Introduction
Preeclampsia is a major disorder of pregnancy and a
leading contributor to maternal and perinatal morbidity and
mortality rates (1). Although
reduced trophoblast proliferation, aberrant trophoblast
differentiation, limited migration and invasion of trophoblasts in
the uterus, poor remodeling of spiral arteries, and excessive
apoptosis have been associated with preeclampsia (2–5), the
molecular mechanisms underlying the onset and progression of
preeclampsia remain to be fully elucidated, therefore, it is
important to investigate these molecular mechanisms of
preeclampsia.
The transforming acidic coiled-coil protein (TACC)
family is characterized by a conserved C-terminal 'TACC domain'
(6). In mammals, three TACC
proteins are expressed from three genes, TACC1, TACC2 (also known
as AZU-1 and ECTACC) and TACC3 (also known as AINT and ERIC1)
(7). During mitosis, the TACC
proteins localize around the centrosomes and are important for the
organization of microtubule organizing centers (8). TACC3, a member of the TACC domain
family, was first identified as a microtubule-binding protein
(9). It has been reported that the
depletion of TACC3 causes growth retardation and embryonic
mortality in mice due to increased apoptosis (10); and TACC3 promotes axon elongation
and regulates microtubule plus end dynamics in multiple embryonic
cell types (11). In addition,
several studies have demonstrated that TACC3 can promote cell
proliferation, metastasis and invasion, and regulate cell cycle
progression and differentiation in a variety of cancer cells
(12–14). However, the role of TACC3 in
trophoblast function during placental development remains to be
fully elucidated. The present study aimed to determine the
expression and function of TACC3 in human placentas and to examine
the underlying mechanisms. The results demonstrated that TACC3
induced trophoblast cell growth, migration and invasion by
activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling
pathway. TACC3 may serve as a novel potential target in the
treatment of preeclampsia.
Materials and methods
Tissue specimens
Human placental tissues at 6–8 weeks were obtained
from 10 healthy women undergoing cesarean section for non-medical
reasons; normal term and preeclamptic placentas were collected
following cesarean section. The fresh tissue specimens were
immediately snap-frozen and stored in liquid nitrogen until use.
The protocol for the use of patient samples was approved by the
Ethics Committee of the School of Medicine, Zhejiang University
(Zhejiang, China) and written informed consent was obtained from
each patient.
Cell culture
The immortalized first trimester trophoblast cell
line, HTR8/SVneo, was obtained from American Type Culture
Collection (Manassas, VA, USA). The cells were cultured in RPMI
1640 medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 100 U/ml penicillin and 100 mg/ml
streptomycin (Sigma-Aldrich; Thermo Fisher Scientific, Inc.) in the
presence of 10% fetal bovine serum (Sigma-Aldrich; Thermo Fisher
Scientific, Inc.). The cells were cultured at 37°C in a 5%
CO2 incubator.
Small interfering (si)RNA knockdown of
TACC3
The siRNA against TACC3 (siRNA-TACC3) and the
control (scramble) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). The HTR8/SVneo cells were transfected
with either siRNA-TACC3 or scramble siRNA using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the HTR8/SVneo cells
using TRIzol reagent (Abcam, Cambridge, UK) according to the
manufacturer's protocol. cDNA was synthesized from the extracted
RNA (4 µg) using the EasyScript First-Strand cDNA Synthesis
SuperMix kit (Invitrogen; Thermo Fisher Scientific, Inc.). The
RT-qPCR was performed in a final volume of 10 µl, containing
5 µl of SsoFast™ EvaGreen Supermix (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), 1 µl cDNA (1:50 dilution), and 2
µl each of the forward and reverse primers (1 mM) with a
Bio-Rad iQ5 Quantitative PCR System (Bio-Rad Laboratories, Inc.).
PCR amplification was performed using the following primers: TACC3,
forward 5′-GAACTGGGGAAGATCATGGA-3′ and reverse
5′-CTCTTCGTTCTTGCGGTAGC-3′; and β-actin, forward
5′-TTAGTTGCGTTACACCCTTTC-3′ and reverse 5′-ACCTTCACCGTTCCAGTTT-3′.
The PCR conditions consisted of an initial preincubation step at
95°C for 30 sec, followed by 39 cycles at 95°C for 5 sec and 60°C
for 30 min. The relative mRNA levels were calculated using the
2−ΔΔCq method (15).
