Introduction
Cardiovascular diseases (CVDs), particularly
ischemic heart disease, are the primary cause of disability and
mortality worldwide. The World Health Organization estimated that
17.5 million people succumbed to CVDs in 2012, and 7.4 million
deaths were attributed to ischemic heart disease (1). The pathogenesis of myocardial
ischemia, which is caused by atherosclerotic plaques or coronary
artery occlusion (2), is
considered to be multifactorial, and is associated with oxidative
stress (3), mitochondrial
dysfunction (4) and apoptotic
cascade activation (5).
Conversely, antioxidants may decrease cellular injury and apoptosis
in ischemic hearts through radical-scavenging activities (6).
Natural products-based therapeutic strategies have
been suggested as a potential option for the treatment of patients
with myocardial ischemia (7).
Dendrobium officinale Kimura et Migo (Orchidaceae family) is
a prized traditional Chinese medicine, which has been used
clinically to promote body fluid production and maintain gastric
tonicity in China and Southeast Asia (8). Dendrobium officinale has been
reported to possess diverse pharmacological properties, including
anti-inflammatory (9),
immunomodulatory (10),
neuroprotective (11) and
antitumor (12) activities.
Dendrobium officinale polysaccharides, which are a potential
candidate for the treatment of Sjögren's syndrome (13), were able to suppress the
overexpression of proinflammatory cytokines, including tumor
necrosis factor-α, interleukin (IL)-1β and IL-6, in a mouse model
of Sjögren's syndrome, and inhibited apoptosis by downregulating
the expression of caspase-3 and decreasing the B-cell lymphoma 2
(Bcl-2)-associated X protein/Bcl-2 ratio (9). Dendrobium officinale
polysaccharides also possess immunomodulatory activity in
vivo and in vitro, which is mediated by nitric oxide
(10). Furthermore, dendrocandins
extracted from Dendrobium officinale have been reported to
promote neurite outgrowth in PC12 cells (14). Considering the antiaggregation
activity of other Dendrobium species (15), the present study hypothesized that
the water extract of Dendrobium officinale (DOE) may exert
cardioprotective effects against myocardial ischemia.
The homeodomain protein Meis1 is a three amino acid
loop extension transcription factor, which participates in various
physiological processes, including growth, proliferation and
prevention of the accumulation of reactive oxygen species (16). Specifically, the Meis1/pre-B-cell
leukemia homeobox 1/homeobox A9 complex is able to activate
oncogenic genes, resulting in cell hyperproliferation (17). Meis1 is also required to establish
definitive hematopoiesis in the mouse embryo, and its deletion
in vivo may lead to agenesis of the megakaryocyte lineage
and localized defects in vascular patterning (18). In terms of normal cardiac
development, Meis1 acts as a regulator of cardiomyocyte cell cycle
arrest and a potential transcriptional regulator of neonatal heart
regeneration (19).
The present study aimed to evaluate the
cardioprotective effects of DOE in vivo. Briefly, myocardial
ischemia was induced in mice following ligation of the left
anterior descending coronary artery (LAD). Subsequently, the
effects of water-soluble components of Dendrobium officinale
were investigated on myocardial injury, and the potential
underlying mechanisms against cardiomyopathy were evaluated. The
results suggested that pretreatment with DOE significantly
decreased infarct size; reduced creatine kinase (CK)-MB and lactate
dehydrogenase (LDH) levels in serum; and attenuated cardiac
oxidative damage.
Materials and methods
Chemicals and materials
CK-MB (cat. no. H197), LDH (cat. no. A020-1), total
superoxide dismutase (SOD; cat. no. A001-1) and malondialdehyde
(MDA; cat. no. A003-1) diagnostic agents were obtained from Nanjing
Jiancheng Bioengineering Research Institute (Nanjing, China). Meis1
antibody (cat. no. BS3488) was obtained from Bioworld Technology,
Co., Ltd. (Nanjing, China). GAPDH polyclonal primary antibody (cat.
no. 10494-1-AP) and goat anti-rabbit horseradish
peroxidase-conjugated immunoglobulin G secondary antibody (cat. no.
SA00001-2) were purchased from Wuhan Sanying Biotechnology (Wuhan,
China). All other reagents used were of commercial analytical
grade.
DOE preparation
The dry stems of Dendrobium officinale were
purchased from Xi'an Xiaocao Botanical Development Co., Ltd.
(Xi'an, China). The voucher specimens were deposited at Southwest
University (Chongqing, China). Briefly, the dry stems were ground
into a fine powder through a 100-mesh filter. The powdered
materials (100 g) were pre-extracted using petroleum ether and the
residues were then extracted three times using hot distilled water.
The crude extracts were filtered through a 0.22 µm microporous
membrane, concentrated via the alcohol precipitation method and
centrifuged at 625 × g for 10 min. The extracts were then
dried by lyophilization, and 23.5 g powder extracts were produced.
The DOE predominantly consisted of polysaccharide, and the
concentration of polysaccharide was determined by the
phenol-sulfuric acid method to be 97.4%.
Experimental animals
A total of 60 Kunming male mice (age, 4 weeks;
weight, 22±2 g) were purchased from Chongqing Tengxin
Bio-technology Co., Ltd. (Chongqing, China). The present study was
carried out according to recommendations of the National Institutes
of Health Guide for the Care and Use of Laboratory Animals (2015).
In addition, the experiments were approved by the Ethical Committee
for Animals of Southwest University. The mice were housed in
standard conditions: Humidity, 50%; temperature, 25±2°C, under a
12:12 h light/dark cycle, with ad libitum access to normal
food and tap water. Mice were allowed to acclimate to the
environment for 1 week prior to experimentation.
Experimental procedure
Mice were randomly assigned to five groups
(n=10/group): Sham group, model group, and LAD + DOE pretreatment
groups (75, 150 and 300 mg/kg DOE; oral administration). DOE was
dissolved in distilled water. Mice were administered normal saline
(10 ml/kg) or DOE (75, 150 or 300 mg/kg) intragastrically once
daily for 2 weeks. Subsequently, the mice were subjected to
coronary artery ligation; LAD occlusion was performed as previously
reported (20,21). Briefly, the left chest cavity of
the mice was opened and the heart was exposed following
anesthetization with sodium pentobarbital (40 mg/kg, i.p.).
Subsequently, in the model and LAD + DOE pretreatment groups, 7–0
silk was used to ligate the LAD for 24 h, after which the heart was
returned to its normal position and the thoracic cavity was closed.
In the sham group, 7–0 silk was passed through the LAD, without
ligation. After 24 h, mice were sacrificed by an overdose of
pentobarbital sodium (100 mg/kg), and blood and heart samples were
collected.
