Introduction
Cancer cachexia (CC) is a clinical syndrome
characterized by progressive loss of the skeletal muscle with or
without fat loss, which cannot be reversed by traditional
nutritional support therapy, leading to progressive body (1). Specifically, CC may give rise to
anorexia, anemia, acceleration of proteolysis and even dysfunction
of the organs, which leads to the progressive loss of skeletal
muscle (2). It has been previously
reported that CC may affect >80% of patients with cancer, which
directly affects the overall survival and the life quality of the
patients, reduces the tolerance to radiotherapy and chemotherapy,
and increases the occurrence rate of complications, thus CC is
regarded as an crucial cause of death in patients with cancer
(3,4). The pathogenesis of CC remains
complex, and there is little information regarding the use of
therapeutic strategies including anti-inflammatories, appetite
stimulation and nutritional support, which may aid in the
prevention of skeletal muscle degeneration, however further
investigation is required (5,6).
As a therapy for liver cancer, the modified Chinese
herbal compound jianpijiedu (MCHCJ; patent no. 2009100420702) may
significantly enhance survival in a hepatic carcinoma mouse model,
postpone the development of tumors and maintain the body weight of
the xenografted mice (7,8). However, the mechanism of the effect
remains unclear, thus, based on previous research and the
characteristics of the ascites-induced hepatic carcinoma cachexia,
the current study used MJPJD and the Traditional Chinese Medicine
principal of warming Yang for diuresis and promoting
Qi for purging fu-organs, in a mouse model of hepatic
carcinoma to investigate the effects and the mechanism of MJPJD on
hepatic carcinoma cachexia.
Materials and methods
Experimental animals and cancer cell
lines
A total of 52 male C57BL/6 mice (certification no.
00014376), aged 4–6 weeks and weighing 14–17 g, were provided by
the Guangdong Province Experimental Animal Center [approval no.
SCXK (Guangdong) 2013-0002]. The H22 ascites-oriented hepatic
carcinoma cells were provided by the Experimental Animal Center of
Sun Yat-sen University [Approval No. SCXK (Guangdong) 2013-0081].
The H22 cell lines were frozen, then while thawing, the cells were
washed by phosphate-buffered saline (PBS; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) three times, then were diluted to
106 cells/ml. All the experimental procedures were
performed in specific pathogen-free animal laboratory,
environmental and facility at 24–26°C, an air pressure of 20–50 Pa
and a relative humidity of 40–70% with a 12/12 h light/dark cycle.
Mice were fed with a diet comprised of water (≤10%), crude protein
(≥18%), crude fat (≥4%), crude fiber (≥5%), crude ash (≤8%),
calcium (1.0–1.8%) and phosphorus (0.6–1.2%) according to the
National Standard of China (GB 14924.3-2001). All the animal
experiments were conducted in strict accordance with the ethical
principles that were approved and supervised by the Ethics
Committee of the First Affiliated Hospital of Sun Yat-sen
University [Approval no. ethical application (2013) no. 149].
Medicines and reagents
MCHCJ (supplied by the Department of Pharmacy of the
First Affiliated Hospital of Sun Yat-sen University, Guangzhou,
China) composed of 30 g Codonopsis, 15 g poria, 15 g
atractylodes, 6 g licorice, 15 g Curcuma, 30 g
Scutellaria barbata, 6 g dried tangerine, 15 g Magnolia
officinalis, 15 g immature bitter orange, 15 g Coptis
chinensis, 15 g Scutellaria baicalensis, 15 g Rhizoma
anemarrhenae, 15 g Grifola, 15 g Rhizoma
alismatis, 6 g Fructus amomi, 15 g turmeric, 15 g
Pinellia ternata and 10 g Semen pharbitidis, were all
dissolved in 75°C distilled water to a concentration of 7.5 g/ml.
The medroxyprogesterone (MPA) (Zhejiang Xianju Pharmaceutical Co.,
Ltd., Taizhou, China) was dissolved to 24 g/ml in 25°C distilled
water. The indometacin (IND) enteric-coated tablets (Zhongsheng
Pharmacy, Guangzhou, China) was dissolved to 0.1 g/ml in 25°C
distilled water and well shaken. All the dilutions were stored at
0°C prior to use.
The interleukin-1α (IL-1α; cat. no. MUE027), the
IL-6 (cat. no. MUE044) and tumor necrosis factor-α (TNF-α; cat. no.
MUE030) enzyme-linked immunosorbent assay (ELISA) kits were
purchased from the ShanghaiBangyi Biotechnology Co., Ltd.
(Shanghai, China). Rabbit anti-F-box protein 32 (also termed
atrogin 1) antibody (cat. no. ab74023) and rabbit anti-muscle
RING-finger protein-1 (MU-RF1) antibody (cat, no. ab77577) were
purchased from Abcam (Cambridge, MA, USA). Mouse anti-β-tubuin
antibody (cat. no. AC008) was purchased from ABclonal Biotech Co.,
Ltd. (Wobrun, MA, USA). Goat anti-rabbit horseradish
peroxidase-conjugated antibody (cat. no. PV-6001) was purchased
from OriGene Technologies, Inc. (Beijing, China).
Devices
The infrared thermometer for animals (model DT-8866)
was purchased from the Shenzhen Kai Heng Jie Tech Co., Ltd.
(Shenzhen, China) and the improved grasping force measuring
instrument (patent no 201030634194.3) was purchased from the
Guangzhou Weiheng Electronics Co., Ltd. (Guangzhou, China).
Method
Model establishment
Two C57BL/6 mice were selected randomly to be
injected with 106 H22 ascitic hepatic carcinoma cells.
On day 7 post-injection, the mice were sacrificed by overdose
anesthesia; 4 ml ether (Tianjin Damao Chemical Reagent Factory,
Tianjin, China) was transferred to a 15-ml centrifuge tube and a
cotton ball was added and left to soak. Following soaking, the
cotton ball was placed in front of the nose of the animal inducing
anesthesia. Subsequent to sterilization, the ascites were extracted
then were centrifuged (600 × g, 2 times for 5 min at 4°C), and the
supernatant was discarded. The survival rate of tumor cells was
>95%, observed by the optical microscopy and trypan blue
staining, the ascites were dissolved in saline and diluted to a
cell suspension of 107/ml, and 0.2 ml of the suspension was
intraperitoneally injected into 40 C57BL/6 mice to establish the
model of ascites-induced hepatic carcinoma cachexia.
Grouping and intervention
C57BL/6 mice which received model establishment
(n=40) were randomly divided into five groups, as follows:
xenograft tumor group (Group B); low concentration of MJPJD
administration group (Group C); high concentration of MJPJD
administration group (Group D); and MPA combined with IND
administration group (Group E). Additionally, 10 mice were used as
the control group (Group A), which received injection of an
equivalent volume of saline. The intervention was initiated on day
6 of model establishment and administered daily for 8 days. Groups
A and B received the intragastric administration of the 0.1 ml/10
kg saline, Groups C and D received the intragastric administration
of 37.5 g/kg and 75 g/kg MJPJD, respectively, and Group E received
120 mg/kg MPA and 0.5 mg/kg IND. Subsequent to 8-days treatment,
the mice were sacrificed by overdose of inhalation anesthesia.
Indices detection
Body weight
The body weight of each experimental animal was
recorded each morning, and the net weight at the end of the
experiment represented the gross weight without the weight of
ascites. The day before the experiment was represented as 'd0' and
'd1' and 'd2' represented the day 1 and 2, respectively.