Western blot analysis
Cell lysate was prepared from the HTR8/SVneo cells
using lysis buffer (Cell Signaling Technology, Inc., Danvers, MA,
USA). Proteins were separated by centrifugation at 6,000 × g for 15
min at 4°C and the supernatant was collected. Protein concentration
was determined by the bicinchoninic acid assay. The proteins (20–30
µg per lane) were separated by 10% SDS-PAGE and transferred
onto a nitrocellulose membrane (EMD Millipore, Boston, MA, USA).
The membranes were blocked with 5% fat-free milk in
phosphate-buffered saline with Tween 20 (PBST) at room temperature
for 1 h, followed by incubation with primary antibodies (all from
Santa Cruz Biotechnology, Inc.) overnight at 4°C. The primary
antibodies were as follows: Mouse monoclonal anti-TACC3 (1:3,000;
cat. no. sc-376883), mouse monoclonal anti-matrix
metalloproteinase-2 (MMP-2; 1:1,000; cat. no. sc-13594), mouse
monoclonal anti-MMP-9 (1:2,500; cat. no. 21733), mouse monoclonal
anti-tissue inhibitor of metalloproteinase 1 (TIMP1; 1:1,500; cat.
no. sc-365905), mouse monoclonal anti-TIMP2 (1:1,500; cat. no.
21735), rabbit polyclonal anti-phosphorylated PI3K (1:2,000; cat.
no. 293115), mouse monoclonal anti-PI3K (1:2,000; cat. no. 365290),
rabbit polyclonal anti-phosphorylated Akt (1:2,000; sc-135650),
mouse monoclonal anti-Akt (1:2,000; cat. no. sc-5298) or mouse
monoclonal anti-GAPDH (1:1,500; cat. no. sc-365062). The membrane
was washed five times with PBST buffer. Goat anti-mouse (1:3,000;
cat. no. sc-2302) and goat anti-rabbit (1:3,000; cat. no. sc-2054)
horseradish peroxidase-conjugated secondary antibodies were added
and incubated at room temperature for 1 h. The protein bands were
evaluated using enhanced chemiluminescence (Thermo Fisher
Scientific, Inc.).
Cell proliferation assay
The HTR8/SVneo cells were plated in 96-well plates
at a density of ~1×104 cells/well. The cells were
transfected with siRNA-TACC3 or scramble. Following incubation for
24 h at 37°C, 20 ml of 5 mg/ml
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT;
Sigma-Aldrich; Thermo Fisher Scientific, Inc.) was added to the
medium and incubated for 4 h at 37°C. The formazan was dissolved in
dimethylsulfoxide (150 µl/well; Sigma-Aldrich; Thermo Fisher
Scientific, Inc.) for 10 min. The absorbance was measured at 570
nm.
Matrigel invasion assay and Transwell
migration assay
Cell invasion was determined by the ability of the
cells to cross the 8-mm pores of polycarbonate membranes (6.5-mm
filter; 8-mm pore size; Corning Costar, Inc., Corning, NY, USA). In
brief, the HTR8/SVneo cells (1.0×105 cells/well)
transfected with siRNA-TACC3 or scramble were plated in the upper
chambers, and 600 µl of RPMI-1640 medium containing 10%
fetal bovine serum was added to the lower chamber. Following
incubation for 24 h under normal conditions, the cells on the upper
surface of the base membrane were removed with a sterile cotton
swab. The cells, which had transferred to the lower surface of the
base membrane were stained with hematoxylin and eosin
(Sigma-Aldrich; Thermo Fisher Scientific, Inc.) and the numbers of
cells were counted under a Leica microscope (Leica Microsystems
GmbH, Wetzlar, Germany). The methods utilized in the Transwell
migration assays were similar to those used in the cell invasion
assays, with the exception that the inserts were not pre-coated
with Matrigel.
Statistical analysis
Data are presented as the mean ± standard deviation.
