Introduction
Gastric cancer has high metastasis and recurrence
rates following curative resection, and is the second leading cause
of cancer-associated mortality worldwide (1–3).
Indeed, the majority of gastric cancer cases are identified at the
advanced stages and often develop recurrence following curative
resection. Thus, the poor prognosis increases the importance of
chemotherapy for treating gastric cancer (4). However, in the clinic, chemotherapy
drugs often cause serious side-effects, including
immunosuppression, gastrointestinal toxicity and body weakness
(5,6). Thus, chemotherapeutic drug candidates
derived from natural compounds with low toxicity and low adverse
effects have attracted increasing attention.
Interleukin-8 (IL-8), a cytokine of the CXC
chemokine family (7), is highly
expressed in numerous tumor tissues (8). Accumulating evidence has indicated
that overexpression of IL-8 is closely associated with increased
adhesion and invasion of human gastric cancer cells, whereas
inhibition of IL-8 expression reduces relevant risks (7–10).
Accordingly, chemical compounds targeting IL-8 may be useful for
controlling the metastasis of gastric cancer.
Berberine hydrochloride (BER), a major active
alkaloid molecule isolated from Coptis Chinensis Franch.
(Huanglian), is typically used to treat infectious
gastrointestinal diseases and bacterial diarrhea in the clinic.
Previous studies demonstrated that BER exerts anti-tumor activity
against various types of cancer cells, including human
hepatocellular carcinoma (SMMC-7721 cells), gastric cancer (AGS
cells, SGC 7901 cells and BGC-823 cells) and colorectal cancer
(SW620 cells and LoVo cells) in vitro and in vivo
(11–14). Similarly, previous investigation
has demonstrated that BER inhibited proliferation and IL-8
expression in AGS cells, a gastric cancer cell line, in
vitro (10,15). However, whether BER can prevent
gastric cancer development and IL-8 secretion in vivo has
not been demonstrated.
BER has previously been demonstrated to modulate
mitogen-activated protein kinase (MAPK) signaling pathways,
including the extracellular signal-regulated kinase 1/2 (ERK1/2),
p38 MAPK and c-Jun N-terminal kinase (JNK) pathways, to exert
anti-cancer effects may be cell-type specific. For instance, BER
activates MAPKs in human colonic carcinoma cells (16), human hepatoma (HepG2) and non-small
cell lung cancer cells (17–19).
Whereas in human cervical carcinoma HeLa cells, BER enhances JNK
and ERK1/2 phosphorylation but inhibits p38 MAPK phosphorylation
(20). Currently, the effect of
BER on MAPK pathways in gastric cancer cells remains poorly
understood.
Thus, in present study, the effects of BER on
gastric cancer MGC 803 cell proliferation and IL-8 secretion were
investigated in vitro and in vivo. Furthermore, the
association between MAPK pathway inactivation and the proliferative
inhibition of BER, and IL-8 secretion in MGC 803 cells was also
examined. The results may provide a novel and safe strategy for the
therapy of gastric cancer using BER.
Materials and methods
Materials and chemicals
BER (purity, 98%), evodiamine (EVO; purity, 98%) and
5-fluorouracil (5-Fu; purity, 98%) were obtained from Melonepharma
Co., Ltd. (Dalian, China). Trypsin and fetal bovine serum (FBS)
were obtained from Gibco (Thermo Fisher Scientific, Inc. Waltham,
MA, USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Molecular Technologies, Inc. (Kumamoto, Japan). IL-8 (cat. no.
88-8086-88) and TNF-α (cat. no. 88-7346-88) enzyme-linked
immunosorbent assay (ELISA) kits were obtained from eBioscience,
Inc., (San Diego, CA, USA). Anti-GAPDH (cat. no. 5174),
anti-phospho (p) p38 MAPK (cat. no. 4511), anti-pERK1/2 (cat. no.
9154), anti-pJNK (cat. no. 4668) and anti-β-actin (cat. no. 12413)
antibodies were supplied by Cell Signaling Technology, Inc.
(Danvers, MA, USA). Enhanced chemiluminescence (ECL) Prime kit was
purchased from GE Healthcare Life Sciences (Chalfont, UK).
SB202190, SP600125 and PD98059 were purchased from Selleck
Chemicals (Houston, TX, USA). Anisomycin was obtained from EMD
Millipore (Billerica, MA, USA).
Cell culture
MGC 803 cells obtained from the Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China) were
cultured in RMPI 1640 medium (Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS. The cells were cultured at 37°C in a
humidified incubator with 5% CO2.
Proliferation assay
Cells were seeded in 100 µl medium at
1.0×104 cells/ml in 96-well culture plates and cultured
overnight. Following pre-incubation with or without inhibitors of
p38 MAPK (25 µM SB202190), ERK1/2 (20 µM PD98059),
JNK (20 µMSP600125) and the activator of MAPKs (0.05
µg/ml anisomycin) for 1 h, cells were treated with BER (0,
7.5, 15, 30 and 60 µM) for 24 or 48 h. The medium was then
removed and replaced with equal volume of fresh medium with
additional 10 µl CCK-8 solution and incubated at 37°C for 20
min. Absorbance of the dissolved solutions was detected at 450 nm
using a Varioskan Flash microplate reader (Thermo Fisher
Scientific, Inc.). Cell viability rate (%) was calculated as
follows: (Absorbance of drug-treated sample/absorbance of control
sample) ×100.
ELISA assay
For in vitro experiments, MGC 803 cells were
seeded in 96-well culture plates and cultured overnight. Following
treatment with BER (0, 15, 30 and 60 µM) for 48 h, culture
medium was collected and subjected to IL-8 and TNF-α ELISA assay
using the respective kits. To identify the involvement of MAPKs in
modulation of IL-8 expression, the cells were pre-treated with
SB202190 (25 µM), SP600125 (20 µM), PD98059 (20
µM) and anisomycin (0.25 µg/ml) for 1 h, then treated
with or without BER (60 µM) for 24 or 48 h. The culture
medium was collected for IL-8 ELISA.
For in vivo experiments, serum and the
supernatant of tumor homogenates from nude mice xenografts were
used for IL-8 ELISA.
Western blotting analysis
Cells or tumor tissues were lysed with CelLytic MT
Cell Lysis Reagent (Sigma-Aldrich, St. Louis, MO, USA) and
sonicated three times, each for 15 sec. The lysate was centrifuged
at 14,000 × g for 15 min at 4°C and the supernatant was collected.
