Introduction
Parkinson's disease (PD) is a progressively
debilitating neurodegenerative disorder (1). The major pathological feature of PD
is the selective degeneration and loss of dopaminergic (DA) neurons
of the substantia nigra leading to a significant reduction in
synthesis of extrapyramidal dopamine (2). Currently available medication only
alleviates symptoms temporarily and cannot cure PD (3). It is therefore imperative to identify
safe and effective methods of treating this disorder, preferably by
supplementing patients with DA neurons. One approach is to
investigate the dynamics of postnatal and adult neurogenesis in
vivo, which if successfully manipulated may succeed in
correcting the dopamine imbalance in PD patients (4,5).
Another approach is to generate healthy DA neurons in vitro
and transplant them into the brains of PD patients to replenish the
loss (6).
The multipotent neural stem cells (NSCs) identified
in the adult hippocampus have the ability to proliferate and
differentiate throughout the lifetime of the individual. Numerous
studies have demonstrated that NSCs secrete various neurotrophic
factors, neurotransmitters and enzymes (7–9). In
response to molecular signals from the microenvironment,
transplanted hippocampal NSCs (Hip-NSCs) differentiate into various
types of neurons and glia in vivo, a property that may be
recapitulated in vitro under appropriate conditions.
Transplanted Hip-NSCs may not only replenish the reservoir of
neurotrophic growth factors in the damaged nervous tissue, but also
expand and generate lost and degenerated neurons to achieve
functional recovery (10,11). The multipotency of NSCs affords
them a broad application in cell replacement therapy and injury
repair in diseases of the central nervous system (12,13).
In the present study, Hip-NSCs were isolated from
postnatal mouse brains and cultured in vitro to monitor
their proliferation, migration and differentiation properties. The
dynamic expression of DA neuronal markers nuclear receptor
related-1 protein (Nurr1) and tyrosine hydroxylase (TH) in the
differentiated neurons was analyzed. Hip-NSCs were observed to
differentiate into neurons with DA characteristics. The results of
the present study provide further evidence of the merits of using
Hip-NSCs as a suitable donor population for stem cell therapy in
PD.
Materials and methods
Animals
All experiments were conducted with C57BL/6 mice
(0–3 days old) provided by the Laboratory Animal Center of Ningxia
Medical University (Yinchuan, China). Mice were housed at 24–25°C
and 50–60% humidity on a 12-h light/dark cycle. Food and water were
provided ad libitum. All experiments were approved by the
Animal Experimentation Ethics Committee of Ningxia Medical
University (Yinchuan, China) and were specifically designed to
minimize the number of animals used.
Hip-NSC culture
Obtained 0–3-day old C57BL/6 mice were sacrificed by
drowning in ethyl alcohol for five min, and were stripped of the
skin and periosteum of the head. Mice were then placed onto a 35-mm
dish in 2 ml Hank's balanced salt solution, the cerebral cortex was
stripped, the bilateral hippocampus was fully exposed and the
intact hippocampal tissues were collected. Following removal of the
blood vessels and fascia of the brain, dissected hippocampal
tissues were finely minced and treated with 1–2 ml
Accutase® (A1110501; Gibc Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C for 10 min, followed by
resuspension in 3–4 ml fresh medium. No additional washes or enzyme
inhibitors were required. Following enzymatic digestion, the
tissues were mechanically dissociated into a single cell suspension
by gentle movement up and down in fresh medium using a pipette, in
order to reduce cell damage and death. The cell suspension was
centrifuged at 179 × g for 5 min at room temperature. The
supernatant was discarded, and the cell pellet resuspended and
seeded at a density of 1×105 cells/tissue culture flask
in 1:1 Dulbecco's modified Eagle's medium (DMEM)/F12 1:1
(11330-032; Gibc Thermo Fisher Scientific, Inc.) supplemented with
2% B-27® (12587-010; Invitrogen; Thermo Fisher
Scientific, Inc.), 20 ng/ml recombinant human epidermal growth
factor (450-02; PeproTech, Inc., Rocky Hill, NJ, USA) and 20 ng/ml
basic fibroblast growth factor (bFGF, 100-18B; PeproTech, Inc.).
Cells were cultured in a humidified 5% CO2/95% air
incubator at 37°C for 5–7 days. During this period, the cells
gradually grew into floating neurospheres. They were maintained in
culture with regular passaging every 5–7 days.
5-bromo-2′-deoxyuridine (BrdU)
immunocytochemistry
Passage (P) 2 Hip-NSCs were treated with the
thymidine analog BrdU (Sigma-Aldrich, St Louis, MO, USA) at 10
µmol/l upon reaching 50% confluency. A total of 48 h later,
the neurospheres derived from the Hip-NSCs were plated on
polylysine-treated sterile glass coverslips and incubated at 37°C
in 5% CO2 for 2–4 h. Following thorough rinsing with
phosphate-buffered saline (PBS), the neurospheres were treated with
2 M hydrochloric acid for 1 h at 37°C and boric acid buffer (pH
8.5) for 10 min at room temperature (RT). BrdU-incorporated cells
were visualized by immunostaining. Briefly, cells were incubated
with blocking solution 0.3% Triton X-100 containing 1% normal
donkey serum (Jackson ImmunoResearch Labortories, Inc., West Grove,
PA, USA) for 30 min. Cells were then incubated with a primary
antibody mixture of mouse anti-BrdU (1:200; ab8152; Abcam,
Cambridge, MA, USA) and rabbit anti-nestin (1:100; N5413;
Sigma-Aldrich) for 24 h at 4°C, followed by the secondary
antibodies (Alexa Fluor 488-conjugated donkey anti-mouse IgG;
1:1,000; R37114; Alexa Fluor 594-conjugated donkey anti-rabbit IgG;
1:1,000; R37119; both Thermo Fisher Scientific, Inc.) for 4 h at
RT. Finally, the immunostained Hip-NSCs were mounted onto glass
slides using VECTASHIELD® antifade fluorescence mounting
medium (Vector Laboratories, Inc., Burlingame, CA, USA), and
observed under a fluorescence microscope.