The differences were analyzed using Student's t-test or
one-way analysis of variance and Student's t-test using SPSS
13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of TACC3 is decreased in
preeclamptic placentas
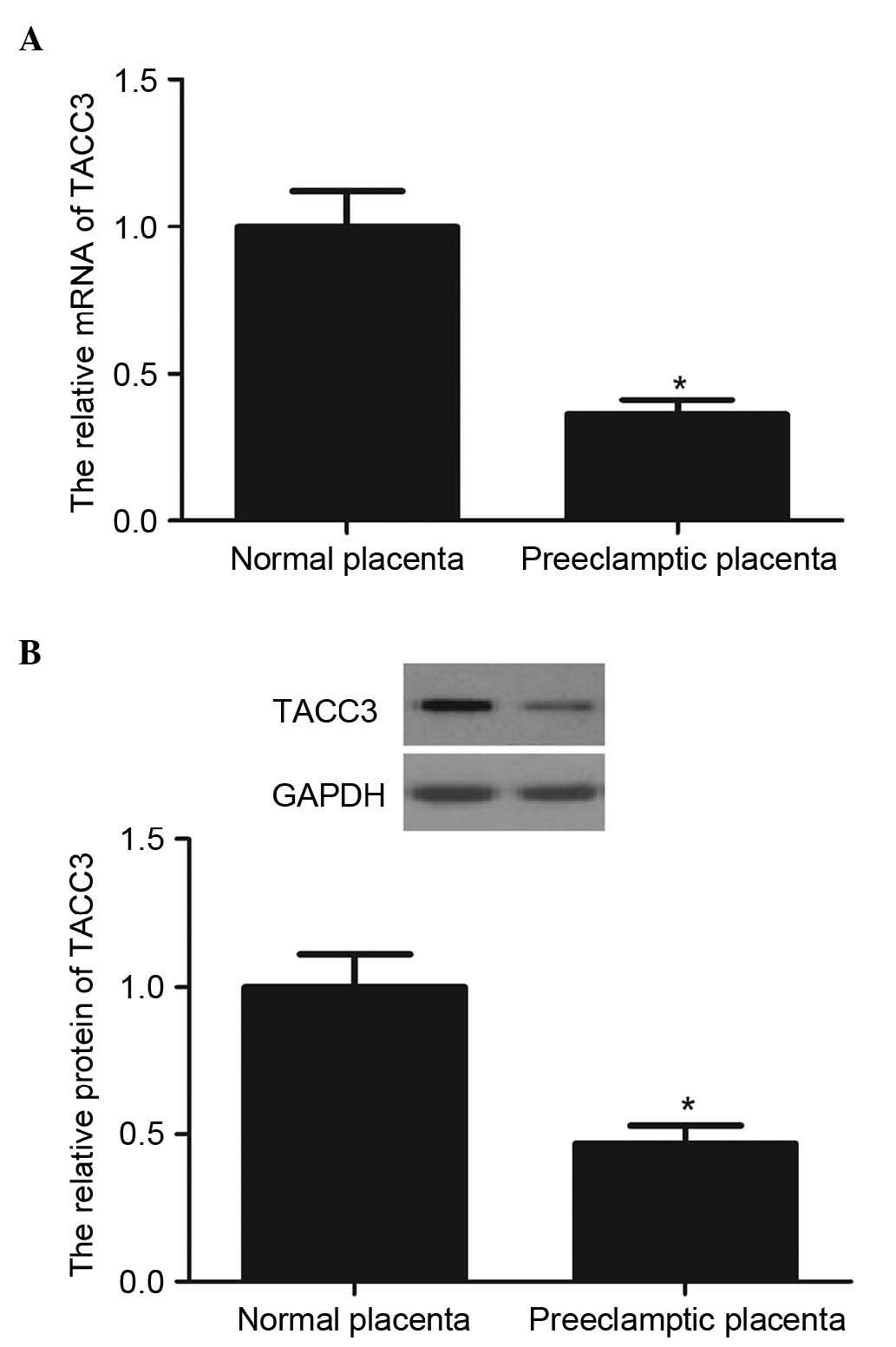
The present study first detected the mRNA levels of
TACC3 in the preeclamptic placentas using RT-qPCR. As shown in
Fig. 1A, the mRNA levels of TACC3
in the preeclamptic placentas were markedly lower, compared with
those in the normal placentas. Western blot analysis showed that
the protein levels of TACC3 were also significantly lower in the
preeclampsia placentas, compared with the control group (Fig. 1B). These results suggested that the
expression of TACC3 was reduced in preeclamptic placentas.