Electrocardiogram (ECG) record
The lead II ECG was recorded using the BL-420F
biological function experiment system (Chengdu Taimeng Technology
Co. Ltd., Chengdu, China). Significant ECG changes, including
elevation of the ST segment and widening of the QRS complex,
indicated successful coronary occlusion.
Determination of infarct size
A total of 24 h after ischemia, the hearts were
collected and dissected at 1.2 mm cross-section. Subsequently, the
heart sections were stained with 1% triphenyl tetrazolium chloride
(TTC) for 15 min at 37°C in 0.2 M phosphate-buffered saline
(22). The viable myocardium was
stained red, whereas the infarcted tissue remained pale. Images
were captured and the infarct size was analyzed using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Determination of serum CK-MB, LDH, SOD
and MDA activity
Blood samples were collected and were centrifuged at
625 × g for 10 min at 4°C to obtain serum samples. The
CK-MB, LDH, SOD and MDA activity levels in the serum samples were
determined using commercially available kits according to the
manufacturer's protocols.
Histopathological examination
Heart tissues harvested from the mice were washed
immediately with ice-cold saline. The biopsies were then fixed in
4% neutral paraformaldehyde and were embedded in paraffin. Heart
tissues were cut into 5 µm sections, which were stained with
hematoxylin and eosin (H&E) for 90 min at room temperature.
Morphological evaluation and observation were performed under a
light microscope.
Terminal deoxynucleotidyl transferase
mediated dUTP nick end labeling (TUNEL) assay
TUNEL assays were performed as previously reported
(23). The total number of
TUNEL-positive cardiomyocyte nuclei in the infarcted zone was
counted. Individual nuclei were visualized at a magnification of
400x (Olympus CKX41; Olympus Corporation, Tokyo, Japan), and the
percentage of apoptotic nuclei (apoptotic nuclei/total nuclei) was
calculated in six randomly selected fields per section and averaged
for statistical analysis.
Western blotting
Heart tissues were lysed in radioimmunoprecipitation
assay buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, 1% SDS, 1 mM phenylmethylsulfonyl fluoride],
and concentration was determined using the bicinchoninic acid
method. Heart tissue lysates (100 µg) were separated by 12%
SDS-PAGE, and the proteins were electrophoretically transferred to
polyvinylidene difluoride membranes. After blocking non-specific
binding sites for 2 h with 5% dried skim milk at room temperature,
the membranes were individually exposed to primary antibodies
(anti-Meis1, 1:6,000; anti-GAPDH, 1:10,000) at 4°C overnight. The
membranes were subsequently incubated for 2 h at room temperature
with HRP-conjugated secondary anti-rabbit antibodies. The
immunoreactive proteins were detected using an enhanced
chemiluminescence reagent (Advansta Inc., Menlo Park, CA, USA). The
proteins were visualized and representative images were obtained.
Densitometric analysis was performed using Quantity One 4.6.2
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and GAPDH
expression was used as an internal standard.
Statistical analysis
All statistical analyses were performed using SPSS
statistical software (version 16.0 for Windows; SPSS Inc., Chicago,
IL, USA). Data are presented as the mean ± standard deviation.
Statistical comparisons were performed using one-way analysis of
variance followed by Tukey's post-hoc test for multiple group
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of DOE on ECG parameters
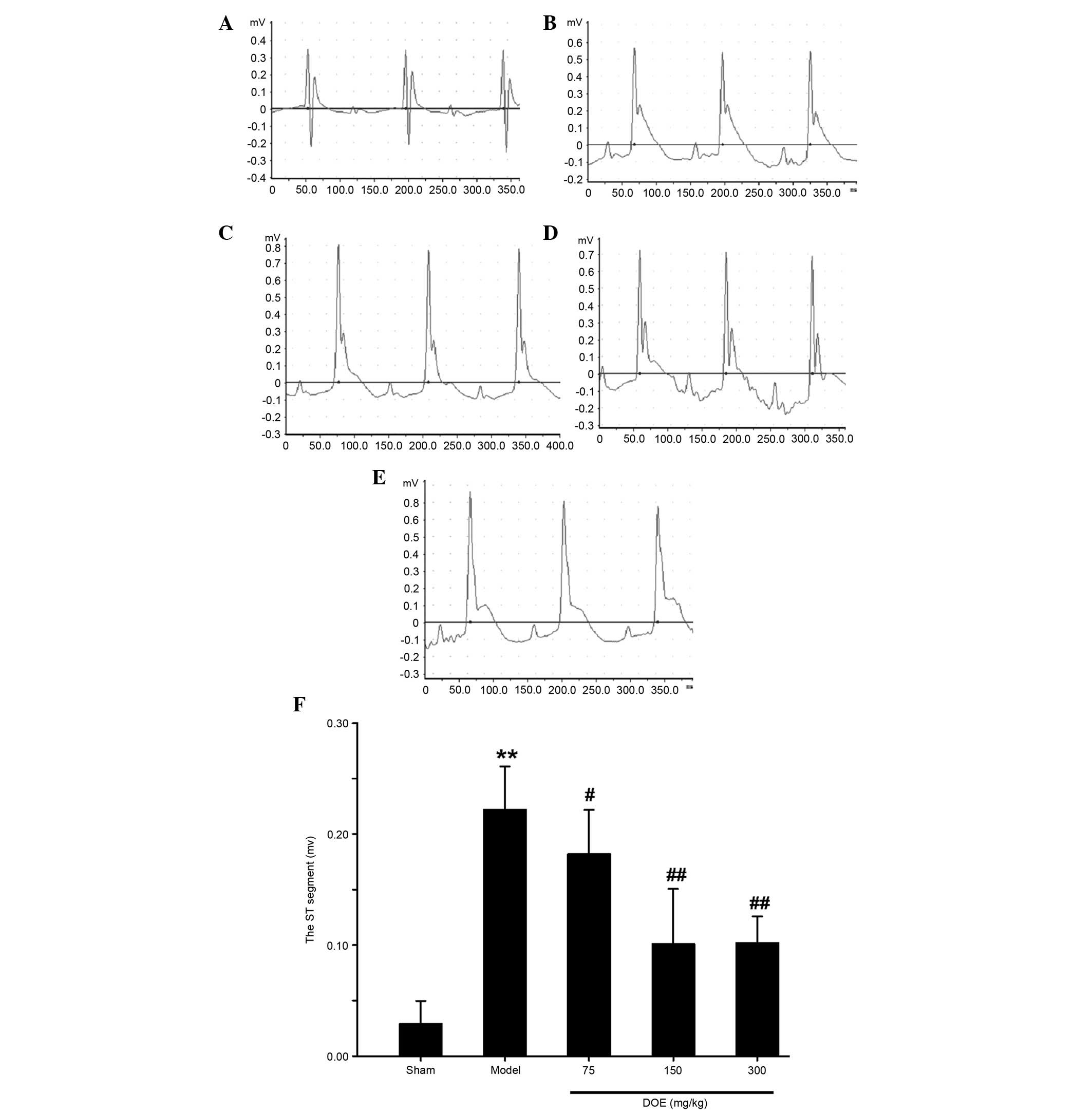
The Lead II ECG is presented in Fig. 1. Marked elevation of the ST segment
was detected in the myocardial ischemia model group, whereas a
normal ECG was observed in the sham group. Ischemia-induced
alterations to the ECG were significantly ameliorated by
pretreatment with DOE, as compared with in the model group.