Protein concentration was determined by the bicinchoninic acid
method. Protein samples (30 µg) were separated by SDS-PAGE
and transferred onto polyvinylidene difluoride (PVDF) membrane
using the wet transfer method. Then, PVDF membranes were blocked
with 5% non-fat milk solution at room temperature for 1 h and
incubated with the different primary antibodies (anti-GAPDH,
1:5,000; anti-p-p 38, 1:1,000; anti-p-ERK, 1:1,000; anti-p-JNK,
1:1,000; and anti-β-actin, 1:2,000) overnight at 4°C. After washing
with 1X phosphate-buffered saline Tween 20 (PBST), PVDF membranes
were incubated with the respective secondary antibodies (1:5,000;
cat. no. 111-035-003; Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA) for 1 h at room temperature. The protein bands
were visualized with the ECL Prime kit and X-ray films.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the MGC 803 cells by
using TRIzol reagent. RT was performed using a Prime-Script RT
reagent kit (Takara Biotechnology Co., Ltd., Dalian, China).
Forward and reverse primers used for qPCR are presented in Table I. qPCR reactions were performed
using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.) under the
following cycling conditions: Initial step of 95°C for 30 sec;
followed by 95°C for 5 sec and 60°C for 34 sec for 40 cycles; with
final steps of 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec.
The relative expression level of IL-8 was normalized to that of
GAPDH in the same sample. Relative expression of target genes was
normalized to GAPDH, analyzed by 2−ΔΔCq method (21) and presented as a ratio compared
with the control.
 | Table ISequences of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
| Interleukin-8 |
CATACTCCAAACCTTTCCACC |
AAACTTCTCCACAACCCTCTG |
Tumor xenograft model in nude mice
Thirty 4-week-old male BALB/C nude mice were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China; license no. SCXK 2014-0008). The tumor xenograft model was
established by subcutaneous injection of MGC 803 cells
(5×106 cells in 200 µl PBS) into the right flank
of the mouse. Animals bearing tumors were randomly divided into
five groups (n=6) as follows: i) Control group; ii) EVO group (45
mg/kg); iii) BER group (15 mg/kg); iv) BER + EVO group (EVO 45
mg/kg, BER 15 mg/kg) and v) 5-Fu group (25 mg/kg). Prior to
injection of the MGC 803 cells, mice were orally administrated with
BER, EVO or 5-Fu, and administration was continued for 23 days.
Body weight and two perpendicular tumor diameters (width, a;
length, b) were recorded every 4 days. The tumor volume was
calculated as ab2/2. Following completion of treatment,
the mice were sacrificed using 1% pentobarbital sodium (DingGuo
Biotech Co., Ltd., Shanghai, China). The tumors were dissected,
weighed, and stored at −80°C for use in ELISA and western
blotting.
All animal experiments were performed according to
the protocols approved by Animal Care and Use Committee of Shanghai
University of Traditional Chinese Medicine, (Shanghai, China) which
complies with international rules and policies. All efforts were
made to minimize suffering and reduce the number of animals
used.
Statistical analysis
Values are presented as the mean ± standard error.
Differences among groups were analyzed by one-way analysis of
variance with Newman-Keuls test using Prism software (version 5;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
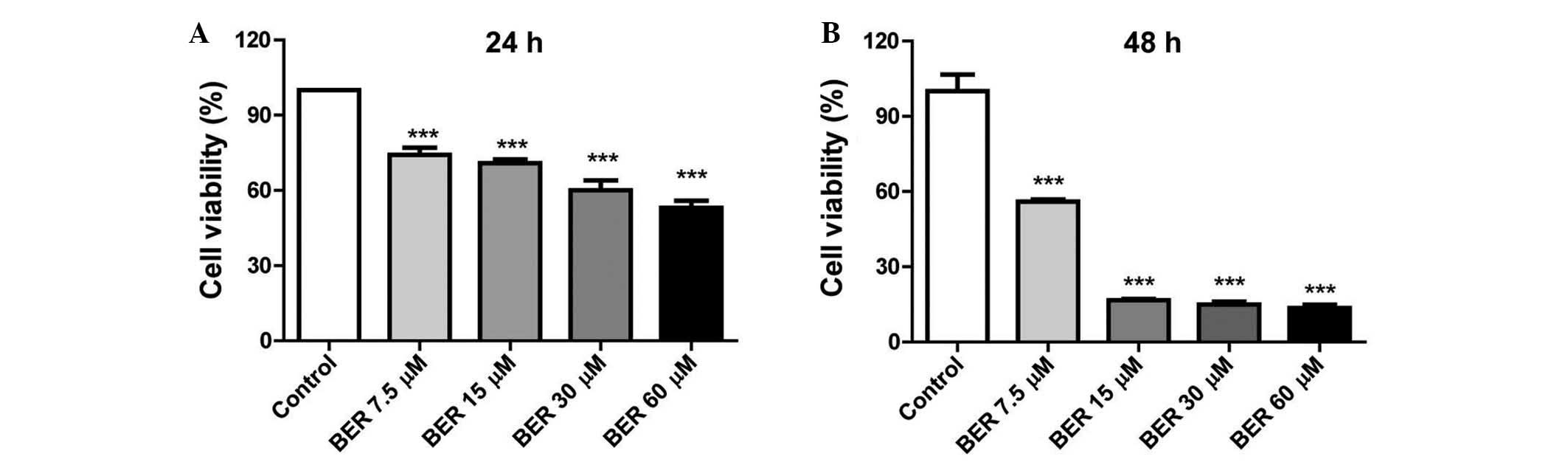
BER suppresses proliferation of MGC 803
cells in vitro
BER demonstrated an inhibitory effect on the
viability of MGC 803 cells in a dose- and time-dependent manner. As
demonstrated in Fig. 1, BER
treatment (7.5–60 µM) significantly decreased the cell
viability compared with the control (P<0.001) at 24 and 48 h.
Furthermore, prolonged BER treatment (48 h) led to increased injury
to the MGC 803 cells as demonstrated by the reduced cell
viability.
