Introduction
Ulcerative colitis (UC) is a non-specific type of
inflammation with an unknown cause. Lesions exhibit continuous and
diffuse distribution, primarily in the rectum and sigmoid colon of
the large intestine (1). Typical
clinical symptoms of UC include abdominal pain and diarrhea
(2). As a systemic disease, it may
lead to further manifestations in certain patients, including
enteropathic arthritis, bowel disease, hepatobiliary diseases
including primary sclerosing cholangitis, and eye and skin damage
(3). UC has a worldwide incidence
of 0.5–24.5 cases per 100,000. Notably, the incidence of UC is
lowest in developing countries, and highest in North America and
Western Europe (4). At present,
the incidence of UC in Central Europe and Eastern Europe is
increasing; however, it is steadily decreasing in Western Europe
and Scandinavia (5). UC was first
identified in 1859, yet its etiology remains unclear. A recent
study on the underlying molecular mechanisms of the disease has
furthered the understanding of the etiology of UC (6).
UC has numerous similarities with infectious
enteritis and can cause microbial inflammation of the intestinal
tract (7). However, no
microorganism has yet been identified to be associated with UC.
There may not be a single cause of the disease, as there is no
evidence of infection in patients with UC. In countries with high
incidences of UC the incidence of bowel infection is low and in
developing countries with poor sanitation, the consumption of
unprocessed food is a protective factor (8). The frequent use of antibiotics in
childhood leads to an increased risk of UC, and antimicrobial
agents are ineffective in the treatment of UC (9). Cultivation of feces from patients
with UC has provided inconsistent results. Increasing evidence has
indicated that there is an abnormal mucosal immune response between
intestinal bacteria and the mucous membrane in patients with UC
(10). Molecular biology
techniques have revealed that the adult intestinal space may
accommodate >50 types of bacteria, that strains gradually
increase in number along the small intestine and that Gram-negative
bacteria predominate (11). There
are up to ~1012 bacteria per cm in the large intestine.
Currently, >50% of strains cannot be cultured by humans
(12). D-limonene (Fig. 1) is a monoterpenoid, present in
citrus and numerous other plants (13). It has been demonstrated that
D-limonene may have broad anticancer properties. A previous study
(14) revealed that D-limonene has
significant inhibitory effects in animal models of breast, liver,
lung, stomach and skin cancers, without clear adverse reactions. In
addition, D-limonene may inhibit gastrointestinal reflux, promote
healthy motility of the intestines, dissolve gallstones, relieve
angina and prevent bacterial infection (15,16).
The present study aimed to investigate the potential
anti-inflammatory and antioxidant effects of D-limonene in a UC rat
model, and the underlying mechanisms.
Materials and methods
Materials
2,4,6-trinitrobenzenesulfonic acid solution and
D-limonene were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). Tumor necrosis factor-α (TNF-α; R019), interleukin
(IL)-1β (H002), IL-6, nuclear factor-κB (NF-κB; H202), superoxide
dismutase (SOD; A001-3), glutathione (GSH; A006-2) and
prostaglandin (PG) E2ELISA kits were obtained from the Nanjing
Jiancheng Bioengineering Institute (Jiangsu, China). A
bicinchoninic acid (BCA) assay kit was obtained from Fermentas;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Animal treatment and grouping
Healthy male Sprague-Dawley rats (weight, 220–300 g;
age, 8–10 weeks; n=32) were purchased from Changzhou Cavens
Laboratory Animal Co., Ltd. (Changzhou, China), housed at 23–24°C,
50–60% humidity, light/dark cycle (7:00-19:00) with free access to
food and water, and randomly divided into control, UC model, and
treatment with 50 or 100 mg/kg D-limonene groups (n=8/group). The
control group rats were subjected to enema and oral gavage with
normal saline. The UC model was established by administration of 2%
DSS for 7 days. For the D-limonene-treated groups, UC model rats
were administered with 50 or 100 mg/kg D-limonene by gastric lavage
for 7 days (17). After treatment
with D-limonene, rats were sacrificed using decapitation under
anesthesia (2% pentobarbital sodium; Sigma-Aldrich; Merck
KGaA).