Effects of DOE on infarct size
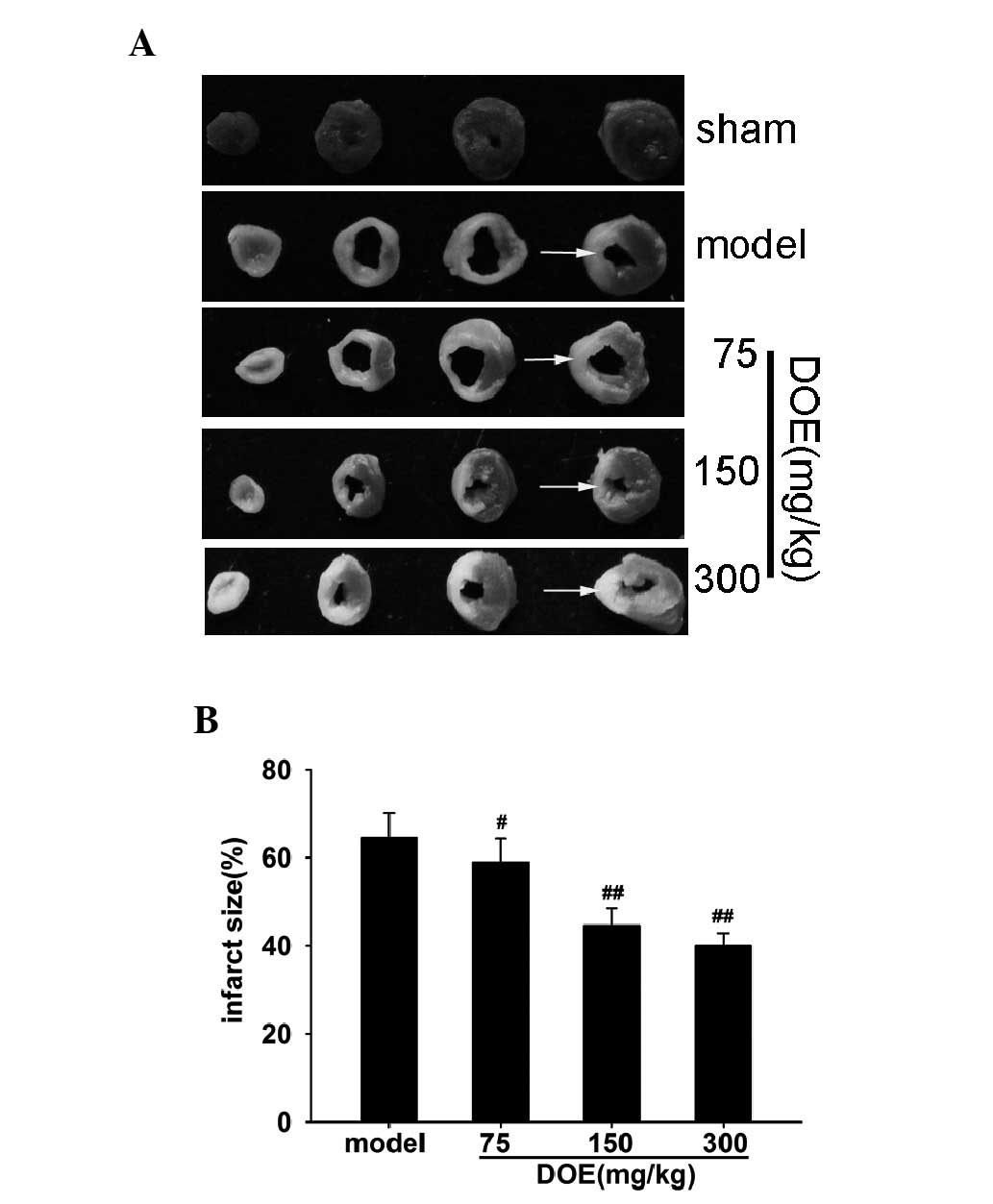
To evaluate infarct size, TTC staining was
performed. The cross-section of the heart stained with TTC is
presented in Fig. 2A. As shown in
Fig. 2B, DOE markedly decreased
infarct size compared with the model group. A modest reduction in
infarct size was detected in mice treated with 75 mg/kg DOE (5.6%
decrease). Notably, pretreatment with 150 mg/kg DOE more
significantly reduced infarct size (10.0% decrease).
