Introduction
Atherosclerosis is a chronic inflammatory disease,
which is associated with several types of infiltrating inflammatory
cell and contributes to mortality rates worldwide (1,2).
Foam cells, which are formed as a result of oxidized low-density
lipoprotein (oxLDL) uptake by macrophage colony-stimulating factor-
or granulocyte-macrophage colony-stimulating factor-differentiated
macrophages, contribute primarily to the formation of a necrotic
core in plaques and thus accelerate the development of
atherosclerosis (3,4). The oxLDL-induced apoptosis of
macrophages is also important in the process of atherosclerosis
(5). It has been shown that
treatment with oxLDL at a high concentration (100 µg/ml)
promotes macrophage apoptosis in vitro by inducing
endoplasmic reticulum (ER) stress (6–9). In
addition, high concentrations of oxLDL result in the activation of
other signaling pathways, including c-Jun N-terminal kinase,
peroxisome proliferator-activated receptor (PPAR)-γ, p38 and p53,
which subsequently contribute to the apoptosis of macrophages
(7,10,11).
This suggests that oxLDL-induced macrophage apoptosis is a complex
and multi-factorial process.
R-spondins are secreted proteins, which have a
thrombospondin type I repeat in their structure and are involved in
activation of the Wnt signaling pathway (12). There are four members in this
group, Rspo1, Rspo2, Rspo3 and Rspo4, each of which is ~35 Kd in
size, and all of which have been shown to be involved in the
regulation of various diseases and biological processes, including
osteoporosis pseudoglioma, skeletal myogenesis, keratinocyte
proliferation, mammary epithelial cell invasiveness and colorectal
cancer (13–17). Preliminary data have suggested that
Rspo2 is the most abundant of the four R-spondins, which is
expressed in the THP-1 macrophage-like cell line. The activation of
Wnt signaling has been implicated in the protection from apoptosis
in pre-adipocytes (18) and, as
R-spondins have been linked with the activation of Wnt signaling,
the present study hypothesized that Rspo2, due to being abundantly
expressed and activating the Wnt pathway, may be involved in
oxLDL-induced macrophage apoptosis. Therefore, in the present
study, the monocytic THP1 cell line was used to confirm the role of
Rspo2 in the ox-LDL-induced apoptosis of macrophages.
Materials and methods
Cell culture
The THP-1 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in RPMI 1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
calf serum (Sigma-Aldrich, St. Louis, MO, USA). Prior to any
treatments, the THP-1 monocytic cells were treated with phorbol
myristate acetate (Sigma-Aldrich) at a concentration of 100 nmol/l
for 24 h at 37°C to differentiate the cells into macrophages-like
sticky cells. The differentiated macrophages (1×106)
were treated with 40 µg/ml oxLDL (Guangzhou Yiyuan
Biological Technology Co., Ltd., Guangzhou, China) or thapsigargin
(Tg; 1 µM; Sigma-Aldrich) for 24 h at 37°C in subsequent
experiments.
Transfection
According to the manufacturer's protocol, 2
µl of small interfering (si)RNA (50 nM) or 2 µg
plasmid were mixed with 5 µl P3000 reagent (Life
Technologies, Grand Island, NY, USA) in 100 µl of opti-MEM
(Gibco; Thermo Fisher Scientific, Inc.) medium. Separately, 3.75
µl of lipo 3000 (Life Technologies) was added to another 100
µl of opti-MEM medium. After 5 min of incubation at room
temperature, the opti-MEM medium was mixed and added to
1×106 cells in a complete medium for 24 h at 37°C. The
siRNA knockdown or plasmid overexpression efficiencies were
analyzed by detecting the mRNA levels of Rspo2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using a total mRNA isolation
kit (Tiangen Biotech Co., Ltd., Beijing, China), according to the
manufacturer's instructions. cDNAs were generated using a
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China). Briefly, 500 ng total mRNA was mixed with 2 µl
PrimeScript RT reagent and added ddH2O to a total volume
of 10 µl. The RT reaction was performed at 37°C for 15 min.
qPCR was conducted using SYBR-Green Premix Ex Taq (Takara
Biotechnology Co., Ltd.) and detected by ABI PRISM 7500 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The cycling condition were 95°C for 15 sec and 60°C for 34
sec, for 40 cycles. The primer sequences used for Rspo2 were:
Forward, 5′-GTCTCCCTCTGAGTCCTCCC-3′; and reverse,
5′-CGTCTCCATCGGTTGCCTT-3′. Analysis of relative gene expression was
perfomed using the 2−ΔΔCq method (19).
Apoptosis assays
The apoptotic rates were measured using annexin V
(BD Biosciences, Franklin Lakes, NJ, USA) and propidium iodide (PI;
BD Biosciences,) staining kits, as previously described (20). Briefly the treated macrophage cells
were incubated with annexin V staining for 15 min at room
temperature, following which the PI solution was added and the
cells were incubated in the dark for another 15 min at room
temperature. Finally, the cells were analyzed using flow
cytometry.
Western blotting
Following treatment, the differentiated THP-1 cells
were lysed using RIPA lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) to extract the total proteins.
Following extraction, 40 µg protein of each sample were
separated by electrophoresis on a 10% polyacrylamide SDS gel and
then transferred onto a PVDF membrane (EMD Millipore, Billerica,
MA, USA). The membrane was then incubated with monoclonal rabbit
anti-Rspo2 antibody (1:1,000; cat. no. ab73761; Abcam, Cambridge,
MA, USA), monoclonal rabbit anti-GAPDH antibody (1:1,000; cat. no.