Differentiation of Hip-NSCs
P2 Hip-NSCs were seeded onto polylysine-treated
coverslips at 30–40 cells/coverslip in a 24-well plate. To induce
differentiation, these cells were cultured in low-serum conditioned
medium containing DMEM/F12 1:1 supplemented with 2% fetal bovine
serum (26140-111, Gibc Thermo Fisher Scientific, Inc.) and 100
ng/ml FGF-8 (100-25, PeproTech, Inc.). The growth medium was
replaced every 2–3 days, and immunocytochemistry and western
blotting were performed following 3–7 days of differentiation.
Immunocytochemistry of differentiated
Hip-NSCs
Following flattening and adhesion, Hip-NSCs and
their differentiated progeny were washed in PBS 2–3 times and
incubated overnight at 4°C with the following primary antibodies:
Rabbit anti-Nurr1 (1:1,000; N4663; Sigma-Aldrich), mouse anti-TH
(1:8,000; T2928; Sigma-Aldrich), mouse anti-glial fibrillary acidic
protein (GFAP; 1:4,000; SAB1405864; Sigma-Aldrich), rabbit
anti-GFAP (1:3,000; ab7779; Abcam), mouse anti-β-Tubulin III (Tuj1;
1:3,000; T5076; Sigma-Aldrich), rabbit anti-Tuj1 (1:3,000;
SAB4300623; Sigma-Aldrich) and mouse anti-2′, 3′-cyclic nucleotide
3′-phosphodiesterase (CNPas 1:500; C5922; Sigma-Aldrich). Cells
were then incubated with the appropriate Alexa Fluor 488 or Alexa
Fluor 555-labeled secondary antibodies (1:500; goat anti-mouse IgG;
A11001; goat anti-rabbit IgG; A31629; Invitrogen; Thermo Fisher
Scientific, Inc.). Nuclei were stained with Hoechst 33342 (1:500;
B2261; Sigma-Aldrich). Coverslips were mounted onto slides, and
observed under a fluorescence microscope.
Western blot analysis
This assay was performed as described previously
(14). Hip-NSCs and their
differentiated progeny were treated with radioimmunoprecipitation
assay lysis buffer containing 2% protease inhibitor (Boehringer
Mannheim, Mannheim, Germany) for 30 min, removed with a cell
scraper (on ice), transferred to 2 ml microcentrifuge tubes and
pelleted by centrifugation at 13,314 × g for 5–10 min at 4°C. The
supernatant was transferred to fresh microcentrifuge tubes and the
total protein concentration was estimated using the bicinchoninic
acid assay according to the manufacturer's instructions. A total of
20–30 µg protein was loaded in each well of a 10% SDS-PAGE
gel, separated by electrophoresis (2 h at 120 mV) and transferred
to polyvinylidene difluoride membranes (100 min at 130 mA). The
membranes were blocked with 5% milk at RT for 1 h and incubated
overnight at 4°C with the following primary antibodies: Rabbit
anti-Nurr1 (1:1,000; N4663; Sigma-Aldrich), mouse anti-TH (1:8,000;
T2928; Sigma-Aldrich) and mouse anti-β-actin (1:2,000; A2228;
Sigma-Aldrich). The following day the membranes were washed in
Tris-buffered saline with 1% Tween 20 three times, and incubated
with horseradish peroxidase HRP-conjugated secondary antibodies
(goat anti-rabbit; 1:3,000; ab6721; or goat anti-mous 1:3,000;
ab97023; Abcam) for 1 h at room temperature. The protein bands were
visualized by incubating the membranes in enhanced
chemiluminescence substrate solution and analyzed using
ImagePro-Plus version 6.0 software (Media Cybernetics, Inc.,
Rockville. MD, USA). The band intensity of Nurr1 and TH was
normalized to β-actin.
Statistical analysis
Data were collected from triplicate samples from
three independent experiments. All data are expressed as the mean ±
standard error. Statistical comparisons were performed using
Student's t-test or one-way analysis of variance, followed
by Dunnett's post-hoc test. Statistical analysis was performed
using SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
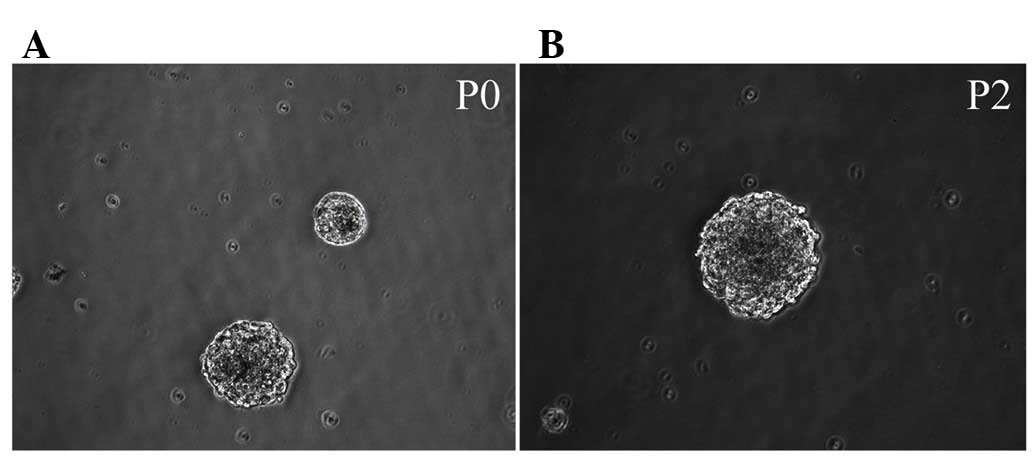
Morphology of Hip-NSCs
Immediately following the seeding of isolated
Hip-NSCs, cells were large and rounded with strong light
refraction. Very little cell debris and few adherent differentiated
cells were observed under a bright-field microscope. Cellular
aggregates with an orbicular morphology started to appear 36 h
later. These resembled neurospheres and their number and size
increased with time (Fig. 1A).
