Introduction
Tuberculosis (TB) is an infectious disease and
remains one of the leading infectious causes of fatality worldwide.
Factors including co-infection with HIV, extending the treatment
(at least 6 months) and the emergence of drug resistant strains put
more burdens on the treatment of TB (1,2). One
of the World Health Organization (WHO) Millennium Development Goals
is to reduce tuberculosis deaths by 90% and to reduce the number of
new cases by 80% between 2015 and 2030. However, >8.5 million
tuberculosis cases were reported in 2011, with an increase in the
number of multidrug-resistant strains (3). Therefore, the WHO target cannot be
reached without the development of a vaccine to limit the spread of
TB.
Currently, Bacille Calmette-Guérin (BCG) is the only
vaccine available against TB and it has been questioned for the low
protective effect against TB in adults and adolescents (3,4).
Therefore, a new vaccine or an alteration of the current BCG
vaccine is urgently required in order to promote cellular immune
responses against Mycobacterium tuberculosis (M.
tuberculosis).
In 2005, Pan et al (5) found a gene in C57BL/6J mice that was
able to promote macrophage resistance to intracellular pathogens
and was designated intracellular pathogen resistance I
(Ipr1). Ipr1 has been shown to promote the activation
of macrophages against M. tuberculosis infection in
vitro (5). However the
mechanisms remained unclear.
In the present study, a recombinant BCG carrying
Ipr1 (BCGi) was constructed and its immune effect against
M. tuberculosis was examined in vivo. Then, the oligo
microarray assay was conducted to further investigate the
mechanisms underlying the effect of Ipr1 on the prevention
of M. tuberculosis infection.
Materials and methods
Construction of BCGi
Coding sequences of Ipr1, green
fluorescent protein (GFP) and OriM (mycobacterial
origin of replication) were simultaneously cloned into pBudCE4.1,
which has multiple promoters to generate the shuttle plasmid
pBudCE4.1/Ipr1/GFP/OriM (pBGOI). The pBGOI was transformed
into BCG. The recombinant BCG carrying Ipr1 (BCGi) had been
successfully constructed previously (6).
Bacterial strains, media and growth
conditions
BCG Pasteur (2003050401) was obtained from the
Shanghai Institute of Biological Products (Shanghai, China). The
M. tuberculosis H37Rv strain was obtained from the
Microbiology Biosafety Laboratory of Tongji Medical College of
HUST, Huazhong University (Wuhan, China). BCG, BCGi and H37Rv were
routinely cultured in Middlebrook 7H9 (Difco; BD Biosciences,
Franklin Lakes, NJ, USA) broth containing 10% OADC, 0.2% (v/v)
glycerol, 0.05% (v/v) Tween 80 (all Difco) and Löwenstein-Jensen
media (produced internally, containing: Malachite green, glycerol,
asparagine, potato starch, coagulated eggs, potassium dihydrogen
phosphate,magnesium sulfate and sodium citrate) for 4 weeks. They
were then harvested and diluted to 5×105 colony-forming
units (cfus)/50 µl, in saline containing 0.05% Tween 80.
Animals
Specific pathogen-free (SPF) 6–8 week old female
C3HeB/FeJ mice were obtained from the Model Animal Research
Institute of Nanjing University (Nanjing, China). Mice were kept in
specific pathogen-free housing in a temperature/humidity-controlled
environment (~24°C), with a 12-h dark:light cycle and free access
to food and water in the Biosafety Laboratory of Tongji Medical
College, Huazhong University (Wuhan, China). The experimental
protocols complied with the ethics guidelines of Chongqing Medical
University and Huazhong University, China.
Intranasal vaccination with BCGi in
vivo
The mice were divided into 3 groups (control group,
BCG group, BCGi group) at random, with 10 mice in each group and
another five normal mice were used as controls. Once anesthetized
for 10 sec with ether, BCGi was administered to mice intranasally.
The BCGi group received BCGi (5×105 cfu/50 µl) a
total of 7 times at 3-day intervals over 3 weeks. The BCG group
received BCG (5×105 cfu/50 µl) and the control
group received 50 µl saline.
Animal challenge study
The experiment started 4 weeks after vaccination and
mice were intranasally challenged with the live H37Rv
(5×105 cfu/50 µl). Successful infection was
confirmed by the bacterial counts of 2 mice sacrificed by cervical
dislocation 3 days after infection. Control mice received 50
µl sterile 0.9% NaCl solution only. The experimental mice
were sacrificed on the 11th week after the first vaccination.
Immunization efficacy
Protection efficacy of the vaccine against M.
tuberculosis was assessed as follows: i) Ipr1 expression in
lung tissues; ii) organ coefficient; iii) M. tuberculosis
load in the lungs and spleen; and iv) histopathological
characterization of the infected organs.
Ipr1 expression in organs
Firstly, the fresh lung and spleen tissues were
lysed by TRIzol (Takara Biotechnology Co., Ltd., Dalian, China) for
5 min at room temperature. Secondly, samples were lysed at 10,000 ×
g for 10 min, and the pellet was discarded. Chloroform was added
and the lysate was placed on ice for 5 min after mixing upside
down, and then centrifuged at 16,000 × g under 4°C for 15 min. The
upper aqueous phase was transferred to another centrifuge tube and
placed on ice for 10 min after mixing with isopropanol. RNA sunk to
the bottom of the tube following centrifugation at 10,000 × g for
10 min at <4°C. Ethanol was added to the centrifuge tube, and
the precipitate was suspended with gentle shaking followed by
centrifugation at 4°C at 8,000 × g for 5 min in order to discard
the supernatant. Once dried at room temperature, using 20 µl
DEPC treated water (Takara Biotechnology Co., Ltd.) to dissolve the
RNA sample. Ipr1 was detected by reverse transcription-polymerase
chain reaction. Total RNA was extracted from fresh lung and spleen
tissues. Total RNA (1 µg) was used for cDNA synthesis, which
was conducted by reverse transcription using the PrimeScript RT
reagent kit Perfect Real time (Takara Biotechnology Co., Ltd.). PCR
the Ipr1 gene with the primers (shown in Table I), with β-actin mRNA as an
endogenous control. It was also examined by western blot analysis.
