Introduction
Bone remodeling is a fundamental mechanism for
removing and replacing bone during skeleton adaptation to
mechanical loads. Osteocytes are the most abundant cells in bone
(1–3), and they are actively involved in the
orchestration of both bone-forming osteoblasts and bone-resorbing
osteoclasts (4,5). Osteocytes implement their role by
activating the signaling pathways involved in the maintenance of
bone homeostasis, specifically the Wnt/β-catenin pathway (6). This pathway has been demonstrated to
be activated by mechanical loading to protect against bone loss
(7) and to lead to the production
of sclerostin, an inhibitor of osteoblast activity in response to
unloading (8). It has been
previously observed that osteocytes express receptor activator of
nuclear factor κB ligand, a pre-osteoclastogenic cytokine involved
in bone resorption activation (5,9,10).
Osteocytes are known as the mechanosensor of bone (11,12)
due to their location deep within the bone matrix and their
dendritic network that allows the detection of variations in the
levels of strain placed upon bone (4,13).
Skeletal loading is fundamental in maintaining osteocyte viability
(14), as demonstrated by
increasing osteocyte apoptosis with disuse (15,16).
Skeletal unloading, as observed in patients
undergoing bed rest, results in imbalanced bone remodeling that
favors bone resorption (16,17).
It has been suggested that the loss of bone observed during disuse
is the result of osteocyte hypoxia, resulting from unloading that
reduces the interstitial fluid flow and, consequentially, reduces
oxygen transport (18,19). It is widely regarded that
disruption of the lacuno-canalicular network, which is necessary
for nutrient and gaseous exchange for osteocytes, results in
localized hypoxia in bone (20,21).
It has been hypothesized that oxygen deprivation,
due to reduced mechanical loading, may be the cause of the
osteocyte apoptosis (22,23) and osteoclast recruitment, in order
to remove bone matrix during hypoxia (24–27).
Hypoxia and oxidative stress increase the expression of different
factors that mediate the adaptive responses to hypoxia/ischemia.
The role of certain molecular endoplasmic reticulum (ER) chaperones
has been extensively investigated, in particular regarding their
functions in the quality control of proteins processed in the ER
and in the regulation of ER signaling in response to stress
(28).
Oxygen-regulated protein 150 (ORP150) protein (150
kDa), a novel endoplasmic-reticulum-associated chaperone induced by
hypoxia/ischemia (29), has been
identified to be highly expressed in osteocytes compared with
osteoblasts (30). ORP150 has been
proposed to be involved in the prevention of apoptosis induced by
oxygen deprivation (31,32).
The cytoprotective role of ORP150 in different cell
types has been previously demonstrated and it has been hypothesized
that ORP150 is required by osteocytes to survive in their reduced
oxygen state into the bone matrix (33–35).
The current study aimed to establish whether ORP150 levels
correlated with osteocyte cell death and apoptosis under hypoxic
conditions.
Materials and methods
Cell culture
The murine long bone osteocyte Y4 (MLO-Y4) cell line
(supplied from University of Missouri, Kanas City, USA) was used
and cultured as previously described (36,37).
Briefly, the cells were cultured on collagen-coated (rat tail type
I collagen; BD Biosciences, Franklin Lakes, NJ, USA) plastic ware
and were grown at 37°C, 5% CO2, 95% air with α-minimal essential
medium (α-MEM; 1X; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 2.5% fetal bovine serum (FBS)
(PAA; GE Healthcare Life Sciences, Pittsburgh, PA, USA), 2.5%
bovine calf serum (BCS; GE Healthcare Life Sciences, Logan, UT,
USA) and 100 U/ml penicillin/streptomycin at (Invitrogen; Thermo
Fisher Scientific, Inc). For the hypoxia experiments, 5,000
cells/cm2 were seeded onto collagen-coated multi-well dishes,
incubated in α-MEM without phenol red, 2.5% FBS + 2.5% BCS and 100
U/ml penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.).
To confirm the data obtained with the MLO-Y4 cells,
the pre-osteoblast MC3T3-E1 cell line was additionally used.
MC3T3-E1 subclone 14 was obtained from the American Type Culture
Collection cell bank (Manassas, VA, USA) and was cultured as
previously described (30).
Hypoxia
Time 0 was designated as 24 h post-seeding, which
was when cell culture under normal conditions (normoxia 20% O2), or
hypoxic conditions (hypoxia 1% O2) was initiated. Cells were
cultured under these conditions for 8, 16, 24, 48 and 72 h. For the
MC3T3-E1 cells, the 16 h time point was not repeated, as this set
of experiments was performed to confirm the result already obtained
with the MLO-Y4 cell line. For hypoxic conditions, the cells were
placed inside a chamber from Billups-Rothenberg, Inc. (San Diego,
CA, USA), where a mixture of gas (95% N2 and 5% CO2) was injected
resulting in 1% O2. The oxygen percentage was controlled by an
Oxygen Analyzer/Monitor (Vascular Technology, Inc., Nashua, NH,
USA).
To evaluate hypoxia of osteocytes and
pre-osteoblasts, pimonidazole hydrochloride (Hypoxyprobe™-1;
Chemicon; EMD Millipore, Billerica, MA, USA) (38) was used. Pimonidazole hydrochloride
is a substance with low-molecular weight that binds only to cells
that have an oxygen tension of 10 mm Hg (pO2~1,2%) or
lower. The cultures were stained for hypoxic cells with
Hypoxyprobe™-1 according to the manufacturer's protocol.
One experiment in quadruplicate was performed for
each cell culture and each time point.
Cell viability and apoptosis
Osteocyte viability and total cell number were
determined by trypan blue staining (39,40).
Three experiments in quadruplicate were performed for each cell
culture and time point. The results were expressed as the number of
trypan blue-positive cells, over the total number of cells.