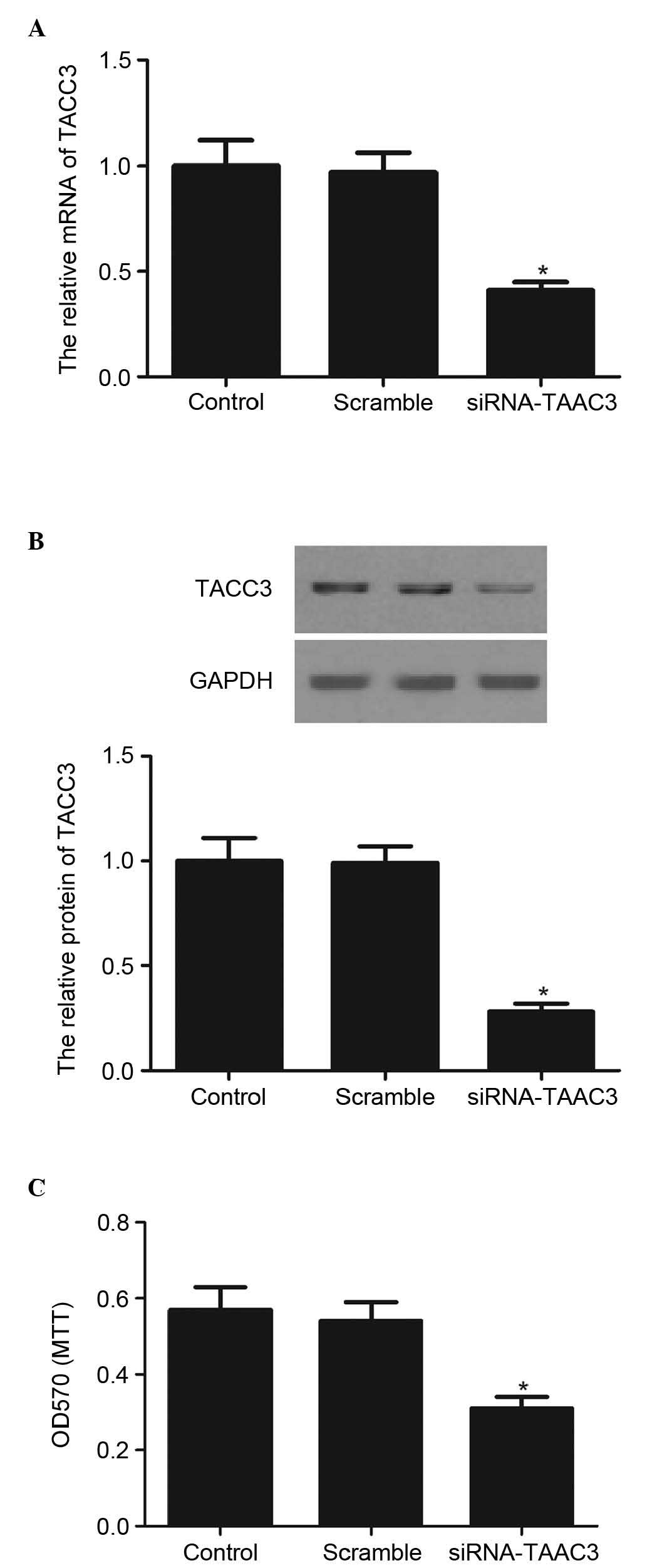
Silencing TACC3 significantly inhibits
the proliferation of HTR8/SVneo cells
To examine the functional roles of TACC3 in the
human placenta, HTR8/SVneo cells were transfected with siRNA-TACC3
or scramble. As shown in Fig. 2A,
the mRNA expression of TACC3 was significantly decreased in the
HTR8/SVneo cells transfected with siRNA-TACC3, compared with the
scramble group. The knockdown of TACC3 also reduced the protein
expression of TACC3 (Fig. 2B). An
MTT assay was then used to measure the effect of TACC3 on
HTR8/SVneo cell proliferation. As shown in Fig. 2C, knockdown of TACC3 significantly
inhibited the proliferation of the HTR8/SVneo cells, compared with
the scramble group.
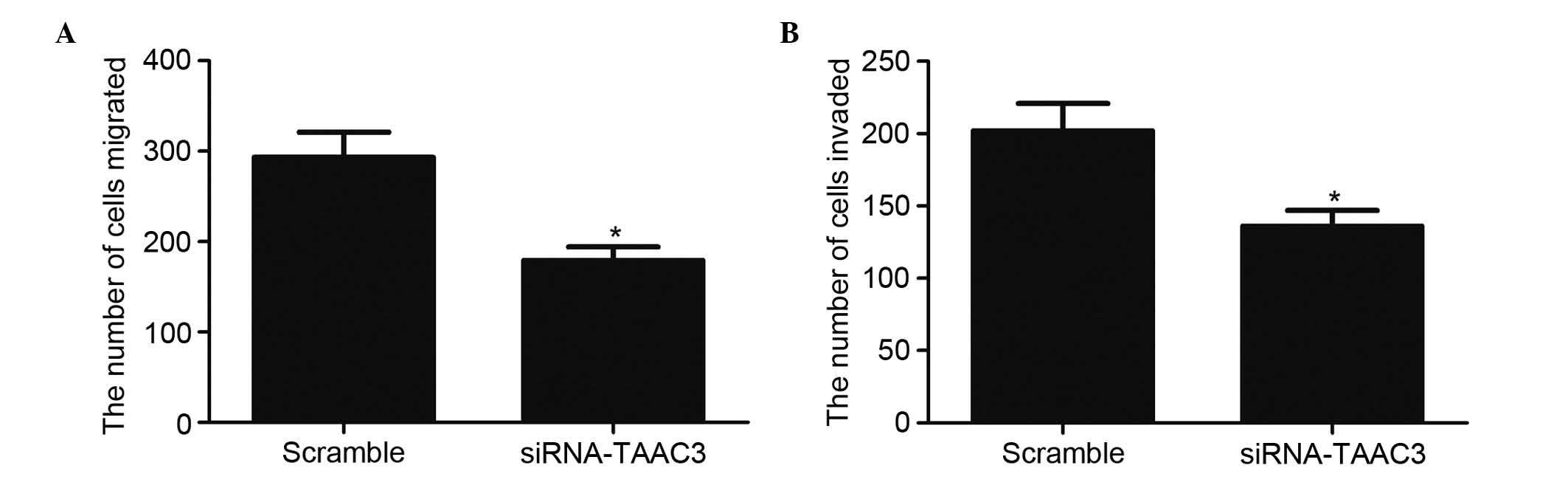
Silencing TACC3 significantly inhibits
the migration and invasion of HTR8/SVneo cells
The migration and invasion of trophoblast cells is
important in the process of preeclampsia. Therefore, the present