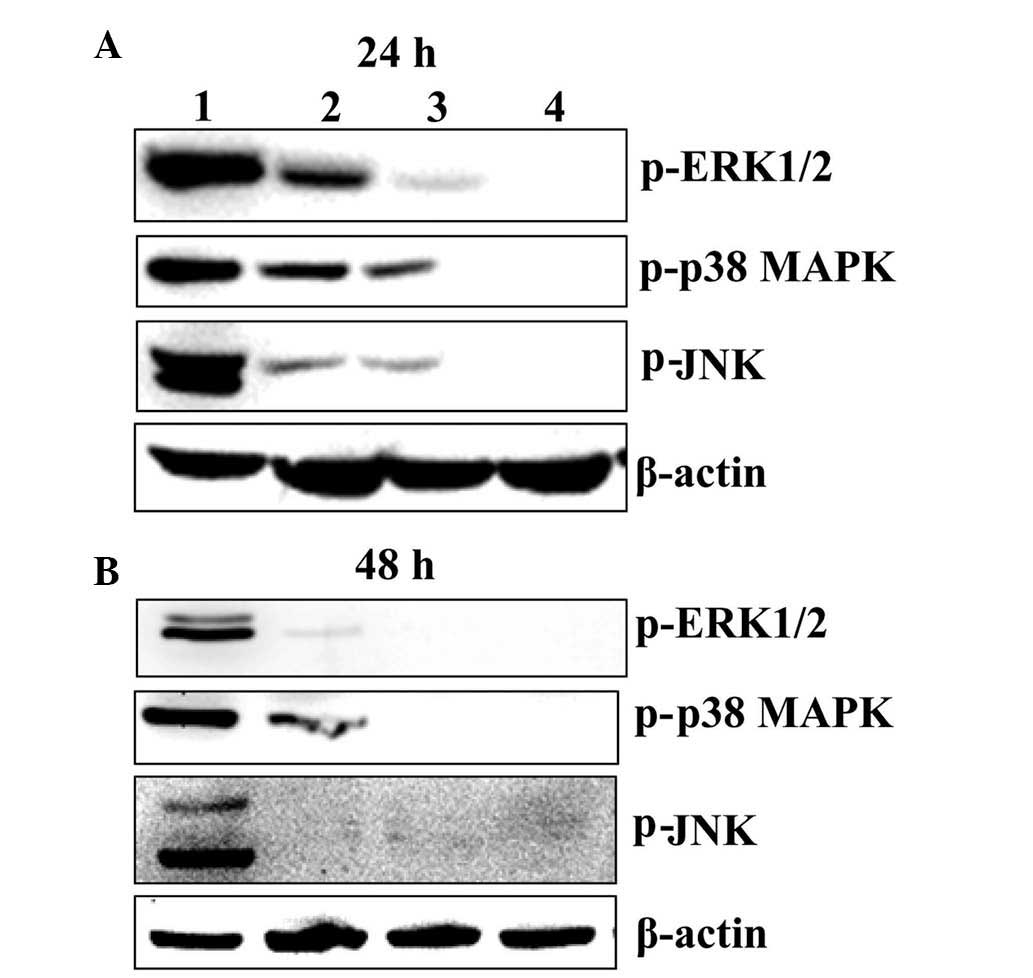
BER inactivates MAPK pathways in MGC 803
cells
To determine the association between MAPK signaling
pathways, including p38 MAPK, ERK1/2 and JNK pathway, and cell
survival of MGC 803 cells, western blot analysis was performed. As
demonstrated in Fig. 2, BER
treatment at 15, 30 and 60 µM for 24 and 48 h reduced the
phosphorylation of p38 MAPK, ERK1/2 and JNK in a dose- and
time-dependent manner.
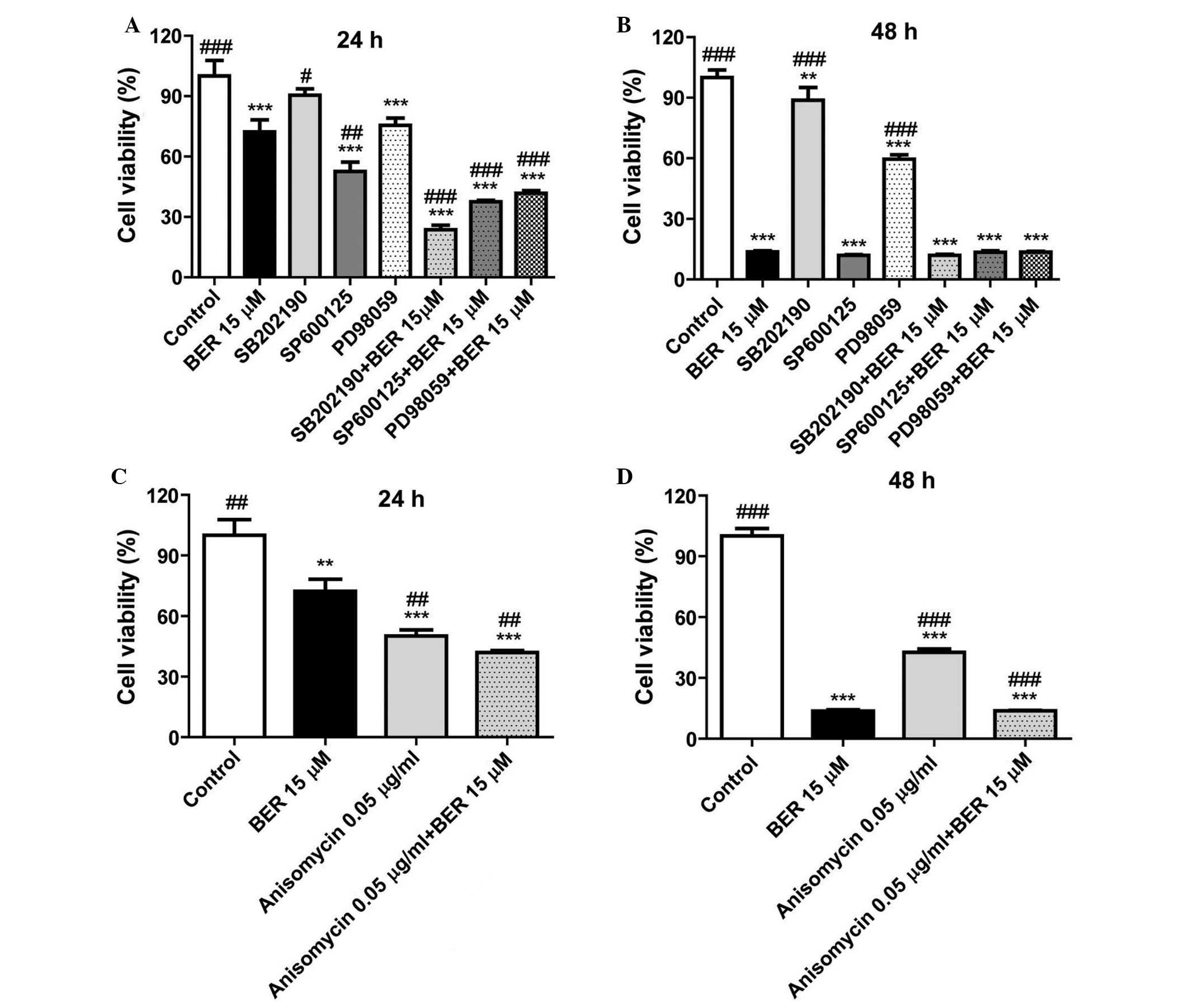
In order to further clarify the importance of MAPK
signaling in the cell proliferation, and whether BER inhibited cell
proliferation of gastric cancer cells via deactivating MAPKs, the
inhibitors of p38 MAPK (SB202190), JNK (SP600125) and ERK1/2
(PD98059) were used in a CCK-8 assay. As demonstrated in Fig. 3A, in MGC 803 cells, these
inhibitors enhanced the inhibitory effect of BER on cell
proliferation compared with BER treatment (P<0.001, P<0.001
and P<0.05). Notably, anisomycin, an activator of p38 MAPK and
JNK, also significantly reduced cell viability of MGC 803 cells
compared with controls (Fig. 3C).