Food intake
The volumes of the food were recorded each morning
prior to feeding to calculate the food intake over time. The
'average food intake' was calculated as: (Volume of food prior to
feeding - volume of the food on the subsequent day)/numbers of
animals.
Body temperature
The body temperature were detected using an infrared
thermometer between 9:00 a.m. and 10:00 a.m. on d0, d4, d8 and d12
according to the instructions, which were detected three times for
an average (median) body temperature.
Grasping force
The grasping forces were detected by the improved
grasping force measuring instrument on d0, d4, d8 and d12 according
to the manufacturer's instructions, and detected three times to
calculate an average (median) grasping force.
Cytokines
The ascitic fluid was collected from animals in
Group B and the net weights were recorded. The suspension was
stored at −80°C following centrifugation at 600 × g for 5 min at
4°C prior to detection of the IL-lα, IL-6 and TNF-α levels using
the ELISA kits.
Tissue preparation
The heart, liver, spleen, lung, kidney (bilateral),
stomach and gastrocnemius [bilateral, left for immunohistochemistry
and right for western blot analysis and reverse
transcription-quantitative-polymerase chain reaction (RT-qPCR)]
were harvested and weighed to calculate the viscera index [organ
weight (g)/gross body weight (g)]. All the harvested tissues were
stored at −80°C prior to analysis.
Western blot analysis
The expression levels of the atrogin 1 and MU-RF1
proteins in the gastrocnemius were examined by western blotting.
The brief steps were as follows: i) Proteins extracted from the
right gastrocnemius using lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China); ii) SDS-PAGE spacer and separation
gels (10 and 5%, respectively) were prepared, with 20 µl
protein added per lane; iii) acrylamide prepared with different
concentrations for sample addition; iv) gel electrophoresis; v)
electrophoretic transfer of target gels according to the reference
protein marker; vi) polyvinylidene fluoride membrane blocked with
5% skimmed milk for 1 h at room temperature; vii) primary
incubation overnight at 4°C and secondary antibody incubation
performed for 2 h at room temperature [dilutions: Reference protein
(β-tubulin), 1:1,000; atrogin 1, 1:500; MU-RF1, 1:100; secondary
antibody, 1:500]; viii) membrane washing with Tris-buffered saline
Tween 20 6×5 min; ix) color rendering with the SuperSignal™
Chemiluminescent Horseradish Peroxidase Substrate kit (Thermo
Fisher Scientific, Inc., Shanghai, China) enhanced
chemiluminescence kit; and x) blots were semi-quantitatively
analyzed for protein expression, calculated as follows: relative
optical density (OD) = integral OD of target band/integral OD of
reference protein. Protein quantification was conducted using the
Bicinchoninic Acid Protein Quantification kit (Wuhan Boster
Biological Technology, Ltd.) and a microplate reader (Thermo Fisher
Scientific, Inc.).
RT-qPCR
The expression levels of atrogin 1 and MU-RF1 mRNA
in gastrocnemius were detected by RT-qPCR. The right gastrocnemius
tissues were lysed in 1 ml TRIzol to extract the total RNA
according to the manufacturer's instructions, and RT was performed.
The atrogin 1, MU-RF1 and GAPDH primers details and products
fragments as listed in Table I.
cDNA was synthesized using the TransScript All-in-One First-Strand
cDNA Synthesis SuperMix for qPCR (One-Step gDNA Removal) kit
(Beijing TransGen Biotech Co., Ltd., Beijing, China), the concs
were 4 µl total RNA; 1.6 µl 5X TransScript All-in-One
SuperMix for qPCR; 0.4 µl gDNA Remover; and 2 µl
RNase-free water, under the conditions of 37°C for 15 min and 85°C
for 5 sec. Subsequently, fluorescence qPCR was performed with
reactions consisting of 18.5 µl H2O, 2.5
µl 10X SYBR green PCR buffer (SYBR® Fast qPCR
Mix; Takara Biotechnology Co., Ltd., Dalian, China), 0.5 µl
dNTPs, 0.5 µl Taq, 0.5 µl forward primer (10
pmol/µl), 0.5 µl reverse primer (10 pmol/µl)
and 2 µl cDNA, and incubation at 93°C for 2 min, followed by
40 cycles of 93°C for 15 sec, 55°C for 25 sec and 72°C for 25 sec.
Finally, the cycle threshold (Cq) values were calculated to analyze
the relative expression level (ΔCq=Cqtarget
gene−Cqreference gene; ΔΔCq=ΔCq−ΔCqmax)
(9).
 | Table IPrimer sequence and the size of
products fragments. |
Table I
Primer sequence and the size of
products fragments.
| Gene | Primer sequence
(5′-3′) | Size of products
(bp) |
|---|
| Atrogin-1 | F:
ATGCACACTGGTGCAGAGAG | |
| R:
TGTAAGCACACAGGCAGGTC | 168 |
| MU-RF1 | F:
AGGATCAGAGCCTCGATGAAGC | |
| R:
AAGTTTGACACCCTCTACGCCA | 107 |
| GAPDH | F:
CCTGCACCACCAACTGCTTAG | |
| R:
TCTTCTGGGTGGCAGTGATG | 108 |
Immunohistochemistry
The expression levels of atrogin 1 and MU-RF1 in
gastrocnemius were detected by immunohistochemistry. The brief
steps were as follows: i) Gastrocnemius tissues were fixed with 10%
neutral formalin solution; ii) embedded with paraffin; iii)
sectioned at 3 µm; iv) dried at 80°C, deparaffinized and
dehydrated; v) infused with H2O2 for 10 min;
vi) antigen retrieval with microwaves; vii) incubated with primary
antibody (dilutions: atrogin 1, 1:500; MU-RF1, 1:100) at 4°C
overnight; viii) washed with PBS 3×2 min; ix) incubated with
secondary antibody at 37°C for 15–20 min; x) washed with PBS for
3×2 min for color rendering with diaminobenzidine; and xi) washed
with distilled water. The negative control samples were incubated
with PBS instead of the primary and secondary antibodies. Finally,
the qualitative principals were established as follows: Positive,
clear particles with yellowish-brown or tan-brown appeared in the
cytoplasm; negative, the number of positive cells were >20% of
total cells in one ×400 magnification field using a DM2500
microscope (Leica Microsystems Shanghai Trading Co., Ltd.,
Shanghai, China).
Statistical analysis
The data were analyzed using SPSS software (version
13.0). The continuous data were expressed as the mean ± standard
deviation. One-way analysis of variance was used to analyze the
normally distributed data, rank sum tests and Kaplan-Meier survival
analysis were used for the non-normally distributed data. Student's
t-test was used for the relative qualitative test, which was
replaced by the corrected t-test when equal variance was not
assumed. P<0.05 was considered to indicate a statistically
significant difference.