Apoptosis was assessed subsequent to 4′,6-diamidino-2-phenylindole
nuclear staining, counting cells exhibiting chromatin condensation
or nuclear blebbing over the total number of cells (41).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA isolation was performed using TRIzol reagent
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Total RNA
(500 ng) was reverse transcribed using the High-Capacity cDNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Gene expression was analyzed using TaqMan Assay
probes and primers for ORP150 (cat. no. Mm00491279) and β2
macroglobulin (cat. no. Mm00437762) with Master Mix TaqMan Gene
Expression reagents (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The cycling condition used were an initial stage of 2 min
incubation at 50°C then 10 min at 95°C, this was followed by 40
cycles of 95 °C for 15 min and 60°C for 1 min. All equipment and
reagents used for the RT-qPCR were purchased from Applied
Biosystems (Thermo Fisher Scientific, Inc.). The four experiments
performed were conducted in triplicate; the quantification cycle
(Cq) value was determined by the default settings, using StepOne
software, version 2.1 (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The relative expression of ORP150 in hypoxic
MLO-Y4 cells was compared with the expression in MLO-Y4 cells grown
for 8 h under normoxic conditions using the 2-∆∆Cq method (42).
Western blotting
Cells cultured under hypoxia or normoxia were lysed
in radioimmunoprecipitation assay buffer complete with a proteinase
inhibitor cocktail (Enzo Life Sciences, Farmingdale, NY, USA). A
total of four experiments for MLO-Y4 and one for MC3T3-E1 were
performed to compare the ORP150 expression in hypoxic and normoxic
conditions at all different time points. A total of three different
experiments were conducted in order to compare ORP150 expression in
normoxic osteocytes and pre-osteoblasts. Due to the fact that the
experiments made with MC3T3-E1 cells were performed to confirm the
results obtained with the MLO-Y4 cells, the 16 h time point for
this cell line was not repeated. Protein concentration was
determined in each cell lysate supernatant using a DC Protein Assay
