Introduction
Osteosarcoma is the most widespread type of
malignant bone tumor in children and teenagers, which accounts for
~2.4% of all pediatric cancers and ~20% of all primary bone
malignancies. Osteosarcoma has been described to be more
predominant in males compared with females, with the highest rates
of occurrence during adolescence (1,2).
Chemotherapy and surgery are the main therapies used in the
management of osteosarcoma; however, there are several difficulties
associated with the high dosages of chemotherapeutic agents that
patients receive. A number of patients with osteosarcoma do not
show any response to chemotherapy due to the development of
multidrug resistance in cancer cells. In addition, there are
several serious side effects associated with chemotherapy,
including decreased renal function and gonadal and cardiac
dysfunction (3–5). Consequently, there is an urgent
requirement for the identification of novel chemotherapeutic agents
with enhanced activity and less likelihood of resistance for the
treatment of osteosarcoma. The main aim of cancer drug research is
to develop therapeutic agents that are effective, safe and
affordable. An integrative approach for managing a patient with
osteosarcoma should target the numerous biochemical and
physiological pathways that support tumour development and minimize
normal-tissue toxicity. In the past 50 years, a large number of
plant-derived bioactive compounds have been isolated that are now
used to treat different types of malignant cancer. A number of
studies have focused on natural products extracted from Chinese
medicinal herbs as anticancer agents in cancer therapy. Over 60% of
the current anticancer drugs originate from natural sources. Nature
continues to be the most productive source of biologically active
and diverse chemotypes (6).
Potentilla Linnaeus (L.) is one of a hundred
genera in the rose family (Rosaceae), subfamily Rosoideae, tribe
Potentilleae (7). The genus name
Potentilla derives from the Latin diminutive of potens
meaning 'powerful' in reference to the medicinal properties of
certain species. The genus Potentilla includes ~500 species
of perennial, biennial, and annual herbs and small shrubs with
rhizomes. In Chinese traditional medicine Potentilla
extracts have been used to treat diarrhea, hepatitis, rheuma and
scabies, as well as a remedy for detoxification (8,9).
Different parts of Potentilla chinensis have been used in
oriental medicine against diseases, such as dysentery and
carbunculosis. Pharmacological studies on Potentilla
chinensis revealed that it has hypoglycemic and
anti-inflammatory activities (10,11).
Chinese authors have attributed significant anticancer activity to
a number of triterpenoid compounds isolated from the aerial parts
of Potentilla chinensis and the roots of Potentilla
multicaulis, which were evaluated for their in vitro
cytotoxic activities against SMMC-7221 human hepatoma and HL-60
human promyelocytic leukemia cells (12,13).
Various previously published phytochemical reports on Potentilla
chinensis have revealed the presence of various triterpenes,
such as 3-hydroxy-11-ursen-28, 13-olide, 11,12-dehydroursolic acid
lactone, 3-O-acetyl pomolic acid, betulinic acid,
3-oxo-12-ursen-28-oic acid, ursolic acid and oleanic acid (14).
The present study aimed to determine the anticancer
effects of the ethanol extract of the roots of Potentilla
chinensis against MG63 osteosarcoma cancer cells by
investigating its effects on apoptosis induction, cell cycle
arrest, inhibition of cell migration and DNA damage, which to the
best of our knowledge constitutes the first such report on this
plant species.
Materials and methods
Plant material and extraction
procedure
Potentilla chinensis was collected during
July–August 2014 from a local region of Henan, China. The plant
material was confirmed by a well-known taxonomist. The roots of
Potentilla chinensis were thoroughly washed with tap water,
shade dried and then chopped into small pieces. Ethanol (95%) was
used for hot extraction, which was conducted for 3 h using a
soxhlet extraction apparatus. The extract was then concentrated
under reduced pressure in a rotary evaporator at 45°C and was then
kept in a refrigerator at 4°C prior to use.
Chemicals and reagents
RPMI-1640 growth medium (Hangzhou Sijiqing
Biological Products Co., Ltd., Hangzhou, China), minimum essential
medium (MEM), fetal calf serum (Gibco, Thermo Fisher Scientific,
Inc., Waltham, MA, USA), trypsin, penicillin, MTT, streptomycin,
dimethyl sulfoxide (DMSO) and phosphate-buffered saline (PBS) were
used in this study. The MTT kit was obtained from Roche Diagnostics
(Indianapolis, IN, USA). Annexin V-Fluorescein Isothiocyanate
(FITC)-Propidium Iodide (PI) Apoptosis Detection kit was purchased
from Sigma-Aldrich (St. Louis, MO, USA). Hoechst dye was purchased
from Sigma-Aldrich. All other chemicals and solvents used were of
the highest purity grade. Cell culture plastic ware was purchased
from BD Biosciences (San Jose, CA, USA).
Cell lines and culture conditions
The MG63 human osteosarcoma cell line and fR-2
normal epithelial cell line were obtained from Shanghai Institute
of Cell Resource Center of Life Science (Shanghai, China). All
cells were grown in a humidified 5% CO2 atmosphere at
37°C in an incubator, and cultured in RPMI-1640 medium supplemented
with 10% heat-inactivated newborn calf serum, 100 IU/ml penicillin
and 100 µg/ml streptomycin.
Analysis of cell viability using an MTT
assay
Inhibition of cell proliferation of the extract was
measured using an MTT assay. Briefly, MG63 and fR-2 cells were
plated in 96-well culture plates (1×105 cells/well)
separately. After 24 h incubation, cells were treated with the
ethyl acetate extract of Potentilla chinensis (EEPC) (0, 5,
10, 20, 40, 80 and 150 µg/ml, eight wells per concentration)
for 24 or 48 h. MTT solution (10 mg/ml) was then added to each
well. After 4 h incubation, the formazan precipitate was dissolved
in dimethyl sulfoxide (100 µl) and then the absorbance was
measured in an enzyme-linked immunosorbent assay reader (Thermo
Molecular Devices Co., Union City, CA, USA) at 570 nm. The cell
viability ratio was calculated by the following formula: Inhibitory
ratio (%) = (OD control−OD treated) / (OD control) × 100.