Results
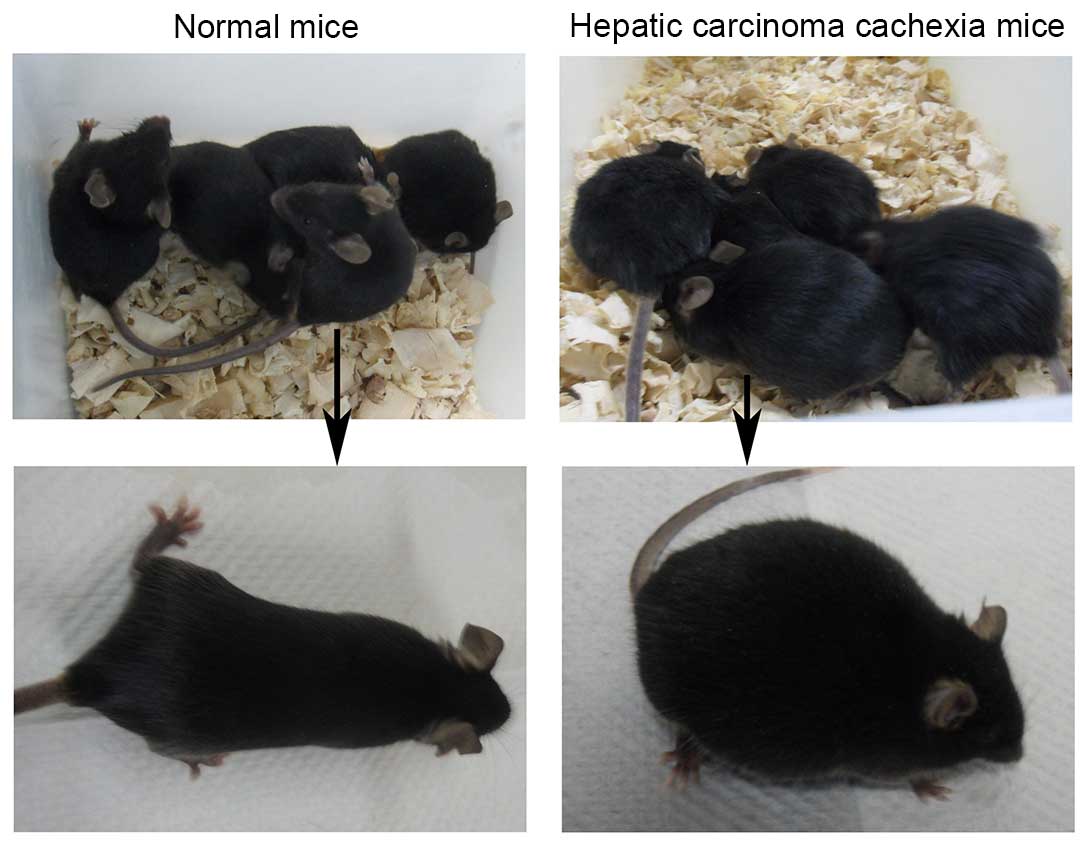
General observation
The animals in the model groups (Groups B, C, D and
E) exhibited normal postures, glossy and compact furs, normal
respiration and well mental state prior to model establishment.
However, compared with mice in Group A, these animal exhibited
distended abdomens, matted furs, passable activities and smooth
respirations at day 6 model establishment. Additionally, two
animals from each of the model groups were selected randomly to
undergo dissection 6 days subsequent to injection of H22 cells and
when the abdominal bulge was observed, in order to confirm the
ascites were developing as expected. At the end of the experiment,
the animals in Group A exhibited almost similar general
characteristics prior to establishment of the model, whereas, the
animals in the other groups demonstrated markedly distended
abdomens, mat and fluffy furs, accidie, cowering together, panting
respiration and reduced body temperature, which indicated
intermediate and advanced cachexia (Fig. 1).
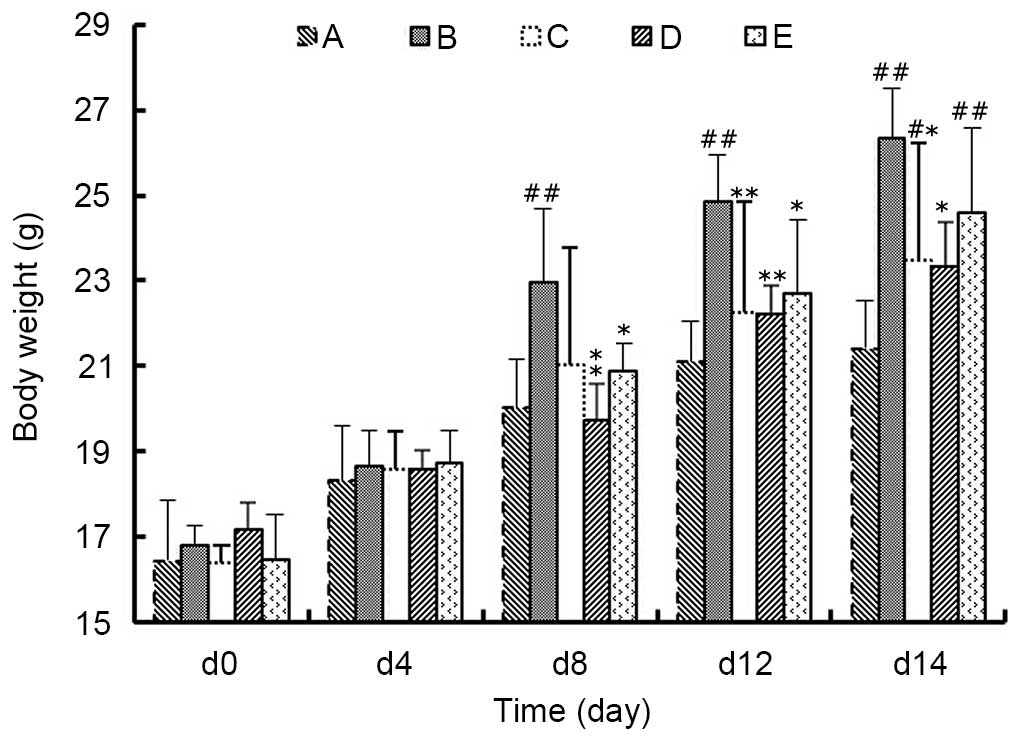
Body weight
As presented in Fig.
2, at day 8 of model establishment, the body weight of animals
in Group B was significantly higher compared with Groups A, D and E
(P<0.05), among which the body weights of Group D were most
similar with that of Group A (P>0.05). Additionally, on day 12
of model establishment, the average body weight in Groups A, C, D
and E were significantly lower compared with the weights in Group B
(P<0.05), among which Group C and D were less lower than Group E
(P>0.05). Similarly, at day 14 the same trend was observed as on
day 12. These data suggested that the rapid weight gain induced by
the mouse model of ascites-induced hepatic carcinoma may be
inhibited by MJPJD, the effect of which was greater compared with
the effect of MPA combined with IND.