Following five days of in vitro culture, cells were passaged
by enzymatic dissociation using Accutase®. Dead cells
were rarely observed at this time point (Fig. 1B). During passaging, 98% of the
cultured Hip-NSCs were dissociated into single cells. A total of 36
h subsequent to passaging, the cells reassembled into
mitotically-active aggregates, exhibiting a rounded shape.
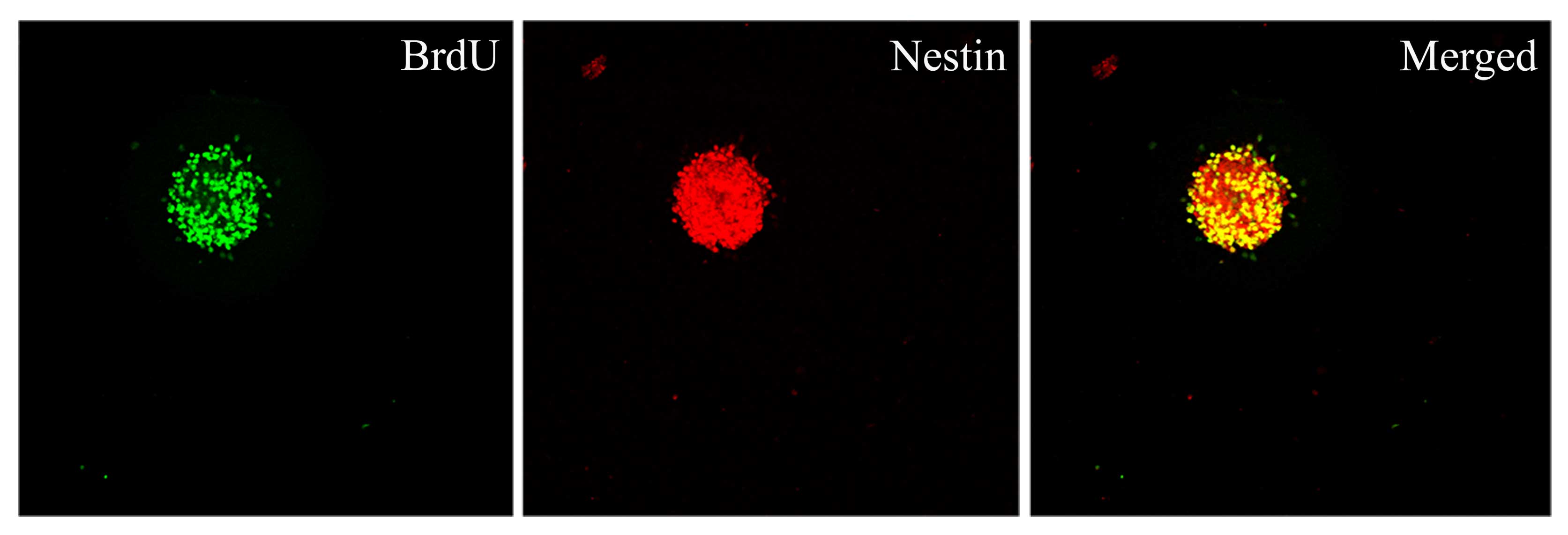
Proliferative capacity of Hip-NSCs in
vitro
To determine the proliferative capacity of Hip-NSCs
in vitro, neurospheres were examined by double
immunofluorescence staining for incorporation of the proliferation
indicator BrdU and the neural progenitor marker nestin. The results
revealed that Hip-NSCs expanded rapidly in culture as visualized by
the extent of BrdU incorporation and that NSCs were abundant within
the neurospheres (Fig. 2).