kit I colorimetric assay (Bio-Rad Laborartories, Inc., Hercules,
CA, USA). Equal amounts of protein (15 µg) were applied and
separated using 10% sodium dodecyl sulfate-polyacrylimide gel
electrophoresis and transferred by electrophoresis to a
nitrocellulose membrane. The membranes were blocked with 2.5%
non-fat dried milk (Bio-Rad Laboratories, Inc.) in 1X
phosphate-buffered saline at room temperature for 30 min and
incubated with rabbit anti-ORP150 (dilution 1:1,000; cat. no.
3905-1; Epitomics, Burlingame, CA, USA) and rabbit anti-β-actin
(dilution 1:1,000; cat. no. LF-PA0207; AbFrontier, Seoul, Korea)
primary antibodies for 2 h at room temperature, with β-actin as the
internal control. Horseradish peroxidase-conjugated anti-rabbit
secondary antibody (dilution 1:1,000; cat. no. HAF008; R&D
Systems, Inc., Minneapolis, MN, USA) was subsequently incubated
with the membranes for 1.5 h at room temperature. Enhanced
chemiluminescent reagents (GE Healthcare Life Sciences) and films
were used to visualize the protein bands and were quantified using
Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA).
Statistical analysis
Data were analyzed for normal distribution using the
Kolmogorov-Smirnov test. Differences between the two oxygen
conditions were assessed using Mann-Whitney U test, and differences
between the experimental times were assessed using the
Kruskal-Wallis test for nonparametric data. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS software, version
14.0 (SPSS, Inc., Chicago, IL, USA). Differences between data
obtained by western blot analysis, comparing ORP150 expression in
MC3T3-E1 and MLO-Y4 cells was assessed using Student's t-test.
Results
Induction of hypoxia
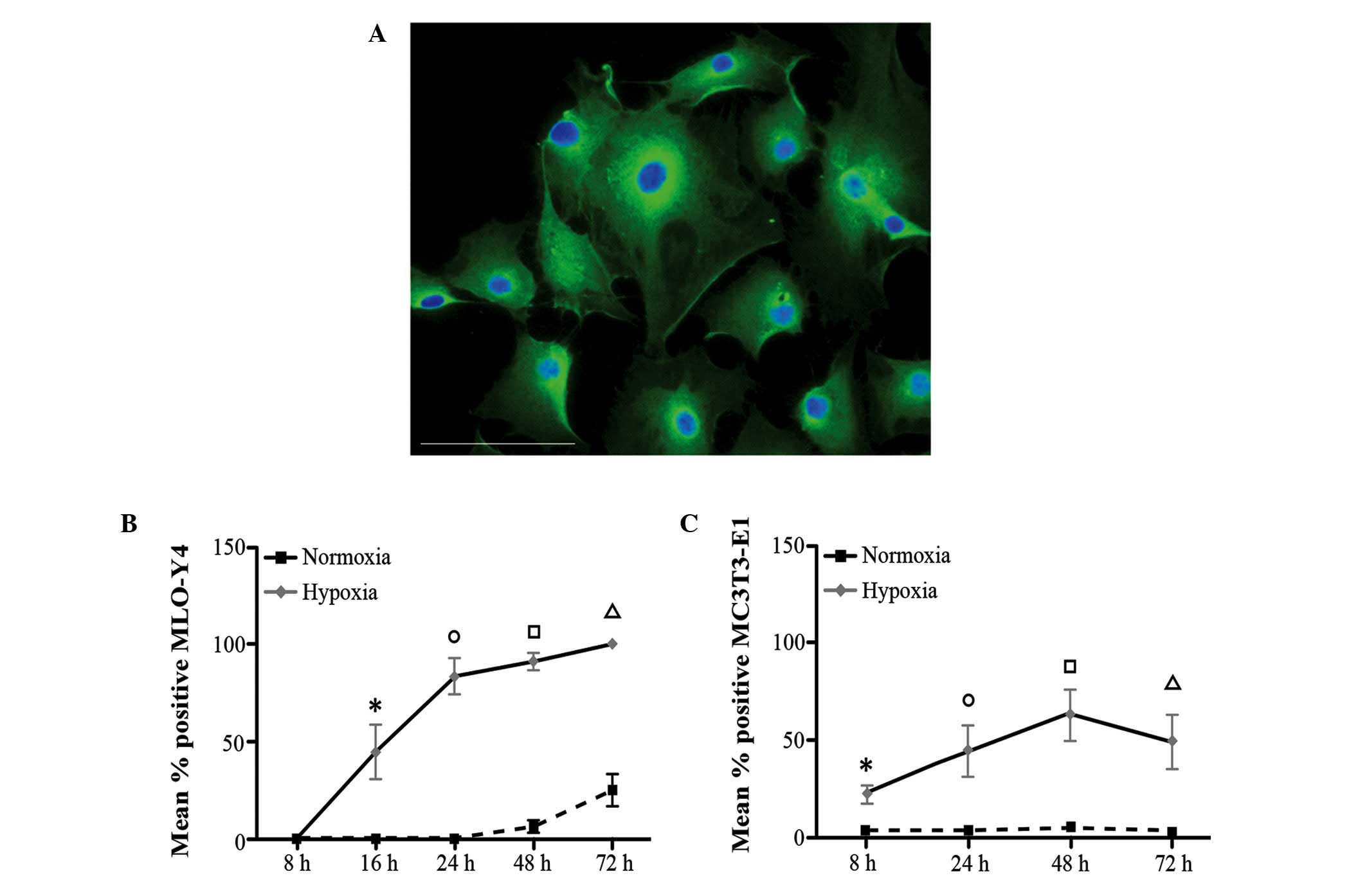
MLO-Y4 cells cultured at 1% O2 exhibited
a significantly increased level of Hypoxyprobe staining (Fig. 1A and B) compared with cells
cultured in normoxic conditions at the same time points: 16, 24, 48
and 72 h (P=0.005, 0.005, 0.009 and 0.007, respectively). In
addition, in hypoxic conditions, the number of Hypoxyprobe-positive
cells significantly increased over time (hypoxic MLO-Y4 P=0001;