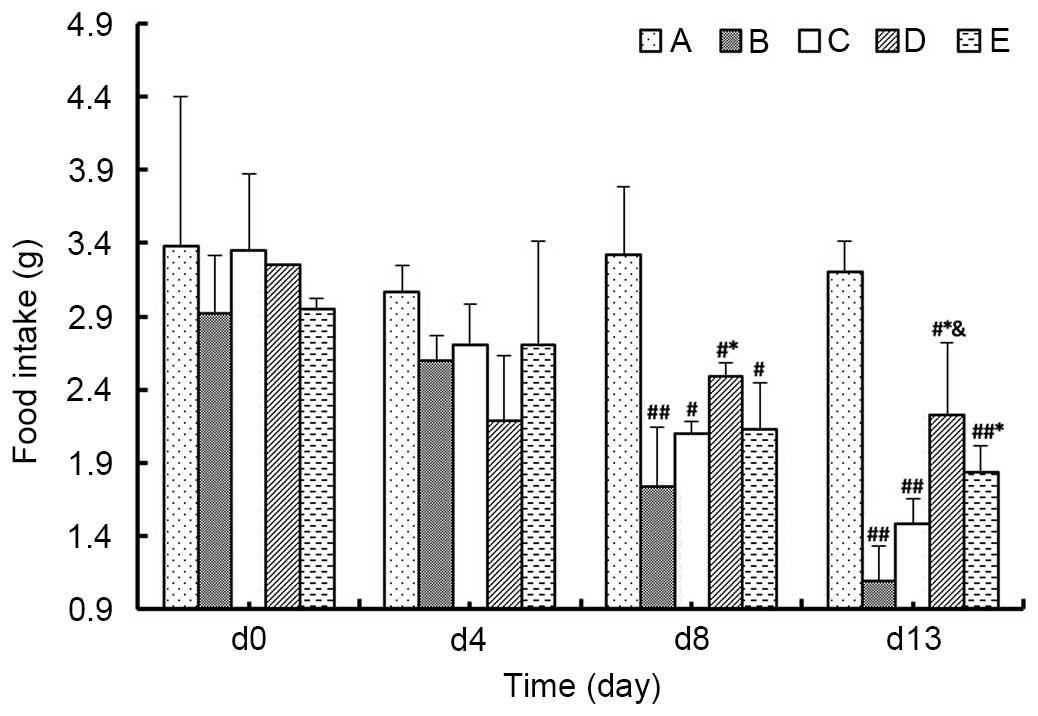
Food intake
As demonstrated in Fig.
3, at day 8 of the experiment, the food intake of the animals
in Group B was significantly lower compared with Group D
(P<0.05), and the average food intake of Group D was marginally
greater than in Groups C and E (P>0.05). At the day 13 of the
experiment, the food intake in model group was again decreased
compared with Group A, among which the average food intake of
Groups D and E were significantly greater than in Group B
(P<0.05), and furthermore, the food intake of Group D was
significantly increased compared with Group C (P<0.05) and
marginally increased compared with Group E (P>0.05). These data
suggested that the food intake of mice in the model of
ascites-induced hepatic carcinoma is improved by MJPJD, and the
effect of MJPJD is greater compared with the effect of MPA combined
with IND.
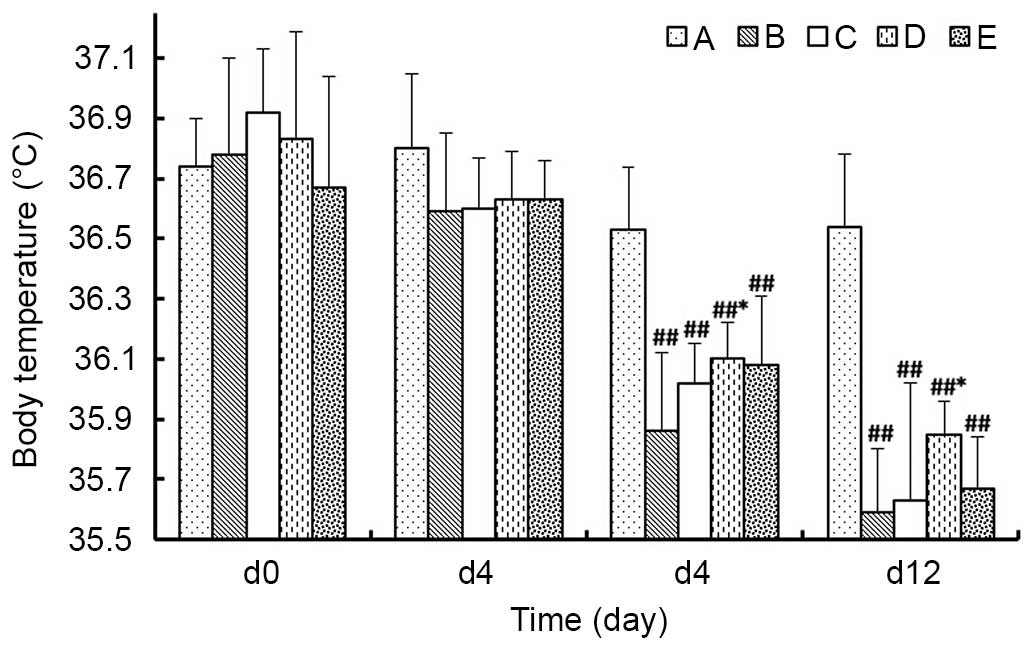
Body temperature
As presented in Fig.
4, on day 4 of the experiment, the body temperature of animals
in each group demonstrated no significant differences compared with
the body temperatures measured that prior to the experiment (day
0). By day 8 of the experiment, the body temperatures in the model
groups were significantly decreased compared with Group A
(P<0.01), among which the temperature in Group D was significant
increased compared with Group B (P<0.05), whereas, Group C and E
were not statistically different from Group B (P>0.05). The
trends were the same at day 12 of the experiment.
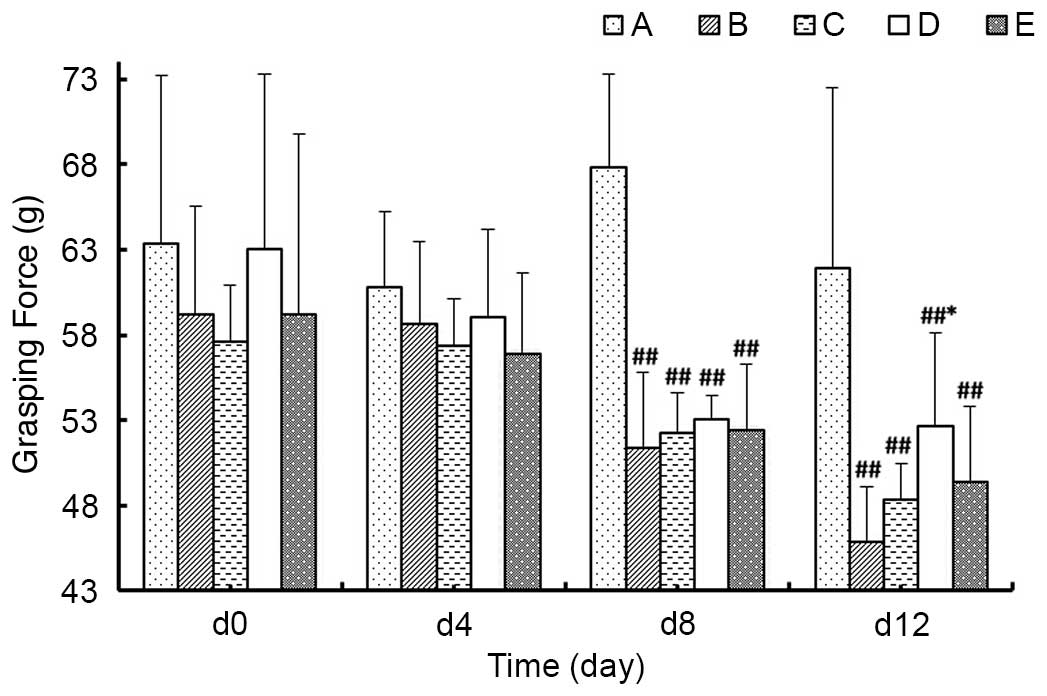
Grasping force
As demonstrated in Fig.