Disease Activity Index (DAI) and
Colonic Mucosa Damage Index (CMDI) scoring
Body weight, stool consistency, behavior and fecal
blood in the stools of the rats were recorded daily. The scores
were assigned as follows: Body weight reduction (0, no alteration;
1, 1–5%; 2, 6–10%; 3, 11–15%; 4, >15%); stool consistency (0,
typical; 2, loose; 4, diarrhea); and the presence of fecal blood
(0, typical; 2, positive occult blood test; 4, visible bleeding).
The DAI was calculated as the sum of these scores. The entire colon
was excised from the cecum of rats, and macroscopic damage was
evaluated using the CMDI scoring system (18), with slight modifications: 0, No
inflammation; 1, local hyperemia without ulcers, and/or stool
consistency; 2, ulceration without hyperemia; 3, ulceration and
adhesions at one site; 4, two or more sites of inflammation and
ulceration extending >1 cm; 5, ulceration >2 cm.
Inflammatory cytokine, antioxidant and
PGE2 production
Serum was obtained from a peripheral vessel and
centrifuged at 1,200 × g for 10 min at room temperature. Serum
protein expression levels of TNF-α, IL-1β, IL-6, NF-κB, SOD, GSH
and PGE2 were measured using ELISA kits, and the absorbance was
measured at a wavelength of 450 nm using an ELISA reader.
Matrix metalloproteinase (MMP)-2, −9
and transforming growth factor-β (TGF-β) gene expression
Total RNA was extracted from colonic mucosa tissue
samples using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Equal
quantities of total RNA were used to synthesize cDNA using an RNA
Polymerase Chain Reaction (PCR) kit (Avian Myeoblastosis Virus 3.0;
Takara Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocol. Following this, quantitative PCR (qPCR)
was performed using a SYBR®-Green JumpStart™ Taq
ReadyMix™ (Sigma-Aldrich; Merck KGaA), SYBR®-Green PCR
Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
iCycler IQ™ Real-Time PCR Detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The sequences for gene-specific primers
are presented in Table I. The
thermocycling conditions for MMP-2 were as follows: Predenaturation
at 95°C for 10 min, followed by 35 cycles of denaturation for 30
sec at 94°C, annealing at 59°C for 30 sec and extension at 72°C for
90 sec. The thermocycling conditions for MMP-9 were as follows:
Predenaturation at 94°C for 10 min, followed by 35 cycles of
denaturation at 94°C for 45 sec, annealing at 62°Cfor 30 sec and
extension at 72°C for 90 sec. The thermocycling conditions for
MMP-2, MMP-9 and TGF-β were as follows: Predenaturation at 94°C for
10 min, followed by 35 cycles of denaturation at 94°C for 45 sec,
annealing at 58°C for 30 sec and extension at 72°C for 90 sec.
Relative quantitation values were calculated using the
2−ΔΔCq method (19).
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Gene | Sequence
(5′-3′) | Product size
(bp) |
|---|
| MMP-2 | F:
ACCATCGCCCATCATCAAGT | 348 |
|
| R:
CGAGCAAAAGCATCATCCAC |
|
| MMP-9 | F:
CCCTGCGTATTTCCATTCAT | 600 |
|
| R:
ACCCCACTTCTTGTCAGCGTC |
|
| TGF-β | F:
TGCTTCAGCTCCACAGAGAA | 284 |
|
| R:
TGGTTGTAGAGGGCAAGGAC |
|
| β-actin | F:
AAGCCTAAGGCCAACCGTGAAAAG | 241 |
|
| R:
TCAATGAGGTAGTCTGTCAGGT |
|
Western blot analysis of inducible
nitric oxide synthase (iNOS), cyclooxygenase (COX)-2 and
extracellular signal-regulated kinase (ERK) 1/2
For western blot analysis, colonic mucosa tissue
samples were obtained and homogenized with radioimmunoprecipitation
assay buffer (EMD Millipore, Billerica, MA, USA). The homogenate
was centrifuged at 1200 × g for 10 min at 4°C and protein
concentrations were measured using a BCA assay kit. A total of 50
mg protein underwent 10% SDS-PAGE and was subsequently transferred
onto nitrocellulose membranes (Merck KGaA). The membranes were
blocked with 5% (w/v) non-fat milk powder in Tris-buffered saline
containing 0.1% Tween-20 (TBST), followed by incubation at 4°C
overnight with the appropriate primary antibody at the following
dilutions: Anti-iNOS (sc-649; 1:2,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-COX-2 (sc-7951; 1:1,000; Santa Cruz
Biotechnology, Inc.) and anti-phosphorylated (p)-ERK1/2 (sc-101760;
1:2,000, Santa Cruz Biotechnology, Inc.), with anti-β-actin
(D110007; 1:5,000; Sangon Biotech, Co., Ltd., Shanghai, China)
serving as the internal control. Following this, membranes were
washed three times in TBST for 1 h and incubated with horseradish
peroxidase (HPR)-conjugated anti-rabbit IgG secondary antibodies
for 2 h at room temperature (sc-2004; 1:5,000; Santa Cruz
Biotechnology, Inc.). Proteins were detected using a SuperSignal™
West Femto Chemiluminescent Substrate (Thermo Fisher Scientific,
Inc.) and calculated using Image-Pro Plus software version 3.0
(Media Cybernetics, Inc., Silver Spring, MD, USA).