Cell morphological study by phase
contrast microscopy
Phase contrast microscopy was conducted to assess
the morphological alterations in the MG63 osteosarcoma cancer
cells. The cells were incubated for 24 h and treated with EEPC
extract at various concentrations (0, 40, 80 and 150 µg/ml).
Control cells treated with 0.5% DMSO alone were also included. The
morphological changes, characteristic of apoptosis or necrosis,
were observed and the images were captured under an inverted light
microscope (Olympus America, Inc., Center Valley, PA, USA) after 24
and 48 h.
Annexin V binding assay/quantification of
apoptotic cell death
To establish and confirm cells undergoing apoptosis,
an Annexin V binding assay was performed through flow cytom-etry.
Briefly, MG63 cancer cells were treated with the EEPC extract (0,
40, 80 and 150 µg/ml) for 48 h, and then treated and
untreated cells were harvested by trypsinization. Harvested cells
were then incubated in Annexin V-FITC (50 ng/ml) and PI (50
µg/ml), at room temperature for 20 min in the dark, and
analyzed using a FACS Calibur flow cytometer (BD Biosciences)
taking a minimum of 20,000 cells in each sample.
Cell cycle analysis
MG63 cells (5×106) were seeded in 60-mm
dishes and subjected to various concentrations (0, 40, 80 and 150
µg/ml) of the EEPC for 48 h. Floating and adherent cells
were collected by trypsinization and washed three times with PBS.
Cells were incubated in 70% ethanol at −20°C overnight, treated
with 20 µg/ml RNase A and stained with 2.0 µg/ml PI.
Finally the stained cells were analyzed by Flow cytometry at a
wavelength of 488 nm using a FACS Calibur instrument (BD
Biosciences) equipped with CellQuest software, version 3.3 (BD
Biosciences).