5, at day 4 of the experiment, the grasping force of animals in
each group exhibited no significant differences compared with day
0. At day 8, the grasping forces of the model groups were
significantly decreased compared with Group A (P<0.01), however,
there were no significant differences among the model groups. At
day 12 of the experiment, the grasping force values of model groups
remained decreased compared with Group A (P<0.01), among which
the grasping force in Group D was significantly higher than Group B
(P<0.05), whereas Groups C and E were similar to Group B
(P>0.05).
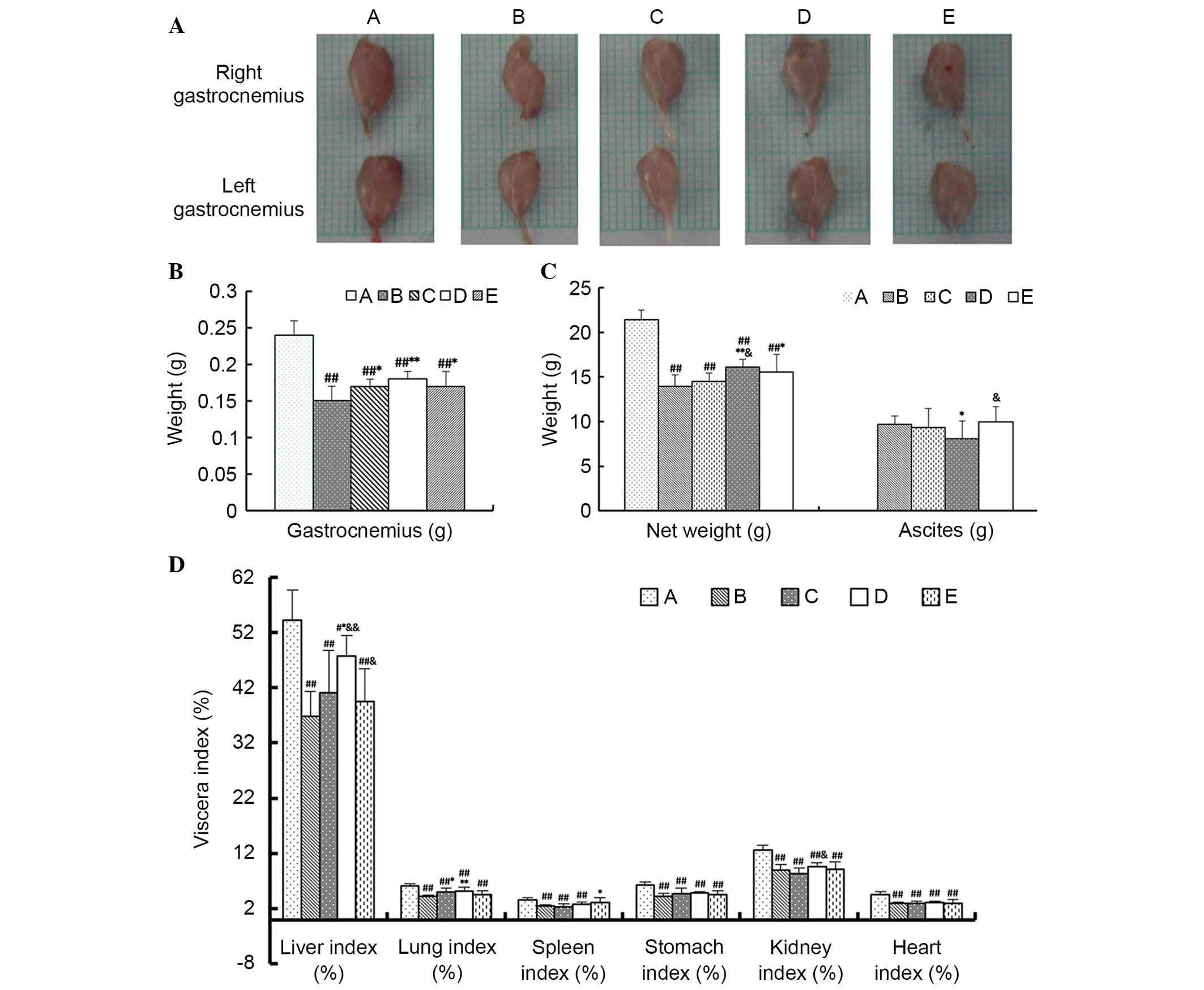
Anatomical data
As demonstrated in Fig.
6, the major findings in terms of the anatomical data were as
follows: i) The organs of model groups exhibited atrophy, indicated
by reduced viscera index, compared with Group A, except for the
spleen (P<0.01); ii) the weight of gastrocnemius in Groups C, D
and E were higher compared with Group B, among which that of Group
D was the highest (P<0.05 or P<0.01); iii) the lung index of
Group C and D were significantly higher than Group B (P<0.05),
whereas there was no significant difference between Group E and
Group B (P>0.05). Furthermore, the effects on the net weight,
liver index and lung index in Group D were significant compared
with Group B (P<0.05 or P<0.01), whereas changes to the
spleen index, stomach index, kidney index and heart index were
marginal and not significant (P>0.05); iv) the volume of the
ascites of Group D was significantly less than Groups B and E
(P<0.05), which indicated that the effects of MJPJD on ascites
was greater than MPA combined with IND.
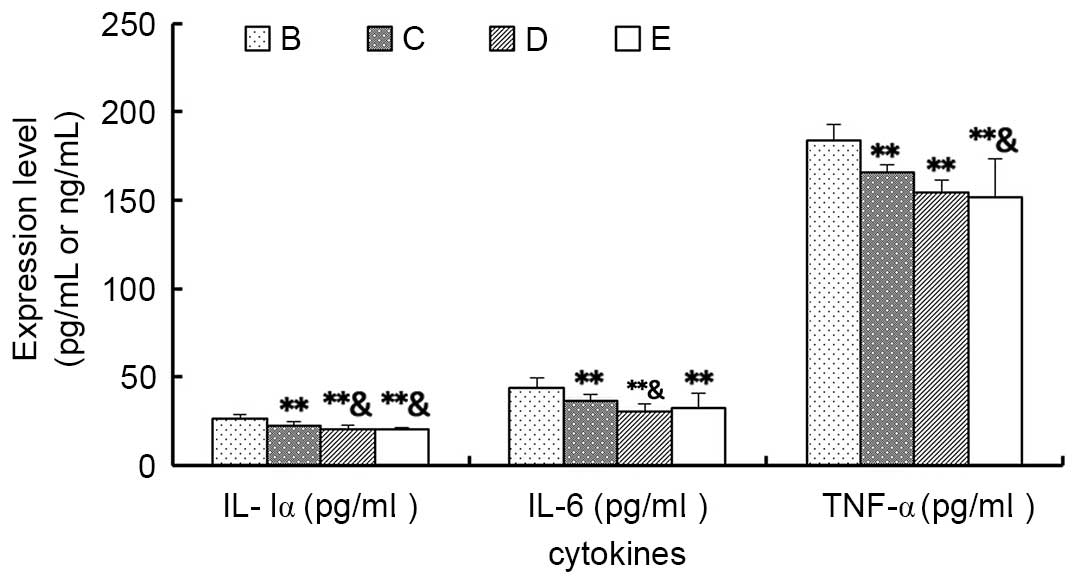
Cytokines
As demonstrated in Fig.
