Introduction
In China, gallbladder carcinoma is the fifth most
common type of cancer of the digestive system (1) and is the most frequent type of cancer
of the biliary system. Early diagnosis and early treatment are
required to cure gallbladder cancer, however, the majority of
patients do not receive medical assistance in time to receive
surgical treatment (2,3). The poor rates of prognosis are
predominantly due to the rapid development of the tumor and
invasion of the surrounding organs. Thus, a therapeutic strategy
aimed at controlling the invasion and metastasis of gallbladder
cancer is necessary.
In 1982, Nusse and Varmus (4) first identified the Wnt gene in mice,
which was termed Int-1. Since then, several members of the Wnt gene
family have been identified. The Wnt gene family is involved in a
series of complex metabolic pathways and is involved in embryonic
development, cell proliferation, migration, differentiation and
other activities (5). In normal
cells, the Wnt pathway is suppressed, and abnormal activation is
associated with several types of malignant tumor (6–9). In
1999, Hsieh et al (10),
reported a novel extracellular inhibitor protein, which can bind to
Wnt proteins and affect their function. This was termed Wnt
inhibitory factor 1 (WIF-1). At present, the gene sequence for
WIF-1 has been determined, and its spatial structure has also been
confirmed (11). WIF-1 belongs to
the secreted Frizzled-related protein family and can inhibit the
classical and non-classical Wnt signaling pathways (12,13).
The abnormal expression of WIF-1 in certain types of tumor has also
been confirmed (14–16). However, the expression of WIF-1 in
gallbladder cancer, the effects of WIF-1 on the biological behavior
of gallbladder cancer and the associated mechanisms remain to be
fully elucidated
In the present study, it was shown in gallbladder
tumors and three gallbladder cancer cell lines that the expression
levels of WIF-1 were low. This low expression was associated with
methylation of the WIF-1 gene promoter. Following treatment of
GBC-SD cells with 5-aza-2-deoxycytidine (5-Aza-dC), the expression
of WIF-1 recovered. The current study aimed to elucidate the
effects of WIF-1 on tumor growth, invasion and metastasis, thus a
GBC-SD cell line was constructed, which stably expressed WIF-1, and
the expression of proteins closely associated with the Wnt
signaling pathway were analyzed. It was found that WIF-1
significantly inhibited tumor cell proliferation, migration and
invasion, and increased the apoptotic rate of the tumor cells.
Protein expression levels were also altered following transfection.
These results showed that WIF-1 markedly inhibited tumor growth,
invasion and metastasis. Therefore, WIF-1 may be an effective
treatment target for gallbladder cancer.
Materials and methods
Case collection and
immunohistochemistry
A total of 40 gallbladder cancer specimens were
collected from the Union Hospital of Fujian Medical University
(Fujian, China), following surgical resection between 2004 and
2011. Of the 40 patients, 18 were male and 22 were female, 19
patients were >60 years old and 21 were <60 years old. All
cases were confirmed by histopathological examinations. In
addition, 50 chronic cholecystitis specimens were collected from
the Union Hospital of Fujian Medical University following surgical
resection in 2012, and were confirmed by histopathological
examinations. Of the 50 patients, 28 were male and 22 were female
and the ages ranged between 42 and 65. The current study was
approved by the ethics committee of the Affiliated Union Hospital
of Fujian Medical University (Fuzhou, China). The rabbit anti-human
monoclonal WIF-1 antibody (#5502; 1:200; incubation at 4°C for 8 h)
was purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA) and a goat anti-rabbit secondary antibody kit (kt-9903; 1:50;
incubation at 37°C for 20 min) was purchased from Beijing ZhongShan
Biotechnology Company (Beijing, China). The expression of WIF-1 was
detected using routine En Vision two-step immunohistochemical
staining (Fuzhou Maxim Biotech, Inc., Fuzhou, China). Selected
paraffin blocks (4 µm thick) were heated (92–98°C) for
antigen retrieval in an alkaline environment following sectioning,
heating, dewaxing and hydration. Subsequently, hydrogen peroxide
was added and incubated at room temperature for 10 min to block
endogenous peroxidase activity. Primary antibody incubation was
performed at room temperature at a dilution of 1:100. Secondary
antibody incubation was then performed, according to the
instructions provided with the secondary antibody kit. The final
step involved diaminobenzidine staining. The sections were then
subjected to a gradient of ethanol dehydration, cleared in xylene
and fixed with neutral balata. The expression of WIF-1 was
predominantly located in the cytoplasm. Staining, which was located
only in the membrane and nucleolus was considered negative using a
BX51 microscope (Olympus Corporation, Tokyo, Japan).
Cell lines, reverse
transcription-polymerase chain reaction (RT-PCR) and western blot
analyses
The GBC-SD gallbladder cancer cell line was
purchased from the Shanghai Institute of Cellular Biology of the
Chinese Academy of Sciences (Shanghai, China). The SGC-996 cell
line was purchased from the Life Science and Technology Institute
of Tongji University (Shanghai, China). The NOZ cell line was
obtained from the Japanese Health Science Research Resources Bank
(Osaka, Japan). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.).
A First-Strand cDNA Synthesis kit was purchased from
Fermentas; Thermo Fisher Scientific, Inc. The primers for WIF-1 and
β-actin were designed using Oligo-6 software. The upstream primer
for WIF-1 was 5′-ATCATCTTCTTAACTGGCATTGTG-3′ and the downstream
primer was 5′-GCTGTAGAGGTTGACTGTGTAG-3′; the product was 328 bp.
The upstream primer for WIF-1 was β-actin
5′-GGCATGGGTCAGAAGGATTCC-3′ and the downstream primer was
5′-ATGTCACDCACGATTTCCCGC-3′; the product was 250 bp. RNA was
extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
from the three tumor cell lines in the logarithmic growth phase,
and the following steps were performed, as described in the kit
instructions. The reaction volume for WIF-1 and β-actin was 25
µl, including 3 µl of template DNA, 1 µl of
the upstream primer and 1 µl of the downstrean primer and
12.5 µl of Taq PCR Mastermix (Bioteke Corp., Beijing,
China); diethyl pyrocarbonate (DEPC) water was added to a final
volume of 25 µl. The reaction conditions were as follows:
Hot start at 94°C for 2 min; 30 cycles of 30 sec at 94°C, 30 sec at
57°C and 60 sec at 72°C, with a final extension step for 10 min at
72°C. The results were assessed using a UV gel imaging system
(JS-2010; Shanghai Peiqing Science and Technology Co., Ltd.,
Shanghai, China).