AG019; Beyotime Institute of Biotechnology, Beijing, China),
monoclonal mouse anti-protein kinase RNA-like ER kinase (PERK)
antibody (1:1,000; cat. no. 12185; Cell Signaling Technology, Inc.,
Danvers, MA, USA), monoclonal rabbit anti lectin-like LDL
receptor-1 (LOX-1) antibody (1:1,000; cat. no. ab60178; Abcam),
monoclonal mouse anti-scavenger receptor A (SRA) antibody (1:1,000;
cat. no. ab36625; Abcam), rabbit anti-CD36 antibody (1:1,000; cat.
no. ab78054; Abcam) and monoclonal rabbit anti-PPAR-γ antibody
(1:1,000; cat. no. 2435; Cell Signaling Technology, Inc.) at 4°C
overnight. The next day, the membranes were washed using
Tris-buffered saline/Tween (TBST) for 15 min, and further incubated
with goat anti-rabbit (cat. no. CW0103M) or goat anti-mouse (cat.
no. CW0102M) specific antibodies (1:5,000; Beijing Kangwei
Biological Technology Co., Ltd., Beijing, China) for 1 h at room
temperature. Finally, following washing with TBST, the protein
signals were detected using ECL reagent (Pierce, Bonn, Germany).
Quantification of western blot analysis was performed using Image
J2x software (National Institutes of Health, Bethesda, MD,
USA).
ROS measurement
ROS measurement was performed using
2,7-dichlorofluorescein diacetate dye, according to the
manufacturer's protocol (Beyotime Institute of Biotechnology). The
cells were incubated with this dye (10 µM) for 30 min to
enable its uptake by the cells, following which the cells were
washed with phosphate-buffered saline (PBS) three times. The dye
inside the cells emitted a fluorescent signal on meeting free
radicals, and images of the fluorescent signal were captured using
an Olympus fluorescence microscope (Olympus Corporation, Tokyo,
Japan).
Detection of liquid uptake
For examining Dil-oxLDL uptake, the cells were
washed twice with RPMI 1640 medium and then incubated with
Dil-oxLDL (10 µg/ml; Guangzhou Yiyuan Biological Technology
Co., Ltd.) for 3 h at 37°C in the dark, according to the
manufacturer's instructions. Subsequently, the cells were washed
with PBS for 15 min in the dark and were observed under an Olympus
fluorescence microscope.
Immunofluorescence staining
Following treatment, the cultured cells were fixed
with 4% paraformaldehyde solution and then permeabilized with 0.5%
TritonX-100 solution. The cells were then incubated with monoclonal
rabbit anti-PPAR-γ antibody (1:100; cat. no. 2435; Cell Signaling
Technology, Inc.) at 37°C for 2 h and washed with PBS. This was
followed by incubation with goat anti-rabbit secondary antibody
(1:200; cat. no. CW0103M; Beijing Kangwei Biological Technology
Co., Ltd.) for another 1 h at 37°C. The cells were then incubated
with DAPI (Guangzhou Yiyuan Biological Technology Co., Ltd.), and
were observed under a Zeiss confocal microscope (Carl Zeiss, Inc.,
Oberkochen, Germany) for visualization of the staining signal.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assays were performed using a kit (Cell
Signaling Technology, Inc.), as described previously (21). In brief, the cells were harvested
and fixed using 4% formaldehyde for 10 min. Following chromosome
shearing with nuclease, smaller DNA fragments were obtained.
Following this, the PPAR-γ protein was immunoprecipitated using
rabbit anti-PPAR-γ antibody and the complex was incubated with NaCl
(5 M) solution at 65°C to reverse crosslinking, following which the
DNA was purified using a DNA purification kit (Cell Signaling
Technology, Inc.). The purified DNA was used for PCR (ABI PRISM
7500 sequence detection system; Applied Biosystems; Thermo Fisher
Scientific, Inc.) to detect the relative expression of target
fragments. SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.) was
used to amplify the DNA (500 ng) with incubation at 95°C for 30
sec, and 40 cycles of 95°C for 5 sec and 60°C for 30 sec. The
results were quantified using the 2−ΔΔCq method. The
specific primers used to amplify the binding site of PPAR-γ to the
CD36 promoter were as follows: Forward 5′-GCGATATCGAGTTATTCCG-3′
and reverse 3′-ACTACAGGTGTGCGCCACCATG-5′.
Statistical analysis
The statistical significance of differences between
groups were calculated with unpaired t-tests using SPSS
software (version 17; SPSS, Inc., Chicago, IL, USA). The values are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Rspo2 inhibits oxLDL-induced apoptosis
under ER stress in macrophages
The present study first compared the expression of
four R-spondinsin THP-1 cells using qPCR. As shown in Fig. 1A, the mRNA expression of Rspo2 was
the highest among all the R-spondins. To identify whether the high
expression level of Rspo2 had any functional role in the
oxLDL-induced apoptosis of macrophages, the expression of Rspo2 was
simultaneously inhibited by siRNA and overexpressed by ectopic
expression, and the apoptotic rates were assessed. As shown in
Fig. 1B, neither treatment with
oxLDL at two concentrations or with the ER stress activator, Tg,
alone resulted in the induction of macrophages apoptosis. However,
the combined treatment of the differentiated THP-1 cells with Tg
and oxLDL significantly induced apoptosis. Notably, following Rspo2
ablation by siRNA in the differentiated THP-1 cells, oxLDL
treatment alone was able to induce the apoptosis of macrophages. In
addition, when the Rspo2-ablated THP-1 cells were treated with a
combination of oxLDL and Tg, an additional increase in the rate of
macrophage apoptosis was observed. Furthermore, the ectopic
overexpression of Rspo2 was able to reverse the induction of
apoptosis by oxLDL and Tg in the differentiated THP-1 cells, as
shown in Fig. 1C.
