
Heiniger A, Torres A and Prosper F: Epigenetic regulation of
Wnt-signaling pathway in acute lymphoblastic leukemia. Blood.
109:3462–3469. 2007. View Article : Google Scholar
|
|
21
|
Liu TH, Raval A, Chen SS, Matkovic JJ,
Byrd JC and Plass C: CpG island methylation and expression of the
secreted frizzled-related protein gene family in chronic
lymphocytic leukemia. Cancer Res. 66:653–658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borssén M, Palmqvist L, Karrman K,
Abrahamsson J, Behrendtz M, Heldrup J, Forestier E, Roos G and
Degerman S: Promoter DNA methylation pattern identifies prognostic
subgroups in childhood T-cell acute lymphoblastic leukemia. PLoS
One. 8:e653732013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Li Q and Chen H: DNA methylation
and histone modifications of Wnt genes by genistein during colon
cancer development. Carcinogenesis. 34:1756–1763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Portela A, Liz J, Nogales V, Setién F,
Villanueva A and Esteller M: DNA methylation determines nucleosome
occupancy in the 5′-CpG islands of tumor suppressor genes.
Oncogene. 32:5421–5428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wodarz D, Boland CR, Goel A and Komarova
NL: Methylation kinetics and CpG-island methylator phenotyope
status in colorectal cancer cell lines. Biol Direct. 8:142013.
View Article : Google Scholar :
|
|
26
|
He W, Li X, Xu S, Ai J, Gong Y, Gregg JL,
Guan R, Qiu W, Xin D, Gingrich JR, et al: Aberrant methylation and
loss of CADM2 tumor suppressor expression is associated with human
renal cell carcinoma tumor progression. Biochem Biophys Res Commun.
435:526–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Twelves D, Nerurkar A, Osin P, Dexter T,
Ward A, Gui GP and Isacke CM: DNA promoter hypermethylation
profiles in breast duct fluid. Breast Cancer Res Treat.
139:341–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, McHale CM, Rothman N, Li G, Ji Z,
Vermeulen R, Hubbard AE, Ren X, Shen M, Rappaport SM, et al:
Systems biology of human benzene exposure. Chem Biol Interact.
184:86–93. 2010. View Article : Google Scholar :
|
|
29
|
Lin X, Wang Z, Wang Y and Feng W: Serum
MicroRNA-370 as a potential diagnostic and prognostic biomarker for
pediatric acute myeloid leukemia. Int J Clin Exp Pathol.
8:14658–14666. 2015.
|
|
30
|
Koon HW, Shih DQ, Hing TC, Yoo JH, Ho S,
Chen X, Kelly CP, Targan SR and Pothoulakis C: Human monoclonal
antibodies against Clostridium difficile toxins A and B inhibit
inflammatory and histologic responses to the toxins in human colon
and peripheral blood monocytes. Antimicrob Agents Chemother.
57:3214–3223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia L, Gong Y, Zhang A, Cai S and Zeng Q:
Loss of GATA5 expression due to gene promoter methylation induces
growth and colony formation of hepatocellular carcinoma cells.
Oncol Lett. 11:861–869. 2016.PubMed/NCBI
|
|
32
|
Li Y, Nagai H, Ohno T, Yuge M, Hatano S,
Ito E, Mori N, Saito H and Kinoshita T: Aberrant DNA methylation of
p57(KIP2) gene in the promoter region in lymphoid malignancies of
B-cell phenotype. Blood. 100:2572–2577. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uchida T, Kinoshita T, Ohno T, Ohashi H,
Nagai H and Saito H: Hypermethylation of p16INK4A gene promoter
during the progression of plasma cell dyscrasia. Leukemia.
15:157–165. 2001. View Article : Google Scholar : PubMed/NCBI
|