Effects of DOE on serum CK-MB and LDH
levels
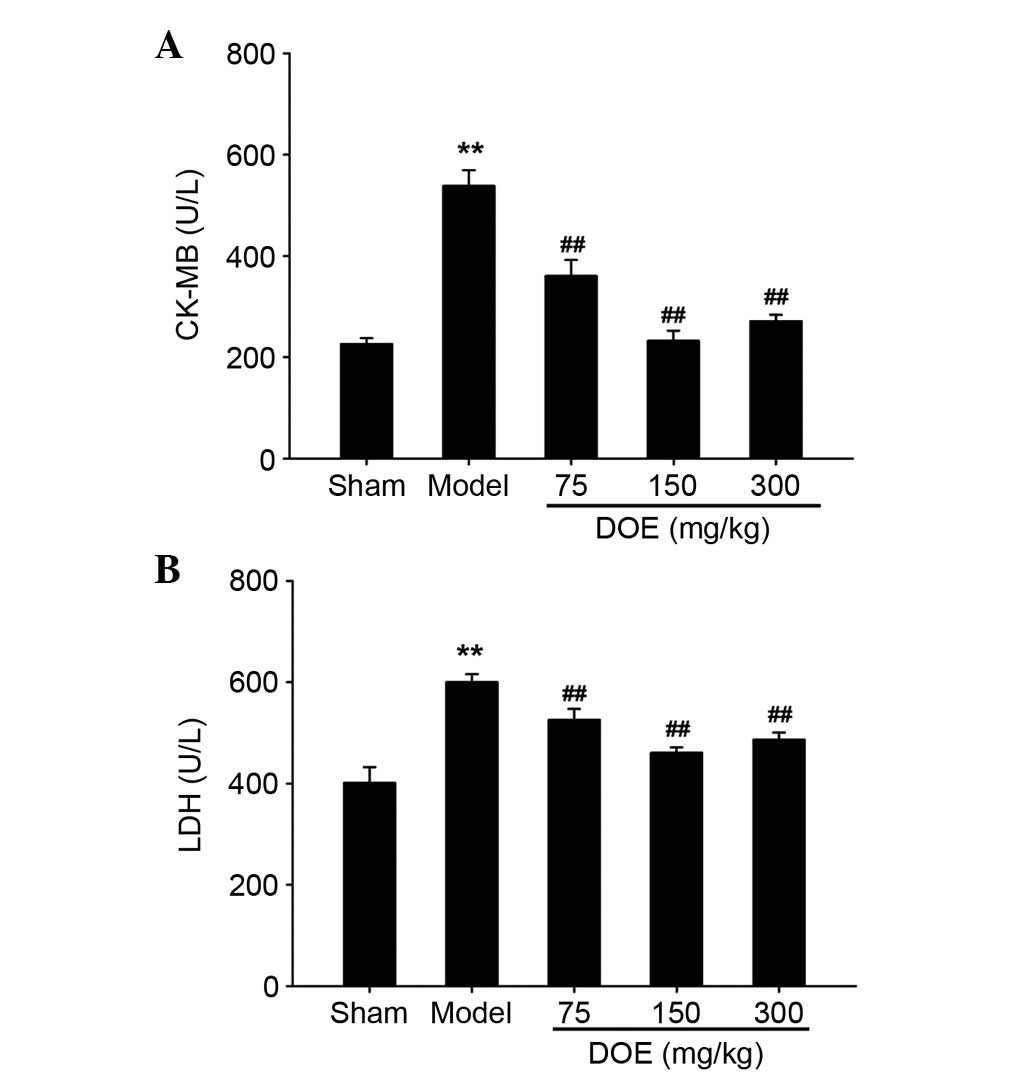
As shown in Fig. 3,
CK-MB and LDH activities, which are indicators of myocardial
ischemia, were significantly increased in the myocardial ischemia
model group compared with the sham group. Conversely, pretreatment
with DOE for 2 weeks decreased myocardial CK-MB and LDH release.
Furthermore, the most obvious reduction was exhibited in mice
pretreated with the middle dose of DOE (150 mg/kg). However, there
was no marked difference in the alterations in CK-MB and LDH levels
between the middle dose group (150 mg/kg) and the high dose group
(300 mg/kg).
Effects of DOE on serum MDA and SOD
levels
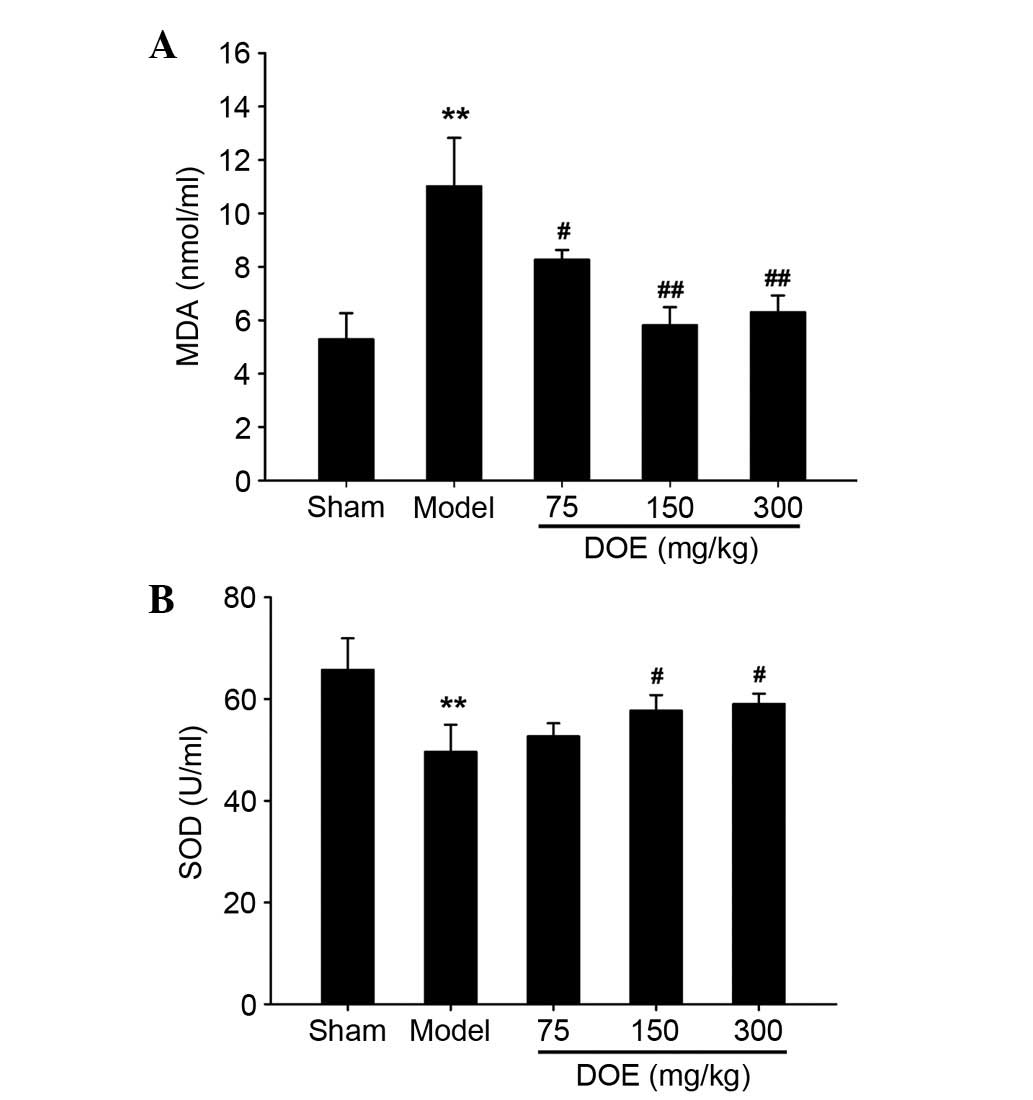
Myocardial ischemia is associated with oxidative
stress (24); therefore, MDA and
SOD levels were measured in the present study. As shown in Fig. 4, MDA activity was markedly
increased in the model group, whereas pretreatment with DOE for 2
weeks decreased serum MDA levels. Furthermore, the most obvious
decrease was observed in the middle dose group (150 mg/kg).