When the cells reached 80% confluency, they were
washed twice with phosphate-buffered saline, following which 300
µl of radioimmunoprecipitation assay lysis buffer containing
1% phenylmethanesulfonyl fluoride was added, and the cells were
then removed using a cell scraper. The samples were transferred
into tubes and lysed on ice for 30 min. The following steps were
performed, according to a standard western blotting protocol. The
proteins extracted from the cells (20 µl; 2
µg/µl) were separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (Beyotime Institute of
Biotechnology, Beijing, China), and electrophoretically transferred
onto polyvinylidene fluoride membranes. The membranes were then
incubated with antibodies against WIF-1, β-catenin and β-actin.
Proteins were revealed by secondary antibody incubation. The final
blots were scanned on an imaging system (JS-2010; Shanghai Peiqing
Science and Technology Co., Ltd.) in order to quantify them, and
the ratio between β-actin and WIF-1 was calculated to show the
different expression.
WIF-1 gene promoter methylation in the
GBC-SD gallbladder carcinoma cell line
The upstream primer for the methylated WIF-1 gene
promoter was 5′-AATTTTATTGGTTGAAAGGGAGAC-3′ and the downstream
primer was 5′-AAAAATAAAAAAAACACGCT-3′. These primers were designed
using Oligo 6 software (Molecular Biology Insights, Inc. Cascade,
CO, USA). The product size was 167 bp. The unmethylated upstream
primer was 5′-GAATTTTATTGGTTGAAAGGGAGAT-3′ and the downstream was
5′-AAAAATAAAAAAAACAAACAA CACT-3′; the product size was 168 bp.
Commercial kits are available to replace the technology of early
standard bisulfite treatment (17). A cell/tissue genomic DNA extraction
kit (centrifugal column type) and EZ DNA Methylation-GoldTM kit
were purchased from Zymo Research (Irvine, CA, USA), and used
according to the manufacturer's recommended protocol. The reaction
was performed with a sample volume of 25 µl, comprising 1.5
µl of template DNA, 0.5 µl of either upstream or
downstream primer, 12.5 µl of Taq PCR Mastermix (Bioteke
Corp.) and DEPC water to 25 µl. The reaction included a hot
start at 95°C for 10 min, and the amplifications were performed in
a thermal cycler for 40 cycles of 45 sec at 95°C, 45 sec at 57°C,
45 sec at 72°C and a final extension step for 10 min at 72°C.
The present study used 5-Aza-dC to investigate the
cause of the decreased expression of WIF-1 in the GBC-SD cell line.
The GBC-SD cells were cultured in DMEM containing 10% fetal bovine
serum. The 5-Aza-dC was added to the culture solution at a final
drug concentration of 1 µg/ml. The drug-containing medium
was replaced every 24 h. After 5 days, the cells were collected for
experiments. GBC-SD cells cultured in DMEM without the presence of
drugs were used as controls. The PCR conditions were the same as
those described above.
Plasmids and stable transfection
procedure
A vector containing WIF-1 and an empty vector
(GV141) were purchased from Shanghai Genechem Co., Ltd. (Shanghai,
China). Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) was used as the transfection reagent. The GBC-SD cells were
diluted to 1,000 cells/ml and incubated at 37°C and 5%
CO2 in 24-well plates. G418 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for selection. Following 1 week of G418
selection, cell line stably expressing WIF-1 (WGBC-SD) and
expressing the empty plasmid (NGBC-SD) were produced, and these
cells were expanded for further experiments.
Detection of the proliferation ability of
GBC-SD, WGBC-SD and NGBC-SD cells
Cell Counting Kit-8 (CCK 8) kits, purchased from
Beyotime Institute of Biotechnology were used to detect the
proliferation of the GBC-SD, WGBC and NGBC cells. The three types
of tumor cells in the logarithmic growth phase were digested with
0.25% trypsin and counted using a cell counting chamber (Shanghai
Baili Science and Technology Co., Ltd., Shanghai, China).
Subsequently, 1.5×103 cells per well were seeded into
96-well plates, with three wells for each group. The cells were
cultured under saturated humidity conditions at 37°C and 5%
CO2. Following culture for 24, 48, 72, 96 and 120 h, the
culture media were replaced with 100 µl fresh culture media
with CCK-8 (90 µl culture medium+10 µl CCK-8). The
plates were incubated for 2 h and the absorbance was measured at
450 nm using a standard instrument (JS-2010; Shanghai Peiqing
Science and Technology Co., Ltd.). On the final day, the data were
analyzed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
Assessment of the invasion and metastatic
abilities of GBC-SD, WGBC-SD and NGBC-SD cells
Transwell plates were purchased from Corning
(Corning, NY, USA). A total of 10 ml frozen fibronectin (FN; Becton
Dickinson; BD Biosciences, San Diego, CA, USA) was applied to the
upper chamber of the Transwell plates, and biological Matrigel
(Becton Dickinson; BD Biosciences) was applied to the lower
chamber. Subsequently, 2×105 of the GBC-SD, WGBC and
NGBC cells were suspended in 200 µl serum-free DMEM, the
suspension was added to the upper well. A total of 0.8 ml DMEM with
10% fetal bovine serum was added to the lower well. The samples
were incubated for 30 h at 37°C and 5% CO2. The
quantification procedure was as follows: Absorbing of the
supernatant; washing once with phosphate-buffered saline (PBS),
fixing with 95% ethanol and 5% acetic acid for 30 min, gently
wiping the upper chamber, washing with PBS and staining with
hematoxylin. The average values from five visual fields
(magnification, ×400) were calculated.
For the migration analysis, the methods of seeding
and cultivation were the same as those described above, with the
exception that 10 µl FN was applied to the upper well. The
cultivation was terminated following 8 h in the incubator at 37°C
and 5% CO2. The methods for fixing, staining and
microscopy were the same as those described above.
The degradation of the extracellular matrix of
tumors is an important process during tumor invasion and
metastasis, and this degradation can be accomplished by the
secretion of matrix metalloproteinases (MMPs). The secretion of
MMPs increases tumor invasive and metastatic abilities. The present
study investigated the activities of MMP-2 and MMP-9 in the three
cell groups using the kits from Beyotime Institute of
Biotechnology.