Statistical analysis
Data were analyzed by one-way analysis of variance,
followed by Student-Newman-Keuls post hoc test, using SPSS version
22.0 (IBM SPSS, Armonk, NY, USA). Data are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
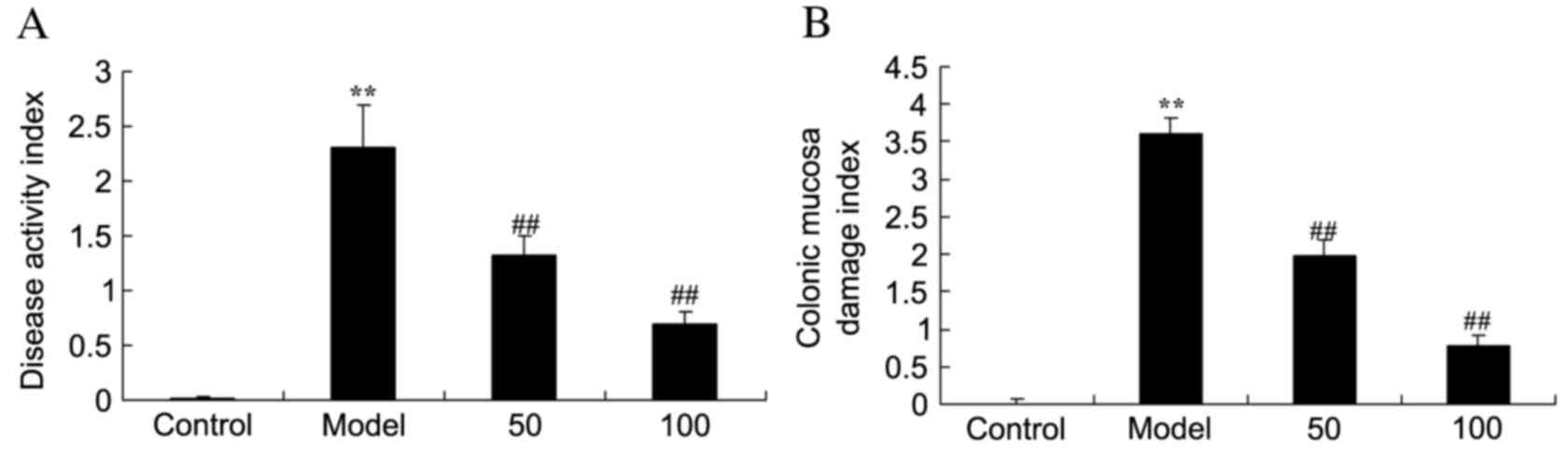
DAI and CMDI scores
In the UC model group, DAI (Fig. 2A) and CMDI (Fig. 2B) scores were significantly
increased compared with the control group (P=0.0011 and 0.0000).
Treatment with 50 or 100 mg/kg D-limonene significantly decreased
these scores compared with untreated UC rats (P=0.0039 and 0.0021;
P=0.0044 and 0.0015).
Inflammatory cytokines
Expression levels of the inflammatory cytokines
NF-κB (Fig. 3A), TNF-α (Fig. 3B), IL-1β (Fig. 3C) and IL-6 (Fig. 3D) were significantly increased in
UC rats, compared with the control group (P=0.0017, 0.0006, 0.0024
and 0.0035), whereas treatment with 50 or 100 mg/kg D-limonene
significantly reduced the expression levels compared with untreated
UC rats (P=0.0079 and 0.0051; P=0.0066 and 0.0049; P=0.0091 and
0.0063; P=0.0082 and 0.0059).