7, the expression levels of the inflammatory cytokines IL-lα,
IL-6 and TNF-α in Groups C, D and E were significantly lower
compared with Group B (P<0.01), among which the levels of IL-lα
and IL-6 in Group D were significantly lower compared with Group C
(P<0.05), and the levels of the IL-lα and TNF-α of Group E were
also significantly lower compared with Group C (P<0.05).
However, there were no significant differences between the levels
in Group D and E (P>0.05).
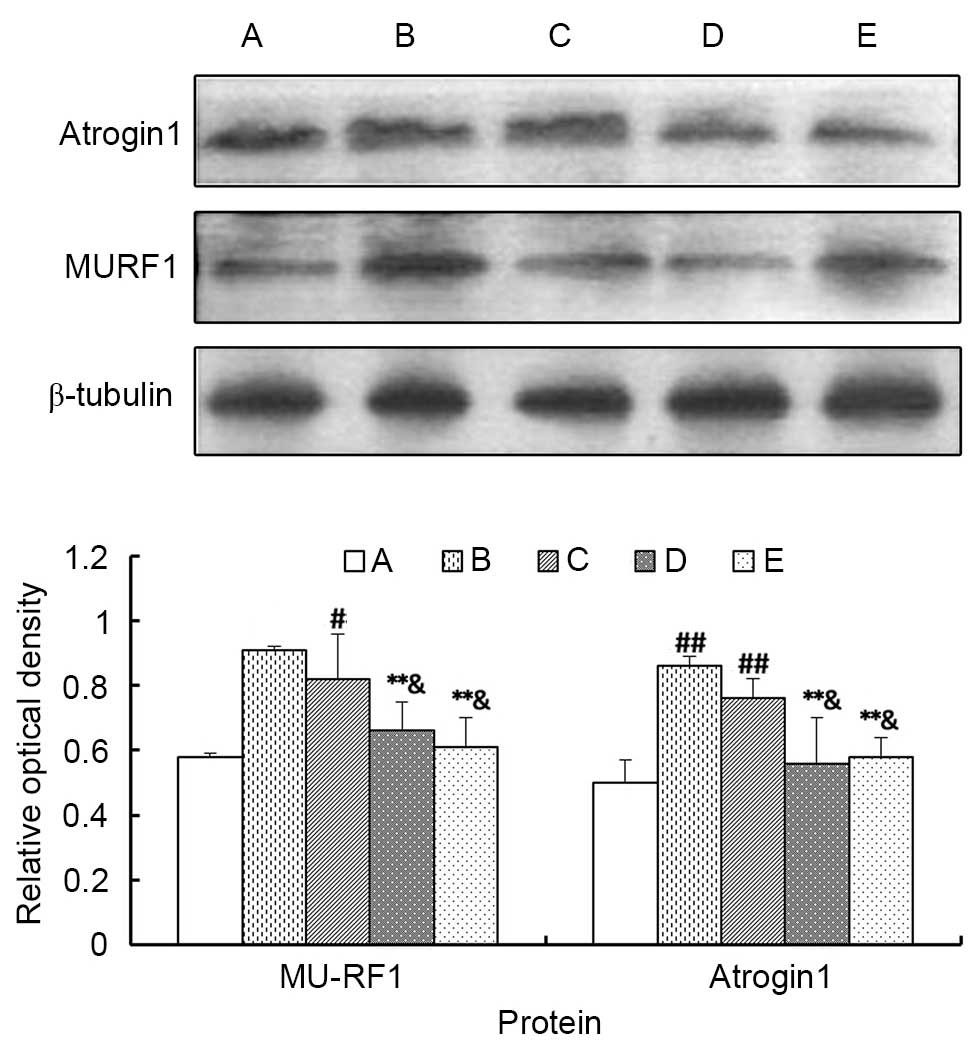
Atrogin 1 and MU-RF1 protein expression
levels detected by western blotting
As demonstrated in Fig.
8, the expression levels of atrogin 1 and MU-RF1 proteins in
the gastrocnemius of Groups B and C were higher compared with Group
A (P<0.05 or P<0.01), whereas there was no significant
difference between Groups D and E (P>0.05). Additionally, the
high concentration of MJPJD (Group D) and the MPA combined with IND
(Group E) significantly reduced the protein expression levels of
atrogin 1 and MU-RF1 compared with Group B (P<0.01), and
compared with the low concentration of MJPJD (Group C; P<0.05).
However, there were no significant differences between the high
concentration of MJPJD (Group D) and the MPA combined with IND
(Group E; P>0.05). These data suggested that the high
concentration of MJPJD significantly reduced the expression of
atrogin 1 and MU-RF1 proteins to inhibit the activation of the
ubiquitin proteasome pathway (UPP).
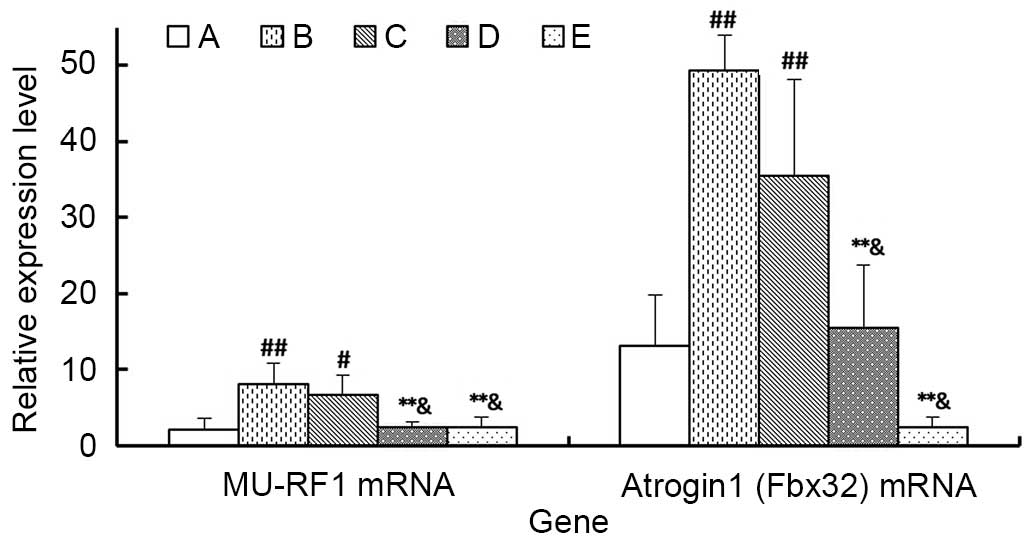
Atrogin 1 and MU-RF1 mRNA expression
levels detected by RT-qPCR
As demonstrated in Fig.
9, the expression levels of atrogin 1 and MU-RF1 mRNA in
gastrocnemius of Group B and C were significantly higher than Group
A (P<0.05). Additionally, the high concentration of MJPJD (Group
D) and the MPA combined with IND (Group E) significantly reduced
the expression level of the atrogin 1 and MU-RF1 mRNA compared with
Group B (P<0.01) and Group C (P<0.05). However, there was no
significant difference between the effects Group d and Group E
(P>0.05).