The proteins were extracted using RIPA buffer (Beyotime Institute
of Biotechnology, Shanghai, China) supplemented with PMSF (Beyotime
Institute of Biotechnology). Protein concentrations were determined
using a BCA protein concentration determination kit (Beyotime
Institute of Biotechnology). Equal amounts of sample were separated
using 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE; Beyotime Institute of Biotechnology) and
transferred onto polyvinylidene fluoride membranes (PVDF; Takara
Biotechnology Co., Ltd.). The PVDF membranes were blocked with 5%
skimmed milk. Subsequent to incubation with polyclonal rabbit
anti-Ipr1 antibody (cat.no. 4407; ProSci, Poway, CA, USA; diluted
1:250) for 12 h, the PVDF membrane was blocked with skimmed milk
after washing three times with TBST buffer. Following this,
membranes were incubated with the monoclonal goat anti-rabbit-IgG
antibody (cat. no. sc-2004; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA; diluted 1:1,000) for 1 h. After washing three times with
TBST buffer, signals were detected using the DAB Horseradish
Peroxidase Color Development kit (Beyotime institute of
Biotechnology). Cells in which cytoplasm stained brown-yellow were
considered positive.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Symbol | Gene ID | Primers | PCR product length
(bp) | Tm (°C) |
|---|
| Gapdh | NM_008084.2 | F:
5′-GTTGTCTCCTGCGACTTCA-3′
R: 5′-GCCCCTCCTGTTATTATGG-3′ | 293 | 59 |
|
Igh-6 | XM_177464 | F:
5′-GGTGCACAGAAATCAAACCC-3′
R: 5′-CCAGGAGCTACTGAATGCGT-3′ | 240 | 59 |
| Lbp | NM_008489 | F:
5′-TCACCGCTCTCCAGTTGCTA-3′
R: 5′-GATGCCGGAGTCATGTGGTA-3′ | 177 | 59 |
| Ltf | NM_008522 | F:
5′-TCAATGGTGTGCTGTGTC-3′
R: 5′-CTGCTACCGCATAGTAGTGA-3′ | 211 | 59 |
| Ly96 | NM_016923 | F:
5′-CTCTTTTCGACGCTGCTTTC-3′
R: 5′-TTCCTTACGCTTCGGCAACT-3′ | 260 | 59 |
| Ncf4 | NM_008677 | F:
5′-CACCAACTGGCTACGATGCT-3′
R: 5′-CATCCTCATCTGACAGCAGC-3′ | 198 | 59 |
| Nfkb1 | NM_008689 | F:
5′-GGCCCATACCTTCAAATATTAGAG-3′
R: 5′-GTGACCAACTGAACGATAACCTT-3′ | 181 | 59 |
| Nfκb2 | NM_019408 | F:
5′-TATGCCATTGTGTTCCGGAC-3′
R: 5′-TCCTCCTTGTCTTCCACCAG-3′ | 148 | 59 |
| Nfκbia | NM_010907 | F:
5′-ACGAGGAGTACGAGCAAATGG-3′
R: 5′-CTCAGGATCACAGCCAGCTT-3′ | 289 | 59 |
| Nos2 | NM_010927 | F:
5′-AGACCCACATCTGGCAGAAT-3′
R: 5′-ACCAGCAGTAGTTGCTCCTCT-3′ | 273 | 59 |
| Prg2 | NM_008920 | F:
5′-GAGAACTTGCCTAGGGATGC-3′
R: 5′-CTGACTAGGAGGTAGCGACAG-3′ | 244 | 59 |
| Sftpd | NM_009160 | F:
5′-GGAGAACGTGGACTAAGTGGA-3′
R: 5′-TCTCCTTTAGGACCTGGTTTGC-3′ | 121 | 59 |
| Stab1 | NM_138672 | F:
5′-GTTCAGTCTGCCAGGAGTGC-3′
R: 5′-TCTTGCTGAGTGTATCCGGG-3′ | 249 | 59 |
| Tlr4 | NM_021297 | F:
5′-TAGAGAATCTGGTGGCTGTG-3′
R: 5′-CCACATGTACTAGGTTCGTC-3′ | 158 | 59 |
| Cd14 | NM_009841 | F:
5′-GGCTTGTTGCTGTTGCTTCT-3′
R: 5′-CGTGTCCACACGCTTTAGAA-3′ | 197 | 59 |
| Cd1d1 | NM_007639 | F:
5′-CGGTACCTACCATGGCTGTT-3′
R: 5′-CTGATGGTGGCTGAGTCATT-3′ | 193 | 59 |
| Chuk | NM_007700 | F:
5′-GAACGTCAGTCTGTACCAGCA-3′
R: 5′-CCTCCAGAACAGTACTCCATTG-3′ | 221 | 59 |
| Csf3 | NM_009971 | F:
5′-GCACTATGGTCAGGACGAGAG-3′
R: 5′-CTGGCTTAGGCACTGTGTCTG-3′ | 272 | 59 |
| Ppbp | NM_023785 | F:
5′-GCTTCAGACTCAGACCTACATC-3′
R: 5′-GGCTATCACTTCCACATCAG-3′ | 250 | 59 |
| Cxcr4 | NM_009911 | F:
5′-TGGAACCGATCAGTGTGAGT-3′
R: 5′-TACTTGTCCGTCATGCTCCT-3′ | 231 | 59 |
| Plunc | NM_011126 | F:
5′-GAGCCTCGTTGTCCTCTGTG-3′
R: 5′-CACCGCTGAGAGCATCTGTG-3′ | 232 | 59 |
Coefficient of lung and spleen
The weight of each mouse was taken at 11 weeks after
the initial vaccination. The lungs and spleens were immediately
removed after the mice were sacrificed. The weight of each lung and
spleen was measured an organ coefficients were determined using the
following formula: Organ coefficient = organ weight (g)/mouse
weight (g) × 100.
M. tuberculosis load in lung and
spleen
The entire left lobe of the lung and one third of
the spleen was weighed accurately and aseptically. These organs
were individually homogenized in sterile saline containing 0.05%
Tween 80. Ten-fold serial dilutions of the homogenates were placed
onto Löwenstein-Jensen media containing 2 mg/l BCG inhibitor TCH
(2-thiophenecarboxylic acid hydrazide; Difco). Bacterial counts in
organs were counted and shown in log10 cfu/g after incubation at
37°C for 4 weeks.