As demonstrated in Fig. 3B and D,
as BER treatment for 48 h killed the majority of the cancer cells,
combination of BER with the inhibitors or activator of MAPKs did
not demonstrate a synergistic effect at this time point.
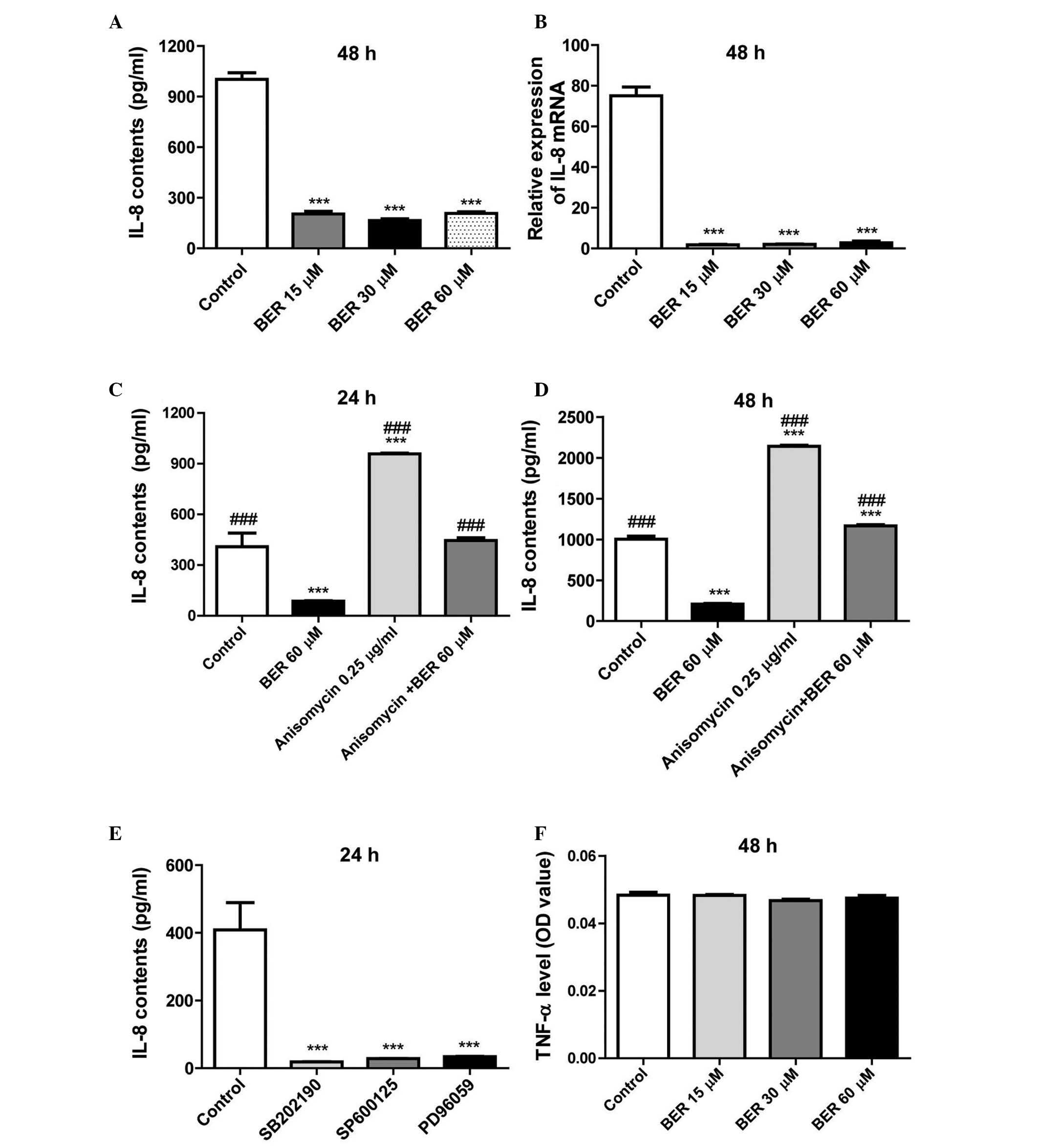
BER decreases IL-8 secretion and gene
expression levels in MGC 803 cells
In previous research, BER was demonstrated to
inhibit IL-8 expression in a dose- and time-dependent manner in AGS
and MDA-MB-231 cells (10,12). However, whether the inhibitory
effect of BER on IL-8 expression is cell-type specific remains
unclear. The present study demonstrated that BER also reduces the
secretion (P<0.001; Fig. 4A)
and gene expression levels (P<0.001; Fig. 4B) of IL-8 in MGC 803 cells compared
with controls. Further investigation demonstrated that anisomycin
significantly increased the secretion of IL-8 and reduced cell
viability of MGC 803 cells compared with controls (P<0.001),
which was completely abolished by combination with BER treatment
(P<0.001; Fig. 4C and D).
Furthermore, as demonstrated in Fig.
4E, treatment with SB202190, SP600125 or PD98059 significantly
decreased IL-8 secretion in MGC 803 cells compared with controls
(P<0.001). However, BER did not alter TNF-α production of the
cells (Fig. 4F). Thus, the results
of the present study indicated that BER specifically affects IL-8
production in MGC 803 cells.
BER inhibits tumor development from MGC
803 cells in vivo
To examine the anti-tumor effect of BER in
vivo, a human gastric cancer xenograft model was used in BALB/C
nude mice. In nude mice transplanted with MGC 803 cells were
treated with the drugs for 23 days. 5-Fu (25 mg/kg), was
administered as a positive control and significantly prevented the
growth of tumor (Fig. 5), with the
tumor weight and volume of mice in this group significantly reduced
compared with the control group (P<0.001). However, long-term
treatment with 5-Fu led to a significant reduction in the body
weight compared with control mice (P<0.001). By contrast, BER
(15 mg/kg daily) significantly reduced the tumor weight and tumor
volume compared with controls (P<0.05 and P<0.001,
respectively), and also did not lead to body weight loss. EVO
treatment also significantly inhibited tumor weight and size
compared with controls (P<0.05 and P<0.01, respectively).
Co-administration of EVO and BER demonstrated a trend to inhibit
tumor weight and volume compared with BER or EVO alone, but without
a statistically significant difference. Both EVO and combination of
BER and EVO demonstrated no significant effect on body weight.