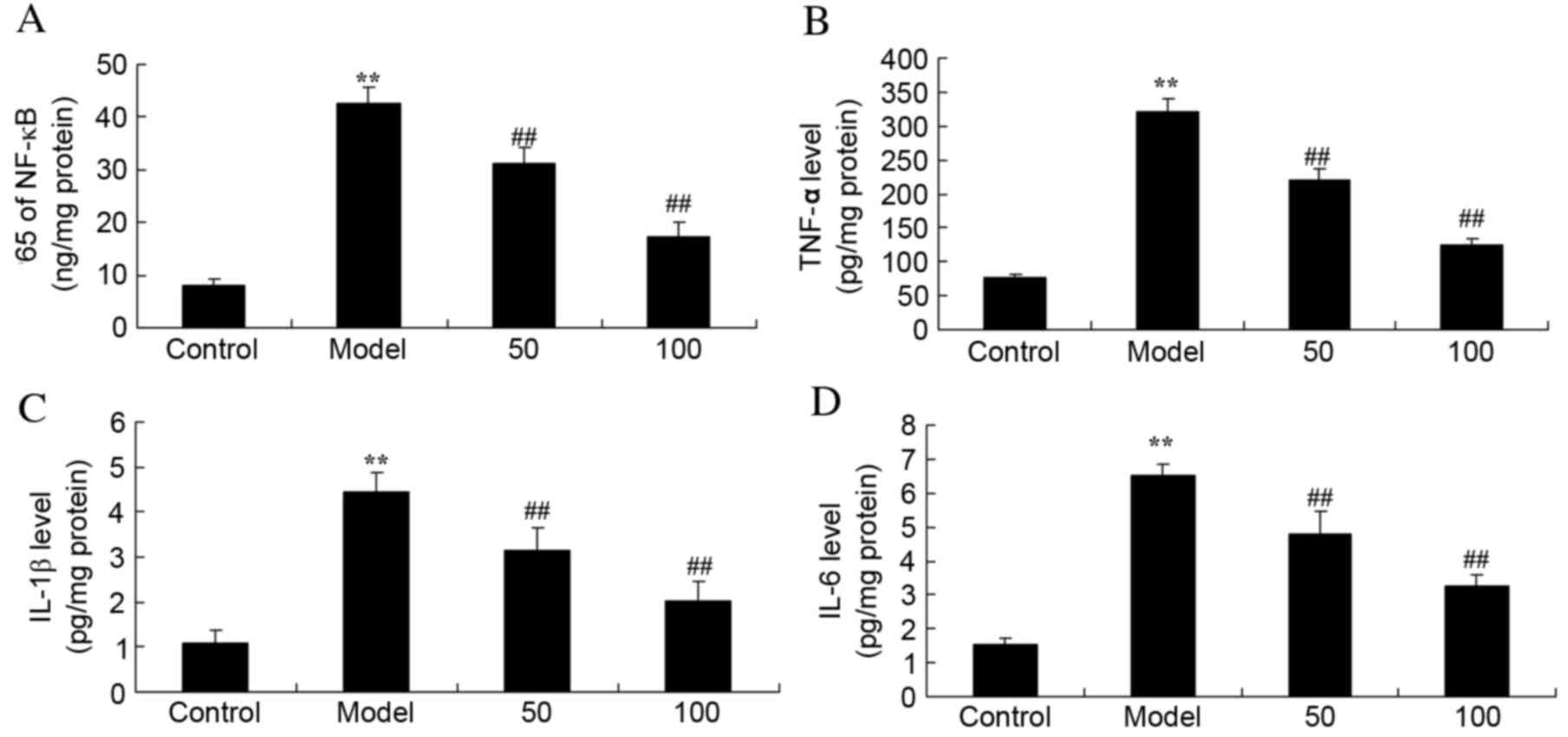 | Figure 3.Inflammatory cytokines. Protein
expression levels of (A) NF-κB p65 subunit, (B) TNF-α, (C) IL-1β
and (D) IL-6 in ulcerative colitis rats, following treatment with
50 or 100 mg/kg D-limonene. Control, control group; model,
ulcerative colitis model; 50, 50 mg/kg D-limonene treated group;
100, 100 mg/kg D-limonene treated group. Data are presented as the
mean ± standard deviation. **P<0.05 vs. control group;
##P<0.05 vs. model group. IL, interleukin; TNF-α,
transforming growth factor-α; NF-κB, nuclear factor κB. |