Immunohistochemistry
The experimental mice were sacrificed at the 11th
week after the first vaccination. Approximately half of the right
lobe of the lung and one third of the spleen were immersed in 4%
paraformaldehyde for 4 h, and transfered to 70% ethanol. Individual
lobes of lung and spleen tissue biopsy material was placed in
processing cassettes, dehydrated through a serial alcohol gradient,
and embedded in paraffin wax blocks. Prior to immunostaining,
5-µm-thick lung/spleen tissue sections were dewaxed in
xylene, rehydrated through decreasing concentrations of ethanol,
and washed in PBS. Sections were then stained with hematoxylin and
eosin. After staining, sections were dehydrated through increasing
concentrations of ethanol and xylene. Using a biological microscope
(OLYMPUS CX23; Olympus Corporation, Tokyo, Japan) to assess the
pathological changes is the tissues.
Western blot analysis
Cells were washed three times by precooling PBS and
add the lysis buffer which contain 1% Phosphatase inhibitors, 0.1%
protease inhibitors and 0.5% PMSF. This was followed by pipetting,
gently shaking at 4°C for 15 min, then centrifugation at the speed
of 12,000 × g at 4°C. Protein concentrations were determined using
a bicinchoninic acid protein concentration determination kit
(Beyotime Institute of Biotechnology). Equal quantities of sample
were separated using 10% SDS-PAGE and transferred onto a PVDF
membrane. The membrane was then blocked with 5% skimmed milk for 1
h at room temperature. After incubation with mouse monoclonal
surfactant protein D (SP-D) antibody (cat. no. sc-59695; Santa
Cruz; diluted 1:500), or rabbit monoclonal anti-β-actin (cat. no.
sc-130656; Santa Cruz; diluted 1:2,500) the membrane was washed
three times. The membranes were then incubated with the required
goat anti-mouse or goat anti-rabbit secondary antibody (Santa Cruz;
diluted 1:2,500) for 1 h. After being washed three times with TBST
buffer, signals were detected using the DAB Horseradish Peroxidase
Color Development kit (Beyotime Institute of Biotechnology).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted by DNA-free RNA mini
extraction kit (Watson, Shanghai, China). Total RNA (1 µg)
was used for cDNA synthesis, which was conducted by reverse
transcription using the PrimeScript RT reagent Kit Perfect Real
time (Takara Biotechnology Co., Ltd.). Relative quantification was
performed using SYBR-Green assays (Roche Diagnostics GmbH,
Mannheim, Germany) for the target genes (mRNA of immune related
genes), with glyceraldehyde 3-phosphate dehydrogenase (gapdh) mRNA
as an endogenous control. The primers were synthetized at
Invitrogen and Applied Biosystems (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and are displayed in Table I. The cycling parameters were as
follows: Initial denaturation at 95°C for 5 min; 40 cycles of
denaturation at 95°C for 10 sec, annealing at 59°C for 15 sec, and
extension at 72°C for 20 sec; 85°C for 5 sec to obtain fluorescence
signals with a final extension at 72°C for 5 min. The thermocycler
was an Applied Biosystems 7300 Quantitative PCR instrument (Applied
Biosystems). The dates were analyzed by 7300 Real Time fluorescence
quantitative PCR analysis system. Expression values of target genes
were calculated using the 2−ΔΔCq method (7).
Oligo microarray of immune-related genes
from murine lung tissue
Total RNA was extracted from 5 individual lung
tissues from each group with TRIzol (Takara Biotechnology Co.,
Ltd.). The total RNA was used for cDNA synthesis and biotin-labeled
cRNA linear synthesis using the TrueLabeling-AMP Linear RNA
Amplification kit (Superarray Bioscience; Qiagen, Hilden, Germany).
Expression profiles of mouse immune responses were determined at
KangChen Corporation (Shanghai, China) using the Oligo Innate &
Adaptive Immune Responses Microarray which contained 113 probes of
immune-related genes from mice. The raw data was analyzed by the GE
Array Expression Analysis Suite (GE Healthcare). The level of cDNA
was determined to be differentially expressed when there was a
>2-fold change compared with control, and it was identified as
significantly changed using the using the Rotor-gene 6.0
Microarrays Analysis method with false discovery rate <0.05.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Differences between groups were assessed by one way
analysis of variance with a Bonferroni post hoc test. P≤0.05
differences were considered to indicate a statistically significant
difference. All analyses were performed using the SPSS statistical
software for Windows, version 10.1.4 (SPSS Inc., Chicago, IL,
USA).
Results
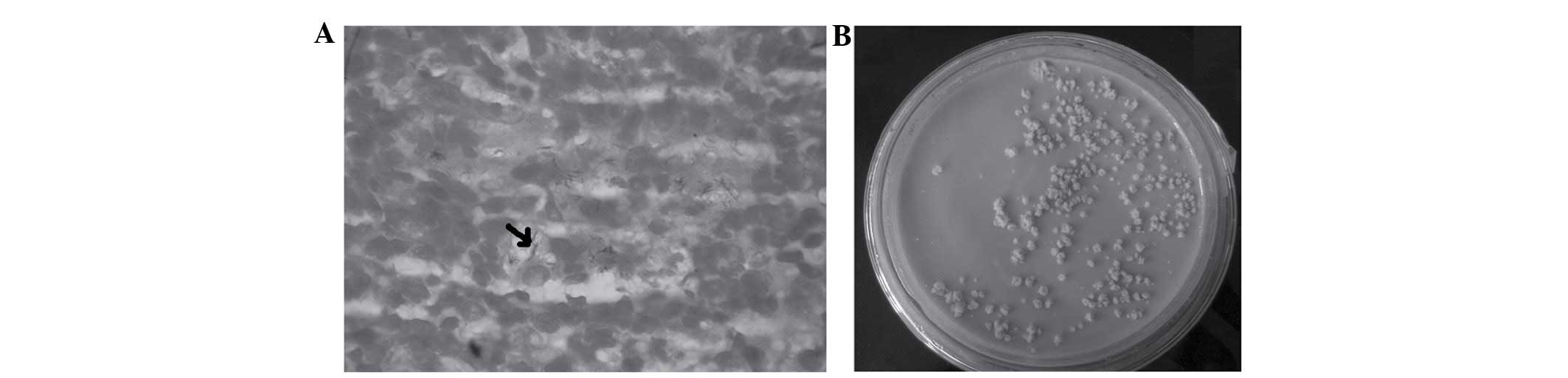
Mice are intranasally infected with
H37Rv
Live H37Rv was intranasally delivered to mice. Lung
counts were conducted 3 days after infection to ensure the mice
were infected with TB. As shown in Fig. 1A, the H37Rv (which has an arrow was
observed in the lung tissues by acid fast staining. A total of 200
µl of the homogenate of lung and spleen tissue (10 g/100 ml)
was added to each of the plates containing Löwenstein-Jensen
medium. Subsequently, these were incubated for 4 weeks, and then
the bacterial colonies were observed in the medium. Bacterial
counts in the organs were shown and counted in Löwenstein-Jensen
medium following the incubation period (Fig. 1B). H37Rv was not identified in the
control mice.