Conversely, SOD activity was reduced in the model group compared
with the sham group, whereas SOD levels were increased by
pretreatment with DOE, as compared with in the model group.
However, no significant change was detected in serum SOD levels
between the low dose DOE group (75 mg/kg) and the model group.
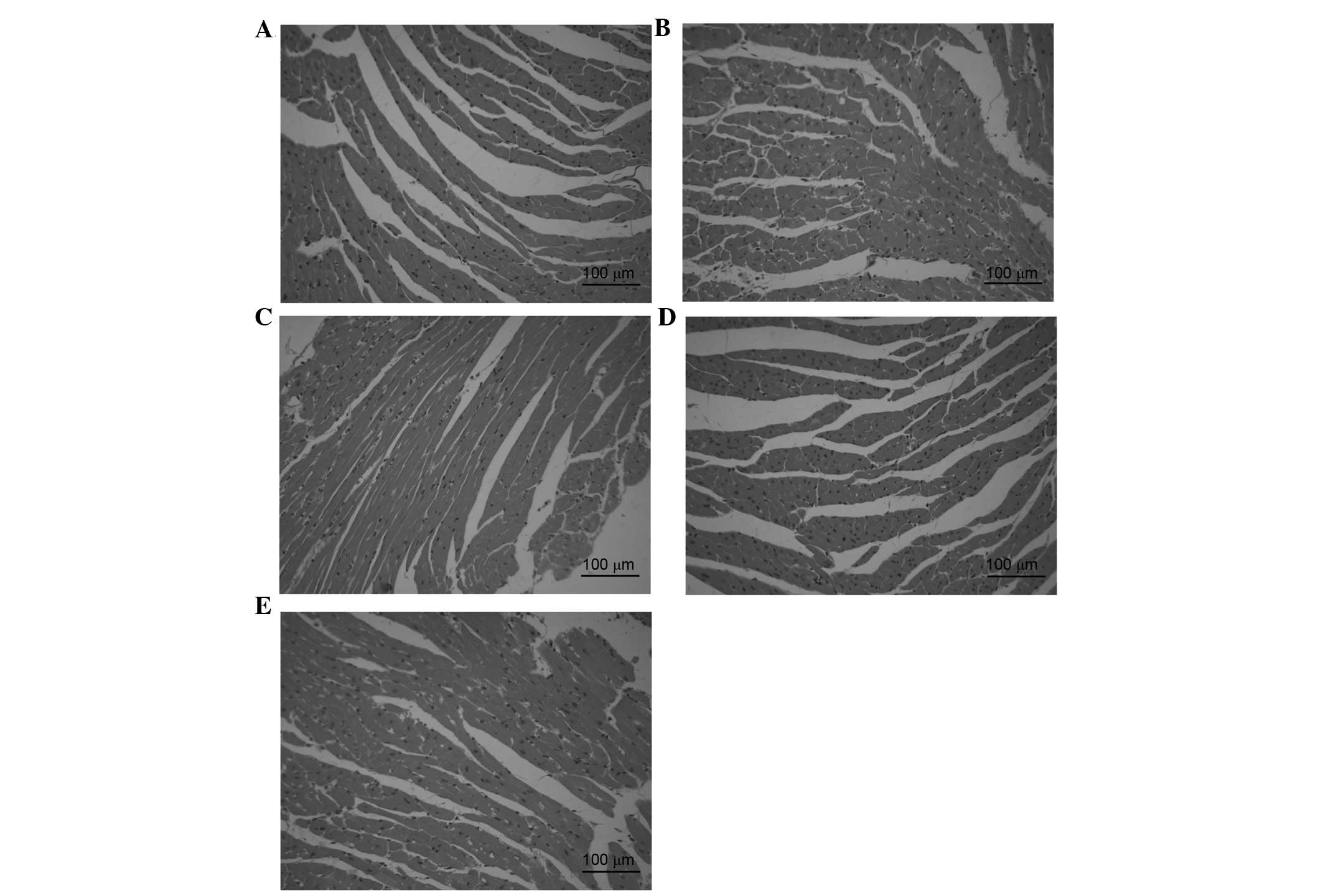
Effects of DOE on histopathological
myocardial alterations
Histopathological evaluation of cardiac tissue was
conducted using H&E staining, as presented in Fig. 5. The cardiac tissue in the sham
group exhibited a clear structure without infiltration of
inflammatory cardiomyocytes (Fig.
5A). However, myocardial structural abnormalities, including
cytoplasmic vacuolization, cardiomyocyte necrosis and inflammatory
infiltration, were detected in the model group (Fig. 5B). Conversely, the presence of
necrotic cardiomyocytes was rare, and vacuolization and
myofibrillar loss were almost undetectable following pretreatment
with DOE (Fig. 5C-E).
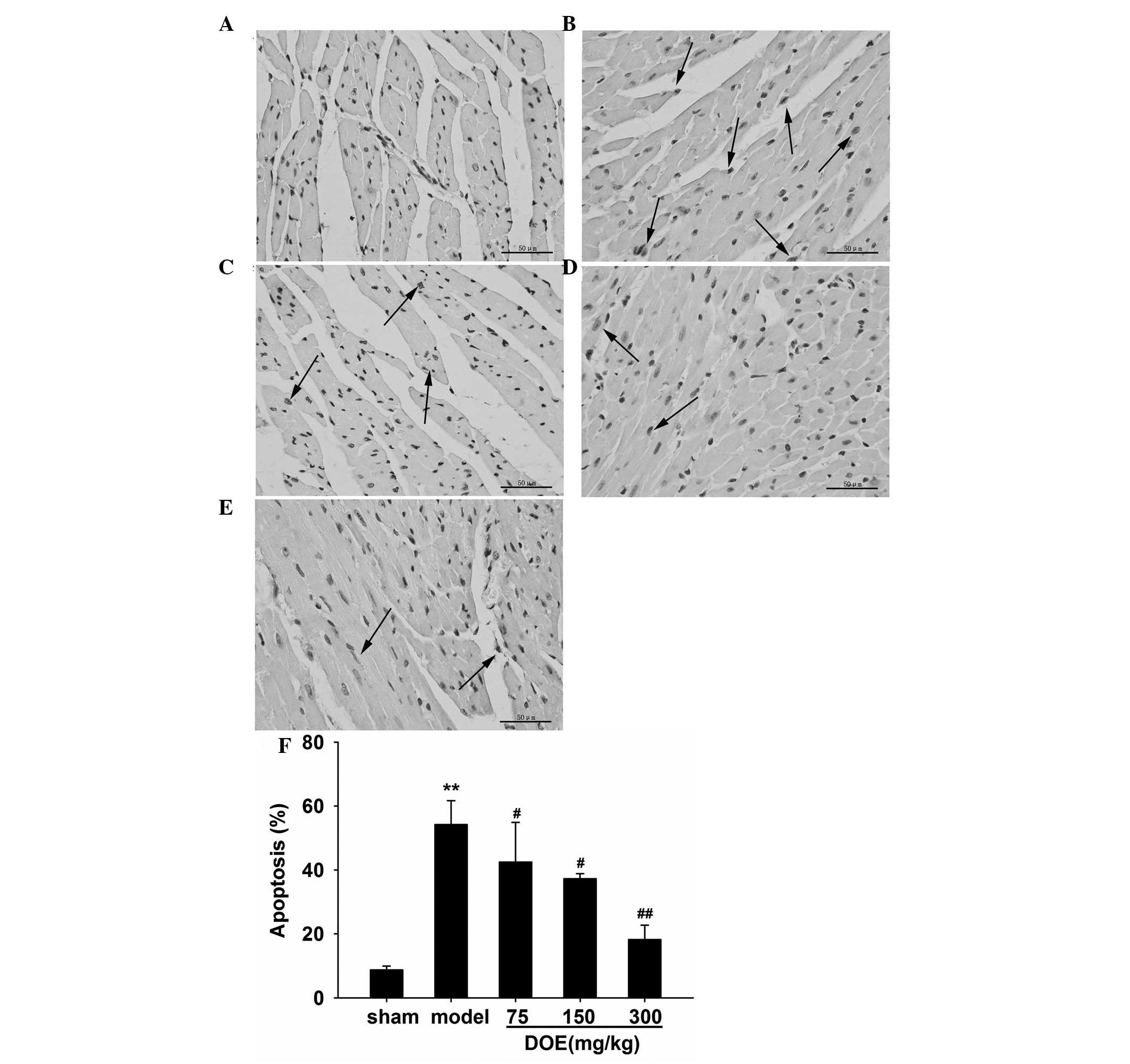
Effects of DOE on myocardial
apoptosis
Apoptotic alterations in cardiac tissue and the
percentage of TUNEL-positive cells are presented in Fig. 6. As shown in Fig. 6A, TUNEL-positive cells were rarely
detected in the sham group. However, the number of TUNEL-positive
cells was markedly increased in the myocardial ischemia model group
(Fig. 6B). The number of
TUNEL-positive cells was significantly decreased (42.5±12.4,
37.3±1.6 and 18.2±4.5%) by pretreatment with DOE (75, 150 and 300