Apoptosis of GBC-SD, WGBC-SD and NGBC-SD
cells
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double staining was used to detect the
apoptotic rates of the three groups of cells. The Annexin V-FITC/PI
kit was purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China). Cells (106) cells in the logarithmic growth were
collected for the analysis of apoptosis, which was performed
according to the kit instructions. The apoptotic rates were
measured using a flow cytometer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). A compensation adjustment of fluorescence was
performed prior to the assessment.
Detection of the oncogenicity of GBC-SD,
WGBC-SD and NGBC-SD in vivo
Specific-Pathogen-Free nude mice were used in the
pilot study, and a total of 15 BALB/C (nu/nu) were used in the
current study (4–6 weeks old; 16–20 grams 12/12 h light/dark cycle;
45–50% humidity; 25–27°C; Shanghai Laboratory Animal Center,
Chinese Academy of Sciences (Shanghai, China. The mice were caged
separately with access to sterilized food and water and were
randomly divided into three groups, each containing five mice. The
GBC-SD, WGBC-SD and NGBC-SD cell lines were inoculated into the
left axilla of the mouse forelimb. The number of injected tumor
cells for each mouse was 107 cells. The mice were
sacrificed by cervical dislocation 5 weeks later. The tumors were
resected completely and weighed, followed by sectioning.
Protein expression in GBC-SD, WGBC-SD and
NGBC-SD cells
An AAH CYT-G10-4 protein chip kit, purchased from
RayBiotech Company (Norcross, GA, USA) was used to detect changes
in protein expression in the three groups of cells. The cells
(>106) were collected following lysis and were
adjusted to the same concentration. The AAH CYT-G10-4 protein chip
kit was used, according to the kit instructions. When all steps
were completed, a laser scanner was used to detect the fluorescent
signal, and the data were analyzed
Statistical analysis
All data were analyzed using SPSS software, version
13.0 (SPSS, Inc., Chicago, IL, USA). The data were analyzed using
the χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
WIF-1 shows reduced expression in
gallbladder cancer and tumor cell lines
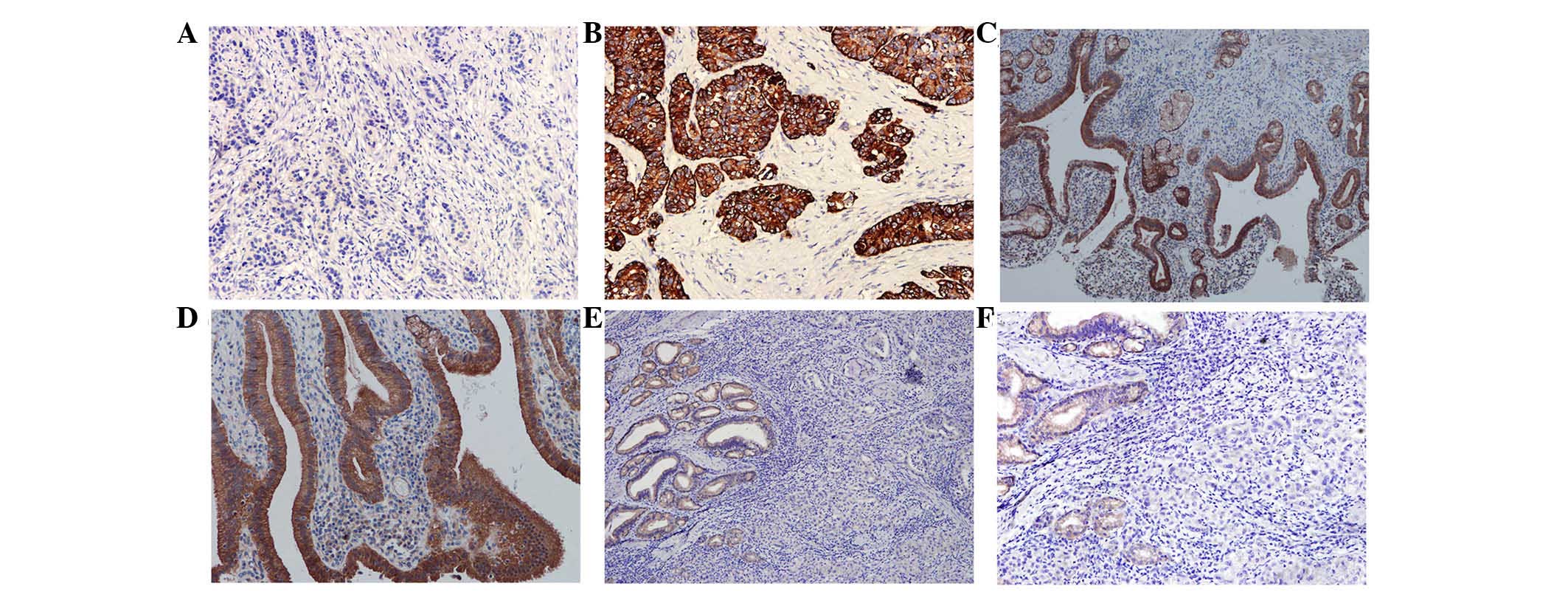
In the 40 clinical cases of gallbladder cancer, only