Kruskal Wallis test), suggesting that the cells became more
sensitive to hypoxia (Fig. 1B).
Conversely, the time-dependent increase of Hypoxyprobe-positive
cells was not observed in normoxic conditions and only following 72
h exposure was a marginal increase detected. A similar trend was
observed for the MC3T3-E1 cells (Fig.
1C); the number of Hypoxyprobe-positive cells was significantly
different at all time points under hypoxic conditions compared with
normal conditions (8, 24, 48 and 72 h; P=0.001, 0.001, 0.009 and
0.001, respectively). In addition, a significant time-dependent
increase in the number of MC3T3-E1 Hypoxyprobe-positive cells was
observed under hypoxia (Kruskal Wallis test; P=0001), however was
not observed under normoxia (Kruskal Wallis test; P=0515).
These results indicate that the two cell lines were
sensitive to hypoxia under the conditions of oxygen
deprivation.
Cell viability and apoptosis
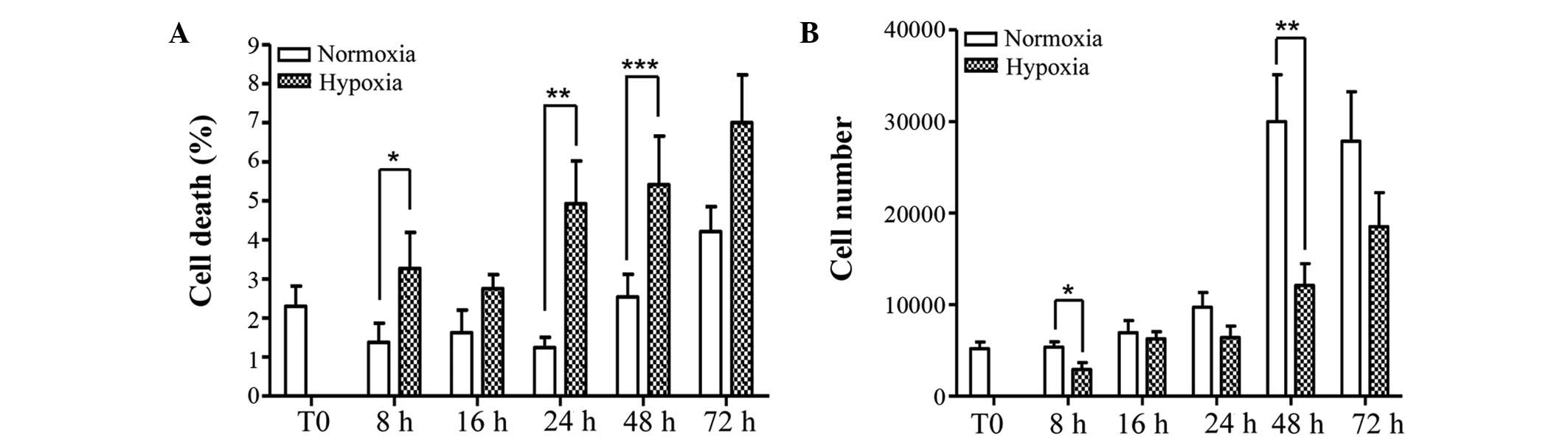
Osteocyte viability, as determined by trypan blue
staining, was compromised by oxygen deprivation and resulted in a
significant increase in cell death at 8, 24 and 48 h (P=0.049;
P=0.002; P=0.043, respectively), however was not significant at 16
and 72 h (P=0.122; P=0.069, respectively) (Fig. 2A). Cell counting observed an
increase in total cell number over time, however, deprivation of
oxygen significantly reduced total number of cells at 8 and 48 h
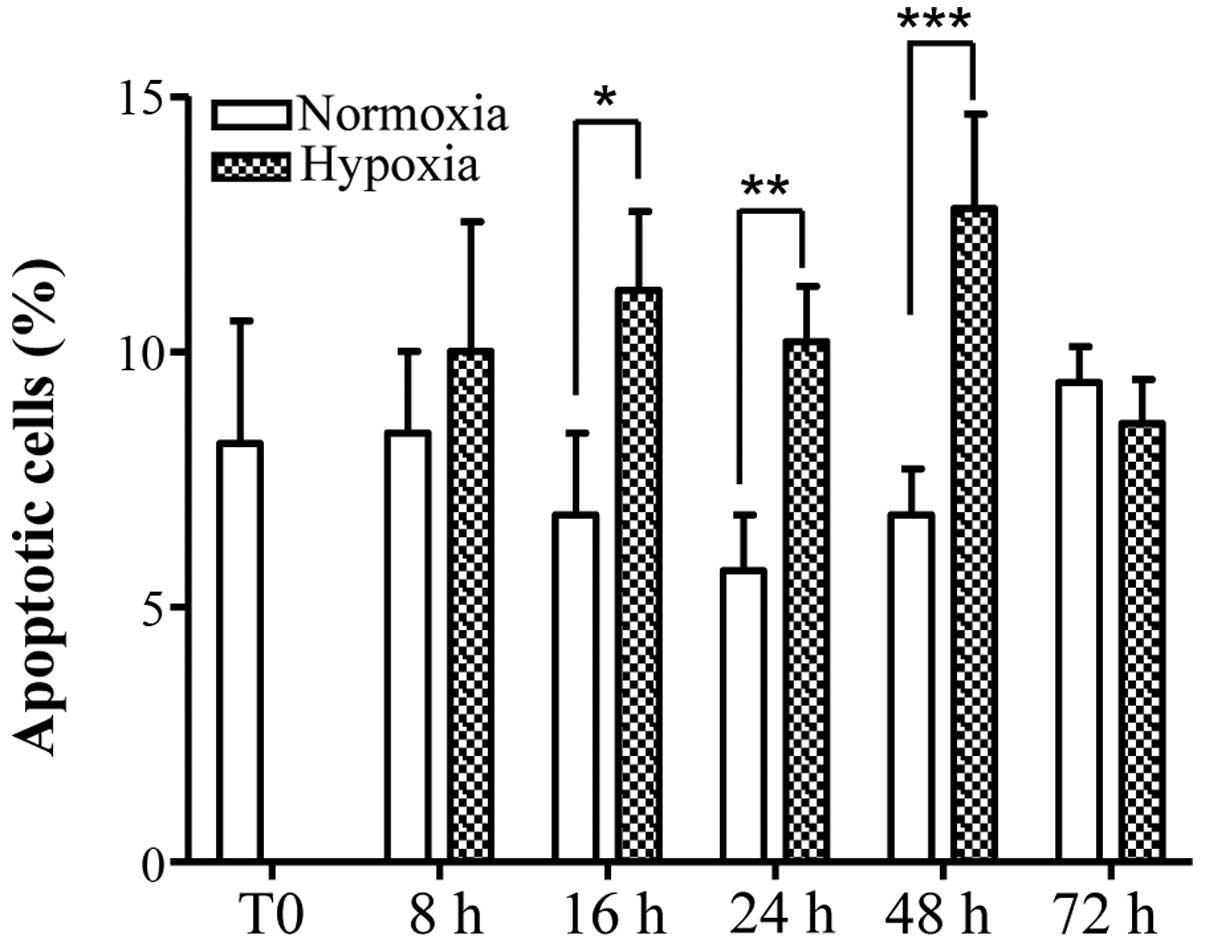
compared with the same time points under normoxia (Fig. 2B). The number of apoptotic
osteocytes, as determined by nuclear fragmentation, was increased
by hypoxia and this increase was identified to be statistically
significant at 16, 24 and 48 h, compared with the same time points
cultured under normoxia. At 72 h, the number of apoptotic cells
cultured under hypoxic conditions was reduced compared with
normoxia, however this difference was identified to not be
significant. The number of apoptotic cells in hypoxia at 72 h was
additionally identified to be marginally reduced compared with the
values observed at 48 h, however this difference was not
statistically significant (Fig.
3).