DNA fragmentation analysis following EEPC
treatment
The MG63 cells (1×105 cells/dish) were
plated in a 6-cm dish and then subjected to treatment with various
concentrations (0, 40, 80 and 150 µg/ml) of the EEPC for 48
h. After drug treatment, the cells were washed with ice-cold PBS
and resuspended in lysis buffer (25 mM Tris-HCl, pH 7.4, 5 mM EDTA
and 0.6% SDS) with 1.0 mg/ml RNase A for 15 min at 50°C. Proteinase
K was added and the cells were incubated overnight. Separation of
DNA was conducted using 2% agarose gel and detected under UV light
after staining with ethidium bromide.
In vitro wound healing assay
This assay was performed using a standard method
(10). Cells (1×105
cells/ml) were seeded in a 6-well plate and incubated at 37°C until
a monolayer of 95–100% confluent cells was obtained. Subsequent to
12 h of starvation, a 500 ml pipette tip was used to create a
straight cell-free wound. Each well was washed twice with PBS to
remove any debris and then exposed to various concentrations of
EEPC extract (0, 40, 80 and 150 µg/ml) in a medium. After 48
h of incubation, the cells were fixed and stained with 5% ethanol
containing 0.5% crystal violet powder for 20 min, and randomly
selected fields were photographed under a light microscope. The
number of cells that migrated into the scratched area were
counted.
Statistical analysis
All data were derived from at least three
independent experiments. The results are expressed as the mean ±
standard deviation. Differences between groups were analyzed using
Student's t-test. GraphPad Prism, version 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA) was used for statistical analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
EEPC exhibits potent and selective