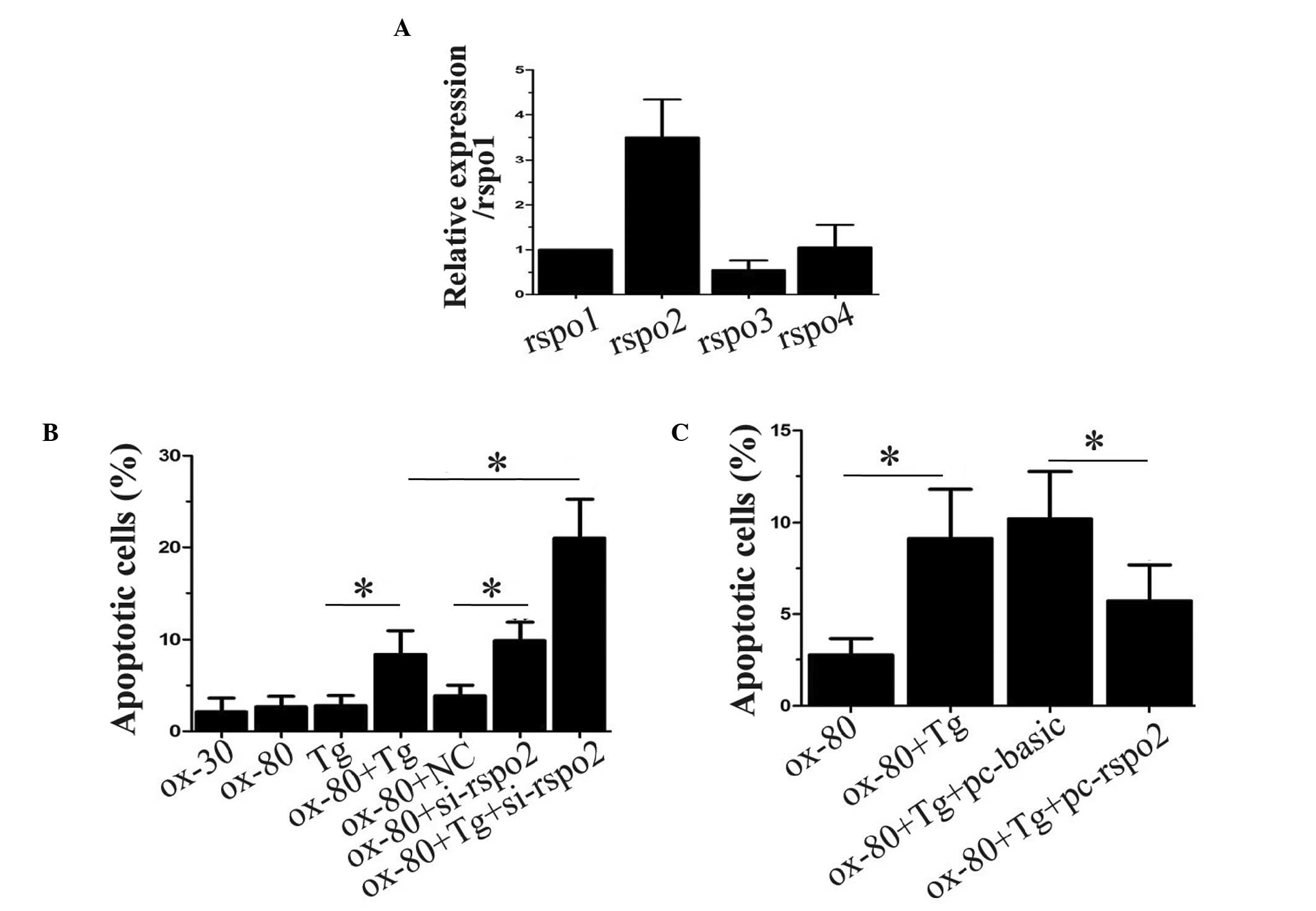 | Figure 1Rspo2 negatively regulates
oxLDL-induced THP-1 cell apoptosis under ER stress. (A) THP-1 cells
were analyzed for the expression of Rspo1, Rspo2, Rspo3 and Rspo4
using reverse transcription-polymerase chain reaction analysis. The
expression of 18S, a ribosomal gene, was used as an endogenous
control for normalization. (B) THP-1 cells, either alone or
transfected with NC or Rspo2 siRNA, were incubated for 24 h with
the indicated concentrations of oxLDL alone or in combination with
Tg. Subsequently, apoptosis was assessed and the percentage of
apoptotic cells were determined. (C) THP-1 cells alone or
overexpressing either the vector (pc-basic) or Rspo2 plasmid
(pc-rspo2), were incubated for 24 h with oxLDL (80 µg/ml)
alone or in combination with Tg. The apoptotic rate was assessed
and presented as a bar graph. Values are expressed as the mean ±
standard deviation. *P<0.05, comparison indicated by
brackets. siRNA, small interfering RNA; oxLDL, oxidized low density
lipoprotein; Rspo, R-spondin; Tg, thapsigargin. |
Rspo2 suppresses ER stress and ROS
production in oxLDL treatment
As it was observed that oxLDL only induced
macrophage apoptosis in the presence of Tg, which increases ER
stress, or under Rspo2 ablation, the present study investigated
whether the expression of Rspo2 is involved in modulating ER
stress. The protein expression of PERK, which is described as an ER
stress marker (22), was
determined by treating the THP-1 cells with increasing
concentrations of oxLDL. As shown in Fig. 2A, the expression of PERK increased
in a dose-dependent manner with increasing concentrations of oxLDL.