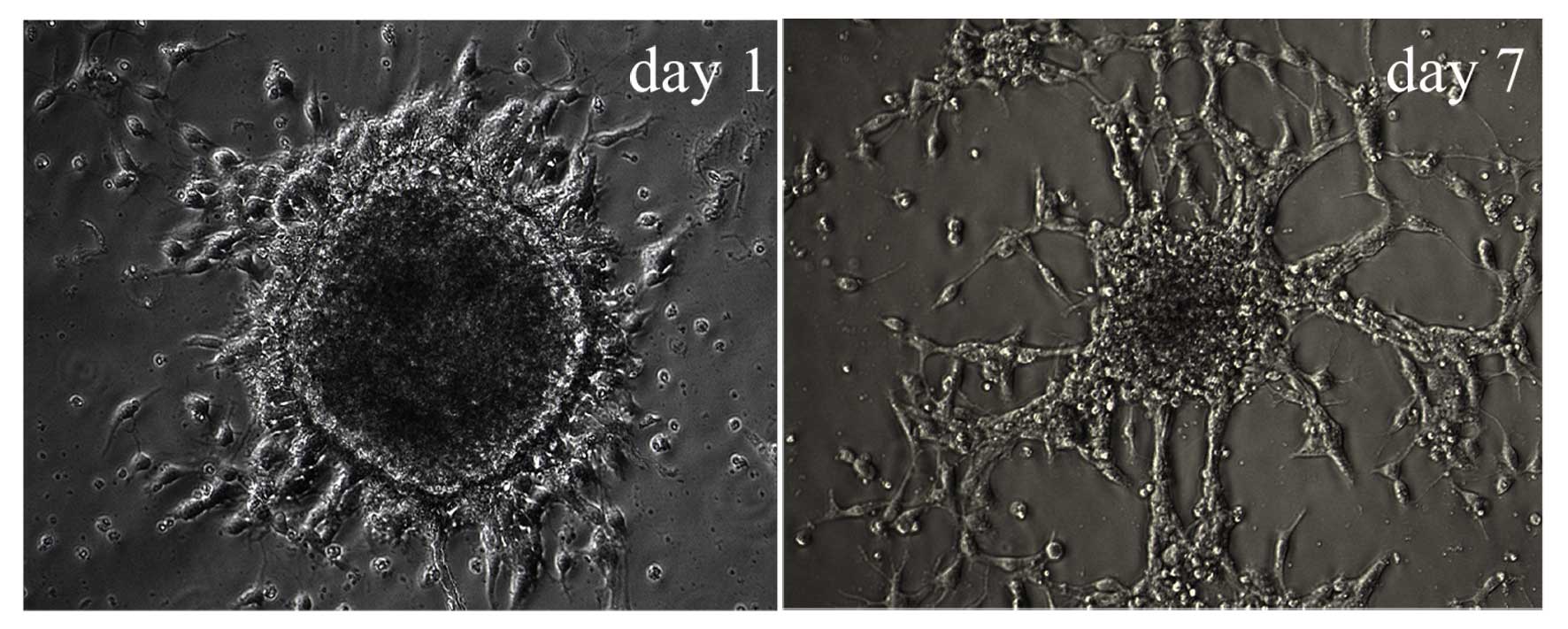
Characteristics of differentiating
Hip-NSCs
Following the second passaging of Hip-NSCs (P2), the
plated cells attached to the surface completely within 4 h, a
feature characteristic of non-proliferative differentiating cells.
Over time, an increasing number of cells migrated out of the
neurospheres. At 12–24 h following passaging, processes began to
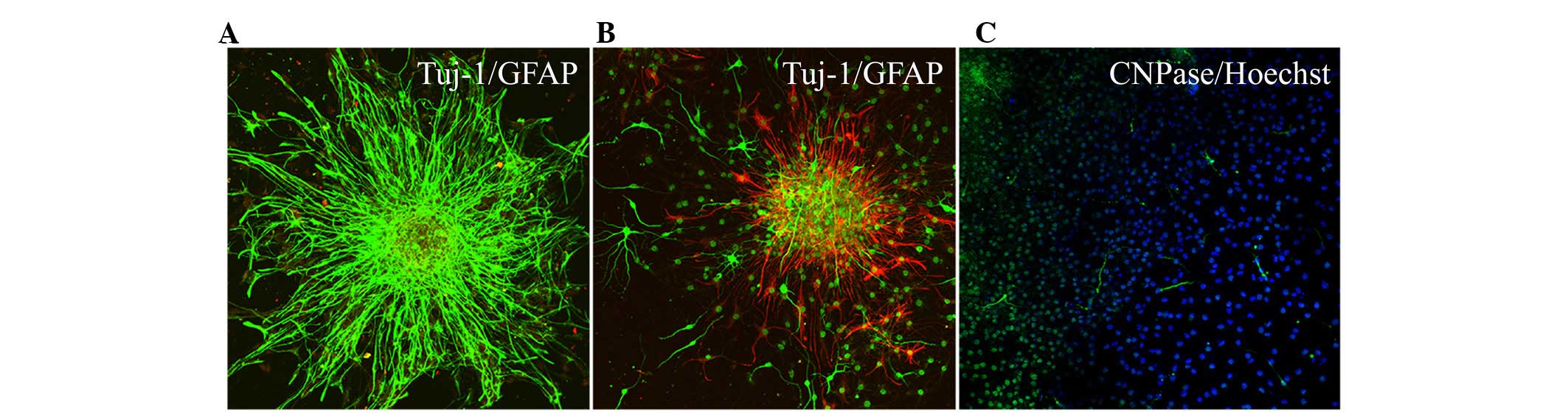
emerge along the edge of the neurospheres (Fig. 3). During the early stages of
differentiation, Hip-NSCs were arranged radially with a tendency to
differentiate into astrocytes without the addition of exogenous
cytokines. The majority of these glial cells stained positive for
GFAP (Fig. 4A). However, when
Hip-NSCs were cultured under low-serum conditions (DMEM/F12 1:1
supplemented with 2% fetal bovine serum and 100 ng/ml FGF-8) there
was an increase in the number of Tuj1-positive neurons (Fig. 4B), with fewer cells differentiating
into CNPase-positive oligodendrocytes (Fig. 4C).