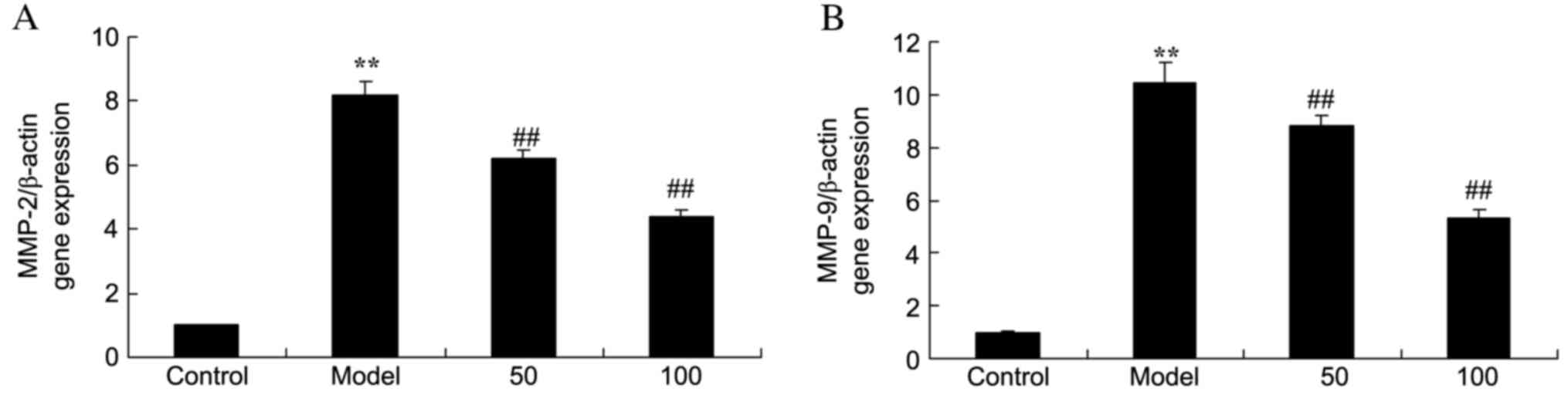
MMP-2 and −9 gene expression
mRNA expression levels of MMP-2 (Fig. 4A) and −9 (Fig. 4B) in the colonic mucosa of UC rats
were markedly increased, compared with the control group (P=0.0007
and 0.0000). By contrast, MMP-2 and −9 mRNA expression levels were
markedly reduced by treatment with 50 or 100 mg/kg D-limonene
compared with untreated UC rats (P=0.0071 and 0.0042; P=0.00097 and
0.0031).
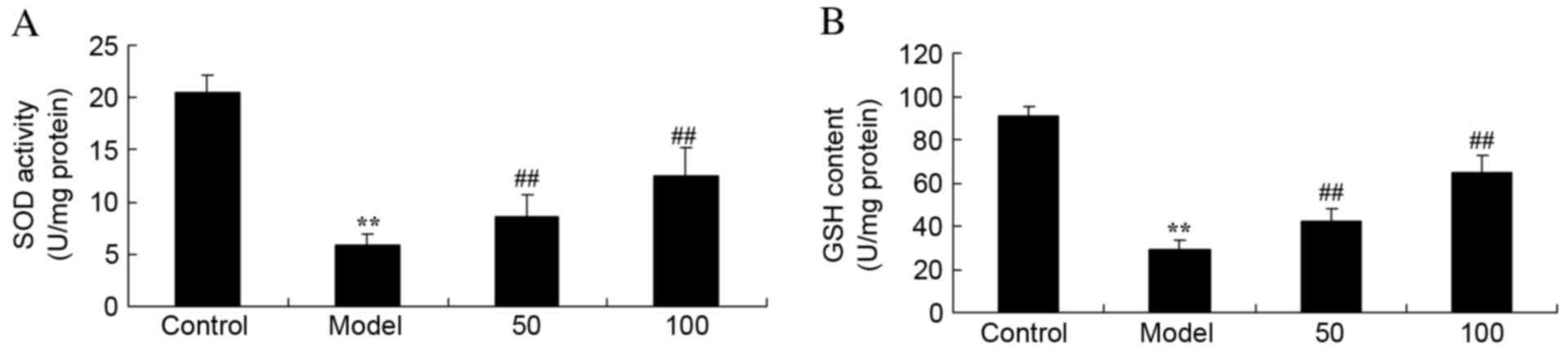
SOD and GSH activities
Activities of SOD (Fig.