anticancer activity
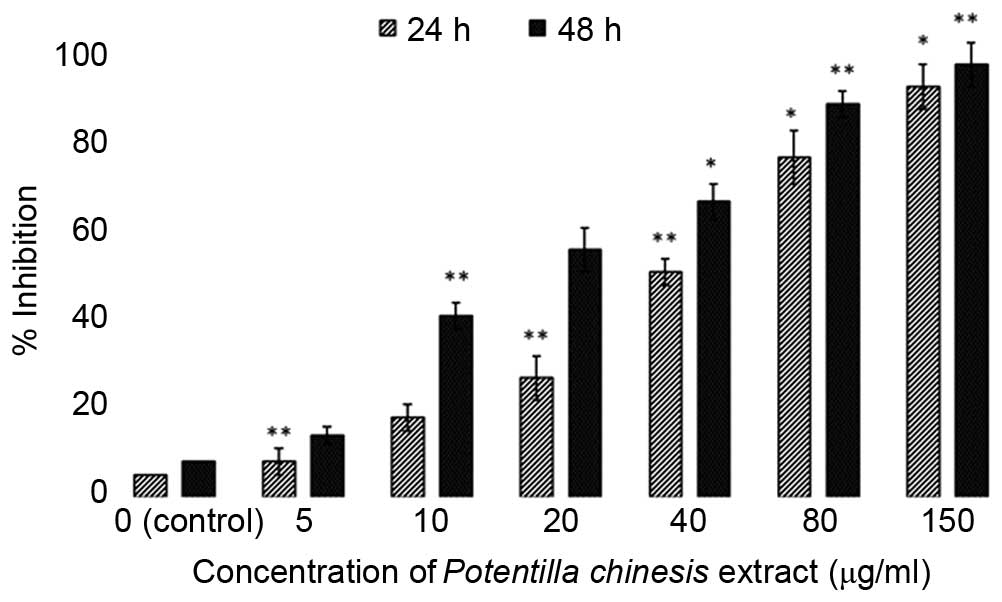
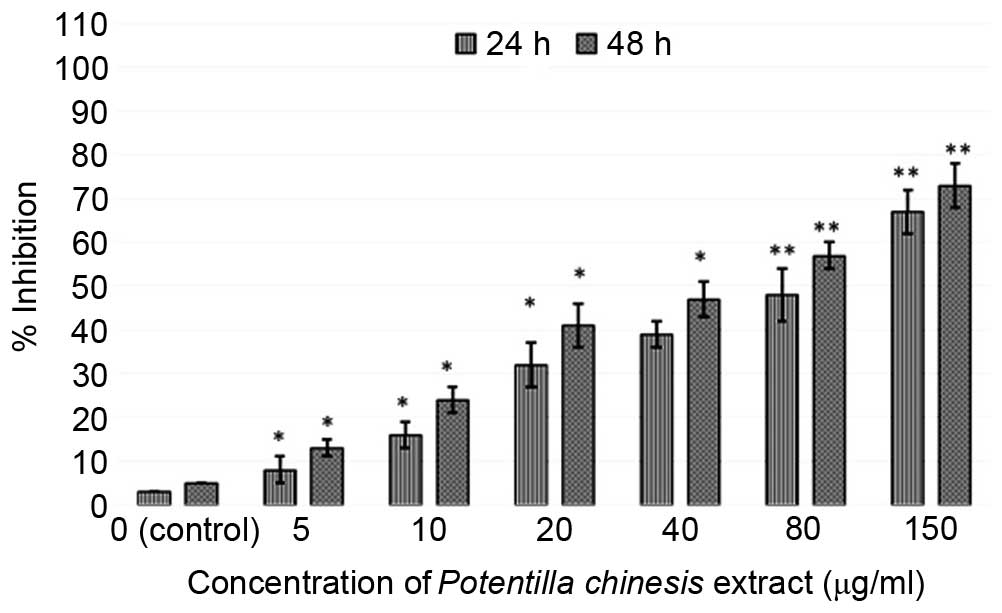
The EEPC was evaluated for antiproliferative
activity using the MTT assay against the MG63 human osteosarcoma
cancer cells and an fR-2 normal cell line (epithelial cell line)
for 24 and 48 h (Figs. 1 and
2). The extract exhibited potent,
dose-dependent and selective cytotoxic activity against MG63 cancer
cells. With the aim of investigating the toxic effects of the
extract on normal cells, the cytotoxic effects of the extract
against fR-2 normal epithelial cells was also assessed (Fig. 2). The results showed that the
extract resulted in decreased cyto-toxicity towards the normal cell
line, and as such was specific towards the cancer cells. The effect
of the EEPC extract on the MG63 osteosarcoma cancer cell growth was
estimated by MTT assay conducted for two different time durations,
24 and 48 h. The results showed that the cytotoxic effect of the
extract showed dose and time-dependence.
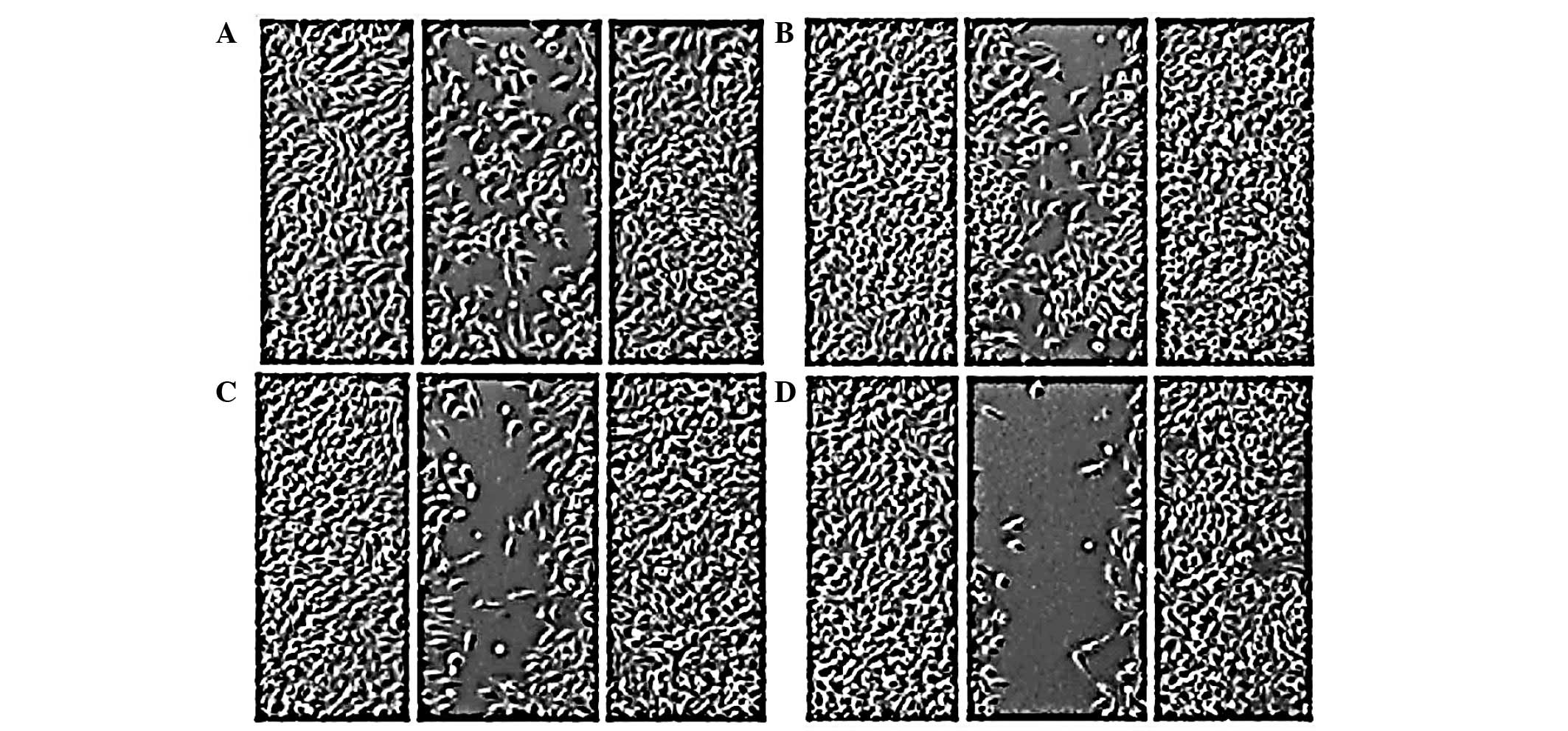
Effect of EEPC extract on cellular
morphology of MG-63 cancer cells using inverted light
microscopy
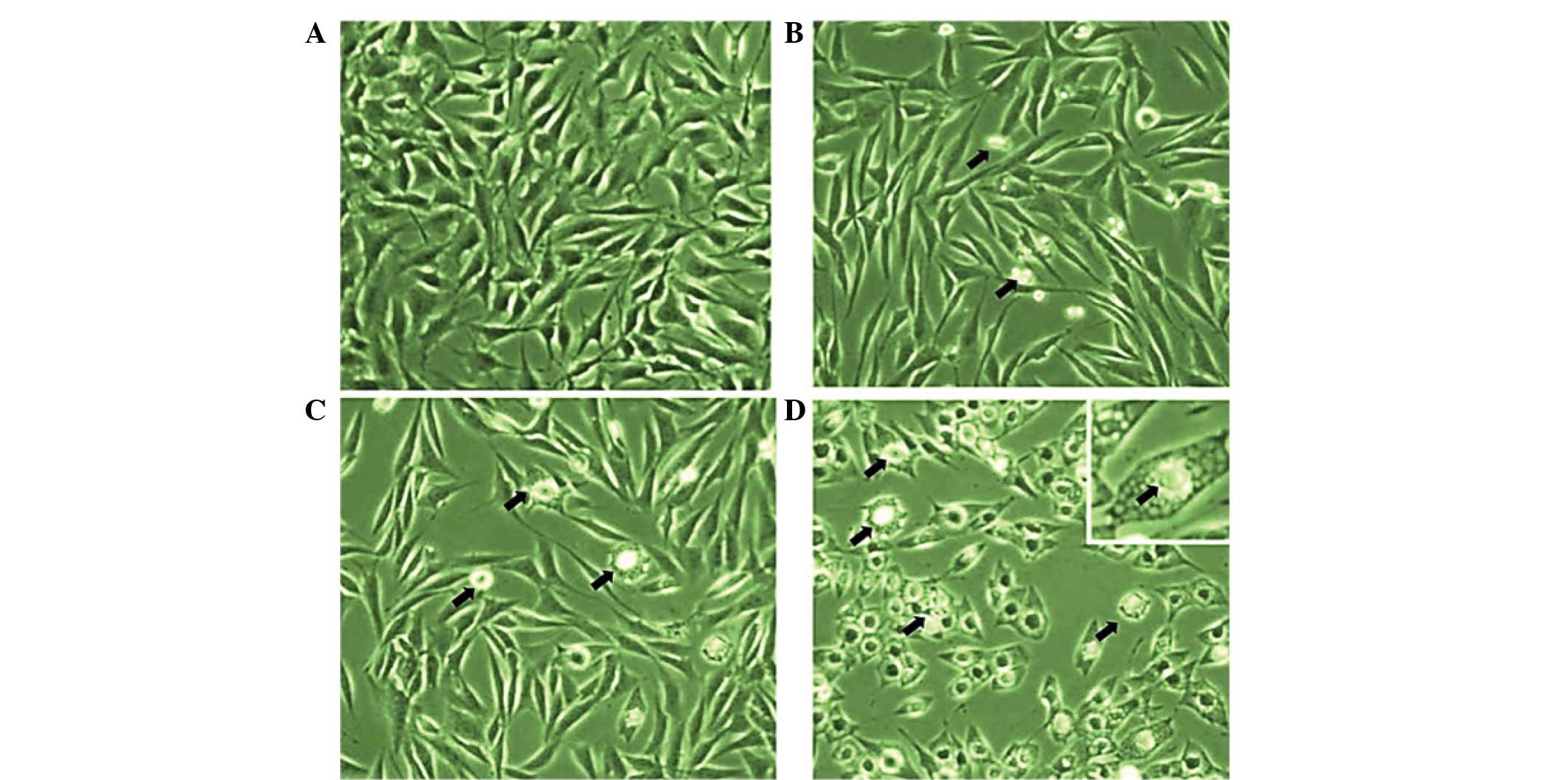
Morphological analysis using inverted light
microscopy revealed that EEPC extract induced growth inhibition and
apoptosis in MG-63 osteosarcoma cancer cells. As shown in Fig. 3A–D, the number of cells in the
control and the ones treated with different concentrations of EEPC
extract increased. The cells with higher doses revealed that
cellular shrinkage and blebbing occurred. This effect was shown to
be associated with EEPC extract dose. The number of cells with
altered morphology increased with EEPC extract concentration.