study investigated the effect of TACC3 on HTR8/SVneo cell migration
and invasion. As shown in Fig. 3,
compared with the scramble group, knockdown of TACC3 significantly
decreased the percentage of cells that migrated (Fig. 3A) or invaded (Fig. 3B) to the other side of the
filter.
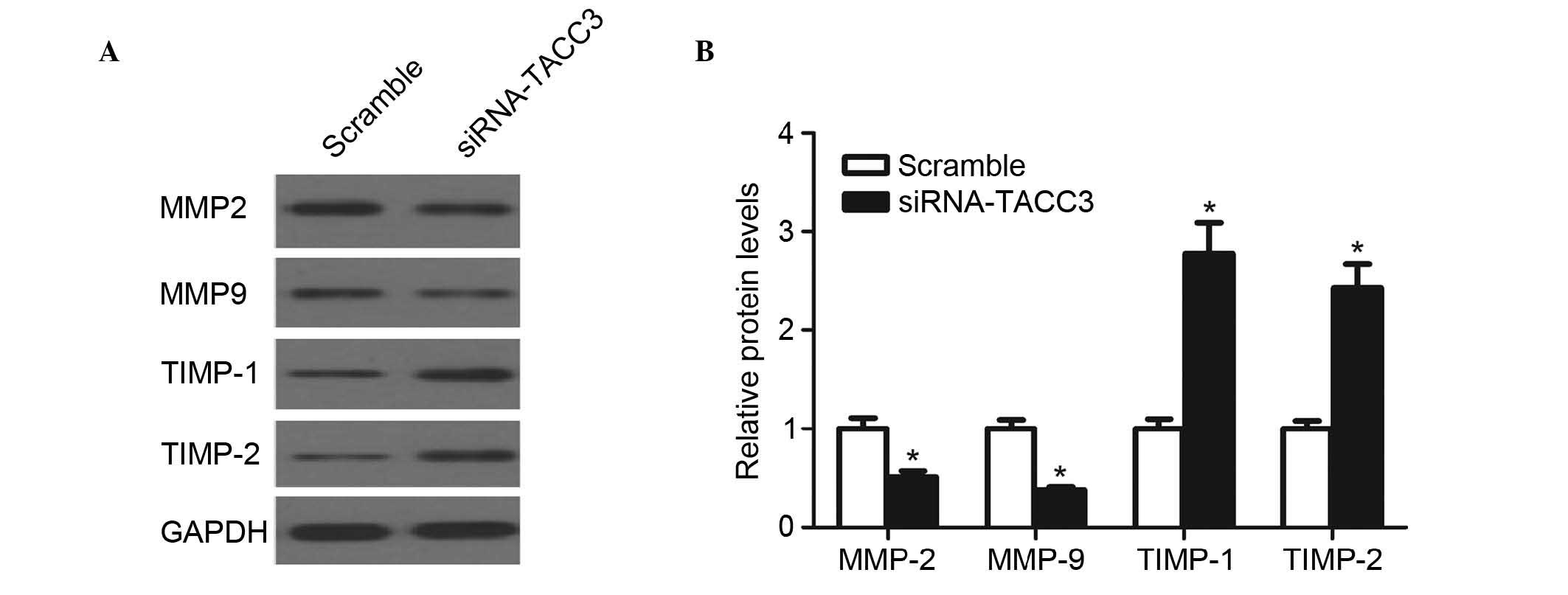
Silencing TACC3 reduces the expression of
MMP2/9 and increases the expression of TIMP1/2 in HTR8/SVneo
cells
It has been reported that MMP2 and MMP9 are critical
in trophoblast invasion by degrading the extracellular matrix (ECM)
(16). Therefore, the present
study determined the effect of TACC3 on the expression of MMPs in
HTR8/SVneo cells. As shown in Fig. 4A
and B, compared with the scramble group, knockdown of TACC3
significantly reduced the expression levels of MMP2 and MMP9. By
contrast, the knockdown of TACC3 significantly increased the
expression levels of TIMP1 and TIMP2 in the HTR8/SVneo cells.