However, the ectopic expression of Rspo2 in THP-1 cells treated
with oxLDL, resulted in a significant reduction in the expression
of PERK (Fig. 2B). These results
suggested that the overexpression of Rspo2 inhibited ER stress. The
present study also measured the production of ROS in the THP-1
cells. As shown in Fig. 2C, the
data revealed that oxLDL treatment induced ROS production, shown as
increased fluorescent signal under the microscope. Similarly, the
overexpression of Rspo2 significantly reduced this signal, thus
supporting the hypothesis that Rspo2 contributes to reductions in
ER stress and ROS production.
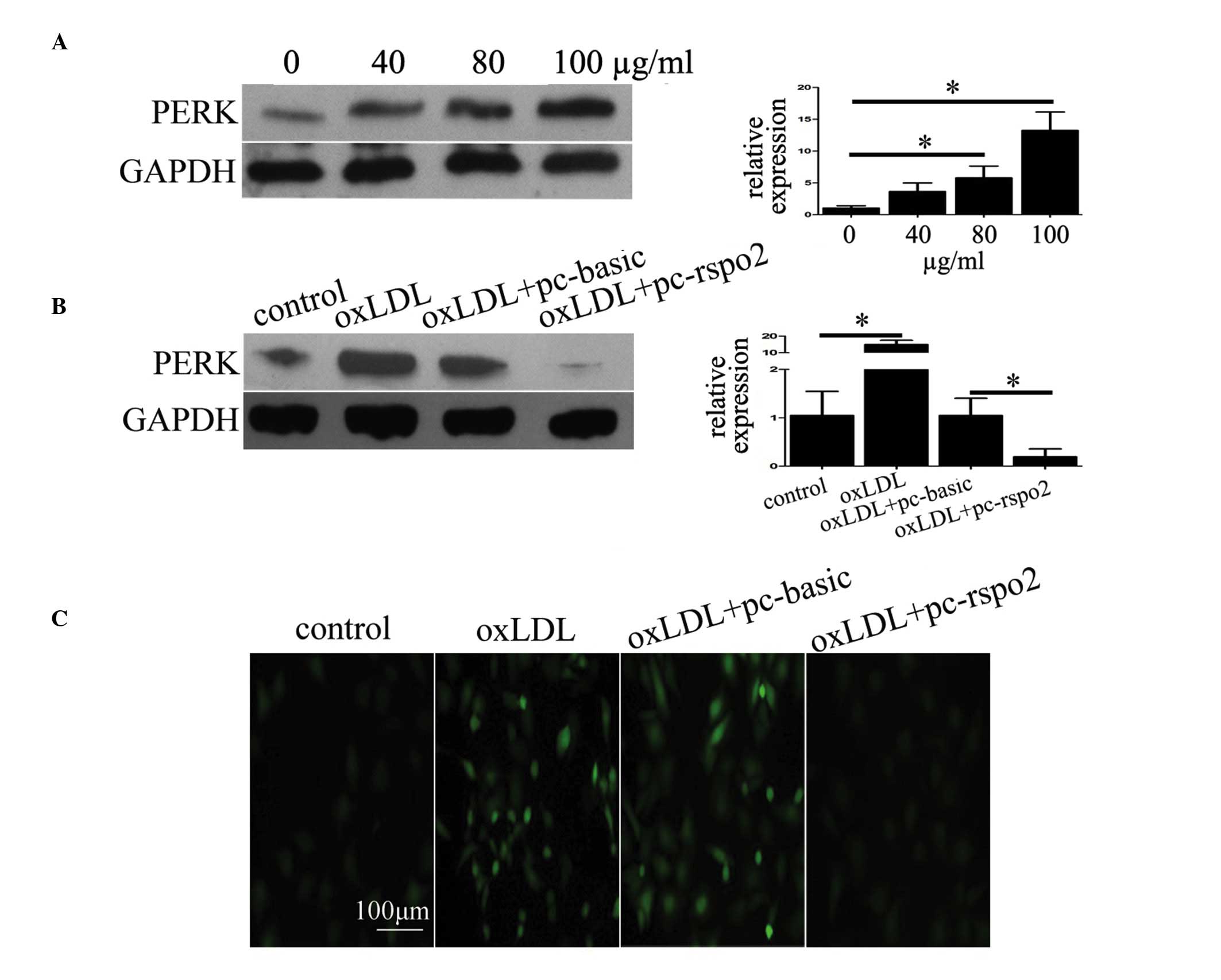 | Figure 2Rspo2 suppresses oxLDL-induced ER
stress and ROS production. (A) Expression of the ER stress marker,
PERK, was assessed using western blotting in THP-1 cells incubated
with the indicated concentrations of oxLDL (0-100 µg/ml) for
24 h. The expression of GAPDH was used as a loading control. (B)
Expression of PERK was assessed in THP-1 cells alone or in those
transfected with pc-basic or pc-rspo2, and treated with or without
oxLDL (100 µg/ml) for 24 h. The expression of GAPDH served
as a loading control. (C) THP-1 cells transfected with pc-basic or
pc-rspo2 and treated with or without oxLDL (100 µg/ml) for
24 h, were labeled with 2,7-dichlorofluorescein diacetate dye to
assess the production of ROS. The labeled cells produced
fluorescent signal proportional to the ROS levels, and this signal
was recorded using an Olympus fluorescence microscope.