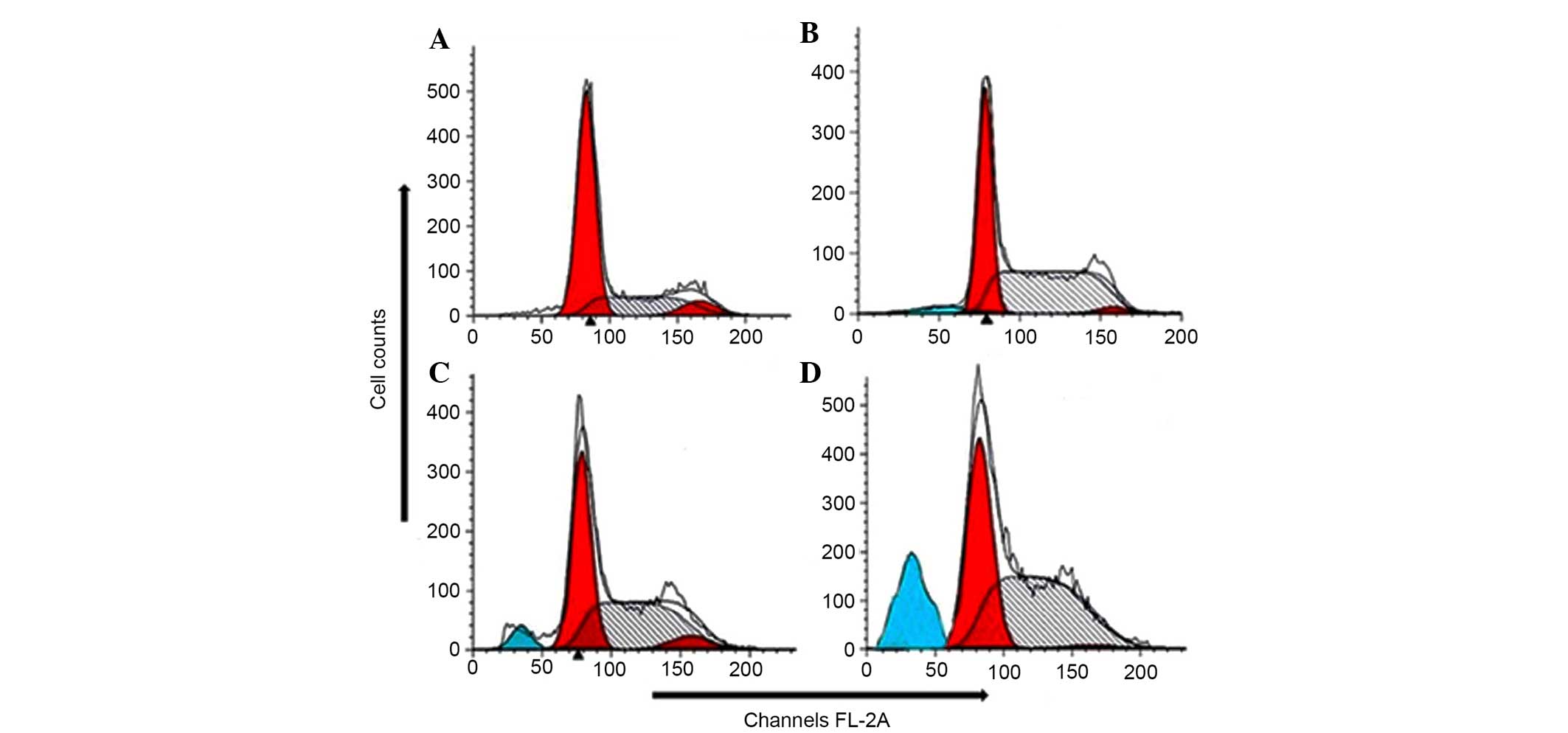
Quantification of apoptotic cell death by
Annexin V binding assay
Translocation of phosphatidylserine to the exterior
surface of the plasma membrane is a unique feature of early
apoptosis, which can be recognized and detected by binding of
Annexin V-FITC. If cell death occurs, fragmented and damaged DNA
becomes permeable for binding with PI. After cells are stained with
Annexin V in tandem with PI, this reagent enters the cell only when
the plasma cell membrane is damaged. In this study, flow cytometry
revealed that in the extract-treated cells, a higher number of
cells were positive for Annexin V compared with control (no drug
treatment) (Fig. 4). The
percentage of viable cells was low at lower concentration of the
extract. However, at higher dosages, the total number of apoptotic
cells considerably increased following treatment with 80 and 150
µg/ml doses of the extract. When the cells were treated with
40, 80 and 150 µg/ml of the EEPC extract for 48 h, the
average proportion of Annexin V-staining positive cells (total
apoptotic cells) markedly increased from 5.6% in control to 24.2,
38.8 and 55.7%, respectively. Thus, this assay allows a
quantitative estimation of the apoptotic cell death following drug
exposure.
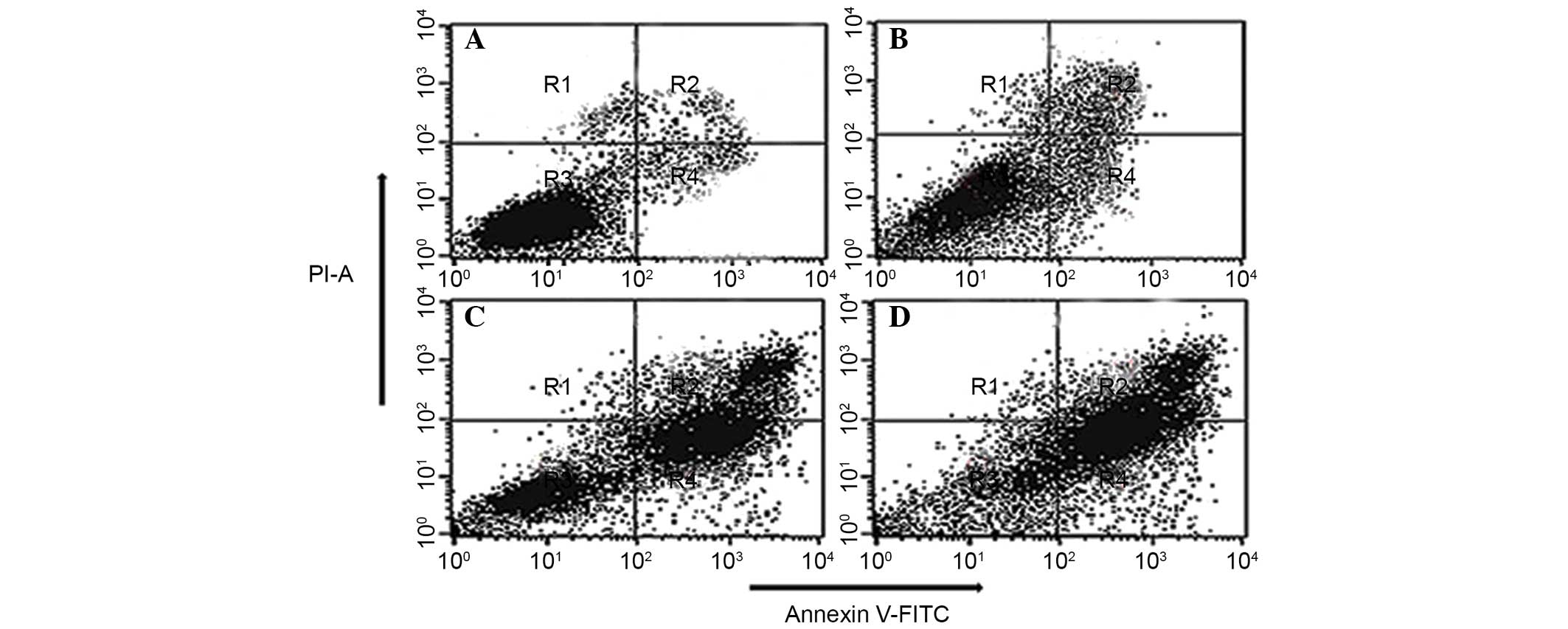 | Figure 4Quantification of apoptosis induced by
EEPC extract in MG63 osteosarcoma cancer cells evaluated by Annexin
V-FITC/PI dual staining. The cells were treated with (A) 0
µg/ml (control), (B) 40 µg/ml, (C) 80 µg/ml
and (D) 150 µg/ml of the EEPC extract for 48 h and analyzed
using fluorescence-activated cell sorting. R1, R2, R3 and R4
quadrants show percentage of necrotic, early apoptotic, normal
healthy and late apoptotic cell populations, respectively. EEPC,
ethyl acetate extract of Potentilla chinensis, FITC,
flusorescein isothiocyanate; PI, propidium iodide. |
Effect of the EEPC extract on cell cycle
phase distribution in MG-63 cancer cells