15 cases were positive for WIF-1, with a rate of 25% (15/40). In
the 50 cases of cholecystitis, WIF-1 was expressed in 100% of the
samples (50/50; Fig. 1). The
difference between the two groups was significant (P<0.001). In
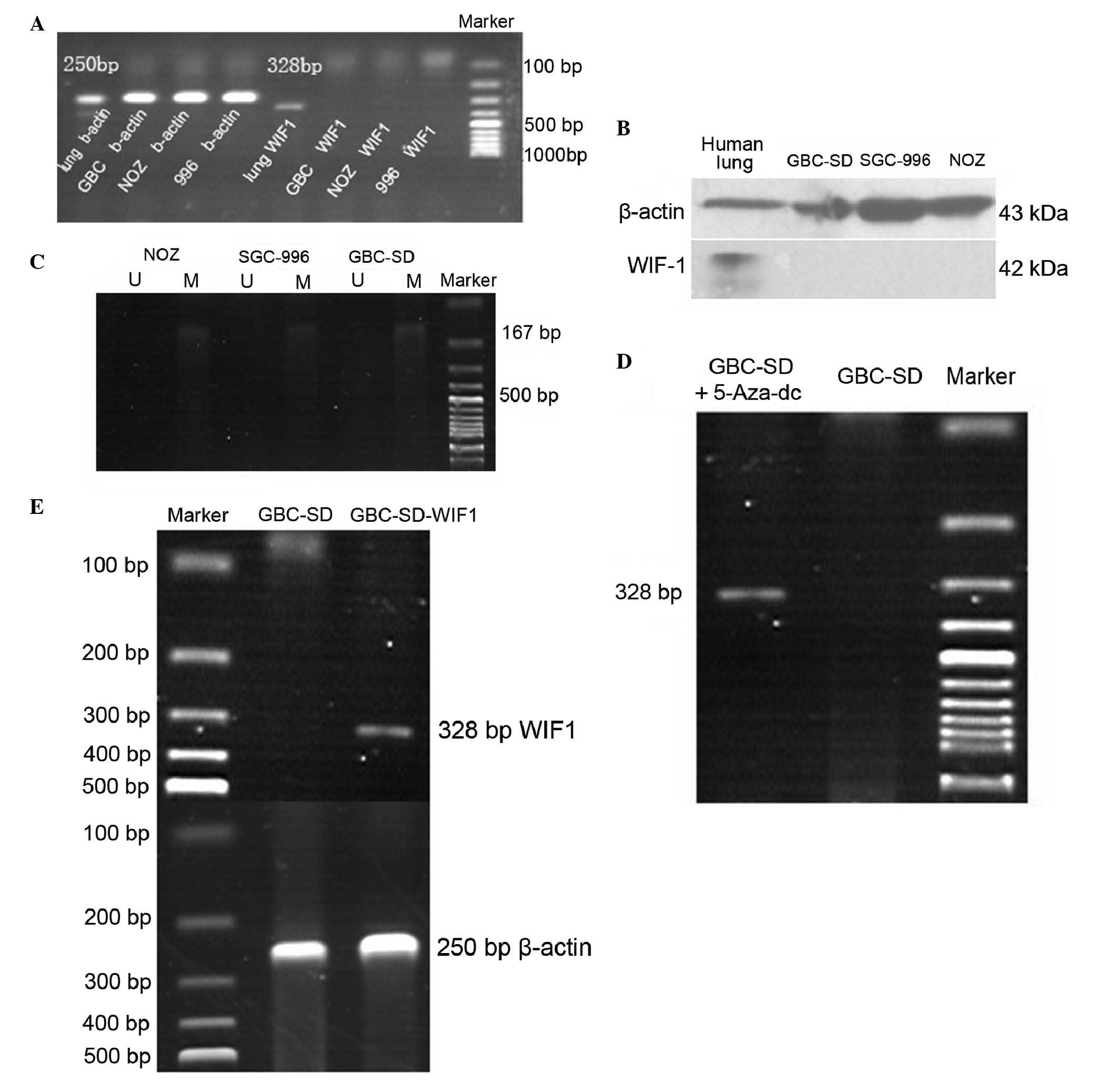
the GBC-SD, NOZ and SGC-996 tumor cell lines, the expression levels
of WIF-1 were similar to the clinical examples, in that neither
mRNA or protein were detected (Fig. 2A
and B).
Loss of the expression of WIF-1 is
associated with promoter methylation and is rescued by
5-Aza-dC
The initial experiment showed that, in the three
tumor cell lines, the mRNA expression of WIF-1 was absent. Using
methylation-specific PCR, it was found that this absence was
associated with gene promoter methylation, which revealed
methylated bands and no unmethylated bands. These data showed that
promoter methylation was high in the gallbladder cancer cell lines
(Fig. 2C). Following treatment
with 5-Aza-dC, the mRNA expression of WIF-1 in the tumor cells
recovered (Fig. 2D). These results
suggested that promoter methylation led to the absence of the mRNA
expression of WIF-1.
WIF-1 suppresses the proliferation,
invasion and metastasis of GBC-SD cells and increases the apoptosis
of the GBC-SD cells
In the present study, a GBC-SD cell line stably
expressing WIF-1 (WGBC-SD) and a control cell line containing an
empty plasmid, (NGBC-SD) were constructed. Although 5-Aza-dC
restored the expression of WIF-1, it also affected other gene
promoters including p16, human mutL homolog 1 and runt-related
transcription factor 3. Therefore, it was necessary to construct a
stable cell line expressing WIF-1. Following transfection and
selection with G418, a confirmation experiment was performed
(Fig. 2E). For this, the GBC-SD
tumor cell line was selected as the transfection target due to its
convenient morphological characteristics.
Based on previous experiments, the present study
examined the effect of the expression of WIF-1 on proliferation,
invasion, metastasis and apoptosis. As an important inhibitor of
the Wnt pathway, recovery of WIF-1 was expected to affect tumor
cell behavior. The results showed that WIF-1 had a marked
suppressive effect on the tumor cells and increased apoptosis
(Fig. 3).