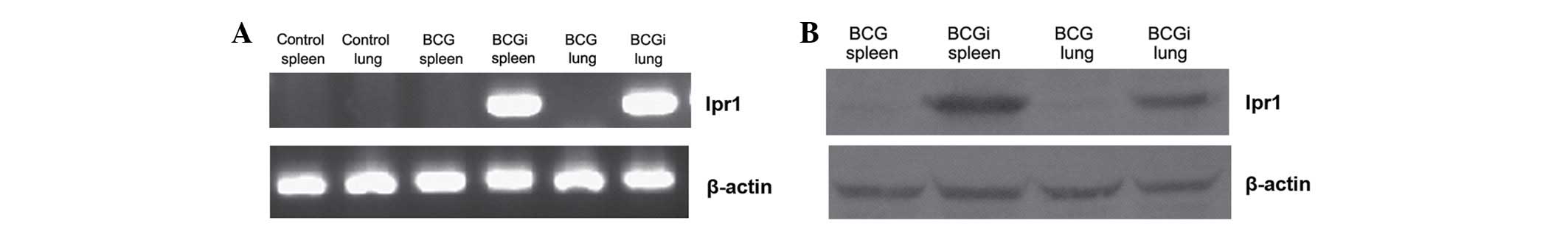
BCGi was successfully delivered to mice
intranasally
Ipr1 mRNA in the lung and spleen tissues was
observed in the BCGi group by RT-PCR (Fig. 2A). Ipr1 expression in the lung and
spleen tissues in BCGi was also conformed by western blot analysis
(Fig. 2B). This result indicated
that the recombinant vaccine BCGi was successfully intranasally
delivered to mice. Furthermore, BCG was shown to be effective in
delivering Ipr1 to target tissues and resulting in its
expression.
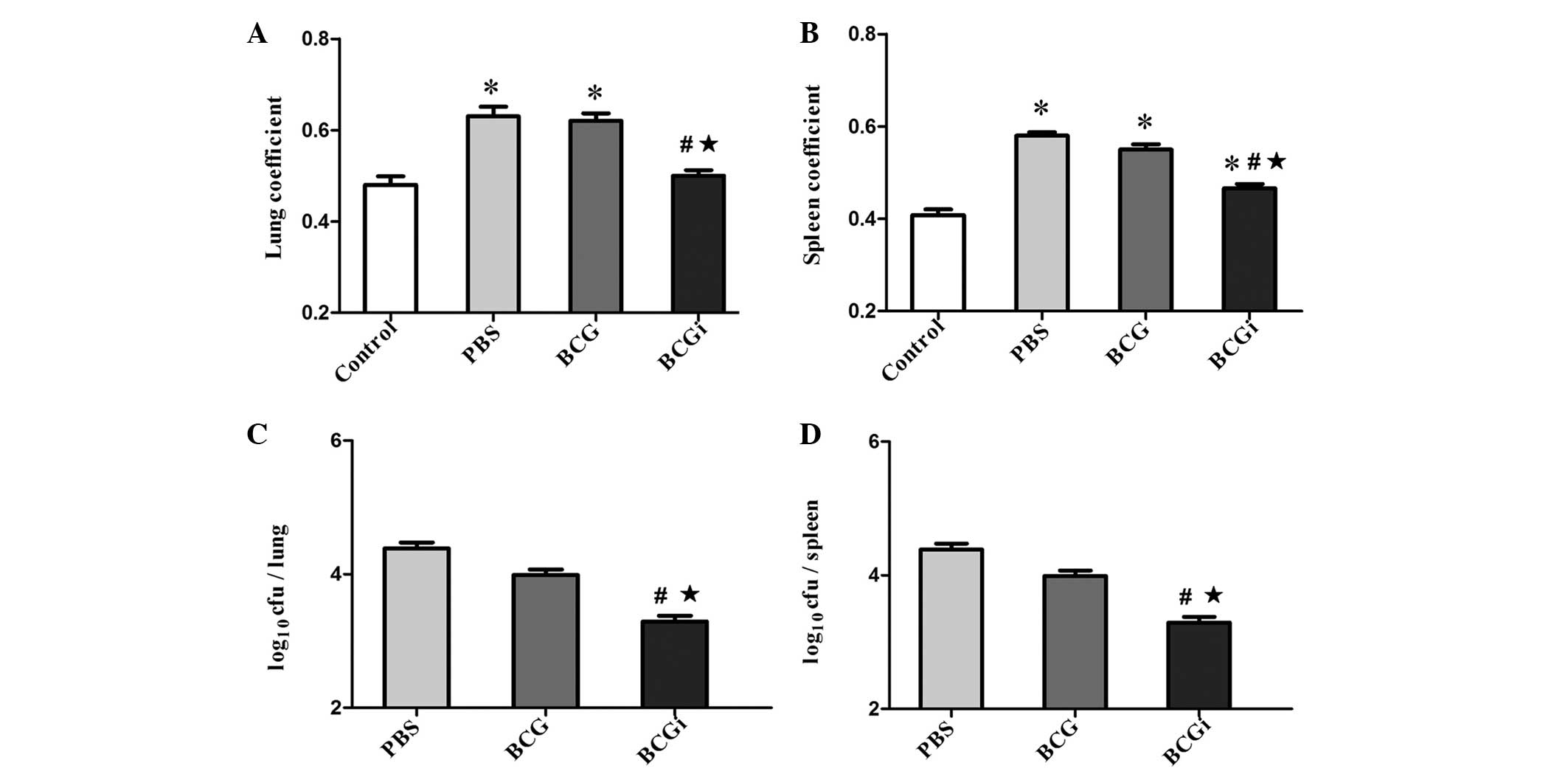
Coefficient of lung and spleen in BCGi
group were lower than that of the BCG group
Compared with the phosphate-buffered saline (PBS)
group, the organ coefficients in the BCGi group were significantly
decreased (P<0.01), while the differences between the PBS and
BCG groups was not significant (P>0.05). Conversely, the lung
and spleen coefficients in the BCGi group were lower than that of
the BCG group (P<0.01; Fig. 3A and
B).