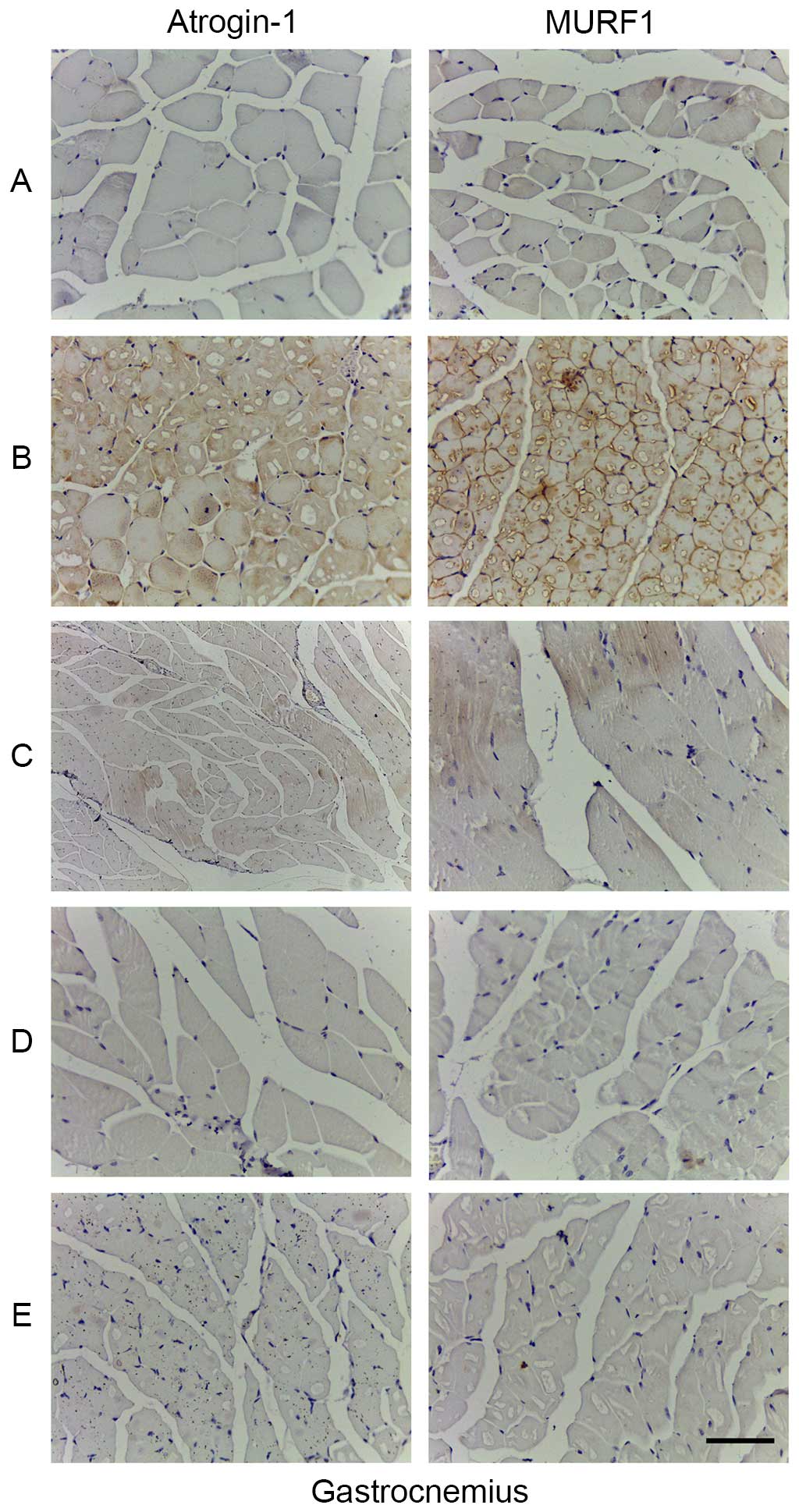
Atrogin 1 and MU-RF1 proteins expression
level detected by immunohistochemistry
Immunohistochemistry demonstrated that atrogin 1 and
MU-RF1 proteins were expressed in the cytoplasm. Specifically, an
increased number of yellow-brown or tan-brown particles were
observed in the gastrocnemius cells of Group B compared with Group
A, which implied that the expression levels of atrogin 1 and MU-RF1
proteins were significantly enhanced. However, an markedly reduced
number of the particles were observed in the gastrocnemius cells of
Group D and E compared with Group B, which implied that the
expression levels of the two proteins were reduced (Fig. 10).
Discussion
The present study demonstrated that when abdominally
injected with H22 hepatic carcinoma cells the animals in the model
groups exhibited the symptoms of intermediate and advanced
cachexia, including rapidly increased body weight, gradually
distended abdomen, mat and fluffy furs, reduced food intake, thin
limbs, lower body temperature, accidie, cowering and panting
respiration, which indicated that the H22 hepatic carcinoma cell
line-induced cachexia model was an effective model for cachexia
research with a short establishment cycle, easy operation and high
tumor formation rate. Treatment with the high concentration of
MJPJD significantly increased food intake compared with the
xenograft tumor group and the low concentration of MJPJD
(P<0.05), however there was no significant difference compared
with MPA combined with IND (P>0.05). The high concentration of
MJPJD significantly reduced body weight that compared with the
xenograft tumor group (P<0.05), and exhibited no significant
difference compared with the control group (P>0.05).
Furthermore, the net weights of animals in the high concentration
of MJPJD group were increased compared with the xenograft tumor
group (P<0.01) and low concentration of MJPJD group (P<0.05).
The high concentration of MJPJD significantly reduced ascites
volume compared with the xenograft tumor group and MPA combined
with IND group (P<0.05). The high concentration of MJPJD
significantly increased the grasping force compared with the
xenograft tumor group (P<0.05). Overall, the high concentration
of MJPJD was demonstrated to improve the food intake, maintain the
net weight, control the rapid increase of ascites, regulate the
metabolic balance and postpone the pathological process of cachexia
in the mouse model of CC.
It was previously reported by Esper et al
(8) that inflammatory cytokines,
including TNF-α, IL-lα, IL-6 and interferon-γ are crucial for the
pathological process of cachexia, and directly influenced the
appetite and metabolism of patients. MPA was observed to interfere
with the synthesis of progesterone, which was demonstrated to
promote appetite, increase body weight and improve the nutrition in
cancer-anorexia-cachexia syndrome (10,11)
by inhibiting the secretion of the inflammatory cytokines IL-6 and
TNF-α (12,13), thus has been commonly used for the
treatment of cancer-associated anorexia and cachexia, and is the
only recommended medication for cachexia in Europe. IND is a
non-steroidal anti-inflammatory drug proven to alleviate chronic
inflammatory reactions and cancer-associated cachexia to
significantly prolong the survival time and improve the life
quality of the patients with metastatic solid tumors (14), the mechanisms of IND have been
previously attributed the inhibition of myoprotein proteolysis
(15), suppressing the synthesis
of cytokines and synthesis and release of prostaglandin E2
(16). MPA combined with IND was
used as the positive control group in the current study, and the
results demonstrated that the high and low concentrations of MJPJD,
and MPA combined with IND all significantly reduced the expression
levels of IL-lα, IL-6 and TNF-α in the ascites (P<0.01), among
which the effects of high concentration of MJPJD on decreasing the
levels of IL-lα, IL-6 were greater than that of low concentration
(P<0.05). However, levels following treatment with the high
concentration of MJPJD were not significantly different compared
with MPA combined with IND. These findings indicated that the
anti-cachexia effects of MJPJD were potentially associated with the
downregulation of the expression levels of the inflammatory
cytokines, IL-lα, IL-6 and TNF-α.