*P<0.05, comparison indicated by brackets. oxLDL,
oxidized low density lipoprotein; Rspo, R-spondin; ER, endoplasmic
reticulum; ROS, reactive oxygen species; PERK, protein kinase
RNA-like ER kinase; siRNA, small interfering RNA. |
Rspo2 negatively regulates lipid uptake
and the expression of scavenger receptor, CD36
As oxLDL-induced macrophage apoptosis is a later
process and is preceded by its uptake by macrophages, the present
study investigated whether Rspo2 is involved in oxLDL uptake. To
determine this, the differentiated THP-1 cells with Rspo2 ablation
or overexpression were treated with Dil-oxLDL. It was observed that
the Rspo2-ablated cells had increased uptake of Dil-oxLDL, whereas
the cells overexpressing Rspo2 had markedly lower levels of lipid
uptake, as shown in Fig. 3A. The
present study also analyzed the regulation of three types of
scavenger receptor, CD36, SRA and LOX-1, which are predominantly
involved in lipid uptake by macrophages (23). Of note, it was observed that Rspo2
negatively regulated the protein expression of CD36, but had no
effect on the expression levels of SRA or LOX-1, as shown in
Fig. 3B. The overexpression of
Rspo2 inhibited the expression of CD36, whereas the ablation of
Rspo2 marginally increased the expression of CD36, compared with
the respective controls.
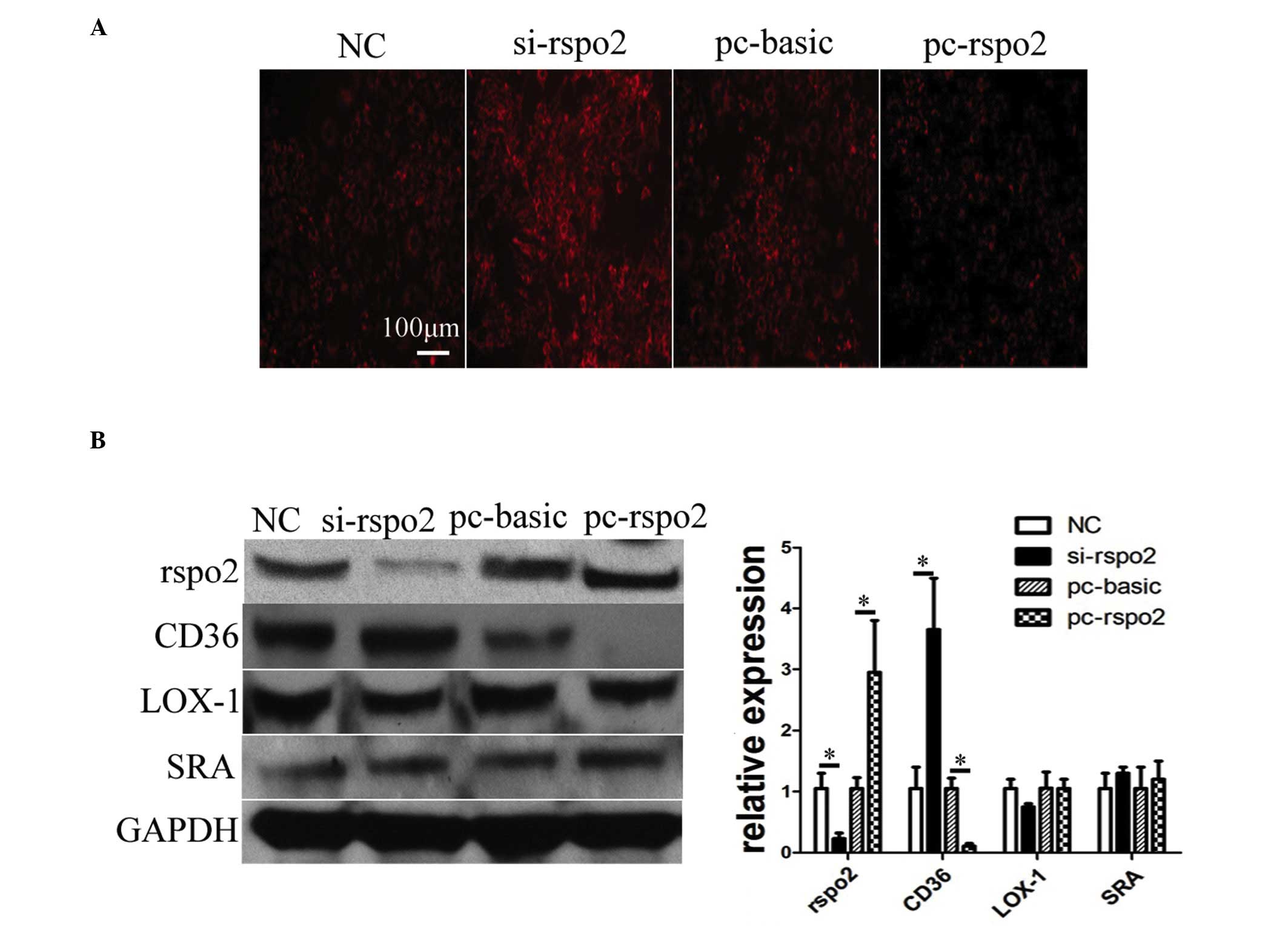 | Figure 3Rspo2 negatively regulates lipid
uptake and the expression of CD36. (A) THP-1 cells were transfected
with pc-basic, pc-rspo2, NC, or si-rspo2 for 24 h. The cells from
the different transfection conditions were then treated with
Dil-oxLDL (10 µg/ml) for 3 h, following which the
fluorescent signal was recorded under a microscope. (B) THP-1 cells
were transfected with NC, si-rspo2, pc-basic or pc-rspo2 for 24 h.
The total proteins extracted from these different groups were
blotted to detect the protein expression of Rspo2, CD36, LOX-1 and
SRA. The expression of GAPDH was used as a loading control.