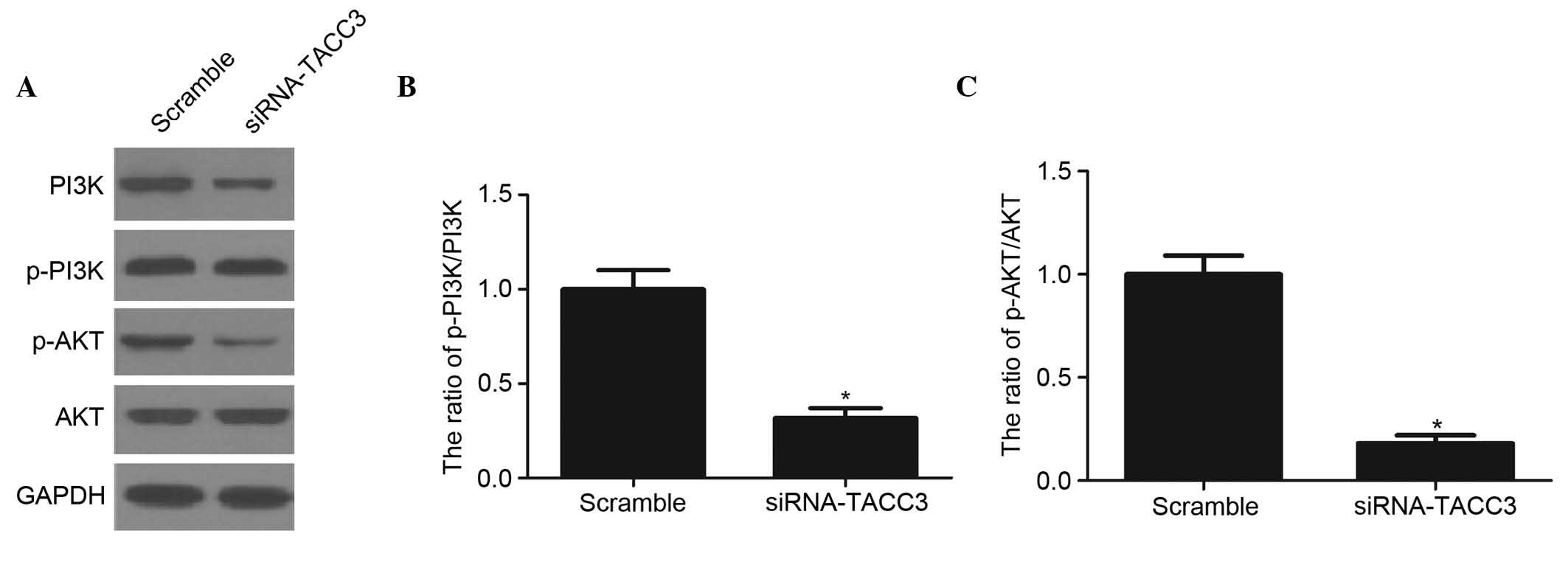
Silencing TACC3 reduces the
phosphorylation levels of PI3K and Akt
The PI3K/Akt pathway is one of the major signaling
pathways associated with trophoblast invasion (17). To examine the involvement of the
signaling pathway in TACC3-induced HTR8/SVneo cell invasion, the
present study evaluated the effects of TACC3 on the PI3K/Akt
signaling pathway. As shown in Fig.
5A–C), compared with the scramble group, the knockdown of TACC3
significantly reduced the levels of phosphorylated PI3K and Akt in
the HTR8/SVneo cells.
Discussion
The present study is the first, to the best of our
knowledge, to demonstrate the expression and function of TACC3 in
human early placental tissues. The results showed that the
expression of TACC3 was downregulated in preeclamptic placentas,
compared with normal placentas. The knockdown of TACC3
significantly inhibited HTR8/SVneo cell proliferation, migration
and invasion, and inhibited the expression of MMPs. In addition,
the knockdown of TACC3 significantly reduced the levels of
phosphorylated PI3K and Akt in the HTR8/SVneo cells.