mg/kg) compared with the model group (54.2±7.5%).
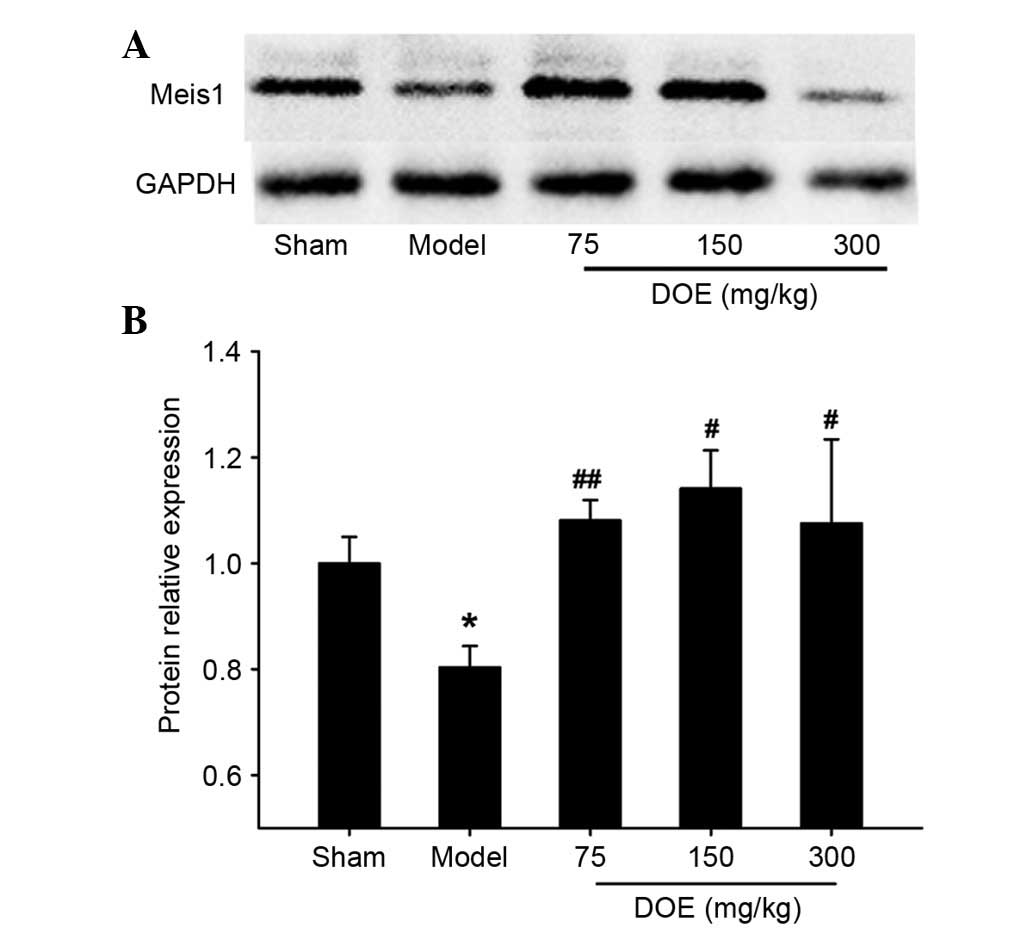
Effects of DOE on the expression of
Meis1
To determine the underlying mechanism of DOE, the
protein expression levels of Meis1 were assessed by western
blotting. The results presented in Fig. 7 indicate that the expression levels
of Meis1 were significantly decreased in the model group compared
with the sham group. Conversely, pretreatment with DOE (75 and 150
mg/kg) markedly upregulated the expression of Meis1.
Discussion
The present study demonstrated that pretreatment
with DOE significantly inhibited ST segment elevation, reduced
infarct size and attenuated cardiomyocyte apoptosis. Furthermore,
DOE prevented CK-MB and LDH release, decreased serum MDA levels,
increased serum SOD levels and upregulated the protein expression
levels of Meis1. These results suggested that DOE may serve a
protective role against ischemic cardiomyopathy, and may be
considered a potential clinical agent in the attenuation and
prevention of ischemic injury.
ECG is an important clinical tool used to evaluate
heart function. ST segment monitoring is sensitive during the
development of ischemia (25). ST
segment elevation, which is a reliable biomarker for the diagnosis
of myocardial ischemia, is associated with continuous damage to the
cell membrane (26). In the
present study, ST segment elevation was observed in the model
group; however, ST segment elevation was alleviated by pretreatment
with DOE. These data indicated that DOE may ameliorate cardiac
ischemia.