*P<0.05, comparison indicated by brackets. Rspo,
R-spondin; pc-basic, empty plasmid pCMV; pc-rspo2, Rspo2 plasmid;
si-rspo2, Rspo2-specific siRNA; NC, negative control; CD36, cluster
of differentiation 36; LOX-1, lectin-like LDL receptor-1; SRA,
scavenger receptor A. |
Rspo2 decreases the expression of CD36
through inhibiting PPAR-γ
In order to understand how Rspo2 mediates the
regulation of the expression of CD36, the present study
investigated the transcription factor, PPAR-γ, which has been
previously demonstrated to regulate the expression of CD36 in
macrophages (24). The expression
of PPAR-γ was analyzed in THP-1 cells transfected with either the
vector or Rspo2 plasmid and treated with oxLDL. As shown in
Fig. 4A, the Rspo2-overexpressing
cells treated with oxLDL had reduced expression of PPAR-γ. This was
consistent with the above-mentioned reduced expression of CD36 in
the Rspo2-overexpressing cells. The nuclear translocation of PPAR-γ
was further examined by analyzing its expression using
immunoflorescence staining. It was observed that oxLDL led to the
translocation of PPAR-γ into the nucleus, however, the
overexpression of Rspo2 reduced its nuclear translocation in THP-1
cells, as seen in Fig. 4B.
Finally, the binding activity of PPAR-γ to the CD36 promoter was
analyzed by ChIP analysis. As seen in Fig. 4C, the binding activity of PPAR-γ
and CD36 increased following oxLDL treatment, but was significantly
reduced in the cells overexpressing Rspo2. This suggested that
PPAR-γ was bound to the CD36 promoter in the presence of ox-LDL,
and that the overexpression of Rspo2 somehow reduced its binding to
the CD36 promoter, and thus reduced its expression.
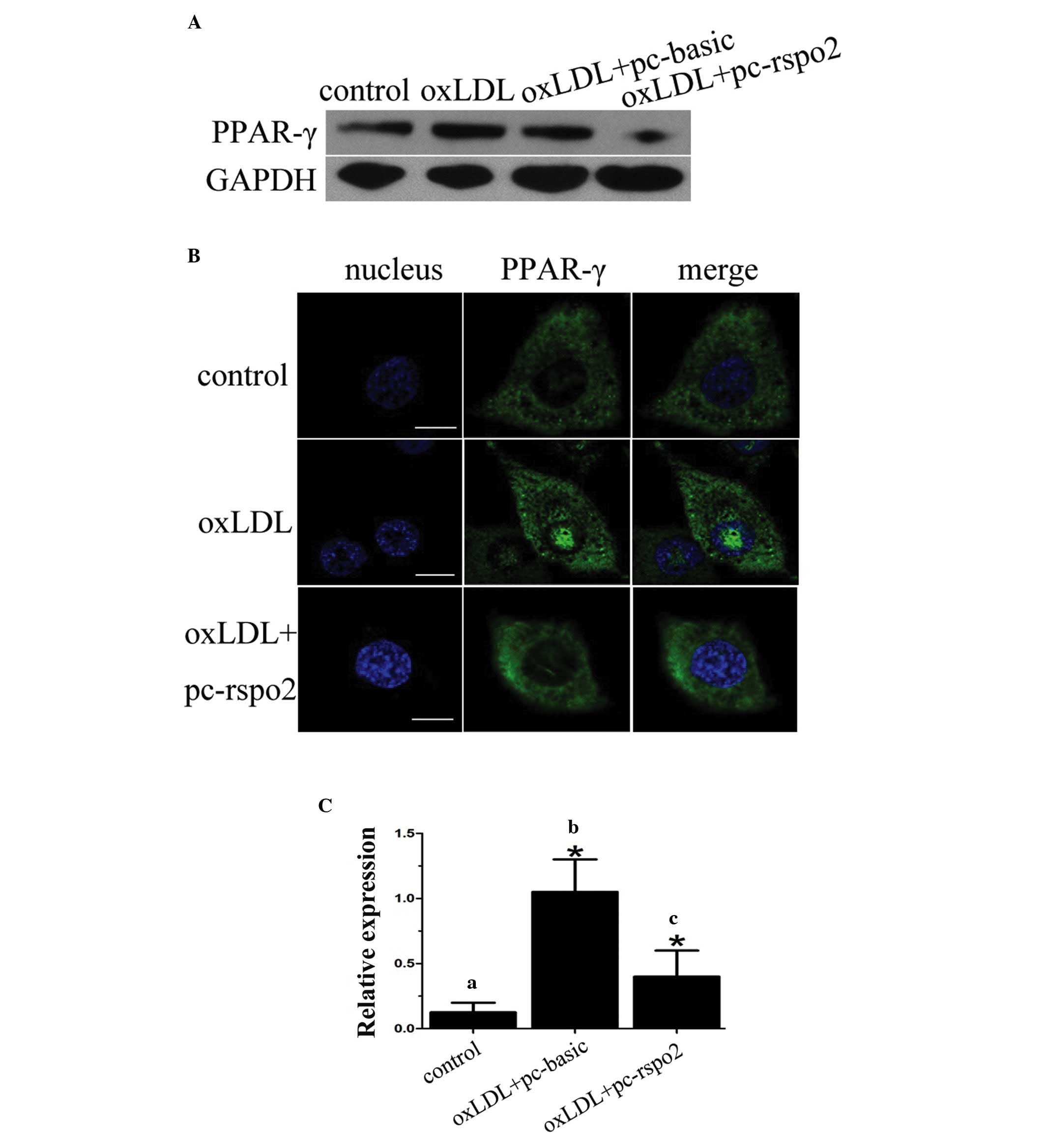 | Figure 4Rspo2 inhibits the expression of
PPAR-γ, and the nuclear translocation and transcriptional activity
of PPAR-γ. (A) Expression of PPAR-γ was assessed in THP-1 cells
alone or transfected with pc-basic or pc-rspo2, and treated with or
without oxLDL (100 µg/ml) for 24 h. GAPDH served as a
loading control. (B) THP-1 cells alone or transfected with pc-rspo2
were treated with or without oxLDL (100 µg/ml) for 24 h.