The UPP was identified to be an important pathway
for selective proteolysis of intracellular proteins. Substrate
proteins are transformed to the ubiquitinated form when the
ubiquitin molecules form a polyubiquitin chain by bioconjugation
with the target proteins through the ubiquitin-activating enzyme,
ubiquitin-conjugating enzyme and ubiquitin-protein ligase. The
target proteins are recognized and proteolysis is performed by the
26S proteasome. Among these enzymes the ubiquitin-protein ligase E3
(UPL-E3) was previously demonstrated to be a crucial enzyme in the
UPP that determines the specificity and speed of the UPP. MU-RF1
and atrogin 1 are the muscle-specific UPL-E3 expressed in the
muscles only. Baracos et al (17) previously established a rat model of
ascites-induced hepatic carcinoma cachexia, in which rats with
tumors demonstrated increased degradation of skeletal muscles and
proteolytic rate, and when the UPP was blocked the proteolysis
returned to normal levels. Other previous studies (18,19)
have demonstrated following abdominal injection rats with
ascites-associated hepatoma carcinoma cells, the weight of the
gastrocnemius and extensor digitorum longus were reduced by
>30%, and the expression levels atrogin 1 and MU-RF1 mRNAs in
gastrocnemius were significantly higher compared with the control
group, which demonstrated that the muscular atrophy of the
cancer-associated cachexia was associated with to the activation of
UPP. In the current study, the expression levels of atrogin 1 and
MU-RF1 protein and mRNA in the high concentration MJPJD group were
significantly lower compared with the xenograft tumor group
(P<0.01) and the low concentration of MJPJD group (P<0.05),
however the levels were similar to those in the MPA combined with
IND group (P>0.05). This indicated that the administration of
the high concentration of MJPJD significantly downregulated the
expression level of atrogin 1 and MU-RF1 protein and mRNA to
inhibit the activation of UPP, by which the proteolysis of
myoproteins may be reduced and the symptoms of patients with
cancer-associated cachexia may be alleviated. Accordingly, the
anti-cachexia effect of MJPJD is potentially attributed to its
action on inhibiting the activation of UPP.
The current study demonstrated that MJPJD exerted
effects at the molecular levels, such as expression of cytokines,
MU-RF1 and atrogin 1, and also effected ascites, body weight,
maintenance of net weight, body temperature and food intake. The
effects were comparable with those of the MPA combined with IND
treatment. Consequently, MJPJD demonstrated anti-cachexia activity
by downregulating the levels of cytokines and inhibiting the
activation of UPP, which reflected the comprehensive treatment
effect of the Chinese herbal compound characterized by the
multi-level, multi-target and multi-level functions. Further
research is required to establish mechanisms of the anti-cachexia
effect of MJPJD.
Acknowledgments
This research was supported by grants from the
National Natural Science Foundation of China for Young Scholar
(grant no. 81102581), the National Natural Science Foundation of
China (grant no. 81373500) and the Administration of Traditional
Chinese Medicine of Guangdong Province (grant no. 20111161).
References
|
1
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mondello P, Mian M, Aloisi C, Famà F,
Mondello S and Pitini V: Cancer cachexia syndrome: Pathogenesis,
diagnosis, and new therapeutic options. Nutr Cancer. 67:12–26.
2015. View Article : Google Scholar
|
|
3
|
Wallengren O, Lundholm K and Bosaeus I:
Diagnostic criteria of cancer cachexia: Relation to quality of
life, exercise capacity and survival in unselected palliative care
patients. Support Care Cancer. 21:1569–1577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von Haehling S and Anker SD: Treatment of
cachexia: An overview of recent developments. J Am Med Dir Assoc.
15:866–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Radbruch L, Elsner F, Trottenberg P,
Strasser F and Fearon K: Clinical practice guidelines on cancer
cachexia in advanced cancer patients. Aachen: Department of
Palliative Medicinen/European Palliative Care Research
Collaborative; 2010
|
|
6
|
Yin LR, Chen ZX, Zhang SJ, Sun BG, Liu YD
and Huang HZ: Expression of phosphatase and tensin homolog deleted
on chromosome ten in liver of athymic mice with hepatocellular
carcinoma and the effect of Fuzheng Jiedu Decoction. World J
Gastroenterol. 14:108–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun BG, Chen ZX, Zhou HM, Zhang SJ and
Huang H: Aftereffect of invigorating spleen and soothing liver and
detoxification and dissipating masses compound recipe of Chinese
crude drug to athymic mice with HCC. Chinese Archives of
Traditional Chinese Medicine. 28:1666–1670. 2010.
|
|
8
|
Esper DH and Harb WA: The cancer cachexia
syndrome: A review of metabolic and clinical manifestations. Nutr
Clin Pract. 20:369–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiefer T, Hirt C, Schüler F, Busemann C,
Wodny M, Jaeger B and Dölken G: Statistical analysis of results
obtained by real-time PCR for improvement of absolute
quantification of target sequences. Clin Lab. 58:465–470.
2012.PubMed/NCBI
|
|
10
|
Gullett NP, Hebbar G and Ziegler TR:
Update on clinical trials of growth factors and anabolic steroids
in cachexia and wasting. Am J Clin Nutr. 91(Suppl): 1143S–1147S.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diament MJ, Peluffo GD, Stillitani I,
Cerchietti LC, Navigante A, Ranuncolo SM and Klein SM: Inhibition
of tumor progression and paraneoplastic syndrome development in a
murine lung adenocarcinoma by medroxyprogesterone acetate and
indomethacin. Cancer Invest. 24:126–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamashita JI and Ogawa M:
Medroxyprogesterone acetate and cancer cachexia: Interleukin-6
involvement. Breast Cancer. 7:130–135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith KL and Tisdale MJ: Mechanism of
muscle protein degradation in cancer cachexia. Br J Cancer.
68:314–318. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lundholm K, Gelin J, Hyhander A, Lönnroth
C, Sandström R, Svaninger G, Körner U, Gülich M, Kärrefors I, Norli
B, et al: Anti-inflammatory treatment may prolong survival in
undernourished patients with metastatic solid tumors. Cancer Res.
54:5602–5606. 1994.PubMed/NCBI
|
|
15
|
Ferreri NR, McGiff JC, Carroll MA and
Quilley J: Renal COX-2, cytokines and 20-HETE: Tubular and vascular
mechanisms. Curr Pharm Des. 10:613–626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Argilés JM, Almendro V, Busquets S and
López-Soriano FJ: The pharmacological treatment of cachexia. Curr
Drug Targets. 5:265–277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baracos VE, DeVivo C, Hoyle DH and
Goldberg AL: Activation of the ATP-ubiquitin-proteasome pathway in
skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol.
268:E996–E1006. 1995.PubMed/NCBI
|
|
18
|
Llovera M, Garcia-Martinez C, Agell N,
Lopez-Soriano FJ and Argiles JM: Muscle wasting associated with
cancer cachexia is linked to an important activation of the
ATP-dependent ubiquitin-mediated proteolysis. Int J Cancer.
61:138–141. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costelli P, Muscaritoli M, Bossola M,
Penna F, Reffo P, Bonetto A, Busquets S, Bonelli G, Lopez-Soriano
FJ, Doglietto GB, et al: IGF-1 is downregulated in experimental
cancer cachexia. Am J Physiol Regul Integr Comp Physiol.
291:R674–R683. 2006. View Article : Google Scholar : PubMed/NCBI
|