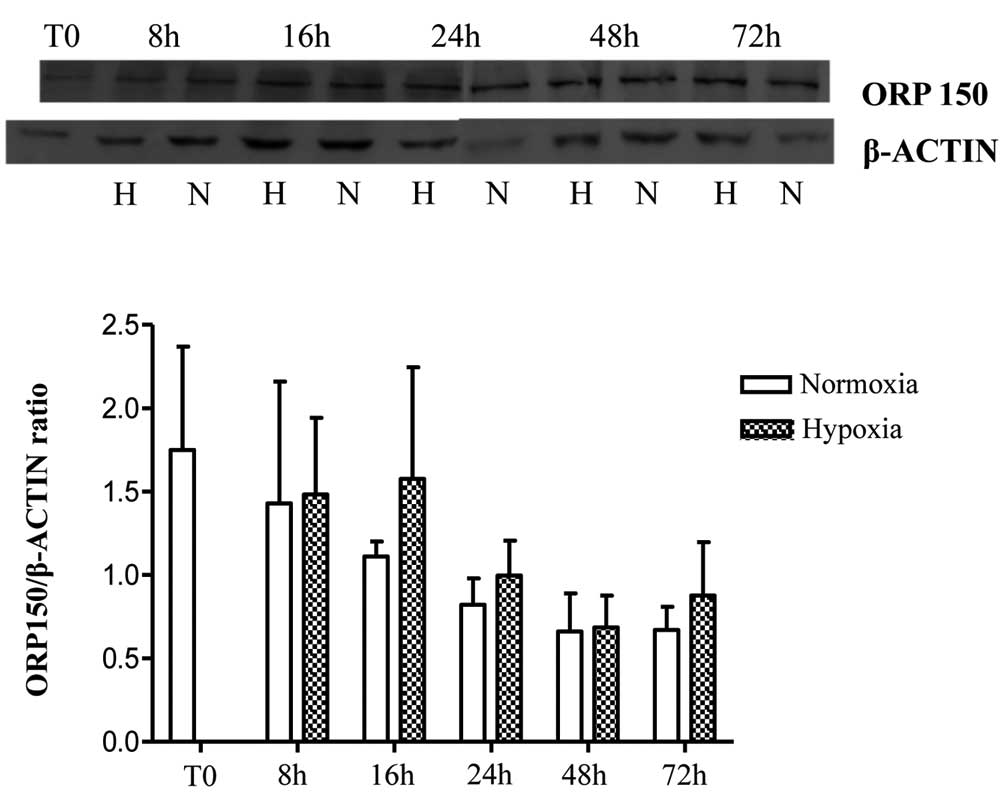
ORP150 expression
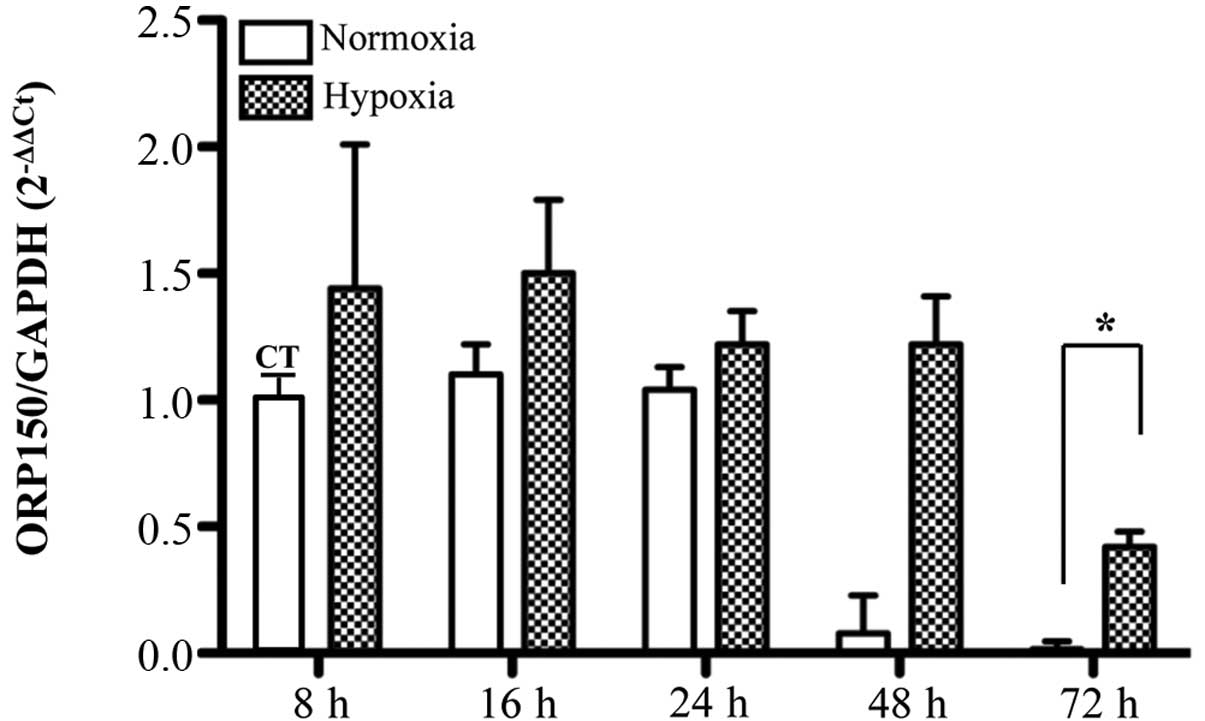
MLO-Y4 ORP150 mRNA expression was significantly
reduced in a time-dependent manner under hypoxic and normoxic
conditions. No significant differences were observed in mRNA levels
under hypoxic conditions compared with normoxia apart from at 72 h,
where the difference was statistically significant with a smaller
reduction under hypoxic conditions (Fig. 4). In particular, there were
statistically significant differences under hypoxia identified
between 72 and 16 h, 24 and 48 h (P=0.001, 0.009 and 0.007,
respectively); similar results were obtained under normoxic
conditions (P=0.000, 0.000 and 0.012, respectively). Western blot
analysis conducted on MLO-Y4 indicated no statistically significant
differences in ORP150 expression at a protein level at any time
point under the same conditions of the mRNA analysis (Fig. 5). It is suggested that additional
later time points would reflect the significant differences in the
72 h mRNA data.
Confirming the ORP150 expression data identified in
MLO-Y4 cells, no differences were observed in ORP150 expression in
MC3T3-E1 cells under normoxic and hypoxic conditions, similarly,
there were no significant differences over time under normoxic and
hypoxic conditions (data not shown). In addition, western blot
analysis indicated that the expression of ORP150 was significantly
reduced in normoxic MC3T3-E1 cells (ORP150/β-actin
ratio=0.531±0.03-mean ± standard deviation-) compared with normoxic
MLO-Y4 cells (ORP150/β-actin ratio=0.845±0.06-mean ± standard
deviation-), with P=0.001, in agreement with a previous study
(30).