Decrease in bacterial load in the lung
and spleen tissues of infected mice following vaccination with
BCGi
The bacterial loads were measured by placing serial
dilutions onto Löwenstein-Jensen media. As shown in Fig. 3C and D, the BCGi group had
significantly fewer cfu/g in the lungs and spleen than the PBS and
BCG groups (P<0.01). Although the BCG group had fewer cfu/g in
organs than the PBS group, the difference was not significant.
Histology result
Different treatments resulted in different
pathological changes of the lung tissues. Subsequent to the primary
vaccination (11 weeks), the lesions of the lung tissues in the PBS
group (vaccinated with PBS) were excessive and extensive, and the
normal alveolar structure had disappeared (Fig. 4A). The lesions in the lung tissues
in the BCG group (vaccinated with BCG) and BCGi group (vaccinated
with BCGi) were decreased compared with the PBS group (Fig. 4B–D). Abundant foamy cytoplasm and
cholesterol crystallization were observed in the BCG group and BCGi
group. Compared with the BCG group, the lesions of the lung tissues
in the BCGi group were fewer and less severe, and the alveoli wall
structure was more defined.
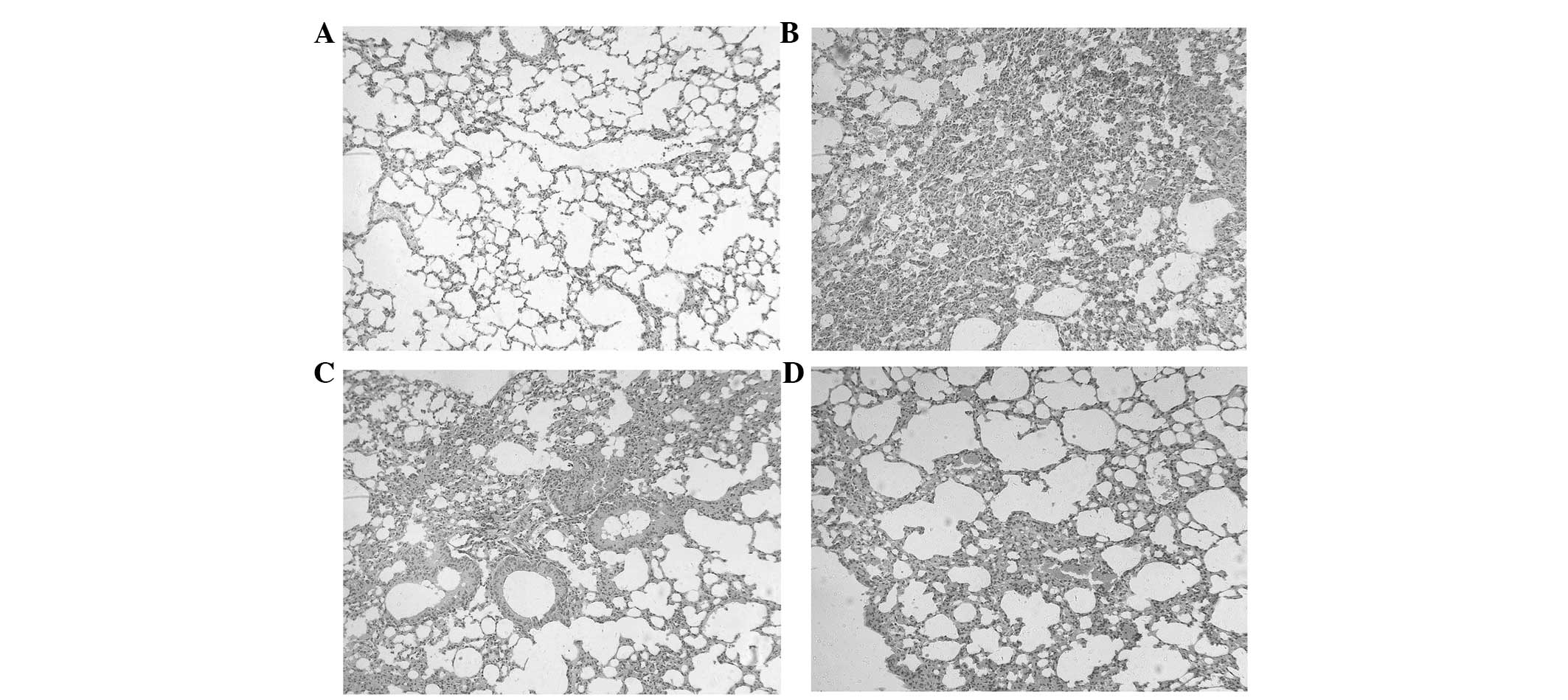 | Figure 4Histology of the lungs of C3HeB/FeJ
mice after 11 weeks of treatment. The C3HeB/FeJ mice were
vaccinated with PBS, BCG or BCGi 7 times at 3-day intervals. The
mice were then intranasally challenged with live H37Rv at 4 weeks
after the last vaccination. The mice were sacrificed 4 weeks after
H37Rv infection. (A) Control group, mice were vaccinated with PBS
but not infected with H37Rv. (B) PBS group, mice infected with
H37Rv after vaccination with PBS. (C) BCG group, mice infected with
H37Rv after vaccination with BCG. (D) BCGi group, mice infected
with H37Rv after vaccination with BCGi. Stain, hematoxylin and
eosin; magnification, ×200. BCG, Bacille Calmette-Guérin; BCGi, BCG
with intracellular pathogen resistance I; PBS, phosphate-buffered
saline. |
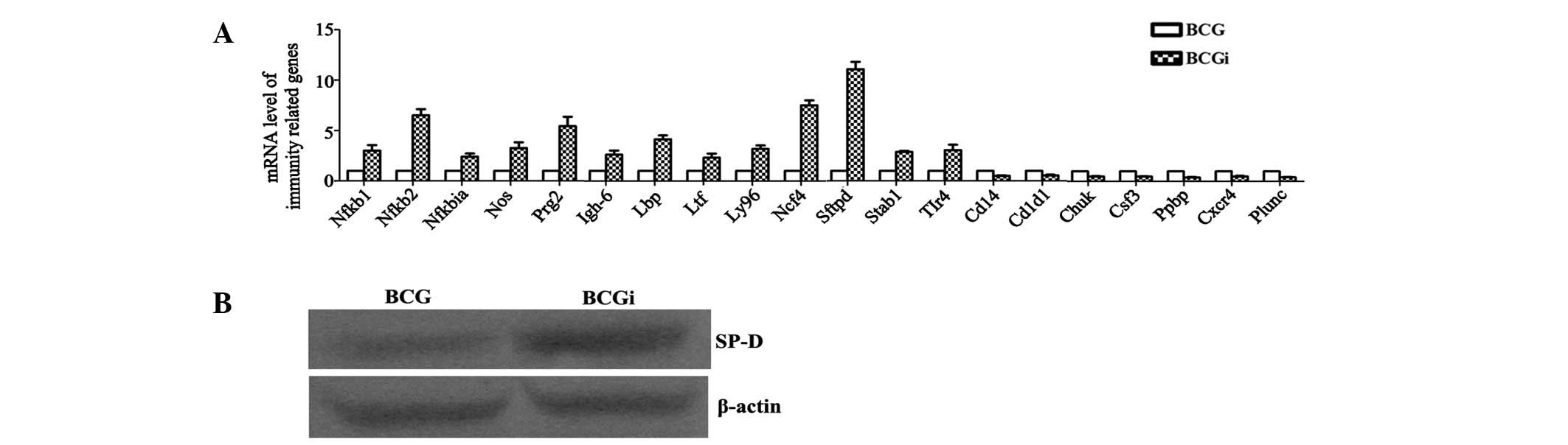
Overexpression of SD-P may be responsible
for the stronger immunity of BCGi against tuberculosis than
BCG
Expression profiles of mouse immune responses were
determined using oligo microarray which contained 113 probes of
immune-related genes from mice (Fig.
5). The result showed that there were 20 differentially
expressed genes with a >2-fold change compared with the BCG
group. Specifically, there were 13 upregulated genes: Igh6,
Lbp, Ltf, Ly96, Ncf4, Nfkb1,
Nfkb2, Nfkbia, Nos2, Prg2,
Sftpd, Stab1 and Tlr4 (Table II); and 7 downregulated genes:
Cd14, Cd1d1, Chuk, Csf3, Ppbp,