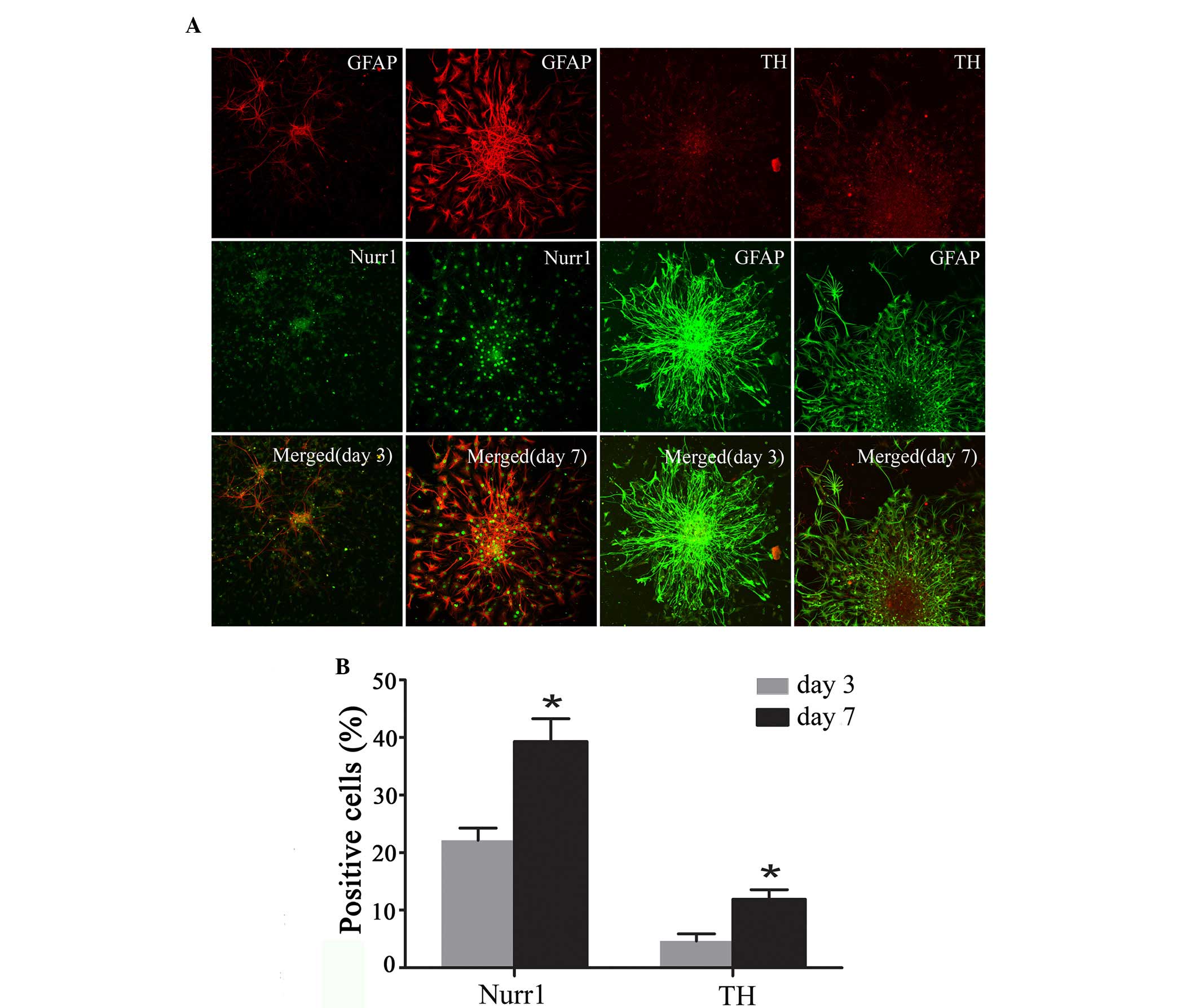
Expression of Nurr1 and TH in
differentiating Hip-NSCs
Immunofluorescent staining was performed to analyze
the expression of the DA neuronal markers Nurr1 and TH at the early
stages of Hip-NSC differentiation in low serum conditions. The
results revealed an increasing number of Nurr1-expressing cells
over time, and increased expression of TH was detected (Fig. 5A). The proportions of
Nurr1-positive cells and TH-positive neurons at day 7 (39.30±3.96
and 11.9±1.68%, respectively) were significantly greater than those
at day 3 (22.23±2.10 and 4.63±1.25%, respectively; P=0.0187 and
0.0254, respectively; Fig.
5B).
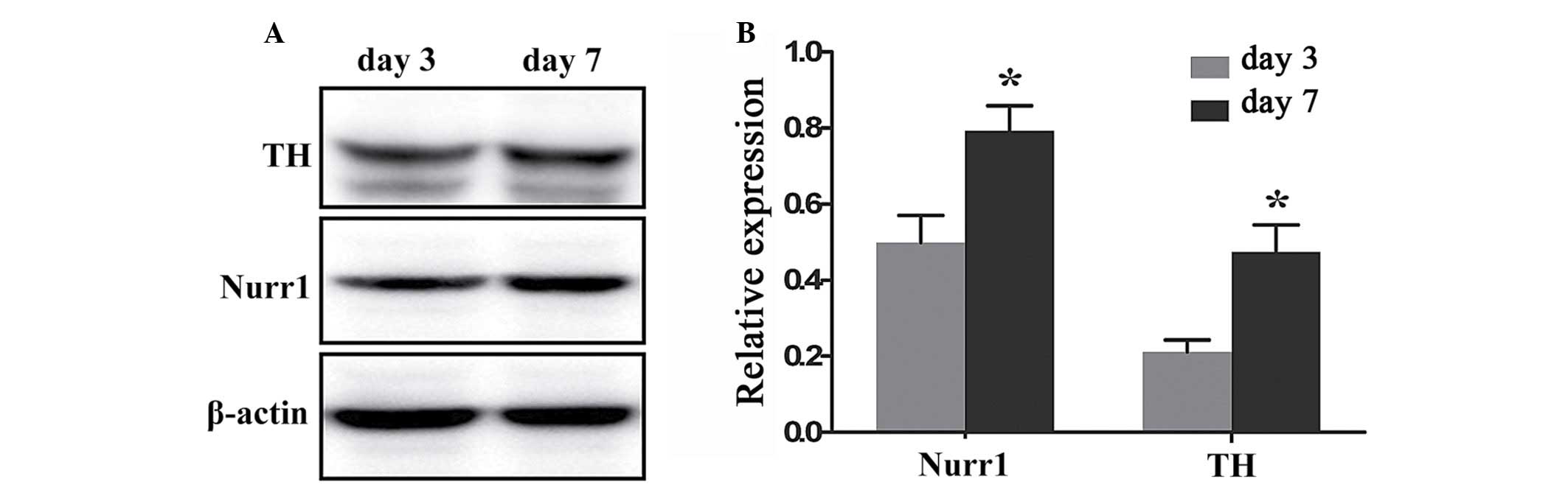
Quantification of Nurr1 and TH expression
in differentiating Hip-NSCs
To quantify and compare the levels of Nurr1 and TH
protein expression at day 3 and 7 of differentiation a western
blotting assay was performed. Nurr1 and TH proteins were expressed
at day 3 (0.499±0.072 and 0.212±0.031, respectively), and their
expression levels increased significantly at day 7 (0.792±0.067 and
0.473±0.072, respectively; P=0.0409 and 0.0292, respectively;
Fig. 6).