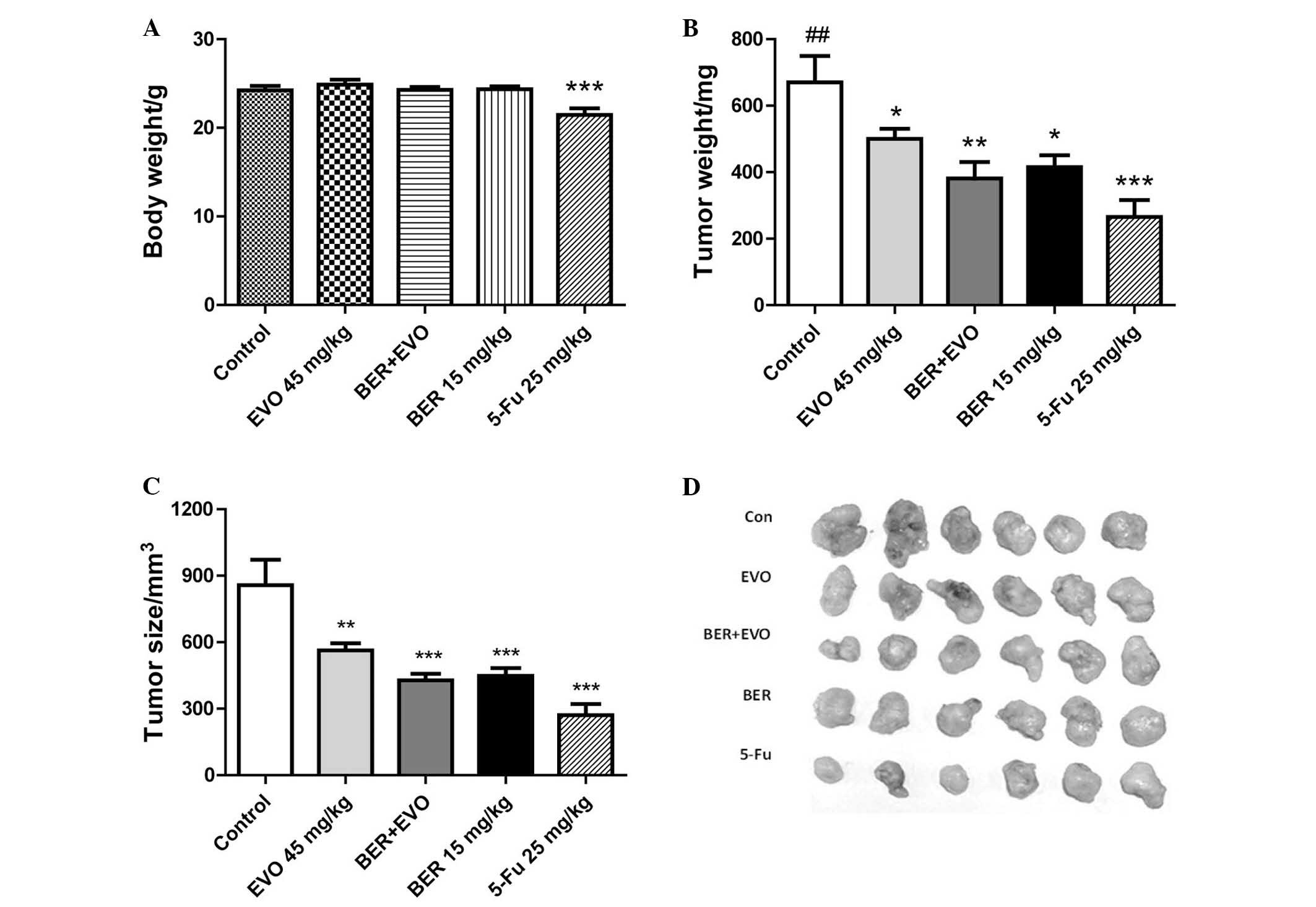 | Figure 5Effect of BER on tumor growth of
human gastric cancer xenograft. (A) BER exhibited no obvious effect
on body weight of nude mice, while 5-Fu induced a significant
weight loss. BER, EVO, 5-Fu and BER + EVO reduced the (B) tumor
weight and (C) tumor size following treatment for 23 days, however,
there was no obvious synergistic effect between BER and EVO. (D)
Comparison of xenograft tumors excised from the mice treated with
control, EVO, BER + EVO, BER and 5-Fu. Data are presented as the
mean ± standard error. *P<0.05,
**P<0.01, ***P<0.001 vs. control;
##P<0.01 vs. BER + EVO. BER, berberine hydrochloride;
EVO, evodiamine; 5-Fu, fluorouracil. |
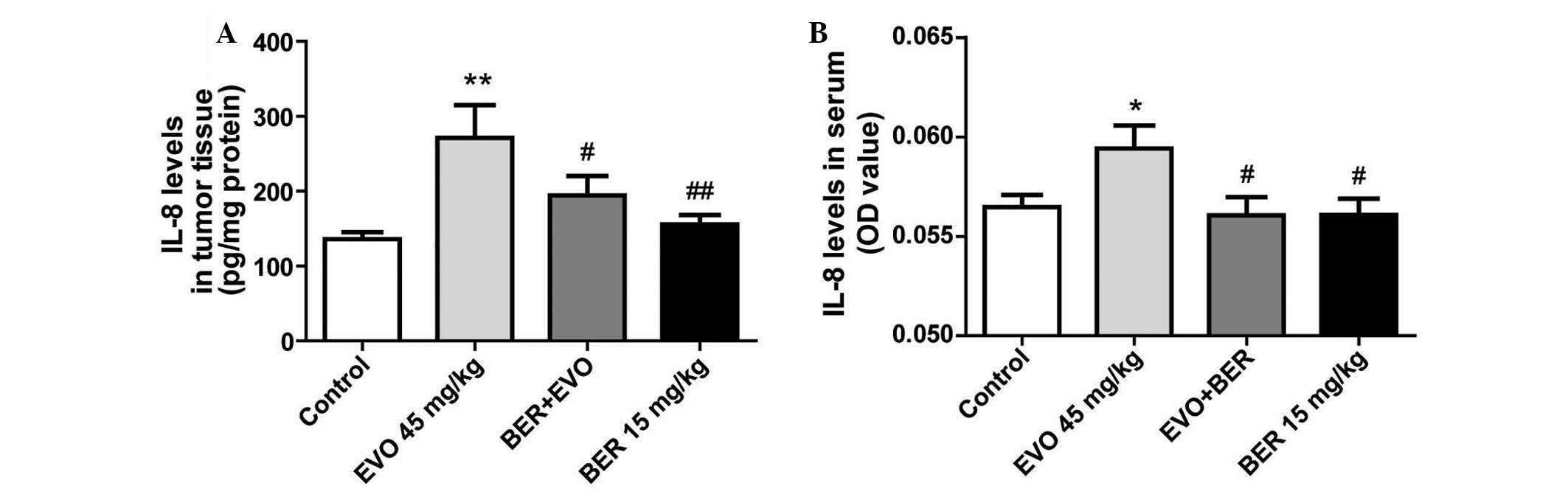
BER inhibits upregulation of IL-8 induced
by EVO in nude mice xenografted with MGC 803 cells
In a previous study, EVO was demonstrated to
upregulate IL-8 expression in AGS cells in vitro (10), whether it induces IL-8 expression
in vivo remains to be determined. In the present study, EVO,
the alkaloid from Evodia fructus, significantly suppressed
MGC 803 tumor development in nude mice compared with controls
(Fig. 5), but increased IL-8
production in tumor tissue (P<0.01; Fig. 6A) and serum (P<0.05; Fig. 6B). BER treatment alone, or in
combination with EVO significantly attenuated the increase of IL-8
in tumor tissue and serum compared with EVO treatment (P<0.01
and P<0.05, respectively).