Trophoblast cells have high rates of cell
proliferation, lack cell-contact inhibition, and are able to evade
effectors of the immune system, particularly during the first
trimester of pregnancy (18). For
trophoblast cells, invasion can ensure that agents can enter the
uterine stroma and gain access to the maternal circulation.
Increasing evidence indicates that the deficient migration and
shallow invasion of trophoblasts may lead to preeclampsia (19–21).
In addition, the invasion/migration properties and the regulatory
mechanisms are similar between trophoblasts and malignant cells
(22). In the present study, the
expression and role of TACC3 in the progression of preeclampsia
were investigated, and it was observed that knockdown of TACC3
significantly inhibited HTR8/SVneo cell proliferation, migration
and invasion. These results are consistent with the previously
reported role of TACC3 in human malignancies (13), and suggest that TACC3 may be
important in the progression of preeclampsia.
MMPs are a family of zinc-dependent endopeptidases
involved in the degradation of the majority of the proteins of the
ECM (23). Previous studies have
reported that MMPs are important in the process of trophoblast
invasion (16,24,25).
It has also been reported that MMP-2 is predominantly expressed in
extravillous trophoblasts in the placenta during the first
trimester (26). In addition,
TIMPs have the ability to inhibit the activity of MMPs in the
extracellular space by binding specifically to active MMPs
(27). In the present study, it
was observed that knockdown of TACC3 significantly inhibited the
expression of MMP-2 and MMP-9, and increased the expression of
TIMP1/2. These results suggested that siRNA-TACC3 may contribute to
the downregulation of the expression of MMP-2 and MMP-9, which may
lead to reduced HTR8/SVneo cell migration and invasion.
Multiple signals have been demonstrated to be
important in trophoblast growth and invasion (28–30).
Among these, the PI3K/Akt signaling pathway is a critical pathway
mediating the growth-factor-dependent regulation of trophoblast
growth and invasion (31). Ha
et al (14) reported that
TACC3 promotes epithelial-mesenchymal transition through activation
of the PI3K/Akt and extracellular signal-regulated kinase signaling
pathways in tumor cells (14). To
further clarify the underlying mechanism involved in
TACC3-inhibited HTR8/SVneo cell growth and invasion, the present
study examined the levels of phosphorylated PI3K and Akt following
siRNA-TACC3 transfection. It was observed that the knockdown of
TACC3 significantly reduced the levels of phosphorylated PI3K and
Akt in the HTR8/SVneo cells. These results suggested that
siRNA-TACC3 inhibited the invasion and downregulated the expression
levels of MMPs in the HTR8/SVneo cells through suppression of the
PI3K/Akt signaling pathway.
Taken together, the results of the present study
demonstrated that the knockdown of TACC3 inhibited the migration
and invasion of HTR8/SVneo cells through suppression of the
PI3K/Akt signaling pathway. Therefore, TACC3 may serve as a novel
potential target in the treatment of preeclampsia.
References
|
1
|
Kanasaki K and Kalluri R: The biology of
preeclampsia. Kidney Int. 76:831–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Redline RW and Patterson P: Pre-eclampsia
is associated with an excess of proliferative immature intermediate
trophoblast. Hum Pathol. 26:594–600. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisher SJ: The placental problem: Linking
abnormal cytotrophoblast differentiation to the maternal symptoms
of preeclampsia. Reprod Biol Endocrinol. 2:532004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui Y, Wang W, Dong N, Lou J, Srinivasan
DK, Cheng W, Huang X, Liu M, Fang C, Peng J, et al: Role of corin
in trophoblast invasion and uterine spiral artery remodelling in
pregnancy. Nature. 484:246–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Myatt L: Role of placenta in preeclampsia.
Endocrine. 19:103–111. 2002. View Article : Google Scholar
|
|
6
|
O'Brien LL, Albee AJ, Liu L, Tao W,
Dobrzyn P, Lizarraga SB and Wiese C: The Xenopus TACC homologue,
maskin, functions in mitotic spindle assembly. Mol Biol Cell.
16:2836–2847. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peset I and Vernos I: The TACC proteins:
TACC-ling microtubule dynamics and centrosome function. Trends Cell
Biol. 18:379–388. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gergely F, Karlsson C, Still I, Cowell J,
Kilmartin J and Raff JW: The TACC domain identifies a family of
centrosomal proteins that can interact with microtubules. Proc Natl
Acad Sci USA. 97:14352–14357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Groisman I, Huang YS, Mendez R, Cao Q,
Theurkauf W and Richter JD: CPEB, maskin, and cyclin B1 mRNA at the
mitotic apparatus: Implications for local translational control of
cell division. Cell. 103:435–447. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piekorz RP, Hoffmeyer A, Duntsch CD, McKay
C, Nakajima H, Sexl V, Snyder L, Rehg J and Ihle JN: The
centrosomal protein TACC3 is essential for hematopoietic stem cell
function and genetically interfaces with p53-regulated apoptosis.