Discussion
Advances in NSC technology have led to novel and
promising therapeutic strategies for PD treatment, including
transplantation of DA neurons derived from NSCs in vitro
into the diseased brain, transplantation of NSCs directly into
injured areas and inducing differentiation into DA neurons in
vivo, and manipulation of endogenous NSCs to differentiate into
DA neurons (15–17). Endogenous NSCs may thus serve as
the ideal donor for cell transplantation therapy (17,18).
However, various studies have demonstrated that, in vitro,
NSCs have an increased tendency to differentiate into glial cells
than neurons, particularly DA neurons (19,20).
The inability to generate large numbers of DA neurons in
vitro poses a major obstacle for the clinical application of
NSCs in a transplantation-based treatment of PD. Therefore, there
is an urgent need to identify the molecular cues and the ideal
microenvironment that would facilitate this process.
Various studies have demonstrated that neurotrophic
factors are important in the regulation of neuron survival, axonal
maturation and neuronal differentiation during the development of
the nervous system (21,22). Growth factors cerebral dopamine
neurotrophic factor and Parkinson disease protein 7 protect
cholinergic and DA neurons against injury (23,24)
and additional investigation of age-associated diseases has
determined that expression levels of neurotrophic factors are
reduced in these conditions (25).
More notably, a significant decline in their function has been
noted in older individuals and patients diagnosed with
neurodegenerative disorders like Alzheimer's disease (26). Hip-NSCs, as all stem cells, have
the ability to proliferate and differentiate into multiple lineages
(27,28). It has been demonstrated that the
propensity of primary NSCs to differentiate into neurons and glial
cells, measured by parameters including the differentiation rate
and characteristics of neural cells generated, varies depending on
the brain region that cells were isolated from; therefore, Hip-NSCs
have regional specificity (14,29).
In the present study, primary Hip-NSCs proliferated
rapidly and aggregated into neurospheres with an orbicular
morphology within 36 h of plating. In addition, during suspension
culture, the passaged Hip-NSCs did not attach to the surface of the
flask and exhibited the characteristics of primary Hip-NSCs.
Following differentiation, cells organized themselves radially
during the early stages, and in the initial three weeks exhibited a
tendency to differentiate into astrocytes in the absence of
exogenous cytokines. Studies have demonstrated that the yield of DA
neurons from NSCs increased significantly in the presence of glial
cell-derived neurotrophic factor (GDNF) and associated cytokines
(6,30,31).
GDNF activates the expression of Nurr1 and pituitary homeobox 3 and
has been used to create an in vitro PD model from NSCs. When
overexpressed, GDNF is able to increase or improve the cognitive
function of aging animals (32,33).
It has been reported that NSCs isolated from the embryonic midbrain
have an increased tendency to differentiate into DA neurons
compared with NSCs from alternative regions of the brain (34). This suggests that NSC
differentiation and cell fate specification is influenced by their
intrinsic properties, in addition to exogenous signaling molecules.
A previous study investigated the molecular mechanism underlying
the generation of DA neurons based on gene expression analysis and
knockout studies identified various transcription factors that
appear to be important in this process (35). A previous study confirmed Nurr1 as
an important factor regulating the differentiation of midbrain DA
neurons, cell survival and acquisition of neuronal properties
(36). In mice, Nurr1 gene
knockout resulted in a reduction in TH expression in ventral
mesencephalon neurons and inhibited production of dopamine in the
striatum. However, the levels of norepinephrine, serotonin and
acetylcholine were not altered (30,37).
A previous study reported that Nurr1-induced differentiation of
TH-positive cells from embryonic stem cells was not completely
neuronogenic (38). Elevated
expression of TH in differentiated glial cells was observed,
indicating that Nurr1 was not a specific inducer of midbrain DA
neuronal fate, and that Nurr1 alone was not sufficient to induce
NSCs to differentiate into mature DA neurons (39). Additional studies confirmed that
expression of forkhead box protein A1/2, orthodenticle homeobox 2,
engrailed-1 and paired-like homeodomain 3 manipulated the
development and differentiation of adult midbrain DA neurons
(39–41).
In the current study, the differentiation of
Hip-NSCs into Nurr1 and TH-positive neurons increased significantly
with time under low serum conditions. Nurr1 expression was detected
during the early phase of differentiation and increased
progressively. Therefore, Nurr1 may be indispensable for the
generation of midbrain DA neurons from Hip-NSCs in vitro
(31). The results of the present
study suggest that the factors controlling in vitro
differentiation of Hip-NSCs into specific neuronal fates include
their intrinsic gene expression profile and their microenvironment.