BER inactivates MAPKs in the tumor tissue
of nude mice xenografted with MGC 803 cells
Following treatment with BER at a dose of 15 mg/kg
for 23 days, the phosphorylation levels of p38 MAPK, ERK1/2 and JNK
in tumor tissue was significantly reduced compared with controls
(Fig. 7). By contrast, EVO alone
did not alleviate the phosphorylation of p38 MAPK, ERK1/2 and JNK
compared with control levels. However, co-treatment with BER and
EVO markedly reduced the phosphorylation of the MAPKs in tumor
tissues compared with control and EVO-treated nude mice.
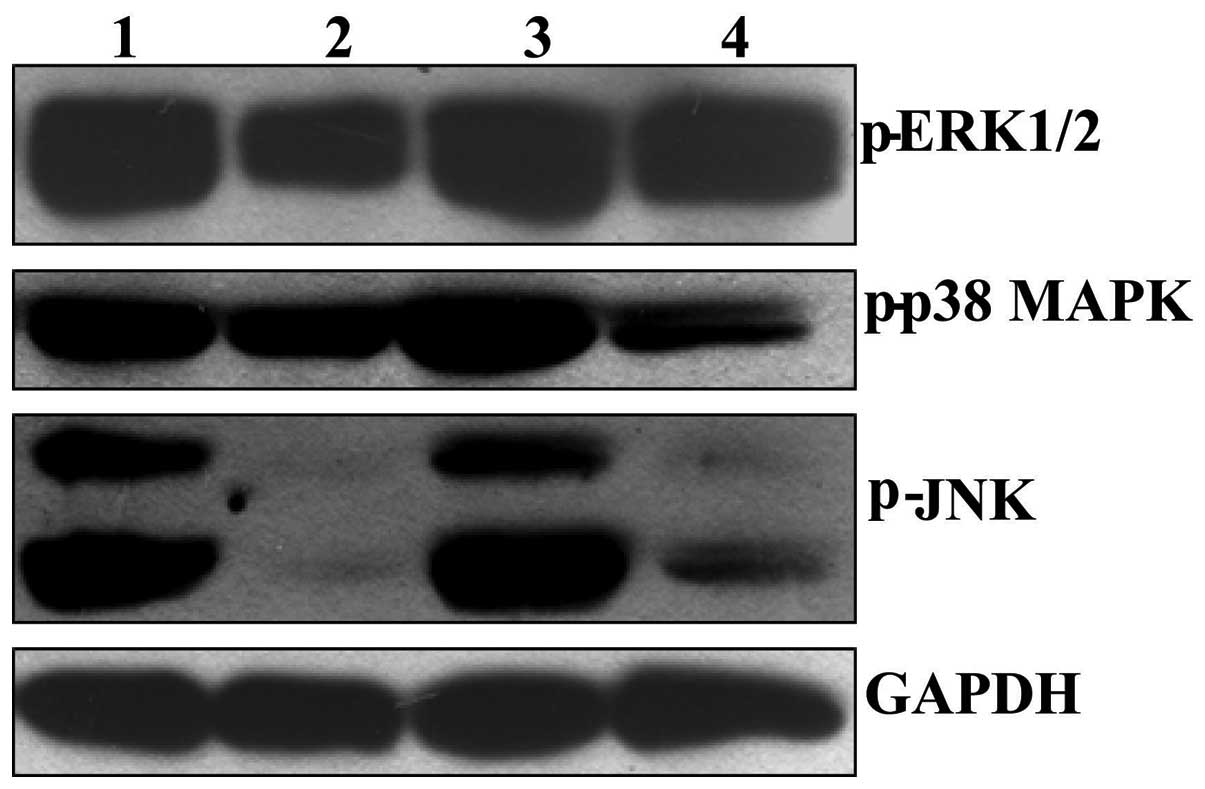 | Figure 7Effect of BER on phosphorylation of
p38 MAPK, ERK1/2 and JNK in tumor tissues of nude mice bearing
gastric cancer. After treatment with drugs for 23 days, the mice
were sacrificed and the tumor were dissected, homogenized and
subjected to western blotting assay. 1: Control; 2: BER, 15 mg/kg;
3: EVO, 45 mg/kg; 4: EVO+BER. MAPK, mitogen-activated protein
kinase; ERK, extracellular signal-regulated kinase; JNK, c-Jun
N-terminal kinase; BER, berberine hydrochloride; EVO,
evodiamine. |
Discussion
Previous studies have demonstrated the inhibitory
effect of BER on the proliferation of cancer cells, and that the
effect is predominantly mediated via the inactivation of the
phosphatidylinositol 3-kinase/AKT serine/threonine kinase 1
signaling pathway (12,13). MAPK pathways have also previously
been indicated to be involved in the anti-cancer effect of BER,
however reports vary depending on the cancer cell type (12,16–20).
Although BER has previously been demonstrated to exert
anti-tumorigenesis functions in various gastric cancer cell lines,
including SGC 7901, BGC 823, AGS, SNU-5, SC-M1 and NUGC-3 cells
(10,13,22,23),
its effect on cell viability, IL-8 expression and MAPK signaling in
MGC 803 cells has not been previously investigated. In the current
study, BER was demonstrated to significantly decrease the cell
viability of MGC 803 cells in a dose- and time-dependent manner.
Further analysis demonstrated that the phosphorylation of p38 MAPK,
ERK1/2 and JNK were inhibited by BER even at a low concentration
(15 µM). Using inhibitors of p38 MAPK (SB202190), ERK1/2
(PD98059) and JNK (SP600125), the present study demonstrated that
the downregulated phosphorylation of p38 MAPK, ERK1/2 and JNK were
involved in the inhibitory effect of BER on the cell viability of
MGC 803 cells. Considering the difference between in vitro
and in vivo settings, the effect of BER on gastric tumors
developed from xenografted MGC 803 cells was also evaluated. The
results demonstrated that 15 mg/kg BER significantly inhibited the
tumor growth and reduced the phosphorylation of p38 MAPK, ERK1/2
and JNK. Thus, inactivation of MAPK signaling is indeed involved in
the anti-tumor activity of BER on MGC 803 cells in vitro and
in vivo. Furthermore, compared with 5-Fu, BER inhibited
tumor growth without affecting body weight, which is a general
side-effect of chemotherapeutic drugs.