5A) and GSH (Fig. 5B) in UC
rats were reduced compared with control rats (P=0.0031 and 0.0023).
Treatment with 50 or 100 mg/kg D-limonene markedly increased
activities of the two antioxidants, SOD and GSH, compared with
untreated UC rats (P=0.0082 and 0.0038; P=0.00090 and 0.0047).
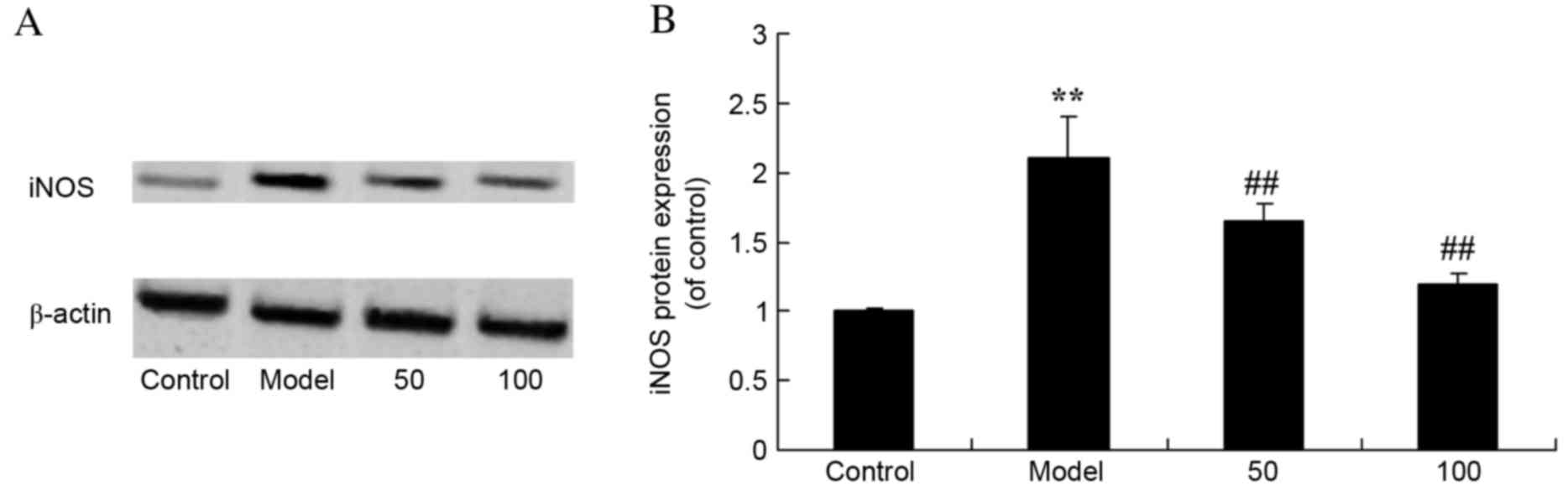
iNOS protein expression levels
As presented in Fig.
6, there was a significant increase in iNOS protein expression
levels in UC rats compared with the control group (P=0.0053).
Treatment with 50 or 100 mg/kg D-limonene significantly reduced
iNOS protein expression levels compared with untreated UC rats
(P=0.0046 and 0.0016).