Furthermore, Hip-NSCs in adherent culture were demonstrated to
generate not only neurons, but also glial cells expressing Nurr1,
indicating that Nurr1 expression is not specific to the development
of midbrain DA neurons. Additional studies are required to identify
alternative transcription factors that may act synergistically with
Nurr1 to induce a DA neuronal fate.
Acknowledgments
The present study was supported by grants from The
National Science Foundation of China (grant nos., 81160169 and
81360062).
References
|
1
|
Yacoubian TA and Standaert DG: Targets for
neuroprotection in Parkinson's disease. Biochim Biophys Acta.
1792:676–687. 2009. View Article : Google Scholar
|
|
2
|
Braak H, Bohl JR, Müller CM, Rüb U, de Vos
RA and Del Tredici K: Stanley fahn lecture 2005: The staging
procedure for the inclusion body pathology associated with sporadic
Parkinson's disease reconsidered. Mov Disord. 21:2042–2051. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diaz NL and Waters CH: Current strategies
in the treatment of Parkinson's disease and a personized approach
to management. Expert Rev Neurother. 9:1781–1789. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reichmann H: New prospects for therapy in
Parkinson's disease. Drug Res (Stuttg). 63(Suppl 1): S232013.
|
|
5
|
Ding YX, Wei LC, Wang YZ, Cao R, Wang X
and Chen LW: Molecular manipulation targeting regulation of
dopaminergic differentiation and proliferation of neural stem cells
or pluripotent stem cells. CNS Neurol Disord Drug Targets.
10:517–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim MS, Chang MY, Kim SM, Yi SH, Suh-Kim
H, Jung SJ, Kim MJ, Kim JH, Lee YS, Lee SY, et al: Generation of
dopamine neurons from rodent fibroblasts through the expandable
neural precursor cell stage. J Biol Chem. 290:17401–17414. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gyárfás T, Knuuttila J, Lindholm P,
Rantamäki T and Castrén E: Regulation of brain-derived neurotrophic
factor (BDNF) and cerebral dopamine neurotrophic factor (CDNF) by
anti-parkinsonian drug therapy in vivo. Cell Mol Neurobiol.
30:361–368. 2010. View Article : Google Scholar
|
|
8
|
Hauser DN and Cookson MR: Astrocytes in
Parkinson's disease and DJ-1. J Neurochem. 117:357–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YH, Yu HT, Pu XP and Du GH: Baicalein
prevents 6-hydroxydopamine-induced mitochondrial dysfunction in
SH-SY5Y cells via inhibition of mitochondrial oxidation and
up-regulation of DJ-1 protein expression. Molecules.
18:14726–14738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okano H and Sawamoto K: Neural stem cells:
Involvement in adult neurogenesis and CNS repair. Philos Trans R
Soc Lond B Biol Sci. 363:2111–2122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muramatsu S, Okuno T, Suzuki Y, Nakayama
T, Kakiuchi T, Takino N, Iida A, Ono F, Terao K, Inoue N, et al:
Multitracer assessment of dopamine function after transplantation
of embryonic stem cell-derived neural stem cells in a primate model
of Parkinson's disease. Synapse. 63:541–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Williams CJ and Dexter DT: Neuroprotective
and symptomatic effects of targeting group III mGlu receptors in
neurodegenerative disease. J Neurochem. 129:4–20. 2014. View Article : Google Scholar
|
|
13
|
Richardson JR and Hossain MM: Microglial
ion channels as potential targets for neuroprotection in
Parkinson's disease. Neural Plast. 2013:5874182013.PubMed/NCBI
|
|
14
|
Wei LC, Ding YX, Liu YH, Duan L, Bai Y,
Shi M and Chen LW: Low-dose radiation stimulates Wnt/β-catenin
signaling, neural stem cell proliferation and neurogenesis of the
mouse hippocampus in vitro and in vivo. Curr Alzheimer Res.
9:278–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kishi Y, Takahashi J, Koyanagi M, Morizane
A, Okamoto Y, Horiguchi S, Tashiro K, Honjo T, Fujii S and
Hashimoto N: Estrogen promotes differentiation and survival of
dopaminergic neurons derived from human neural stem cells. J
Neurosci Res. 79:279–286. 2005. View Article : Google Scholar
|
|
16
|
Zuo FX, Bao XJ, Sun XC, Wu J, Bai QR, Chen
G, Li XY, Zhou QY, Yang YF, Shen Q and Wang RZ: Transplantation of
human neural stem cells in a Parkinsonian model exerts
neuroprotection via regulation of the host microenvironment. Int J
Mol Sci. 16:26473–26492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cave JW, Wang M and Baker H: Adult
subventricular zone neural stem cells as a potential source of
dopaminergic replacement neurons. Front Neurosci. 8:162014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamashita T and Abe K: Direct reprogrammed
neuronal cells as a novel resource for cell transplantation
therapy. Cell Transplant. 23:435–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trueman RC, Klein A, Lindgren HS, Lelos MJ
and Dunnett SB: Repair of the CNS using endogenous and transplanted
neural stem cells. Curr Top Behav Neurosci. 15:357–398. 2013.
View Article : Google Scholar
|
|
20
|
Buttery PC and Barker RA: Treating
Parkinson's disease in the 21st century: Can stem cell
transplantation compete? J Comp Neurol. 522:2802–2816. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sadan O, Bahat-Stromza M, Barhum Y, Levy
YS, Pisnevsky A, Peretz H, Ilan AB, Bulvik S, Shemesh N, Krepel D,
et al: Protective effects of neurotrophic factor-secreting cells in
a 6-OHDA rat model of Parkinson disease. Stem Cells Dev.