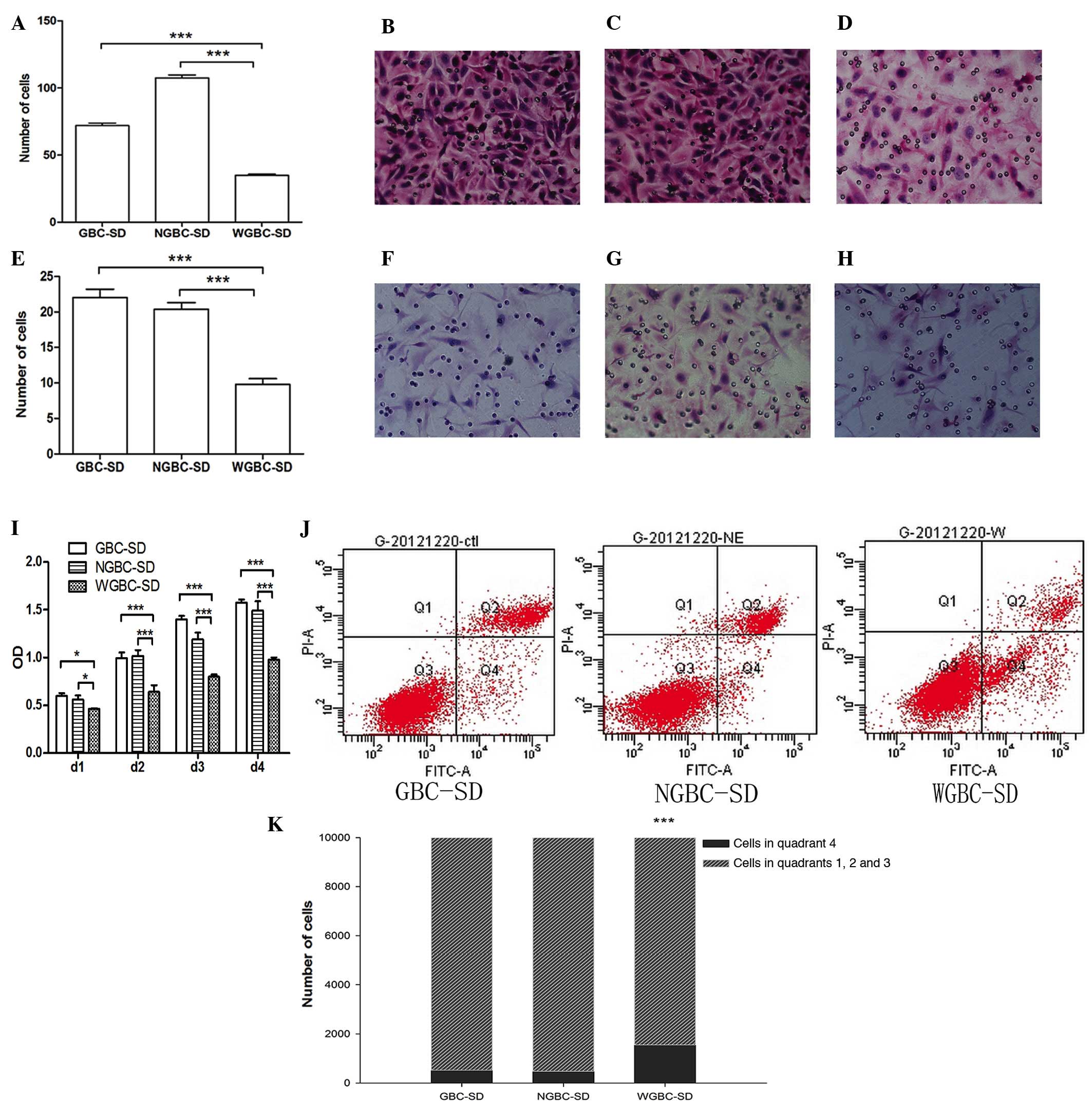 | Figure 3(A) Significant differences were
observed in the invasive ability of the (B) GBC-SD, (C) NGBC-SD and
(D) WGBC-SD cells (magnification, ×400). (E) In an experiment
measuring the metastatic ability of (F) GBC-SD, (G) NGBC-SD and (H)
WGBC-SD cells, the results corresponded to those of the invasion
experiment. (I) GBC-SD, NGBC-SD and WGBC-SD cells exhibited
different proliferation rates. (J and K) Flow cytometry was used to
detect apoptosis. The cells in quadrant (Q)4 were counted as
apoptotic cells. The rate of apoptosis in the WGBC-SD cells was
significantly higher, compared with the rates in the GBC-SD and
NGBC-SD cells (P<0.001). No significant difference in the
apoptotic rate was found between the GBC-SD and NGBC-SD cells
(P>0.05). *P<0.05 and ***P<0.001. WGBC-SD,
GBC-SD cells stably expressing WIF-1; NGBC-SD, empty
vector-transfected GBC-SD cells; OD, optical density; FITC,
fluorescein isothiocyanate; PI, propidium iodide. |
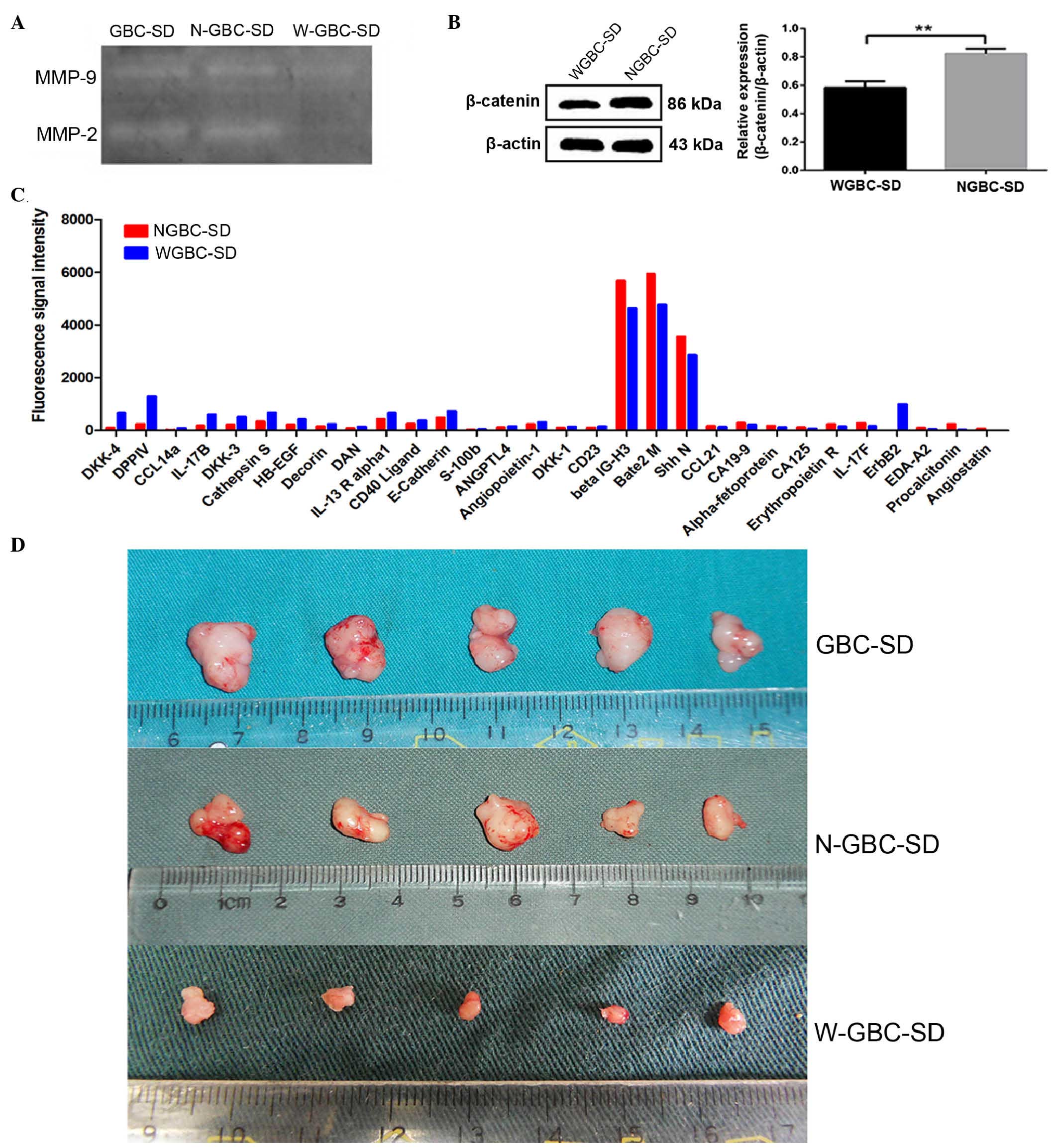
WIF-1 regulates protein expression
To explain the inhibited tumor cell invasion and
metastasis, the present study measured the expression levels of
MMP-2 and MMP-9 in the GBC-SD, NGBC-SD and WGBC-SD cells. MMP-2 and
MMP-9 degrade the extracellular matrix and thus are involved in the
spread of cancer. The expression levels of MMP-2 and MMP-9 were
significantly lower in the WGBC-SD cells (Fig. 4A), compared with those in the
GBC-SD and NGBC-SD cells (P<0.001).