PPAR-γ immunostaining was then performed to assess its localization
and images were captured under a Zeiss confocal microscope. (C)
THP-1 cells (a) alone, (b) transfected with pc-basic or (c)
transfected with pc-rspo2, were treated with oxLDL (100
µg/ml) for 24 h. Chromatin immunoprecipitation assays were
performed using antibodies against PPAR-γ and the expression of
CD36 was analyzed using polymerase chain reaction analysis. Total
chromatin gathered prior to immunoprecipitation served as an input
control. Values are expressed as the mean ± standard deviation.
*P<0.05, comparison indicated by brackets. PPAR-γ,
peroxisome proliferator-activated receptor; Rspo, R-spondin;
pc-basic, empty plasmid pCMV; pc-rspo2, Rspo2 plasmid; si-rspo2,
Rspo2-specific siRNA; NC, negative control; CD36, cluster of
differentiation 36. |
Discussion
The present study was performed to understand the
role of Rspo2 in the oxLDL-induced apoptosis of macrophages. It was
demonstrated that Rspo2 had a functional role in oxLDL-mediated
macrophage apoptosis and is the first report, to the best of our
knowledge, linking Rspo2 to the regulation of macrophage biology,
particularly apoptosis. In another study, Rspo2 knockdown has been
linked with reduced cell viability of human Schwann tumors,
however, this involved the regulation of Wnt signaling (25). Until now, the majority of studies
have suggested that the functional activity of R-spondin proteins
has been via the regulation of Wnt signaling in all biological
pathways, including cancer (16,26).
However, the present study is the first to establish the role of
R-spondin proteins in the regulation of atherosclerosis.
According to previous literature, lipid uptake in
macrophages is a key factor during the process of cardiovascular
disease (5). Of note, the present
study found that Rspo2 protein negatively regulated the uptake of
oxLDL by macrophages and that this was mediated through regulation
of the expression of the scavenger receptor protein, CD36, which is
involved in liquid uptake by macrophages. The effect of Rspo2 on
CD36 was mediated through regulation of the nuclear translocation
of PPAR-γ. This particular observation has an implication in
preventing oxLDL uptake by macrophages if Rspo2 is overexpressed,
ultimately leading to the inhibition of foam cell formation and
finally reducing the overall ability of necrotic plaque formation
in atherosclerosis.
The scavenger receptor, CD36, is predominantly
distributed on different immune cell subsets, including macrophages
and dendritic cells, and other cell types, including platelets,
endothelial cells, adipocytes and muscle cells, and has been shown
to be important in fatty acid metabolism (27), heart disease (28) and the processing of dietary fat
(29). Thus, it has implications
in glucose tolerance, hypertension, diabetes, cardiovascular
problems, Alzheimer's disease and lipoprotein endocytosis in
macrophages (23,30). In this context, the data obtained
in the present study, which suggested that Rspo2 has the ability to
regulate the expression of CD36, indicates the potential of Rspo2
as a suitable target for other diseases, which requires
investigation.
Our previous study showed that Wnt pathway
positively regulated the expression of CD36 (21), whereas Rspo2, a recognized Wnt
pathway activator, was observed to inhibit the expression of CD36.
At present, this differential effect remains to be fully
elucidated, however, it may be that Rspo2 inhibited PPAR-γ nuclear
translocation through another mechanism in the macrophages. In
addition, there are further questions remaining, including whether
oxLDL can also regulate the expression of Rspo2, and whether Rspo2
is involved in the regulation of liquid outflow from the
macrophages. Further investigations are required to address these
questions of interest.
In conclusion, the present study showed that the
Rspo2 protein protected against the oxLDL-induced apoptosis of
macrophages through negative regulation of the expression of the
scavenger receptor protein, CD36. In addition Rspo2 decreased
oxLDL-induced ER stress and ROS production in the differentiated
macrophages. The findings of the current study provide novel
insight on lipid overload-associated disease, including
atherosclerosis, and may be aid the development of improved
therapies.
Acknowledgments
This work was funded by the Natural Science
Foundation of China (grant nos. 81200214/H0215 81400205 and
LQ14H020001).
References
|
1
|
Braunwald E: Shattuck
lecture-cardiovascular medicine at the turn of the millennium:
Triumphs, concerns and opportunities. N Engl J Med. 337:1360–1369.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lloyd-Jones D, Adams RJ, Brown TM,
Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K,
Gillespie C, et al: Executive summary: Heart disease and stroke
statistics-2010 update: A report from the American Heart
Association. Circulation. 121:948–954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waldo SW, Li Y, Buono C, Zhao B, Billings
EM, Chang J and Kruth HS: Heterogeneity of human macrophages in
culture and in atherosclerotic plaques. Am J Pathol. 172:1112–1126.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moore KJ and Tabas I: Macrophages in the
pathogenesis of atherosclerosis. Cell. 145:341–355. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang YC, Huang KX, Huang AC, Ho YC and
Wang CJ: Hibiscus anthocyanins-rich extract inhibited LDL oxidation
and oxLDL-mediated macrophages apoptosis. Food Chem Toxicol.