18:1179–1190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li F, Wang M, Zhu S, Li L, Xiong Y and Gao
DS: The potential neuroprotection mechanism of GDNF in the
6-OHDA-induced cellular models of Parkinson's disease. Cell Mol
Neurobiol. 33:907–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren X, Zhang T, Gong X, Hu G, Ding W and
Wang X: AAV2-mediated striatum delivery of human CDNF prevents the
deterioration of midbrain dopamine neurons in a 6-hydroxydopamine
induced parkinsonian rat model. Exp Neurol. 248:148–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogawa I, Saito Y, Saigoh K, Hosoi Y,
Mitsui Y, Noguchi N and Kusunoki S: The significance of oxidized
DJ-1 protein (oxDJ-1) as a biomarker for Parkinson's disease. Brain
Nerve. 66:471–477. 2014.In Japanese. PubMed/NCBI
|
|
25
|
Kahle PJ, Waak J and Gasser T: DJ-1 and
prevention of oxidative stress in Parkinson's disease and other
age-related disorders. Free Radic Biol Med. 47:1354–1361. 2009.
View Article : Google Scholar
|
|
26
|
Hebsgaard JB, Nelander J, Sabelström H,
Jönsson ME, Stott S and Parmar M: Dopamine neuron precursors within
the developing human mesencephalon show radial glial
characteristics. Glia. 57:1648–1658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding YX, Wei LC, Wang YZ, Cao R, Wang X
and Chen LW: Molecular manipulation targeting regulation of
dopaminergic differentiation and proliferation of neural stem cells
or pluripotent stem cells. CNS Neurol Disord Drug Targets.
10:517–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carrillo-García C, Suh Y, Obernier K,
Hölzl-Wenig G, Mandl C and Ciccolini F: Multipotent precursors in
the anterior and hippocampal subventricular zone display similar
transcription factor signatures but their proliferation and
maintenance are differentially regulated. Mol Cell Neurosci.
44:318–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ambasudhan R, Dolatabadi N, Nutter A,
Masliah E, Mckercher SR and Lipton SA: Potential for cell therapy
in Parkinson's disease using genetically-programmed human embryonic
stem cell-derived neural progenitor cells. J Comp Neurol.
522:2845–2856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park CH, Lim MS, Rhee YH, Yi SH, Kim BK,
Shim JW, Kim YH, Jung SJ and Lee SH: In vitro generation of mature
dopamine neurons by decreasing and delaying the expression of
exogenous Nurr1. Development. 139:2447–2451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lei Z, Jiang Y, Li T, Zhu J and Zeng S:
Signaling of glial cell line-derived neurotrophic factor and its
receptor GFRα1 induce Nurr1 and Pitx3 to promote survival of
grafted midbrain-derived neural stem cells in a rat model of
Parkinson disease. J Neuropathol Exp Neurol. 70:736–747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alvarez-Castelao B, Losada F, Ahicart P
and Castaño JG: The N-terminal region of Nurr1 (a.a 1-31) is
essential for its efficient degradation by the ubiquitin proteasome
pathway. PloS One. 8:e559992013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bang SY, Kwon SH, Yi SH, Yi SA, Park EK,
Lee JC, Jang CG, You JS, Lee SH and Han JW: Epigenetic activation
of the Foxa2 gene is required for maintaining the potential of
neural precursor cells to differentiate into dopaminergic neurons
after expansion. Stem Cells Dev. 24:520–533. 2015. View Article : Google Scholar
|
|
35
|
Moreno-Bravo JA, Martinez-Lopez JE and
Puelles E: Mesencephalic neuronal populations: New insights on the
ventral differentiation programs. Histol Histopathol. 27:1529–1538.
2012.PubMed/NCBI
|
|
36
|
Barneda-Zahonero B, Servitja JM, Badiola
N, Miñano-Molina AJ, Fadó R, Saura CA and Rodríguez-Alvarez J:
Nurr1 protein is required for N-methyl-D-aspartic acid (NMDA)
receptor-mediated neuronal survival. J Biol Chem. 287:11351–11362.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang C, Wan X, He Y, Pan T, Jankovic J
and Le W: Age-dependent dopaminergic dysfunction in Nurr1 knockout
mice. Exp Neurol. 191:154–162. 2005. View Article : Google Scholar
|
|
38
|
Sonntag KC, Simantov R, Kim KS and Isacson
O: Temporally induced Nurr1 can induce a non-neuronal dopaminergic
cell type in embryonic stem celt differentiation. Eur J Nearosci.
19:1141–1152. 2004. View Article : Google Scholar
|
|
39
|
Kittappa R, Chang WW, Awatramani RB and
McKay RD: The foxa2 gene controls the birth and spontaneous
degenerate of dopamine neurons in old age. PLoS Biol. 5:e3252007.
View Article : Google Scholar
|
|
40
|
Veenvliet JV, Dos Santos MT, Kouwenhoven
WM, von Oerthel L, Lim JL, van der Linden AJ, Koerkamp MJ, Holstege
FC and Smidt MP: Specification of dopaminergic subsets involves
interplay of En1 and Pitx3. Development. 140:3373–3384. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Panman L, Papathanou M, Laguna A,
Oosterveen T, Volakakis N, Acampora D, Kurtsdotter I, Yoshitake T,
Kehr J, Joodmardi E, et al: Sox6 and Otx2 control the specification
of substantia nigra and ventral tegmental area dopamine neurons.
Cell Rep. 8:1018–1025. 2014. View Article : Google Scholar : PubMed/NCBI
|