β-catenin is key in Wnt signaling, and controls
cellular proliferation, invasion and apoptosis. The present study
performed western blot analysis to analyze the expression of
β-catenin in the three cell lines. The expression of β-catenin was
downregulated in the WGBC-SD cells, compared with the GBC-SD and
NGBC-SD cells (P<0.001). No difference in expression levels were
observed between the GBC-SD and NGBC-SD cells (P>0.05; Fig. 4B).
The present study subsequently detected the
expression of proteins using a protein chip to identify novel
proteins, which may be possible WIF-1 targets, including proteins
involved in cell motility and tumor angiogenesis. Compared with the
NGBC-SD cells, the expression levels of DKK-4, DPPIV, E-cadherin
and certain other proteins were increased in the WGBC-SD cells, and
the expression levels of other proteins, including CA199, CA125 and
angiostatin were decreased (Fig.
4C). This was the first time, to the best of out knowledge that
these changes have been reported in gallbladder cancer. It was
concluded that these changes were associated with the behavior of
GBC-SD, NGBC-SD and WGBC-SD cells.
WIF-1 inhibits the oncogenicity of GBC-SD
cells in vivo
In the in vivo experiments, 15 nude mice were
successfully inoculated, and the tumors were excised from the mice
in the different groups and were then weighed. No lymph node
metastases were observed in the three groups of nude mice. There
was a significant difference in tumor weight between the WGBC-SD
group and the GBC-SD group, and between the WGBC-SD group and the
NGBC-SD group (P<0.001). No significant difference in tumor
weight was observed between the GBC-SD group and the NGBC-SD group
(P>0.05). The results indicated that WIF-1 inhibited the
oncogenicity of the GBC-SD cells in vivo and in vitro
(Fig. 4D).
Discussion
In vivo, the Wnt pathway controls cell
proliferation and differentiation, and abnormal activation often
leads to tumor development (18).
The inhibition of this abnormally activated signaling pathway is an
area of interest. The expression of WIF-1, a potent Wnt signaling
pathway inhibitor, is low in a several types of tumor. The present
study showed that, in gallbladder cancer, the expression of WIF-1
was significantly decreased. WIF-1 was positive in only 10 cases
(25% of the cases). In 50 cases of chronic cholecystitis, the
expression rate of WIF-1 was 100%. This result suggested that, in
the development of gallbladder cancer, WIF-1 inactivation may occur
early in the process. The inactivation is likely to be the same as
that in gastrointestinal tumors (19), which occurs due to abnormal
methylation of the WIF-1 gene promoter. The inactivation of WIF-1
activates the Wnt pathway, which then leads to abnormal cellular
proliferation and eventual tumor formation.
In the present study, WIF-1 was not expressed in the
GBC-SD, SGC-996 or NOZ cell lines, as determined using PCR and
western blot analyses. A GBC-SD cell line stably expressing WIF-1
was constructed for further investigation, as well as a negative
control.
Following a study in 1997 by Schroeder et al
(20), which reported that CPG
island methylation of the P53 tumor suppressor gene affects its
transcriptional activity, numerous studies have shown that gene
promoter hypermethylation is an important factor in gene expression
(21–28). The methylation of tumor suppressor
gene promoters often leads to the inactivation of tumor suppressor
genes and tumorigenesis (22). The
present study performed WIF-1 gene promoter methylation analysis in
the three gallbladder cancer cell lines. The results showed that,
similar to other types of tumor (23–26),
the WIF-1 gene promotor in the gallbladder cancer cell lines was
completely methylated. This methylation likely caused the lack of
WIF-1 expressed in the tumor cell lines. Detecting the promoter
methylation of genes in tumors is significant, and this technology
has been used in the diagnosis of lung cancer (27,28).
Promoter methylation of the WIF-1 gene in the diagnosis of
gallbladder cancer has not been reported previously, and further
investigation is required prior to the clinical application of such
diagnostic assessment.
To confirm that the lack in the expression of WIF-1
was caused by promoter methylation, the present study treated the
GBC-SD cells with 5-Aza-dC, a potent demethylating drug. As an
inhibitor of gene promoter methylation, 5-Aza-dC binds to DNA
methylation enzymes and inhibits their activity. The results of the
present study confirmed that, following treatment, the mRNA
expression of WIF-1 was recovered. Thus, in the GBC-SD cells, the
absence of the expression of WIF-1 was associated with promoter
methylation. 5-Aza-dC has been used in leukemia (29), melanoma and renal cell carcinoma as
an adjuvant drug (30), however,
its use in gallbladder cancer has not been reported. Future
investigations may determine whether 5-Aza-dC can improve the
prognosis of patients with gallbladder cancer.
The inhibition of WIF-1 in tumor cells has been
confirmed in a series of previous studies (31–33).