44:1015–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deigner HP, Claus R, Bonaterra GA, Gehrke
C, Bibak N, Blaess M, Cantz M, Metz J and Kinscherf R: Ceramide
induces aSMase expression: Implications for oxLDL-induced
apoptosis. FASEB J. 15:807–814. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seimon TA, Nadolski MJ, Liao X, Magallon
J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL,
Tsimikas S, et al: Atherogenic lipids and lipoproteins trigger
CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic
reticulum stress. Cell Metab. 12:467–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erbay E, Babaev VR, Mayers JR, Makowski L,
Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF and
Hotamisligil GS: Reducing endoplasmic reticulum stress through a
macrophage lipid chaperone alleviates atherosclerosis. Nat Med.
15:1383–1391. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oh J, Riek AE, Weng S, Petty M, Kim D,
Colonna M, Cella M and Bernal-Mizrachi C: Endoplasmic reticulum
stress controls M2 macrophage differentiation and foam cell
formation. J Biol Chem. 287:11629–11641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giovannini C, Varì R, Scazzocchio B,
Sanchez M, Santangelo C, Filesi C, D'Archivio M and Masella R:
OxLDL induced p53-dependent apoptosis by activating p38MAPK and
PKCδ signaling pathways in J774A.1 macrophage cells. J Mol Cell
Biol. 3:316–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoon JK and Lee JS: Cellular signaling and
biological functions of R-spondins. Cell Signal. 24:369–377. 2012.
View Article : Google Scholar
|
|
13
|
Han XH, Jin YR, Seto M and Yoon JK: A
WNT/beta-catenin signaling activator, R-spondin, plays positive
regulatory roles during skeletal myogenesis. J Biol Chem.
286:10649–10659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boyden LM, Mao J, Belsky J, Mitzner L,
Farhi A, Mitnick MA, Wu D, Insogna K and Lifton RP: High bone
density due to a mutation in LDL-receptor-related protein 5. N Engl
J Med. 346:1513–1521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chua AW, Ma D, Gan SU, Fu Z, Han HC, Song
C, Sabapathy K and Phan TT: The role of R-spondin2 in keratinocyte
proliferation and epidermal thickening in keloid scarring. J Invest
Dermatol. 131:644–654. 2011. View Article : Google Scholar
|
|
16
|
Wu C, Qiu S, Lu L, Zou J, Li WF, Wang O,
Zhao H, Wang H, Tang J, Chen L, et al: RSPO2-LGR5 signaling has
tumour-suppressive activity in colorectal cancer. Nat Commun.
5:31492014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klauzinska M, Baljinnyam B, Raafat A,
Rodriguez-Canales J, Strizzi L, Greer YE, Rubin JS and Callahan R:
Rspo2/Int7 regulates invasiveness and tumorigenic properties of
mammary epithelial cells. J Cell Physiol. 227:1960–1971. 2012.
View Article : Google Scholar
|
|
18
|
Longo KA, Kennell JA, Ochocinska MJ, Ross
SE, Wright WS and MacDougald OA: Wnt signaling protects 3T3-L1
preadipocytes from apoptosis through induction of insulin-like
growth factors. J Biol Chem. 277:38239–38244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Devries-Seimon T, Li Y, Yao PM, Stone E,
Wang Y, Davis RJ, Flavell R and Tabas I: Cholesterol-induced
macrophage apoptosis requires ER stress pathways and engagement of
the type A scavenger receptor. J Cell Biol. 171:61–73. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang S, Sun Z, Zhang X, Li Z, Wu M, Zhao
W, Wang H, Chen T, Yan H and Zhu J: Wnt1 positively regulates CD36
expression via TCF4 and PPAR-γ in macrophages. Cell Physiol
Biochem. 35:1289–1302. 2015. View Article : Google Scholar
|
|
22
|
Liu J, Lee J, Salazar Hernandez MA,
Mazitschek R and Ozcan U: Treatment of obesity with celastrol.
Cell. 161:999–1011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murphy JE, Tedbury PR, Homer-Vanniasinkam
S, Walker JH and Ponnambalam S: Biochemistry and cell biology of
mammalian scavenger receptors. Atherosclerosis. 182:1–15. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tontonoz P, Nagy L, Alvarez JG, Thomazy VA
and Evans RM: PPARgamma promotes monocyte/macrophage
differentiation and uptake of oxidized LDL. Cell. 93:241–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watson AL, Rahrmann EP, Moriarity BS, Choi
K, Conboy CB, Greeley AD, Halfond AL, Anderson LK, Wahl BR, Keng
VW, et al: Canonical Wnt/β-catenin signaling drives human schwann
cell transformation, progression and tumor maintenance. Cancer
Discov. 3:674–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chartier C, Raval J, Axelrod F, Bond C,
Cain J, Dee-Hoskins C, Ma S, Fischer MM, Shah J, Wei J, et al:
Therapeutic targeting of tumor-derived R-spondin attenuates
β-catenin signaling and tumorigenesis in multiple cancer types.
Cancer Res. 76:713–723. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hajri T, Han XX, Bonen A and Abumrad NA:
Defective fatty acid uptake modulates insulin responsiveness and
metabolic responses to diet in CD36-null mice. J Clin Invest.
109:1381–1389. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Febbraio M, Podrez EA, Smith JD, Hajjar
DP, Hazen SL, Hoff HF, Sharma K and Silverstein RL: Targeted
disruption of the class B scavenger receptor CD36 protects against
atherosclerotic lesion development in mice. J Clin Invest.
105:1049–1056. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Drover VA, Ajmal M, Nassir F, Davidson NO,
Nauli AM, Sahoo D, Tso P and Abumrad NA: CD36 deficiency impairs
intestinal lipid secretion and clearance of chylomicrons from the
blood. J Clin Invest. 115:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rać ME, Safranow K and Poncyljusz W:
Molecular basis of human CD36 gene mutations. Mol Med. 13:288–296.
2007. View Article : Google Scholar
|


