Discussion
The objective of the current study was to determine
whether the expression of ORP150 in osteocytes is regulated in
response to oxygen deprivation.
Hypoxia is a normal physiological condition for
osteocytes. A concentration of approximately 5% O2 is
more likely to be physiological for osteocytes than a concentration
of 20%O2, as osteocytes are embedded deep inside the
mineralized bone matrix and their nutrient availability is greatly
dependent on diffusion (43–45).
The actual concentration of oxygen inside of the osteocyte lacunae
during skeletal unloading remains unknown. A previous study has
measured pO2 values of approximately 6% in normal human
bone marrow aspirates (46). On
the basis of this information, 1% O2 was selected as the
hypoxic condition and 20% O2 as the normoxic condition
(representing the atmospheric oxygen levels); although it is widely
accepted that a pO2 of 20% corresponds to hyperoxia
(44).
On the basis of this assumption, the data of the
present study indicate that 1% O2 induces hypoxia and
significantly compromises cellular viability. The results obtained
additionally demonstrate that apoptosis serves a role in oxygen
deprivation-mediated induction of cell death. Despite the fact that
osteocytes were sensitive to hypoxia from 16 to 72 h, as
demonstrated by Hypoxyprobe staining (Fig. 1), the difference in the rate of
apoptosis in hypoxia compared with normoxia, remained significant
until the 48 h time point. Following that, the apoptotic stimuli
induced by hypoxia appear to be inhibited at 72 h, where the
percentage of apoptotic cells was not identified to be
significantly different in hypoxia compared with normoxia. It is
hypothesized that, in the early stages, additional mechanisms of
protection against oxygen deprivation-induced cell death may occur
(47,48).
No significant differences were observed in ORP150
mRNA levels until the 72 h time point, and protein levels at any
time point, however, it is suggested that the protein levels may
have been greater at later time points. This suggests that ORP150
mRNA expression is delayed, as based on the Hypoxyprobe results,
the cells become hypoxic from 16 h. Notably, ORP150 expression
reduced over time under normoxic and hypoxic conditions. Unlike
additionally cell types including Hela cells (49), neuronal cells (50–52),
renal cells (53) and
cardiomyocytes (54,55), in MLO-Y4 cells ORP150 does not
appear to be regulated by hypoxia at early time points. Similar
results were identified for the ORP150 protein expression in
pre-osteoblasts grown under hypoxic conditions compared with
normoxic conditions. The ORP150 protein levels were increased in
MLO-Y4 osteocytes compared with MC3T3-E1 pre-osteoblasts grown
under normoxic conditions. These observations are in agreement with
previous studies demonstrating that low oxygen tension serves an
important role in the differentiation of osteoblasts into
osteocytes (45,56). Guo et al (30) demonstrated that a greater amount of
hypoxia-associated proteins in osteocytes (such as ORP150),
compared with in osteoblasts. The higher level of ORP150 protein
may be a possible explanation for the capacity of osteocytes to
adapt to their hypoxic environment.
In conclusion, ORP150 is commonly highly expressed
in osteocytes as compared with osteoblasts, and mRNA expression is
not elevated compared with the control until late stages of hypoxic
culture conditions. Significant cell death and apoptosis precede
the significant differences in ORP150 mRNA in hypoxia compared with
normoxia. It is hypothesized that the amount of ORP150 in MLO-Y4 is
sufficient to protect the cells from hypoxia up to 48 h, however
longer exposure to oxygen deprivation may lead to upregulation of
ORP150 expression. After 72 h, the significant increase in ORP150
mRNA, identified in hypoxia compared with normoxia, strongly
support the hypothesis that longer exposure to hypoxia may
additionally induce an increase of ORP150 protein levels. It is
notable that at present the actual concentration of oxygen inside
of the osteocyte lacunae remains unknown, and this should be
considered a limitation. However, in order to understand the
behavior of osteocytes in response to the oxygen deprivation and to
increase knowledge of this complex pathway, evaluation of the
hypoxic condition at early time points is scientifically valuable.
It is thus important to further investigate the role of ORP150 in
the apoptotic process under hypoxic conditions for longer time
periods, and to block ORP150 expression, using ORP150 small
interfering RNA or knockout mice, to more fully elucidate the
protective role of this protein.
Acknowledgements
The authors would like to thank Dr Luca Dalle
Carbonare, of the Policlinico G.B. Rossi (Verona, Italy), for
providing the MLO-Y4 cell line. The authors would additionally like
to thank Dr Lucia Mancini for helping with the revision of the
manuscript, and Mr. Luigi Lena for the illustrations.
References
|
1
|
Bianconi E, Piovesan A, Facchin F, Beraudi
A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F,
et al: An estimation of the number of cells in the human body. Ann
Hum Biol. 40:463–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bonewald LF: Osteocytes as dynamic
multifunctional cells. Ann N Y Acad Sci. 1116:281–290. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Busse B, Djonic D, Milovanovic P, Hahn M,
Püschel K, Ritchie RO, Djuric M and Amling M: Decrease in the
osteocyte lacunar density accompanied by hypermineralized lacunar
occlusion reveals failure and delay of remodeling in aged human
bone. Aging Cell. 9:1065–1075. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonewald LF: The amazing osteocyte. J Bone
Miner Res. 26:229–238. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakashima T, Hayashi M, Fukunaga T, Kurata
K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et
al: Evidence for osteocyte regulation of bone homeostasis through
RANKL expression. Nat Med. 17:1231–1234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: From human mutations to treatments.
Nat Med. 19:179–192. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Javaheri B, Stern AR, Lara N, Dallas M,
Zhao H, Liu Y, Bonewald LF and Johnson ML: Deletion of a single
b-catenin allele in osteocytes abolishes the bone anabolic response
to loading. J Bone Miner Res. 29:705–715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robling AG, Niziolek PJ, Baldridge LA,
Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido
TM, Harris SE and Turner CH: Mechanical stimulation of bone in vivo
reduces osteocyte expression of Sost/sclerostin. J Biol Chem.