Cxcr4 and Plunc in the BCGi group compared with the
BCG group (Table III). The
expression level of these 20 genes was further determined by
RT-qPCR (Fig. 6A), which showed
the same trend as the microarray. In particular, the expression
level of Sftbp, an immune-related gene, increased
>10-fold in BCGi group compared with the BCG group. SP-D was
encoded by the Sftbp gene and its expression level in the
BCGi group was significantly higher than that of the BCG group as
determined by western blot analysis (Fig. 6B).
 | Table IIUpregulated expression genes
comparison BCGi group with BCG group. |
Table II
Upregulated expression genes
comparison BCGi group with BCG group.
| Symbol | Gene ID | Relative level of
gene expression in group BCGi | Relative level of
gene expression in group BCG | Gene expression
ratio of group BCGi to BCG |
|---|
|
Igh-6 | XM_177464 | 1.827041 | 0.801591 | 2.3 |
| Lbp | NM_008489 | 1.180695 | 0.512301 | 2.3 |
| Ltf | NM_008522 | 0.772659 | 0.343613 | 2.2 |
| Ly96 | NM_016923 | 1.061771 | 0.492027 | 2.2 |
| Ncf4 | NM_008677 | 2.166263 | 0.446541 | 4.9 |
| Nfκb1 | NM_008689 | 7.759150 | 2.590354 | 3.0 |
| Nfκb2 | NM_019408 | 5.276989 | 1.089581 | 4.8 |
| Nfκbia | NM_010907 | 7.424547 | 3.152819 | 2.4 |
| Nos2 | NM_010927 | 2.641727 | 0.794053 | 3.3 |
| Prg2 | NM_008920 | 1.436093 | 0.340494 | 4.2 |
| Sftpd | NM_009160 | 4.856252 | 0.517239 | 9.4 |
| Stab1 | NM_138672 | 1.390140 | 0.485789 | 2.9 |
| Tlr4 | NM_021297 | 1.866528 | 0.528936 | 3.5 |
 | Table IIIDownregulated genes comparison BCGi
group with BCG group. |
Table III
Downregulated genes comparison BCGi
group with BCG group.
| Symbol | Gene ID | Relative level of
gene expression in group BCGi | Relative level of
gene expression in group BCG | Gene expression
ratio of group BCGi to BCG |
|---|
| Cd14 | NM_009841 | 0.657430 | 1.817355 | 0.36 |
| Cd1d1 | NM_007639 | 0.237848 | 0.523217 | 0.45 |
| Chuk | NM_007700 | 0.626025 | 2.156289 | 0.29 |
| Csf3 | NM_009971 | 0.565524 | 1.676739 | 0.34 |
| Ppbp | NM_023785 | 0.725782 | 2.579958 | 0.28 |
| Cxcr4 | NM_009911 | 0.822538 | 4.387695 | 0.19 |
| Plunc | NM_011126 | 1.071701 | 5.285196 | 0.20 |
Discussion
Tuberculosis is a major challenge to global public
health. However, research has indicated that only 10% of
individuals infected with M. tuberculosis will develop
disease (8) and there are factors
that determine the susceptibility in individuals (9). Association studies have identified
various host genetic factors that affect susceptibility to TB. The
mouse gene Ipr1 within the sst1 locus has been shown to
contribute to innate immunity in a murine model of M.
tuberculosis infection (5).