TTC staining was used to evaluate infarct size. TTC
is considered a proton acceptor for pyridine
nucleotide-linked-dehydrogenases together with cytochromes, which
comprise an integral part of the inner mitochondrial membrane and
form the electron transport chain (27). Tetrazolium salt is reduced by these
enzymes into lipid formazan in cardiac tissue, resulting in red
staining. Therefore, viable tissue is stained red, whereas
infarcted tissue is stained white. The present study demonstrated
that DOE administration significantly reduced LAD ligation-induced
infarct size.
The cytosolic enzymes CK-MB and LDH are sensitive
markers used to diagnosis myocardial ischemia (28). When the cell is damaged, the cell
membrane may be ruptured or become permeable, resulting in the
leakage of CK-MB and LDH from the damaged cardiomyocytes to the
bloodstream (29). The results of
the present study detected a marked increase in CK-MB and LDH in
the mouse model of myocardial ischemia. A previous study indicated
that increases in serum CK-MB and LDH were associated with severe
damage to myocardial tissue (30).
Notably, the elevated levels of serum CK-MB and LDH in the model
group were significantly reduced by pretreatment with DOE. These
results indicated that DOE may attenuate damage to cardiomyocytes,
reduce the leakage of cardiac markers and suppress the development
of myocardial ischemia.
Free radicals are generated and accumulated during
acute myocardial ischemia. MDA, which is a lipid peroxidation
product, has been used to determinate the levels of oxygen free
radicals mediated by myocardial injury (31). The present study demonstrated that
DOE significantly reduced serum MDA levels, thus indicating that
DOE may exert its protective effects against myocardial ischemia by
reducing lipid peroxidation. SOD, which is an antioxidant enzyme,
is beneficial in the scavenging of free radicals (32,33).
Pretreatment with 150 mg/kg DOE induced a significant increase in
serum SOD activity, as compared with in the model group. These
results indicated that DOE may affect endogenous antioxidants or
oxidative stress. Previous studies reported that oxidative stress
serves important roles in myocardial ischemia (3,24);
therefore, the antioxidant effects of DOE may prevent the
deleterious effects of oxidative stress on the development of
ischemia.
The protective effects of DOE against myocardial
ischemia were further confirmed by histopathological examination.
Basophilic components, including the nucleus and endoplasmic
reticulum, are stained blue by hematoxylin, whereas
eosinophilic-like proteins are stained pink by eosin (34). Mild cardiomyocyte necrosis and
reduced inflammatory infiltration were detected in the DOE
pretreatment groups, thus suggesting that DOE may alleviate cardiac
infarction and decrease inflammatory cell recruitment. Apoptosis
had been observed in cardiac pathologies, including hypoxia,
ischemia reperfusion and myocardial ischemia (5). In the present study, TUNEL assays
demonstrated that pretreatment with DOE decreased the degree of
apoptosis in the heart. These data suggested that DOE may attenuate
cardiac injury through inhibiting myocardial apoptosis.
Meis1 is a critical transcriptional regulator in
cardiomyocyte proliferation, which may be considered a potential
therapeutic target for heart regeneration (19). Stankunas et al examined
cardiac development in mice lacking Meis1 and demonstrated that
embryos without Meis1 displayed subcutaneous hemorrhage and died
between embryonic day 14.5 and 15.5, confirming that Meis1 is
essential for cardiac development (35). In the present study, the expression
levels of Meis1 were decreased in the model group. However, this
decrease was markedly ameliorated by pretreatment with DOE (75 and
150 mg/kg); this may be a potential mechanism underlying the
protective effects of DOE against myocardial ischemia. Therefore,
Meis1 may be a novel therapeutic target for the development of
anti-ischemia drugs. However, further studies are required to
confirm this hypothesis.
In conclusion, the present study demonstrated that
DOE possessed cardioprotective potential against LAD
ligation-induced myocardial ischemia. The underlying mechanism is
possibly associated with restoration of antioxidant enzyme
activity, inhibition of apoptosis and upregulation of Meis1
expression. These findings may be beneficial for patients with
myocardial ischemia; however, further studies are required to
elucidate the precise molecular mechanisms underlying the effects
of DOE.
Acknowledgements
The present study was supported by the Chongqing
Science and Technology Commission (no. cstc2016jcyjA0296), the
Fundamental Research Funds for the Central University (nos.
XDJK2014B024 and XDJK2016E137), and the Innovative Project on
Designing and Screening Drug Candidates of Chongqing (no.
cstc2015zdcy-ztzx120003).
References
|
1
|
World Health Organization, . Global status
report on noncommunicable disease 2014. World Health Organization;
Geneva, Switzerland: pp. 9–14. 2014
|
|
2
|
Gao E, Lei YH, Shang X, Huang ZM, Zuo L,
Boucher M, Fan Q, Chuprun JK, Ma XL and Koch WJ: A novel and
efficient model of coronary artery ligation and myocardial
infarction in the mouse. Circ Res. 107:1445–1453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hori M and Nishida K: Oxidative stress and
left ventricular remodelling after myocardial infarction.
Cardiovasc Res. 81:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun J, Nguyen T, Aponte AM, Menazza S,
Kohr MJ, Roth DM, Patel HH, Murphy E and Steenbergen C: Ischaemic
preconditioning preferentially increases protein S-nitrosylation in
subsarcolemmal mitochondria. Cardiovasc Res. 106:227–236. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abbate A, Bussani R, Amin MS, Vetrovec GW
and Baldi A: Acute myocardial infarction and heart failure: Role of
apoptosis. Int J Biochem Cell Biol. 38:1834–1840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han SY, Li HX, Ma X, Zhang K, Ma ZZ and Tu
PF: Protective effects of purified safflower extract on myocardial
ischemia in vivo and in vitro. Phytomedicine. 16:694–702. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma X, Zhang K, Li H, Han S, Ma Z and Tu P:
Extracts from Astragalus membranaceus limit myocardial cell death
and improve cardiac function in a rat model of myocardial ischemia.
J Ethnopharmacol. 149:720–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ng TB, Liu J, Wong JH, Ye X, Sze SC Wing,
Tong Y and Zhang KY: Review of research on Dendrobium, a prized
folk medicine. Appl Microbiol Biotechnol. 93:1795–1803. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin X, Shaw PC, Sze SC, Tong Y and Zhang
Y: Dendrobium officinale polysaccharides ameliorate the abnormality
of aquaporin 5, pro-inflammatory cytokines and inhibit apoptosis in
the experimental Sjögren's syndrome mice. Int Immunopharmacol.