283:5866–5875. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paszty C, Turner CH and Robinson MK:
Sclerostin: A gem from the genome leads to bone-building
antibodies. J Bone Miner Res. 25:1897–1904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong J and O'Brien CA: Osteocyte RANKL:
New insights into the control of bone remodeling. J Bone Miner Res.
27:499–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burgers TA and Williams BO: Regulation of
Wnt/b-catenin signaling within and from osteocytes. Bone.
54:244–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tate ML Knothe, Niederer P and Knothe U:
In vivo tracer transport through the lacunocanalicular system of
rat bone in an environment devoid of mechanical loading. Bone.
22:107–117. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gortazar AR, Martin-Millan M, Bravo B,
Plotkin LI and Bellido T: Crosstalk between
caveolin-1/extracellular signal-regulated kinase (ERK) and
b-catenin survival pathways in osteocyte mechanotransduction. J
Biol Chem. 288:8168–8175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tate ML Knothe: ‘Whither flows the fluid
in bone?’ An osteocyte's perspective. J Biomech. 36:1409–1424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dufour C, Holy X and Marie PJ:
Transforming growth factor-beta prevents osteoblast apoptosis
induced by skeletal unloading via PI3K/Akt, Bcl-2 and phospho-Bad
signaling. Am J Physiol Endocrinol Metab. 294:E794–E801. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jilka RL, Noble B and Weinstein RS:
Osteocyte apoptosis. Bone. 54:264–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bikle DD and Halloran BP: The response of
bone to unloading. J Bone Miner Metab. 17:233–244. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ontiveros C, Irwin R, Wiseman RW and
McCabe LR: Hypoxia suppresses runx2 independent of modeled
microgravity. J Cell Physiol. 200:169–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stevens HY, Meays DR and Frangos JA:
Pressure gradients and transport in the murine femur upon hindlimb
suspension. Bone. 39:565–572. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dodd JS, Raleigh JA and Gross TS:
Osteocyte hypoxia: A novel mechanotransduction pathway. Am J
Physiol. 277:C598–C602. 1999.PubMed/NCBI
|
|
21
|
Hinoi E, Ochi H, Takarada T, Nakatani E,
Iezaki T, Nakajima H, Fujita H, Takahata Y, Hidano S, Kobayashi T,
et al: Positive regulation of osteoclastic differentiation by
growth differentiation factor 15 upregulated in osteocytic cells
under hypoxia. J Bone Miner Res. 27:938–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Plotkin LI, Mathov I, Aguirre JI, Parfitt
AM, Manolagas SC and Bellido T: Mechanical stimulation prevents
osteocyte apoptosis: Requirement of integrins, Src kinases, and
ERKs. Am J Physiol Cell Physiol. 289:C633–C643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Ji B, Liu XS, Nakatani E, Iezaki
T, Nakajima H, Fujita H, Takahata Y, Hidano S and Kobayashi T:
Osteocyte-viability-based simulations of trabecular bone loss and
recovery in disuse and reloading. Biomech Model Mechanobiol.
13:153–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aguirre JI, Plotkin LI, Stewart SA,
Weinstein RS, Parfitt AM, Manolagas SC and Bellido T: Osteocyte
apoptosis is induced by weightlessness in mice and precedes
osteoclast recruitment and bone loss. J Bone Miner Res. 21:605–615.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bellido T: Osteocyte-driven bone
remodeling. Calcif Tissue Int. 94:25–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gross TS, Akeno N, Clemens TL, Komarova S,
Srinivasan S, Weimer DA and Mayorov S: Selected contribution:
Osteocytes upregulate HIF-1alpha in response to acute disuse and
oxygen deprivation. J Appl Physiol (1985). 90:2514–2519.
2001.PubMed/NCBI
|
|
27
|
Noble BS, Peet N, Stevens HY, Brabbs A,
Mosley JR, Reilly GC, Reeve J, Skerry TM and Lanyon LE: Mechanical
loading: Biphasic osteocyte survival and targeting of osteoclasts
for bone destruction in rat cortical bone. Am J Physiol Cell
Physiol. 284:C934–C943. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jung TW, Lee KT, Lee MW and Ka KH: SIRT1
attenuates palmitate-induced endoplasmic reticulum stress and
insulin resistance in HepG2 cells via induction of oxygen-regulated
protein 150. Biochem Biophys Res Commun. 422:229–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuwabara K, Matsumoto M, Ikeda J, Hori O,
Ogawa S, Maeda Y, Kitagawa K, Imuta N, Kinoshita T, Stern DM, et
al: Purification and characterization of a novel stress protein,
the 150-kDa oxygen-regulated protein (ORP150), from cultured rat
astrocytes and its expression in ischemic mouse brain. J Biol Chem.
271:5025–5032. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo D, Keightley A, Guthrie J, Veno PA,
Harris SE and Bonewald LF: Identification of osteocyte-selective
proteins. Proteomics. 10:3688–3698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boraldi F, Annovi G, Carraro F, Naldini A,
Tiozzo R, Sommer P and Quaglino D: Hypoxia influences the cellular
cross-talk of human dermal fibroblasts. A proteomic approach.
Biochim Biophys Acta. 1774:1402–1413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ozawa K, Kuwabara K, Tamatani M, Takatsuji
K, Tsukamoto Y, Kaneda S, Yanagi H, Stern DM, Eguchi Y, Tsujimoto
Y, et al: 150-kDa oxygen-regulated protein (ORP150) suppresses
hypoxia-induced apoptotic cell death. J Biol Chem. 274:6397–6404.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kretowski R, Borzym-Kluczyk M and
Cechowska-Pasko M: Hypoxia enhances the senescence effect of
bortezomib-the proteasome inhibitor-on human skin fibroblasts.