The human homologue of Ipr1 is SP110, which may be a
promising candidate for controlling M. tuberculosis
infections. The genotypes and haplotypes of SP110 have been
widely investigated in recent years, yet the results were somewhat
contradictory and indeterminate (10,11).
In 2011, Liang et al (12)
demonstrated that the genotypes and haplotypes of SP110 may
be associated with susceptibility to TB in the Chinese population.
Previous studies indicated that Ipr1 could enhance the effect of
macrophages against intracellular pathogens, such as mycobacterium
(13). Macrophages are key in
innate immunity; however, the intrinsic link between this gene and
macrophages or the innate immune system was unclear. Thus, the
present study investigated the underlying mechanism using IPR1 to
transform BCG and determined its effect on M. tuberculosis
infection.
In the present study, BCG was altered and carried
the Ipr1 gene into macrophages in vivo. C3HeB/FeJ
mice were selected as the test animal as they do not contain the
Ipr1 gene in its genome and are sensitive to M.
tuberculosis. The immune effect of this recombinant vaccine
BCGi was evaluated in the mice by determining the organ
coefficient, bacterial load and the histopathological
characterization of the infected organs. It was shown that the
bacterial load in the lung and spleen was significantly reduced in
the BCGi group compared with the PBS and BCG groups. Bacterial load
was shown to be an important indicator of immune efficacy against
M. tuberculosis infection in animal studies (14). Also known as the organ coefficient
is the ratio of the weight of the organs and the experimental
animals. The normal organ coefficient is relatively constant.
However, an increase in the organ coefficient is suggestive of
organ congestion, edema, or hypertrophy and therefore may reflect
the intensity of infection and inflammation to a certain extent.
The coefficient of the lung and spleen in the BCGi group were lower
than those in the BCG group and PBS group, and closer to the state
of the mice without infection. This result indicated that BCGi as a
vaccine resulted in a greater reduction in bacterium infection and
the inflammatory response compared with BGC. Histopathological
results showed that the lesions in the lungs of the BCG group less
severe slighter than those in the PBS group, but more advanced than
in the BCGi group. These results confirmed that the recombinant
vaccine BCGi had a stronger protective effect against M.
tuberculosis than BCG.
Studies have shown that the TLR signaling pathway is
important in resistance to M. tuberculosis infection by
macrophages. TLR1, TLR6, TLR2, TLR4, TRIF, Irak, Traf6, Myd88 and
NF-κB are involved in the TLR signaling pathway (15). Certain surface structures of M.
tuberculosis are able to combine with the ligands of TLR2 and
TLR4, such as LAM (lipoarabinomannan) a 19 kD lipoprotein, and the
38 kD sugar lipoprotein, HSP70 and so on (16). After TLR2 or TLR4 bind to the
corresponding ligands, NF-κB is activated by the MyD88-dependent
and non-dependent pathways, which caused the release of a variety
of inflammatory cytokines and upregulation of the expression of
CD80, CD86 and other co-stimulatory molecules on the surface of
antigen-presenting cells. These changes improve the innate immune
ability to eliminate the pathogens at an early stage (17). The result of oligo microarray
showed that the gene expression of TLR4 increased >3
times in the BCGi group compared with the BCG group, while no
change in the levels of TLR2, Irak, Traf6,
Myd88 was identified. It indicated that Ipr1 enhancing the
innate immune against M. tuberculosis may occur through the
Myd88-independent pathway of TLR4. In addition, TLRs signaling
pathway could also activate other mechanisms against M.
tuberculosis infection, including apoptosis and NO production.
In this study, the expression of Nos2 was notably increased
in BCGi group. This suggested that Ipr1 may promote the
production of NO by macrophages through the TLR signaling pathway,
and NO was able to damage the structure of M. tuberculosis
so that the bacteria may be eliminated. In total, 113
immune-related genes from mice were detected by oligo microarray,
however, only 20 of these genes showed >2-fold change in
expression compared with the BCG group. Notably, among these 20
genes, stfpd exhibited the greatest change in expression
(>10-fold) in the BCGi group, compared with the BCG group. SP-D
was encoded by the stfpd gene, is a component of the lung
innate immunity that enhances clearance of pathogens and modulates
inflammatory responses (18). The
lung is continuously exposed to inhaled pollutants, microbes and
allergens. Thus, the pulmonary immune system has to defend against
harmful pathogens, but not produce an inappropriate inflammatory
response to harmless particles. In the bronchoalveolar space this
critical balance is maintained by innate immune proteins, termed
surfactant proteins, such as surfactant SP-D, which is central to
the pulmonary host defense. SP-D is an epithelial-cell-derived
immune modulator that belongs to the small family of structurally
related Ca (2+)-dependent C-type collagen-like lectins.
Although collectins can be detected on mucosal surfaces of various
organs, SP-D is constitutively expressed in the lung at high
concentrations (19). Research has
demonstrated that SP-D binds carbohydrates, lipids, lipoteichoic
acid and nucleic acids with a broad spectrum specificity and
initiates phagocytosis of inhaled pathogens as well as apoptotic
cells (20). Therefore, pathogen
binding and aggregation by lung collectins is an method of
phagocytic elimination. Early studies showed that SP-A (similar to
SP-D) enhanced the phagocytosis of M. tuberculosis and M.
bovis by alveolar macrophages (21,22)
However, the details of the innate or adaptive immunity modulation
by SP-D are not well understood. Certain studies have demonstrated
that SP-D could recognize, bind and modulate the function of CD14
(23), TLR-2 (24) and TLR4 (25). Lack of SP-D in gene-deficient mice
resulted in a failure to increase TLR4 expression during allergic
inflammation in comparison with wild-type (26). In addition, SP-D was shown to
enhance the amount of oxygen radicals produced by alveolar
macrophages and neutrophils (27).
In conclusion, the Ipr1 modified BCG has stronger
immunity than BCG against tuberculosis infection in mice, and could
be a candidate vaccine against M. tuberculosis. Ipr1 was
shown to activate the TLR4 signaling pathway to increase
inflammatory cytokine release, co-stimulatory molecule expression
on the surface of APCs, apoptosis and NO production by macrophages.
Conversely, Ipr1 could improve SP-D expression in lung tissues,
which could bind and initiate the phagocytosis of pathogens.
Acknowledgments
This study was supported by grants from the National
Science Foundation of China (grant no. 30901280) and Chongqing
Municipal Commission of Health and Family Planning
(2015MSXM081).