EMBO J. 21:653–664. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nwagbara BU, Faris AE, Bearce EA, Erdogan
B, Ebbert PT, Evans MF, Rutherford EL, Enzenbacher TB and Lowery
LA: TACC3 is a microtubule plus end-tracking protein that promotes
axon elongation and also regulates microtubule plus end dynamics in
multiple embryonic cell types. Mol Biol Cell. 25:3350–3362. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang ZL, Lin ZR, Xiao YR, Cao X, Zhu LC,
Zeng MS, Zhong Q and Wen ZS: High expression of TACC3 in esophageal
squamous cell carcinoma correlates with poor prognosis. Oncotarget.
6:6850–6861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ha GH, Kim JL and Breuer EK: TACC3 is
essential for EGF-mediated EMT in cervical cancer. PLoS One.
8:e703532013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ha GH, Park JS and Breuer EK: TACC3
promotes epithelial-mesenchymal transition (EMT) through the
activation of PI3K/Akt and ERK signaling pathways. Cancer Lett.
332:63–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Cohen M, Meisser A and Bischof P:
Metalloproteinases and human placental invasiveness. Placenta.
27:783–793. 2006. View Article : Google Scholar
|
|
17
|
Qiu Q, Yang M, Tsang BK and Gruslin A:
Both mitogen-activated protein kinase and phosphatidylinositol
3-kinase signalling are required in epidermal growth factor-induced
human trophoblast migration. Mol Hum Reprod. 10:677–684. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferretti C, Bruni L, Dangles-Marie V,
Pecking A and Bellet D: Molecular circuits shared by placental and
cancer cells and their implications in the proliferative, invasive
and migratory capacities of trophoblasts. Hum Reprod Update.
13:121–141. 2007. View Article : Google Scholar
|
|
19
|
Kaufmann P, Black S and Huppertz B:
Endovascular trophoblast invasion: Implications for the
pathogenesis of intrauterine growth retardation and preeclampsia.
Biol Reprod. 69:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davison JM, Homuth V, Jeyabalan A, Conrad
KP, Karumanchi SA, Quaggin S, Dechend R and Luft FC: New aspects in
the pathophysiology of preeclampsia. J Am Soc Nephrol.
15:2440–2448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pijnenborg R, Vercruysse L and Hanssens M:
Fetal-maternal conflict, trophoblast invasion, preeclampsia, and
the red queen. Hypertens Pregnancy. 27:183–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mullen CA: Review: Analogies between
trophoblastic and malignant cells. Am J Reprod Immunol. 39:41–49.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
24
|
Staun-Ram E, Goldman S, Gabarin D and
Shalev E: Expression and importance of matrix metalloproteinase 2
and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol
Endocrinol. 2:592004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hulboy DL, Rudolph LA and Matrisian LM:
Matrix metalloproteinases as mediators of reproductive function.
Mol Hum Reprod. 3:27–45. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Isaka K, Usuda S, Ito H, Sagawa Y,
Nakamura H, Nishi H, Suzuki Y, Li Y and Takayama M: Expression and
activity of matrix metalloproteinase 2 and 9 in human trophoblasts.
Placenta. 24:53–64. 2003. View Article : Google Scholar
|
|
27
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prast J, Saleh L, Husslein H, Sonderegger
S, Helmer H and Knöfler M: Human chorionic gonadotropin stimulates
trophoblast invasion through extracellularly regulated kinase and
AKT signaling. Endocrinology. 149:979–987. 2008. View Article : Google Scholar
|
|
29
|
Chakraborty C, Gleeson LM, McKinnon T and
Lala PK: Regulation of human trophoblast migration and
invasiveness. Can J Physiol Pharmacol. 80:116–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fitzgerald JS, Poehlmann TG, Schleussner E
and Markert UR: Trophoblast invasion: The role of intracellular
cytokine signalling via signal transducer and activator of
transcription 3 (STAT3). Hum Reprod Update. 14:335–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Knöfler M and Pollheimer J: IFPA Award in
Placentology lecture: Molecular regulation of human trophoblast
invasion. Placenta. 33(Suppl): S55–S62. 2012. View Article : Google Scholar :
|