Apoptosis and the cell cycle are closely related
biochemical processes, and any disruption in cell cycle progression
may finally lead to apoptotic cell death. In order to have a
mechanistic indication of the growth inhibitory effect exerted by
the extract in MG-63 cancer cells, flow cytometry analysis was
conducted to identify whether the extract induces cell cycle arrest
in this cell line. The results revealed that EEPC extract induces
sub-G1 cell cycle arrest and increases the fraction of MG-63
apoptotic cells. To determine the distribution of EEPC
extract-treated MG-63 cells in different phases of the cell cycle,
DNA content in cells was detected by PI staining and flow
cytometry. The extract induced sub-G1 cell cycle arrest with a
substantial increase in the number of apoptotic cells. The results
showed that treatment with different concentrations of the extract
for 48 h led to an increase in the population of cells in the
sub-G0/G1 phase (apoptotic population) (P<0.01) (Fig. 5A–D). This upsurge in the sub-G1
population was accompanied by a corresponding reduction of the
cells in the s-phase and G2/M phases of the cell cycle. As compared
with the control (Fig. 5A),
extract treated (Fig. 5B–D) cells
showed a significant proportion of apoptotic cells.
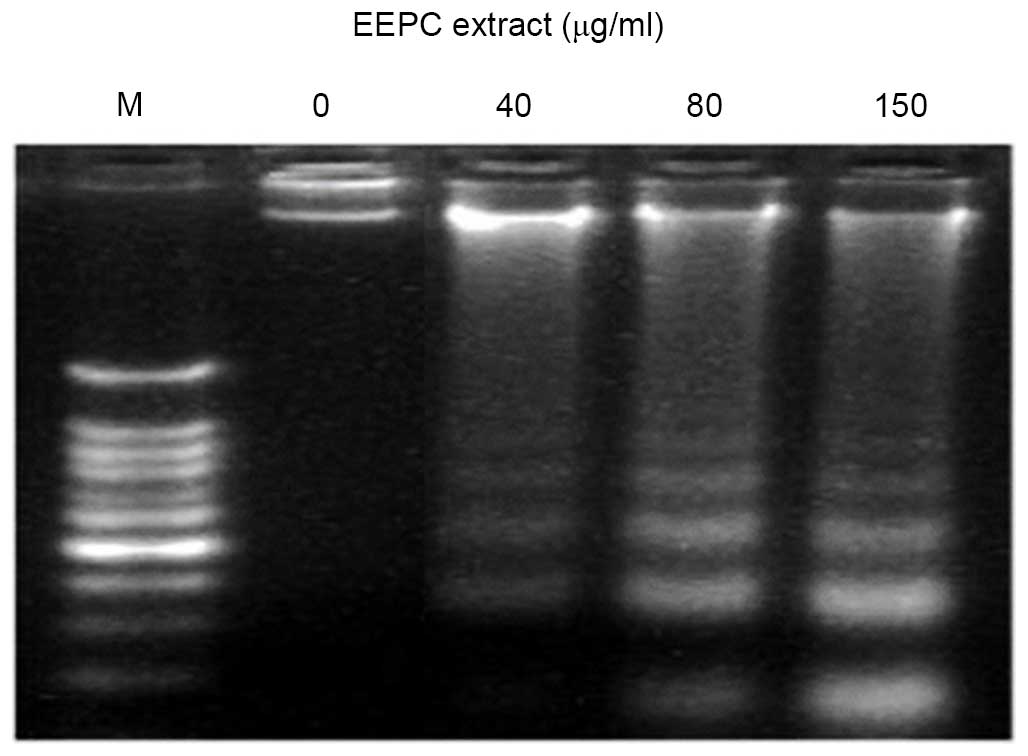
Effect of EEPC extract on DNA
fragmentation in MG-63 human osteosarcoma cancer cells
Besides the morphological changes to extract-treated
MG-63 cells, DNA fragmentation was also examined by observation of
the formation of DNA ladder. As shown in Fig. 6, the DNA ladder appeared to be more
evident with the increasing extract concentration; however, no DNA
fragments were observed in the control group (Fig. 6, 0 µg/ml). However, 40, 80
and 150 µg/ml doses of the extract after 48 h exposure led
to a substantial increase in DNA fragmentation (Fig. 6, right panel). The DNA
fragmentation is a hallmark of apoptosis, further confirming that
the EEPC extract induced cell death through apoptosis.