References
|
1
|
Global tuberculosis control: surveillance,
planning, financing. (WHO report 2007). Geneva: World Health
Organization (WHO/HTM/TB/2007.376);
|
|
2
|
Dos Santos JL, Lima MM, Trindade AB,
Carnavalli F, Melchior AC and Chin CM: Tuberculosis: Challenges to
improve the treatment. Curr Clin Pharmacol. 2013.Epub ahead of
print.
|
|
3
|
Montagnani C, Chiappini E, Galli L and de
Martino M: Vaccine against tuberculosis: What's new? BMC Infect
Dis. 14(Suppl 1): S22014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin C: Tuberculosis vaccines: Past,
present and future. Curr Opin Pulm Med. 12:186–191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan H, Yan BS, Rojas M, Shebzukhov YV,
Zhou H, Kobzik L, Higgins DE, Daly MJ, Bloom BR and Kramnik I: Ipr1
gene mediates innate immunity to tuberculosis. Nature. 434:767–772.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YW, Xu L, Zhang L, He YL, Yang C and
Huang AL: Construction the recombinant BCG targeting delivering
IprI into macrophages: A new strategy of vaccine against
tuberculosis. African Journal of Microbiology Research. 7:533–540.
2013.
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
8
|
Fang R, Li X, Li J, Wu J, Shen X, Gui X,
DeRiemer K, Liu L, Mei J and Gao Q: Mixed infections of
Mycobacterium tuberculosis in tuberculosis patients in Shanghai,
China. Tuberculosis (Edinb). 88:469–73. 2008. View Article : Google Scholar
|
|
9
|
Bellamy R: Genetic susceptibility to
tuberculosis. Clin Chest Med. 26:233–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Png E, Alisjahbana B, Sahiratmadja E,
Marzuki S, Nelwan R, Adnan I, van de Vosse E, Hibberd M, van Crevel
R, Ottenhoff TH and Seielstad M: Polymorphisms in SP110 are not
associated with pulmonary tuberculosis in Indonesians. Infect Genet
Evol. 12:1319–1323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lei X, Zhu H, Zha L and Wang Y: SP110 gene
polymorphisms and tuberculosis susceptibility: A systematic review
and meta-analysis based on 10 624 subjects. Infect Genet Evol.
12:1473–1480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang L, Zhao YL, Yue J, Liu JF, Han M,
Wang H and Xiao H: Association of SP110 gene polymorphisms with
susceptibility to tuberculosis in a Chinese population. Infect
Genet Evol. 11:934–939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kramnik I: Genetic dissection of host
resistance to Mycobacterium tuberculosis: The sst1 locus and the
Ipr1 gene. Curr Top Microbiol Immunol. 321:123–148. 2008.PubMed/NCBI
|
|
14
|
Orme IM, McMurray DN and Belisle JT:
Tuberculosis vaccine development: Recent progress. Trends
Microbiol. 9:115–118. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quesniaux V, Fremond C, Jacobs M, Parida
S, Nicolle D, Yeremeev V, Bihl F, Erard F, Botha T, Drennan M, et
al: Toll-like receptor pathways in the immune responses to
mycobacteria. Microbes Infect. 6:946–959. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung SB, Yang CS, Lee JS, Shin AR, Jung
SS, Son JW, Harding CV, Kim HJ, Park JK, Paik TH, et al: The
mycobacterial 38-kilodalton glycolipoprotein antigen activates the
mitogen-activated protein kinase pathway and release of
proinflammatory cytokines through Toll-like receptors 2 and 4 in
human monocytes. Infect Immun. 74:2686–2696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ospelt C and Gay S: TLRs and chronic
inflammation. Int J Biochem Cell Biol. 42:495–505. 2010. View Article : Google Scholar
|
|
18
|
Hartl D and Griese M: Surfactant protein D
in human lung diseases. Eur J Clin Invest. 36:423–435. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haczku A: Protective role of the lung
collectins surfactant protein A and surfactant protein D in airway
inflammation. J Allergy Clin Immunol. 122:861–879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Forbes LR and Haczku A: SP-D and
regulation of the pulmonary innate immune system in allergic airway
changes. Clin Exp Allergy. 40:547–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaynor CD, McCormack FX, Voelker DR,
McGowan SE and Schlesinger LS: Pulmonary surfactant protein A
mediates enhanced phagocytosis of Mycobacterium tuberculosis by a
direct interaction with human macrophages. J Immunol.
155:5343–5351. 1995.PubMed/NCBI
|
|
22
|
Weikert LF, Edwards K, Chroneos ZC, Hager
C, Hoffman L and Shepherd VL: SP-A enhances uptake of bacillus
Calmette-Guerin by macrophages through a specific SP-A receptor. Am
J Physiol. 272:L989–L995. 1997.PubMed/NCBI
|
|
23
|
Chiba H, Sano H, Iwaki D, Murakami S,
Mitsuzawa H, Takahashi T, Konishi M, Takahashi H and Kuroki Y: Rat
mannose-binding protein a binds CD14. Infect Immun. 69:1587–1592.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohya M, Nishitani C, Sano H, Yamada C,
Mitsuzawa H, Shimizu T, Saito T, Smith K, Crouch E and Kuroki Y:
Human pulmonary surfactant protein D binds the extracellular
domains of Toll-like receptors 2 and 4 through the carbohydrate
recognition domain by a mechanism different from its binding to
phosphatidylinositol and lipopolysaccharide. Biochemistry.
45:8657–8664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guillot L, Balloy V, McCormack FX,
Golenbock DT, Chignard M and Si-Tahar M: Cutting edge: The
immunostimulatory activity of the lung surfactant protein-A
involves toll-like receptor 4. J Immunol. 168:5989–5992. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Ikegami M, Crouch EC, Korfhagen
TR and Whitsett JA: Activity of pulmonary surfactant protein-D
(SP-D) in vivo is dependent on oligomeric structure. J Biol Chem.
276:19214–19219. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Iwaarden JF, Shimizu H, Van Golde PH,
Voelker DR and Van Golde LM: Rat surfactant protein D enhances the
production of oxygen radicals by rat alveolar macrophages. Biochem
J. 286:5–8. 1992. View Article : Google Scholar : PubMed/NCBI
|