Biomed Res Int. 2014:1962492014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kretowski R, Stypulkowska A and
Cechowska-Pasko M: Low-glucose medium induces ORP150 expression and
exerts inhibitory effect on apoptosis and senescence of human
breast MCF7 cells. Acta Biochim Pol. 60:167–173. 2013.PubMed/NCBI
|
|
35
|
Tamatani M, Matsuyama T, Yamaguchi A,
Mitsuda N, Tsukamoto Y, Taniguchi M, Che YH, Ozawa K, Hori O,
Nishimura H, et al: ORP150 protects against
hypoxia/ischemia-induced neuronal death. Nat Med. 7:317–323. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bonewald LF: Establishment and
characterization of an osteocyte-like cell line, MLO-Y4. J Bone
Miner Metab. 17:61–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kato Y, Windle JJ, Koop BA, Mundy GR and
Bonewald LF: Establishment of an osteocyte-like cell line, MLO-Y4.
J Bone Miner Res. 12:2014–2023. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Raleigh JA, Chou SC, Bono EL, Thrall DE
and Varia MA: Semiquantitative immunohistochemical analysis for
hypoxia in human tumors. Int J Radiat Oncol Biol Phys. 49:569–574.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahuja SS, Zhao S, Bellido T, Plotkin LI,
Jimenez F and Bonewald LF: CD40 ligand blocks apoptosis induced by
tumor necrosis factor alpha, glucocorticoids, and etoposide in
osteoblasts and the osteocyte-like cell line murine long bone
osteocyte-Y4. Endocrinology. 144:1761–1769. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jilka RL, Weinstein RS, Bellido T, Parfitt
AM and Manolagas SC: Osteoblast programmed cell death (apoptosis):
Modulation by growth factors and cytokines. J Bone Miner Res.
13:793–802. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Plotkin LI, Weinstein RS, Parfitt AM,
Roberson PK, Manolagas SC and Bellido T: Prevention of osteocyte
and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin
Invest. 104:1363–1374. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Al Hadi H, Smerdon GR and Fox SW:
Hyperbaric oxygen therapy suppresses osteoclast formation and bone
resorption. J Orthop Res. 31:1839–1844. 2013.PubMed/NCBI
|
|
44
|
Arnett TR: Acidosis, hypoxia and bone.
Arch Biochem Biophys. 503:103–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hirao M, Hashimoto J, Yamasaki N, Ando W,
Tsuboi H, Myoui A and Yoshikawa H: Oxygen tension is an important
mediator of the transformation of osteoblasts to osteocytes. J Bone
Miner Metab. 25:266–276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Harrison JS, Rameshwar P, Chang V and
Bandari P: Oxygen saturation in the bone marrow of healthy
volunteers. Blood. 99:3942002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hou M, Liu J, Liu F, Liu K and Yu B: C1q
tumor necrosis factor-related protein-3 protects mesenchymal stem
cells against hypoxia- and serum deprivation-induced apoptosis
through the phosphoinositide 3-kinase/Akt pathway. Int J Mol Med.
33:97–104. 2014.PubMed/NCBI
|
|
48
|
Rouault-Pierre K, Lopez-Onieva L, Foster
K, Anjos-Afonso F, Lamrissi-Garcia I, Serrano-Sanchez M, Mitter R,
Ivanovic Z, de Verneuil H, Gribben J, et al: HIF-2α protects human
hematopoietic stem/progenitors and acute myeloid leukemic cells
from apoptosis induced by endoplasmic reticulum stress. Cell Stem
Cell. 13:549–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cechowska-Pasko M, Bankowski E and Chene
P: The effect of hypoxia on the expression of 150 kDa
oxygen-regulated protein (ORP 150) in HeLa cells. Cell Physiol
Biochem. 17:89–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kitano H, Nishimura H, Tachibana H,
Yoshikawa H and Matsuyama T: ORP150 ameliorates
ischemia/reperfusion injury from middle cerebral artery occlusion
in mouse brain. Brain Res. 1015:122–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ogawa S: ORP150 (150 kDa oxygen regulated
protein) suppressed neuronal cell death. Nihon Yakurigaku Zasshi.
121:43–48. 2003.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Riezzo I, Neri M, De Stefano F, Fulcheri
E, Ventura F, Pomara C, Rabozzi R, Turillazzi E and Fineschi V: The
timing of perinatal hypoxia/ischemia events in term neonates: A
retrospective autopsy study. HSPs, ORP-150 and COX2 are reliable
markers to classify acute, perinatal events. Diagn Pathol.
5:492010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Arrington DD and Schnellmann RG: Targeting
of the molecular chaperone oxygen-regulated protein 150 (ORP150) to
mitochondria and its induction by cellular stress. Am J Physiol
Cell Physiol. 294:C641–C650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Aleshin AN, Sawa Y, Kitagawa-Sakakida S,
Bando Y, Ono M, Memon IA, Tohyama M, Ogawa S and Matsuda H: 150-kDa
oxygen-regulated protein attenuates myocardial ischemia-reperfusion
injury in rat heart. J Mol Cell Cardiol. 38:517–525. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Matsusue A, Hara K, Kageura M, Kashiwagi
M, Lu W, Ishigami A, Gotohda T, Tokunaga I, Nisimura A, Sugimura T
and Kubo S: An autopsy case of rhabdomyolysis related to vegetamin
and genetic analysis of the rhabdomyolysis-associated genes. J
Forensic Leg Med. 17:46–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dallas SL and Bonewald LF: Dynamics of the
transition from osteoblast to osteocyte. Ann N Y Acad Sci.
1192:437–443. 2010. View Article : Google Scholar : PubMed/NCBI
|