11:2025–2032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia L, Liu X, Guo H, Zhang H, Zhu J and
Ren F: Partial characterization and immunomodulatory activity of
polysaccharides from the stem of Dendrobium officinale (Tiepishihu)
in vitro. J Funct Foods. 4:294–301. 2012. View Article : Google Scholar
|
|
11
|
Wang Q, Gong Q, Wu Q and Shi J:
Neuroprotective effects of Dendrobium alkaloids on rat cortical
neurons injured by oxygen-glucose deprivation and reperfusion.
Phytomedicine. 17:108–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao X, Sun P, Qian Y and Suo H: D.
candidum has in vitro anticancer effects in HCT-116 cancer cells
and exerts in vivo anti-metastatic effects in mice. Nutr Res Pract.
8:487–493. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin X, Sze SCW, Tong Y, Zhang Z, Feng Y,
Chen JP, Ng TB, Lin X, Shaw PC and Zhang KY: Protective effect of
Dendrobium officinale polysaccharides on experimental Sjögren's
syndrome. J Complement Integr Med. 7(1)2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Liu SJ, Luo HR, Cui J, Zhou J,
Wang XJ, Sheng J and Hu JM: Two new dendrocandins with neurite
outgrowth-promoting activity from Dendrobium officinale. J Asian
Nat Prod Res. 17:125–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CC, Wu LG, Ko FN and Teng CM:
Antiplatelet aggregation principles of Dendrobium Loddigesii. J Nat
Prod. 57:1271–1274. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui L, Li M, Feng F, Yang Y, Hang X, Cui J
and Gao J: Meis1 functions as a potential AR negative regulator.
Exp Cell Res. 328:58–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan X and Braun T: An unexpected switch:
Regulation of cardiomyocyte proliferation by the homeobox gene
meis1. Circ Res. 113:245–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azcoitia V, Aracil M, Martinez-A C and
Torres M: The homeodomain protein Meis1 is essential for definitive
hematopoiesis and vascular patterning in the mouse embryo. Dev
Biol. 280:307–320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahmoud AI, Kocabas F, Muralidhar SA,
Kimura W, Koura AS, Thet S, Porrello ER and Sadek HA: Meis1
regulates postnatal cardiomyocyte cell cycle arrest. Nature.
497:249–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahn D, Cheng L, Moon C, Spurgeon H,
Lakatta EG and Talan MI: Induction of myocardial infarcts of a
predictable size and location by branch pattern
probability-assisted coronary ligation in C57BL/6 mice. Am J
Physiol Heart Circ Physiol. 286:H1201–H1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Samsamshariat SA, Samsamshariat ZA and
Movahed MR: A novel method for safe and accurate left anterior
descending coronary artery ligation for research in rats.
Cardiovasc Revasc Med. 6:121–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chu W, Qiao G, Bai Y, Pan Z, Li G, Piao X,
Wu L, Lu Y and Yang B: Flavonoids from Chinese Viscum coloratum
produce cytoprotective effects against ischemic myocardial
injuries: Inhibitory effect of flavonoids on PAF-induced
Ca2+ overload. Phytother Res. 22:134–137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao XY, Li GY, Liu Y, Chai LM, Chen JX,
Zhang Y, Du ZM, Lu YJ and Yang BF: Resveratrol protects against
arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br J
Pharmacol. 154:105–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Plummer BN, Liu H, Wan X, Deschênes I and
Laurita KR: Targeted antioxidant treatment decreases cardiac
alternans associated with chronic myocardial infarction. Circ
Arrhythm Electrophysiol. 8:165–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Queenthy SS and John B: Diosmin exhibits
anti-hyperlipidemic effects in isoproterenol induced myocardial
infarcted rats. Eur J Pharmacol. 718:213–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gong LL, Fang LH, Wang SB, Sun JL, Qin HL,
Li XX, Wang SB and Du GH: Coptisine exert cardioprotective effect
through anti-oxidative and inhibition of RhoA/Rho kinase pathway on
isoproterenol-induced myocardial infarction in rats.
Atherosclerosis. 222:50–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Radhiga T, Rajamanickam C, Senthil S and
Pugalendi KV: Effect of ursolic acid on cardiac marker enzymes,
lipid profile and macroscopic enzyme mapping assay in
isoproterenol-induced myocardial ischemic rats. Food Chem Toxicol.
50:3971–3977. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Derbali A, Mnafgui K, Affes M, Derbali F,
Hajji R, Gharsallah N, Allouche N and El Feki A: Cardioprotective
effect of linseed oil against isoproterenol-induced myocardial
infarction in Wistar rats: A biochemical and electrocardiographic
study. J Physiol Biochem. 71:281–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He H, Xu J, Xu Y, Zhang C, Wang H, He Y,
Wang T and Yuan D: Cardioprotective effects of saponins from Panax
japonicus on acute myocardial ischemia against oxidative
stress-triggered damage and cardiac cell death in rats. J
Ethnopharmacol. 140:73–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quan W, Wu B, Bai Y, Zhang X, Yin J, Xi M,
Guan Y, Shao Q, Chen Y, Wu Q and Wen A: Magnesium lithospermate B
improves myocardial function and prevents simulated
ischemia/reperfusion injury-induced H9c2 cardiomyocytes apoptosis
through Akt-dependent pathway. J Ethnopharmacol. 151:714–721. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu L, Qiao H, Li Y and Li L: Protective
roles of puerarin and Danshensu on acute ischemic myocardial injury
in rats. Phytomedicine. 14:652–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qin F, Liu YX, Zhao HW, Huang X, Ren P and
Zhu ZY: Chinese medicinal formula Guan-Xin-Er-Hao protects the
heart against oxidative stress induced by acute ischemic myocardial
injury in rats. Phytomedicine. 16:215–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu C, Fu F, Yu X, Han B and Zhu M:
Cardioprotective effect of Ocotillol, a derivate of
pseudoginsenoside F11, on myocardial injury induced by
isoproterenol in rats. Arzneimittelforschung. 57:568–572.
2007.PubMed/NCBI
|
|
34
|
Borst O, Ochmann C, Schönberger T, Jacoby
C, Stellos K, Seizer P, Flögel U, Lang F and Gawaz M: Methods
employed for induction and analysis of experimental myocardial
infarction in mice. Cell Physiol Biochem. 28:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stankunas K, Shang C, Twu KY, Kao SC,
Jenkins NA, Copeland NG, Sanyal M, Selleri L, Cleary ML and Chang
CP: Pbx/Meis deficiencies demonstrate multigenetic origins of
congenital heart disease. Circ Res. 103:702–709. 2008. View Article : Google Scholar : PubMed/NCBI
|