It is well established that various chemotherapeutic
agents induce upregulation of IL-8 levels in tumor cells, which is
closely associated with chemotherapy resistance and cancer
metastasis (7,9,10,12,24–30).
Overexpression of IL-8 in cancer cells is known to be important for
the tumor microenvironment via binding to CXC motif chemokine
receptor 1 (CXCR1) and CXCR2 receptors on tumor cells,
neutrophils/tumor-associated macrophages and endothelial cells, and
promotes angiogenesis and metastasis (7,9,24,28,31).
By contrast, depletion of IL-8 induces cell cycle arrest and
increases the efficacy of chemotherapeutic agents in breast cancer
cells (32). Thus,
chemotherapeutic agents with an inhibitory effect on IL-8
production may have increased efficacy. In the present
investigation, BER significantly decreased IL-8 secretion in MGC
803 cells in vitro and in vivo, demonstrating its
safety and efficacy in inhibiting gastric cancer cells.
MAPKs have previously been demonstrated to be
involved in the modulation of IL-8 production (25,28,33).
In accordance with the previous reports, the current results
indicated that activation of the ERK1/2, JNK and p38 MAPK signaling
pathways was closely associated with constitutive IL-8 secretion in
MGC 803 cells. Additionally, inhibitors of p38 MAPK, JNK and ERK
significantly decreased IL-8 expression in MGC 803 cells. Whereas,
when the MAPK agonist, anisomycin, was applied, IL-8 secretion was
upregulated. BER, similarly to the MAPK inhibitors, reduced the
phosphorylation of ERK1/2, JNK and p38 MAPK, and counteracted the
increased IL-8 secretion induced by anisomycin. Activation of MAPKs
by anisomycin also decreased cell viability of MGC 803 cells.
However, in accordance with the findings of the current study,
curcumin has previously been reported to induce apoptosis through
activation of ERK1/2 in AGS cells (34), whereas apoptosis was enhanced by
capsaicin through inhibition of MAPKs in AGS cells (35). These results reflect the complex
functions of MAPKs in cancer cells.
In a previous study in AGS cells, EVO was
demonstrated to significantly enhance IL-8 expression, the effect
of which was counteracted by BER (10). Consistent with its in vitro
effects, the results of the current study demonstrated that in
addition to the inhibition of tumor growth, EVO upregulated IL-8
secretion in serum and tumor tissue of tumor xenografted nude mice,
which was also inhibited by BER. As it is not constitutively
expressed in mice (36), the
levels of IL-8 in nude mouse serum secreted from tumor tissue was
relatively low, the optical density value of which was beyond the
limit of detection of the ELISA kit. Compared with control mice,
EVO-treated mice did not demonstrate significant changes in the
levels of phosphorylated p38 MAPK, ERK1/2 and JNK. However, when
BER was added, the level of phosphorylation of MAPKs in MGC 803
cell-derived tumors was markedly reduced. These results indicated
that co-administration of EVO with BER may be a safer therapy for
gastric cancer without reduction of its efficacy.
In conclusion, BER reduced the growth of MGC 803
cells and IL-8 expression levels in vitro and in
vivo, which was associated with deactivation of p38 MAPK,
ERK1/2 and JNK signaling pathways. Furthermore, BER significantly
counteracted the upregulation of IL-8 production induced by EVO
in vivo. The findings of the present study demonstrated the
potential safety and efficacy of BER in the clinical therapy of
gastric cancer.
Acknowledgments
This work was supported by the Educational
Commission of Shanghai of China (grant no. 2012JW19), Key Research
Innovation Project (grant no. 13ZZ099), Key Project from Department
of Education of China (grant no. 20123107130002), Shanghai Eastern
Scholar Program (grant no. 2013–59) and Shanghai E-research
Institute of Bioactive Constituent in TCM plan.
References
|
1
|
Macdonald JS: Treatment of localized
gastric cancer. Semin Oncol. 31:566–573. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
3
|
Lurje G, Husain H, Power DG, Yang D,
Groshen S, Pohl A, Zhang W, Ning Y, Manegold PC, EI-Khoueiry A, et
al: Genetic variations in angiogenesis pathway genes associated
with clinical outcome in localized gastric adenocarcinoma. Ann
Oncol. 21:78–86. 2010. View Article : Google Scholar
|
|
4
|
Zheng L, Wang L, Ajani J and Xie K:
Molecular basis of gastric cancer development and progression.
Gastric Cancer. 7:61–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitchell MS and DeConti RC:
Immunosuppression by 5-fluoro-uracil. Cancer. 26:884–889. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Justino PF, Melo LF, Nogueira AF, Morais
CM, Mendes WO, Franco AX, Souza EP, Ribeiro RA, Souza MH and Soares
PM: Regulatory role of Lactobacillus acidophilus on inflammation
and gastric dysmotility in intestinal mucositis induced by
5-fluo-rouracil in mice. Cancer Chemother Pharmacol. 75:559–567.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuai WX, Wang Q, Yang XZ, Zhao Y, Yu R and
Tang XJ: Interleukin-8 associates with adhesion, migration,
invasion and chemosensitivity of human gastric cancer cells. World
J Gastroenterol. 18:979–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bünger S, Haug U, Kelly FM,
Klempt-Giessing K, Cart-wright A, Posorski N, Dibbelt L, Fitzgerald
SP, Bruch HP, Roblick UJ, et al: Toward standardized
high-throughput serum diagnostics: Multiplex-protein array
identifies IL-8 and VEGF as serum markers for colon cancer. J
Biomol Screen. 16:1018–1026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ju D, Sun D, Xiu L, Meng X, Zhang C and
Wei P: Interleukin-8 is associated with adhesion, migration and
invasion in human gastric cancer SCG-7901 cells. Med Oncol.
29:91–99. 2012. View Article : Google Scholar
|
|
10
|
Shi HL, Wu XJ, Liu Y and Xie JQ: Berberine
counteracts enhanced IL-8 expression of AGS cells induced by
evodiamine. Life Sci. 93:830–839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XN, Han X, Xu LN, Yin LH, Xu YW, Qi Y
and Peng JY: Enhancement of apoptosis of human hepatocellular
carcinoma SMMC-7721 cells through synergy of berberine and
evodiamine. Phytomedicine. 15:1062–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Zhao SJ, Shi HL, Qiu SP, Xie JQ, Wu
H, Zhang BB, Wang ZT, Yuan JY and Wu XJ: Berberine hydrochloride
IL-8 dependently inhibits invasion and IL-8-independently promotes
cell apoptosis in MDA-MB-231 cells. Oncol Rep. 32:2777–2788.