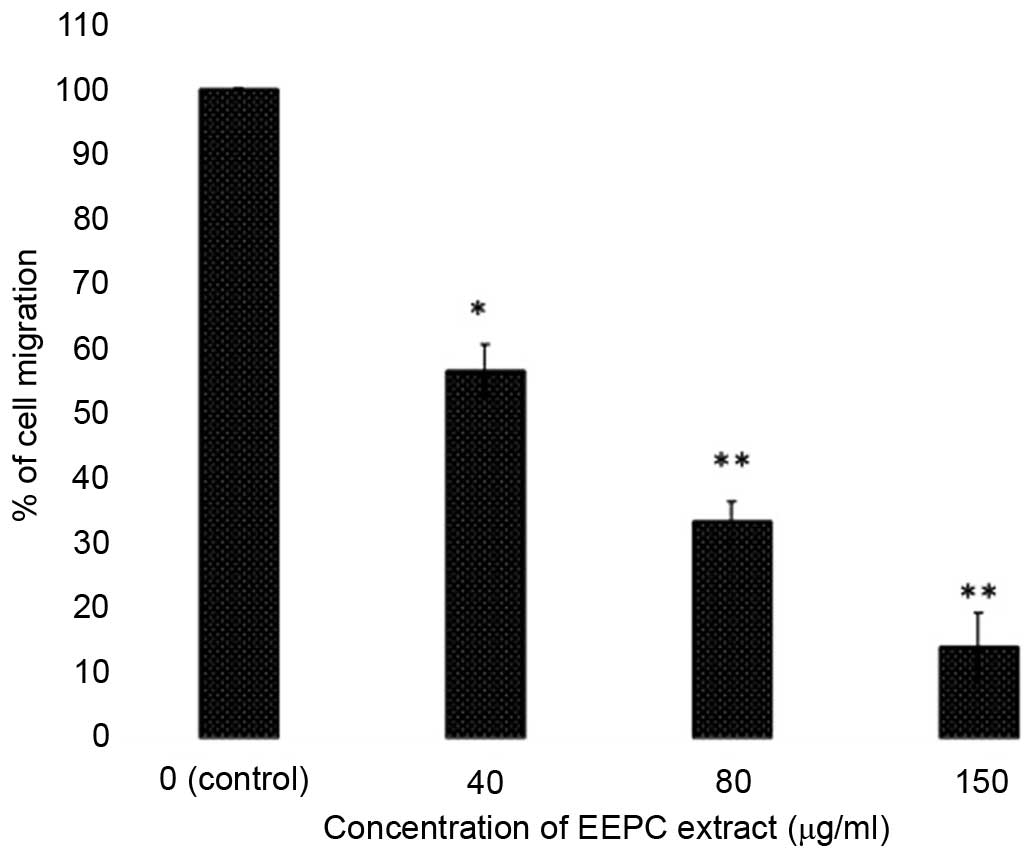
Effects of EEPC extract on the migration
of MG-63 osteosarcoma cancer cells
In this experiment, the effect of EEPC extract on
the migration in MG-63 osteosarcoma cells was examined. Confluent
cells were scratched and then treated with EEPC extract in complete
medium for 48 h. The number of cells that migrated into the
scratched area was captured (magnification, ×40; inverted light
microscope; Olympus America, Inc., Center Valley, PA, USA) and
calculated as a percentage (%) of migration. As shown in Figs. 7 and 8, EEPC extract significantly reduced
MG-63 cell migration in a concentration-dependent manner.
Discussion
Apoptosis or programmed cell death is a key strategy
for eliminating cancerous cells. It acts as a protective mechanism
that abolishes potentially harmful or damaged cells before the
appearance of malignancy, without activating the inflammatory
responses (15,16). Several chemotherapeutic drugs
including doxorubicin (17),
cisplatin (18) and tamoxifen
(19) cause cell cycle arrest and
induce apoptosis to eradicate the cancerous cells. This process is
characterized by distinct morphological changes, including membrane
blebbing, cell shrinkage, loss of mitochondrial membrane potential
(ΔΨm), chromatin condensation and DNA fragmentation. Two distinct
pathways of apoptosis in living cells have been identified, the
extrinsic and intrinsic pathways (20): The extrinsic pathway is arbitrated
through cell surface death receptor, resulting in the activation of
caspase-8. Conversely, the intrinsic pathway is reliant on numerous
cell stress stimuli, leading to altered ratios of Bcl-2 family
members, which affect cytochrome c, Smac and apoptotic
protease activating factor-1 release, resulting in caspase-9 and -3
activation. Besides apoptosis, deregulations of cell cycle
checkpoints, including those of the G1/S and G2/M phases have been
reported to be connected with cancer development (21). Cell cycle arrest offers a chance
for DNA repair to take place, therefore inhibiting replication of
the damaged template. It is regarded as one of the effective
strategies for eliminating cancer cells. Several natural plants
have been investigated for their cytotoxicity in cancer targeting
apoptosis (22).
The current study demonstrated the antitumor effects
of the EEPC, which is a well-known Chinese medicinal plant with
wide range of traditional uses. An MTT assay was used to evaluate
the cell viability against human MG-63 osteosarcoma cancer cells as
well as the fR-2 normal epithelial cell line in order to determine
whether the extract exerts a selective cytotoxic effect. The
extract exhibited potent, dose-dependent and selective cytotoxic
activity against MG63 cancer cells. The results also showed that
the extract displayed decreased cytotoxicity towards the normal
cell line, and as such was specific only towards cancer cells.
Furthermore, the effect of the extract on cell morphology and
apoptosis induction was also evaluated using phase contrast
microscopy and an Annexin V assay. The extract induced
characteristic morphological changes in cancer cells, such as
altered cell shape, shrinkage and blebbing. When the cells were
treated with 40, 80 and 150 µg/ml of the EEPC extract for 48
h, the average proportion of Annexin V-staining positive cells
(total apoptotic cells) significantly increased from 5.6% in
control to 24.2, 38.8 and 55.7% respectively. The extract induced
early and late apoptosis in the cancer cells. The effect of the
extract on cell cycle arrest using flow cytometry was further
evaluated. The results revealed that EEPC induced cell cycle arrest
at the sub-G1 phase in MG-63 human osteosarcoma cancer cells. This
increase in the sub-G1 population was attended by a corresponding
decline of the cells in s-phase and G2/M phase of the cell cycle.