The investigation of the biological behavior of gallbladder cancer
cell lines in the present study also showed that WIF-1 slowed down
cell replication and inhibited tumor cell proliferation. As a
particularly invasive type of cancer, invasion is an important
factor leading to gallbladder cancer-associated mortality (34). In the present study, a plasmid
expressing WIF-1 was transfected into GBC-SD cells, and it was
found that the ability of the cells to invade and metastasize
decreased significantly. The results for MMP were similar. MMP-2
and MMP-9 are involved, not only in physiological processes,
including tissue repair, but are also involved in degradation of
the extracellular matrix, which promotes tumor invasion and
metastasis (35). The experimental
results in the present study showed that WIF-1 reduced the
secretion of these two enzymes. This result may explain why WIF-1
reduced the invasion and metastasis of gallbladder cancer. Taken
together, these results indicated that WIF-1 markedly suppressed
gallbladder cancer.
To validate the inhibitory effects noted in the
tumor cell lines, the present study also performed animal
experiments. The tumorigenicity of the transfected GBC-SD cells was
significantly lower, compared with the other two groups of cells.
The average weight of the tumors was only 1/10 of the weight of
those in the control groups.
As a widely influential factor, which inhibits the
Wnt/β-catenin signaling pathway, the expression of WIF-1 in the
tumor inevitably leads to changes in a series of downstream genes.
In the present study, downregulation of β-catenin at the protein
level was observed in the WGBC-SD cells, with downregulation of
pathway activity. Protein chip technology was also used to identify
the expression of proteins, which are important in the
physiological and pathological course of gallbladder cancer.
Protein chips are a relatively novel type of high-throughput
detection technology, which can rapidly detect protein expression
(36,37). The results showed alterations in
the expression levels of a number of proteins involved in cell
cycle and apoptosis. The expression levels of Cripto-1, DKK-4,
CCL14a, DPPIV, Cathepsin S, EpCAM, E-Cadherin, IL-17B, DKK-3,
Decorin, IL-17C, CA19-9, HB-EGF, IL-13 R alpha1, EG-VEGF, CA125,
CEACAM-1, HVEM and PSA-free increased, and the expression levels of
EDA-A2, Carbonic Anhydrase IX, Angiostatin, and Procalcitonin
decreased. This may have led to the changes in tumor behavior.
As an important signaling molecule in the Wnt
signaling pathway, WIF-1 can effectively inhibit this signaling
pathway, however, it is not the only inhibitor (38–40).
Investigating the mechanism of signal inhibiting factors in the Wnt
signaling pathway may assist in prolonging survival rates and
improving patient quality of life. As Wnt signal transduction is
complex and affects a wide range of other metabolic pathways,
further investigation of its mechanisms is warranted to improve
therapeutic efficacy in the treatment of gallbladder cancer.
In conclusion, the present study demonstrated that
the expression levels of WIF-1 were low in gallbladder cancer tumor
tissues and the GBC-SD, SGC-996 and NOZ gallbladder cancer cell
lines. This low expression was associated with the methylation
status of the WIF-1 gene promotor. Following treatment of the
GBC-SD cell line with the demethylation agent, 5-Aza-dC, the
expression of WIF-1 recovered. Following establishment of cell
lines stably expressing WIF-1, it was found that the proliferation,
invasion, metastasis and tumorigenicity of the established cell
lines were significantly decreased, and the apoptotic rates were
increased. Protein expression levels were also altered in the
modified cells. These results suggest that WIF-1 may be an
effective treatment target for gallbladder cancer.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81272373).
References
|
1
|
Donohue JH, Stewart AK and Menck HR: The
national cancer data base report on carcinoma of the gallbladder,
1989–1995. Cancer. 83:2618–2628. 1998. View Article : Google Scholar
|
|
2
|
Coburn NG, Cleary SP, Tan JC and Law CH:
Surgery for gallbladder cancer: A population-based analysis. J Am
Coll Surg. 207:371–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu AX, Hong TS, Hezel AF and Kooby DA:
Current management of gallbladder carcinoma. Oncologist.
15:168–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wodarz A and Nusse R: Mechanisms of Wnt
signaling in development. Annu Rev Cell Dev Biol. 14:59–88. 1998.
View Article : Google Scholar
|
|
6
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taketo MM: Wnt signaling and
gastrointestinal tumorigenesis in mouse models. Oncogene.
25:7522–7530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Scoyk M, Randall J, Sergew A, Williams
LM, Tennis M and Winn RA: Wnt signaling pathway and lung disease.
Transl Res. 151:175–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karamboulas C and Ailles L: Developmental
signaling pathways in cancer stem cells of solid tumors. Biochim
Biophys Acta. 1830:2481–2495. 2013. View Article : Google Scholar
|
|
10
|
Hsieh JC, Kodjabachian L, Rebbert ML,
Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB and Nathans J:
A new secreted protein that binds to Wnt proteins and inhibits
their activities. Nature. 398:431–436. 1999. View Article : Google Scholar
|
|
11
|
Liepinsh E, Bányai L, Patthy L and Otting
G: NMR structure of the WIF domain of the human Wnt-inhibitory
factor-1. J Mol Biol. 357:942–950. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signaling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim AS, Lowenstein DH and Pleasure SJ: Wnt
receptors and Wnt inhibitors are expressed in gradients in the
developing telencephalon. Mech Dev. 103:167–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wissmann C, Wild PJ, Kaiser S, Roepcke S,
Stoehr R, Woenckhaus M, Kristiansen G, Hsieh JC, Hofstaedter F,
Hartmann A, et al: WIF1, a component of the Wnt pathway, is
downregulated in prostate, breast, lung and bladder cancer. J
Pathol. 201:204–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Licchesi JD, Westra WH, Hooker CM, Machida
EO, Baylin SB and Herman JG: Epigenetic alteration of Wnt pathway
antagonists in progressive glandular neoplasia of the lung.
Carcinogenesis. 29:895–904. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Byun T, Karimi M, Marsh JL, Milovanovic T,
Lin F and Holcombe RF: Expression of secreted Wnt antagonists in
gastrointestinal tissues: Potential role in stem cell homeostasis.
J Clin Pathol. 58:515–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frommer M, McDonald LE, Millar DS, Collis
CM, Watt F, Grigg GW, Molloy PL and Paul CL: A genomic sequencing
protocol that yields a positive display of 5-methylcytosine
residues in individual DNA strands. Proc Natl Acad Sci USA.