2014.PubMed/NCBI
|
|
13
|
Yi T, Zhuang L, Song G, Zhang B, Li G and
Hu T: Akt signaling is associated with the berberine-induced
apoptosis of human gastric cancer cells. Nutr Cancer. 67:523–531.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H,
Fan Z, Cai J and Li Q: Berberine inhibits invasion and metastasis
of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3
signaling pathway. PLoS One. 10:e01234782015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi HL, Xie JQ and Wu DZ: Effect of
berberine on cell proliferation and IL-8 expression in AGS cells.
Zhong Yao Yao Li Yu Lin Chuang Bian Ji Bu. 28:45–48. 2012.
|
|
16
|
Hsu WH, Hsieh YS, Kuo HC, Teng CY, Huang
HI, Wang CJ, Yang SF, Liou YS and Kuo WH: Berberine induces
apoptosis in SW620 human colonic carcinoma cells through generation
of reactive oxygen species and activation of JNK/p38 MAPK and FasL.
Arch Toxicol. 81:719–728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng F, Tang Q, Wu J, Zhao S, Liang Z, Li
L, Wu W and Hann S: P38α MAPK-mediated induction and interaction of
FOXO3a and p53 contribute to the inhibited-growth and
induced-apoptosis of human lung adenocarcinoma cells by berberine.
J Exp Clin Cancer Res. 33:362014. View Article : Google Scholar
|
|
18
|
Hyun MS, Hur JM, Mun YJ, Kim D and Woo WH:
BBR induces apoptosis in HepG2 cell through an Akt-ASK1-ROS-
p38MAPKs-linked cascade. J Cell Biochem. 109:329–338. 2010.
|
|
19
|
Hur JM, Hyun MS, Lim SY, Lee WY and Kim D:
The combination of berberine and irradiation enhances anti-cancer
effects via activation of p38 MAPK pathway and ROS generation in
human hepatoma cells. J Cell Biochem. 107:955–964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu B, Hu M, Liu K and Peng J: Cytotoxicity
of berberine on human cervical carcinoma HeLa cells through
mitochondria, death receptor and MAPK pathways, and in-silico
drug-target prediction. Toxicol In Vitro. 24:1482–1490. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Lin HL, Liu TY, Wu CW and Chi CW:
Berberine modulates expression of mdr1 gene product and the
responses of digestive track cancer cells to Paclitaxel. Br J
Cancer. 81:416–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin JP, Yang JS, Lee JH, Hsieh WT and
Chung JG: Berberine induces cell cycle arrest and apoptosis in
human gastric carcinoma SNU-5 cell line. World J Gastroenterol.
12:21–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kitadai Y, Takahashi Y, Haruma K, Naka K,
Sumii K, Yokozaki H, Yasui W, Mukaida N, Ohmoto Y, Kajiyama G, et
al: Transfection of interleukin-8 increases angiogenesis and
tumorigenesis of human gastric carcinoma cells in nude mice. Br J
Cancer. 81:647–653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Collins TS, Lee LF and Ting JP: Paclitaxel
up-regulates interleukin-8 synthesis in human lung carcinoma
through an NF-kappaB-and AP-1-dependent mechanism. Cancer Immunol
Immunother. 49:78–84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lev DC, Onn A, Melinkova VO, Miller C,
Stone V, Ruiz M, McGary EC, Ananthaswamy HN, Price JE and Bar-Eli
M: Exposure of melanoma cells to dacarbazine results in enhanced
tumor growth and metastasis in vivo. J Clin Oncol. 22:2092–2100.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kishida O, Miyazaki Y, Murayama Y, Ogasa
M, Miyazaki T, Yamamoto T, Watabe K, Tsutsui S, Kiyohara T,
Shimomura I and Shinomura Y: Gefitinib (Iressa, ZD1839) inhibits
SN38-triggered EGF signals and IL-8 production in gastric cancer
cells. Cancer Chemother Pharmacol. 55:584–594. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Britschgi A, Andraos R, Brinkhaus H,
Klebba I, Romanet V, Müller U, Murakami M, Radimerski T and
Bentires-Alj M: JAK2/STAT5 inhibition circumvents resistance to PI3
K/mTOR blockade: A rationale for cotargeting these pathways in
metastatic breast cancer. Cancer Cell. 22:796–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Feng M, Zheng G, Chen Y, Wang X,
Pen B, Yin J, Yu Y and He Z: Chemoresistance to 5-fluorouracil
induces epithelial-mesenchymal transition via up-regulation of
Snail in MCF7 human breast cancer cells. Biochem Biophys Res
Commun. 417:679–685. 2012. View Article : Google Scholar
|
|
31
|
Matsuo Y, Ochi N, Sawai H, Yasuda A,
Takahashi H, Funahashi H, Takeyama H, Tong Z and Guha S: CXCL8/IL-8
and CXCL12/SDF-1alpha co-operatively promote invasiveness and
angiogenesis in pancreatic cancer. Int J Cancer. 124:853–861. 2009.
View Article : Google Scholar :
|
|
32
|
Shao N, Chen LH, Ye RY, Lin Y and Wang SM:
The depletion of interleukin-8 causes cell cycle arrest and
increases the efficacy of docetaxel in breast cancer cells. Biochem
Biophys Res Commun. 431:535–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bezzerri V, Borgatti M, Finotti A,
Tamanini A, Gambari R and Cabrini G: Mapping the transcriptional
machinery of the IL-8 gene in human bronchial epithelial cells. J
Immunol. 187:6069–6081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao AL, Tang QF, Zhou WC, Qiu YY, Hu SJ
and Yin PH: Ras/ERK signaling pathway is involved in
curcumin-induced cell cycle arrest and apoptosis in human gastric
carcinoma AGS cells. J Asian Nat Prod Res. 17:56–63. 2015.
View Article : Google Scholar
|
|
35
|
Park SY, Kim JY, Lee SM, Jun CH, Cho SB,
Park CH, Joo YE, Kim HS, Choi SK and Rew JS: Capsaicin induces
apoptosis and modulates MAPK signaling in human gastric cancer
cells. Mol Med Rep. 9:499–502. 2014.
|
|
36
|
Asfaha S, Dubeykovskiy AN, Tomita H, Yang
X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA,
Muthupalani S, et al: Mice that express human interleukin-8 have
increased mobilization of immature myeloid cells, which exacerbates
inflammation and accelerates colon carcinogenesis.
Gastroenterology. 144:155–166. 2013. View Article : Google Scholar
|