As compared with the control, extract treated cells showed a
significant proportion of apoptotic cells. The DNA ladder appeared
to be more evident with the increasing extract concentration,
however, no DNA fragments were observed in the control groups.
However, 40, 80 and 150 µg/ml doses of the extract after 48
h exposure led to a substantial increase in DNA fragmentation. The
DNA fragmentation is a hallmark of apoptosis, further confirming
that the EEPC extract induced cell death through apoptosis.
Migration and invasion are hallmarks of malignancy.
Inhibition of migration and invasion of cancer may be expected to
be associated with a reduction of the malignancy grade of cancers.
Therefore, the effect of the extract on cell migration in MG-63
osteosarcoma cancer cells was also assessed using an in
vitro wound healing assay. It was demonstrated that EEPC
extract evidently reduced MG-63 cell migration in a
concentration-dependent manner.
In conclusion, the present study reported promising
anticancer effects of EEPC, which were mediated through apoptosis
induction, cell cycle arrest, DNA damage and inhibition of cell
migration. Notably, the extract exhibited a selective cytotoxic
effect against MG-63 osteosarcoma cells, while the normal
epithelial cells were less susceptible to the different extract
doses. This study also confirms the use of EEPC as an anticancer
agent. Considering the potential cytotoxic effects of the EEPC
extract, further studies are required to investigate its cytotoxic
potential in addition to its toxicity profile using different in
vivo models and further mechanisms of action, so that it may
serve as a novel therapeutic agent against osteosarcoma.
References
|
1
|
Enneking WF and Springfield DS:
Osteosarcoma. Orthop Clin North Am. 8:785–803. 1977.PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
Ferguson WS and Goorin AM: Current
treatment of osteosarcoma. Cancer Invest. 19:292–315. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bacci G and Lari S: Adjuvant and
neoadjuvant chemotherapy in osteosarcoma. Chir Organi Mov.
86:253–268. 2001.In English and Italian.
|
|
5
|
La Quaglia MP: Osteosarcoma. Specific
tumor management and results. Chest Surg Clin N Am. 8:77–95.
1998.PubMed/NCBI
|
|
6
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaoluan L, Ikeda H and Ohba H: Potentilla
Linnaeus, Sp. Pl. 1: 495. 1753. Flora of China. 9:pp. 291–327.
2003, http://www.efloras.org.
|
|
8
|
Xue PF, Luo G, Zeng WZ, Zhao YY and Liang
H: Secondary metabolites from Potentilla multifida L. (Rosaceae).
Biochem Syst Ecol. 33:725–728. 2005. View Article : Google Scholar
|
|
9
|
Xue PF, Yin T, Liang H and Zhao YY: Study
on chemical constituents of Potentilla discolor. Chin Pharm J.
40:1052–1054. 2005.
|
|
10
|
Lu LU, Sujun LI, Zonglin LIU, Bo YU, Huan
GUO and Jiguo HE: Study on hypoglycemic mechanism of Potentilla
chinensis extracts to diabetic mice. Food Sci. 29:387–391.
2008.
|
|
11
|
Lim JP, Lee HG, Jeon H and Bora L:
Anti-inflammatory effect of Potentilla chinensis herbal water
extract on the proteinase activated receptor-2-mediated paw edema.
Korean J Orient Physiol Pathol. 23:1444–83. 2009.
|
|
12
|
Liu P, Duan HQ, Pan Q, Zhang YW and Yao Z:
Triterpenes from herb of Potentilla chinensis. Zhongguo Zhong Yao
Za Zhi. 31:1875–1879. 2006.In Chinese.
|
|
13
|
Li PL, Lin CJ, Zhang ZX and Jia ZJ: Three
new triterpenoids from Potentilla multicaulis. Chem Biodivers.
4:17–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang QH, Li ZY, Shen Y, Lin HW, Shu W and
Zhou JB: Studies on triterpenoids from Potentilla chinensis.
Zhongguo Zhong Yao Za Zhi. 31:1434–1436. 2006.In Chinese.
PubMed/NCBI
|
|
15
|
Kaufmann SH and Hengartner MO: Programmed
cell death: Alive and well in the new millennium. Trends Cell Biol.
11:526–534. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reed JC: Apoptosis-regulating proteins as
targets for drug discovery. Trends Mol Med. 7:314–319. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lüpertz R, Wätjen W, Kahl R and Chovolou
Y: Dose and time-dependent effects of doxorubicin on cytotoxicity,
cell cycle and apoptotic cell death in human colon cancer cells.
Toxicology. 271:115–121. 2010. View Article : Google Scholar
|
|
18
|
He G, Kuang J, Khokhar AR and Siddik ZH:
The impact of S- and G2-checkpoint response on the fidelity of
G1-arrest by cisplatin and its comparison to a non-cross-resistant
platinum (IV) analog. Gynecol Oncol. 122:402–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han P, Kang JH, Li HL, Hu SX, Lian HH, Qiu
PP, Zhang J, Li WG and Chen QX: Antiproliferation and apoptosis
induced by tamoxifen in human bile duct carcinoma QBC939 cells via
upregulated p53 expression. Biochem Biophys Res Commun.
385:251–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: Structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar
|
|
21
|
Sun XM, MacFarlane M, Zhuang J, Wolf BB,
Green DR and Cohen GM: Distinct caspase cascades are initiated in
receptor-mediated and chemical-induced apoptosis. J Biol Chem.
274:5053–5060. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buolamwini JK: Cell cycle molecular
targets in novel anticancer drug discovery. Curr Pharm Des.
6:379–392. 2000. View Article : Google Scholar : PubMed/NCBI
|