89:1827–1831. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Behrens J and Lustig B: The Wnt connection
to tumorigenesis. Int J Dev Biol. 48:477–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taniguchi H, Yamamoto H, Hirata T,
Miyamoto N, Oki M, Nosho K, Adachi Y, Endo T, Imai K and Shinomura
Y: Frequent epigenetic inactivation of Wnt inhibitory factor-1 in
human gastrointestinal cancers. Oncogene. 24:7946–7952. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schroeder M and Mass MJ: CpG methylation
inactivates the transcriptional activity of the promoter of the
human p53 tumor suppressor gene. Biochem Biophys Res Commun.
235:403–406. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holmes R and Soloway PD: Regulation of
imprinted DNA methylation. Cytogenet Genome Res. 113:122–129. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Das PM and Singal R: DNA methylation and
cancer. J Clin Oncol. 22:4632–4642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Batra S, Shi Y, Kuchenbecker KM, He B,
Reguart N, Mikami I, You L, Xu Z, Lin YC, Clément G and Jablons DM:
Wnt inhibitory factor-1, a Wnt antagonist, is silenced by promoter
hypermethylation in malignant pleural mesothelioma. Biochem Biophys
Res Commun. 342:1228–1232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Zhu CS, Bi KH, Xu WW, Dong L and
Hou M: Study of WIF-1 promoter methylation with expressions of
β-catenin in acute leukemia. Zhonghua Yi Xue Za Zhi. 91:2858–2860.
2011.In Chinese.
|
|
25
|
Alvarez C, Tapia T, Cornejo V, Fernandez
W, Muñoz A, Camus M, Alvarez M, Devoto L and Carvallo P: Silencing
of tumor suppressor genes RASSF1A, SLIT2 and WIF1 by promoter
hypermethylation in hereditary breast cancer. Mol Carcinog.
52:475–487. 2013. View
Article : Google Scholar
|
|
26
|
Chan SL, Cui Y, van Hasselt A, Li H,
Srivastava G, Jin H, Ng KM, Wang Y, Lee KY, Tsao GS, et al: The
tumor suppressor Wnt inhibitory factor 1 is frequently methylated
in nasopharyngeal and esophageal carcinomas. Lab Invest.
87:644–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palmisano WA, Divine KK, Saccomanno G,
Gilliland FD, Baylin SB, Herman JG and Belinsky SA: Predicting lung
cancer by detecting aberrant promoter methylation in sputum. Cancer
Res. 60:5954–5958. 2000.PubMed/NCBI
|
|
28
|
Belinsky SA, Nikula KJ, Palmisano WA,
Michels R, Saccomanno G, Gabrielson E, Baylin SB and Herman JG:
Aberrant methylation of p16 (INK4a) is an early event in lung
cancer and a potential biomarker for early diagnosis. Proc Natl
Acad Sci USA. 95:11891–11896. 1998. View Article : Google Scholar
|
|
29
|
Bryan J, Kantarjian H, Garcia-Manero G and
Jabbour E: Pharmacokinetic evaluation of decitabine for the
treatment of leukemia. Expert Opin Drug Metab Toxicol. 7:661–672.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gollob JA, Sciambi CJ, Peterson BL,
Richmond T, Thoreson M, Moran K, Dressman HK, Jelinek J and Issa
JP: Phase I trial of sequential low-dose 5-aza-2′-deoxycytidine
plus high-dose intravenous bolus interleukin-2 in patients with
melanoma or renal cell carcinoma. Clin Cancer Res. 12:4619–4627.
2006. View Article : Google Scholar
|
|
31
|
Kawakami K, Hirata H, Yamamura S, Kikuno
N, Saini S, Majid S, Tanaka Y, Kawamoto K, Enokida H, Nakagawa M
and Dahiya R: Functional significance of Wnt inhibitory factor-1
gene in kidney cancer. Cancer Res. 69:8603–8610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rubin EM, Guo Y, Tu K, Xie J, Zi X and
Hoang BH: Wnt inhibitory factor 1 decreases tumorigenesis and
metastasis in osteosarcoma. Mol Cancer Ther. 9:731–741. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu J, Fang J, Yang Z, Chen F, Liu J and
Wang Y: Wnt inhibitory factor-1 regulates glioblastoma cell cycle
and proliferation. J Clin Neurosci. 19:1428–1432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaushik SP: Current perspectives in
gallbladder carcinoma. J Gastroenterol Hepatol. 16:848–854. 2001.
View Article : Google Scholar
|
|
35
|
Benaud C, Dickson RB and Thompson EW:
Roles of the matrix metalloproteinases in mammary gland development
and cancer. Breast Cancer Res Treat. 50:97–116. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim HB, Kim CK, Iijima K, Kobayashi T and
Kita H: Protein microarray analysis in patients with asthma:
Elevation of the chemokine PARC/CCL18 in sputum. Chest.
135:295–302. 2009. View Article : Google Scholar
|
|
37
|
Wu DJ, Qian MJ, Rong RM, Xu M and Zhu TY:
Expression of inflammation cytokines and network analysis in acute
rejection of renal transplantation. Zhonghua Yi Xue Za Zhi.
92:2976–2979. 2012.In Chinese.
|
|
38
|
Enomoto-Iwamoto M, Kitagaki J, Koyama E,
Tamamura Y, Wu C, Kanatani N, Koike T, Okada H, Komori T, Yoneda T,
et al: The Wnt antagonist Frzb-1 regulates chondrocyte maturation
and long bone development during limb skeletogenesis. Dev Biol.
251:142–156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dun Y, Yang Y, Xiong Z, Feng M, Zhang Y,
Wang M, Xiang J, Li G and Ma R: Induction of Dickkopf-1 contributes
to the neurotoxicity of MPP (+) in PC12 cells via inhibition of the
canonical Wnt signaling pathway. Neuropharmacology. 67:168–175.
2013. View Article : Google Scholar
|
|
40
|
Chen B, Ma X, Liu S, Zhao W and Wu J:
Inhibition of lung cancer cells growth, motility and induction of
apoptosis by Klotho, a novel secreted Wnt antagonist, in a
dose-dependent manner. Cancer Biol Ther. 13:1221–1228. 2012.
View Article : Google Scholar : PubMed/NCBI
|