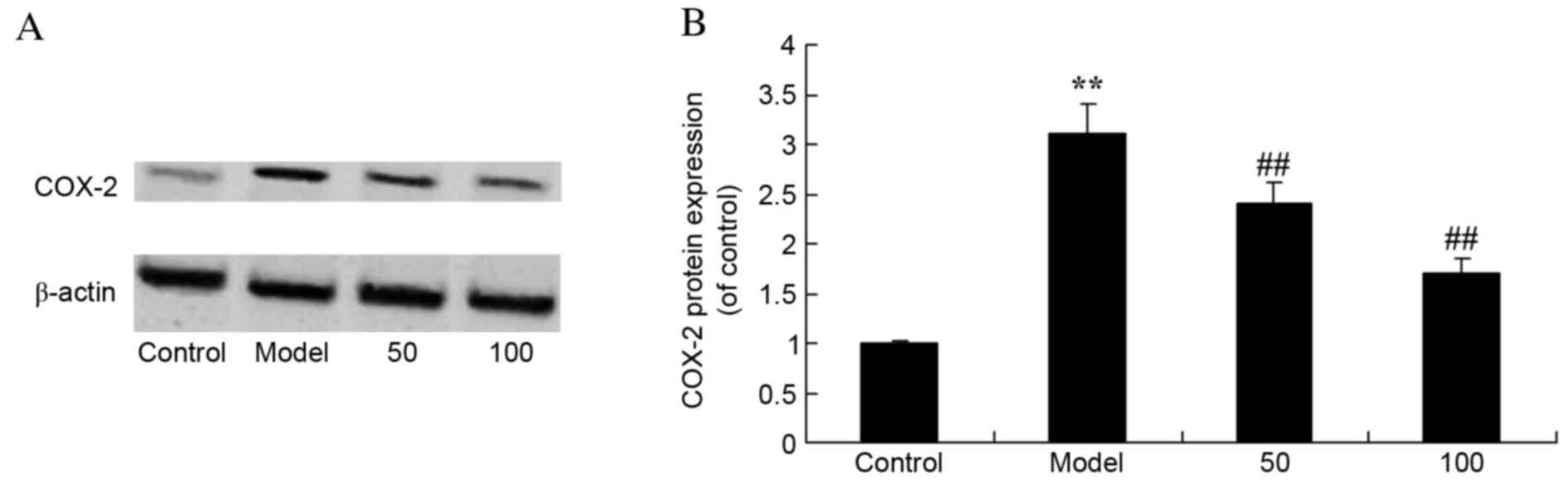
COX-2 protein expression levels
UC rats exhibited increased protein expression
levels of COX-2 compared with control rats (P=0.0078; Fig. 7). Treatment with 50 or 100 mg/kg
D-limonene significantly decreased COX-2 protein expression levels
compared with untreated UC rats (P=0.0062 and 0.0029).
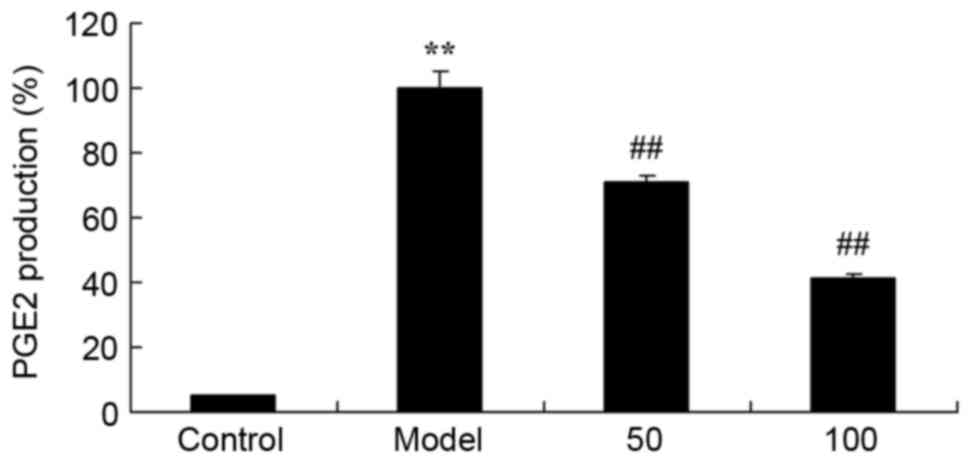
PGE2 production
The effect of D-limonene on PGE2 production was
assessed in UC rats. There was a significant increase in PGE2
production in UC rats compared with the control group (P=0.0012;
Fig. 8). Treatment with 50 or 100
mg/kg D-limonene significantly reduced PGE2 production compared
with untreated UC rats (P=0.0071 and 0.0033).
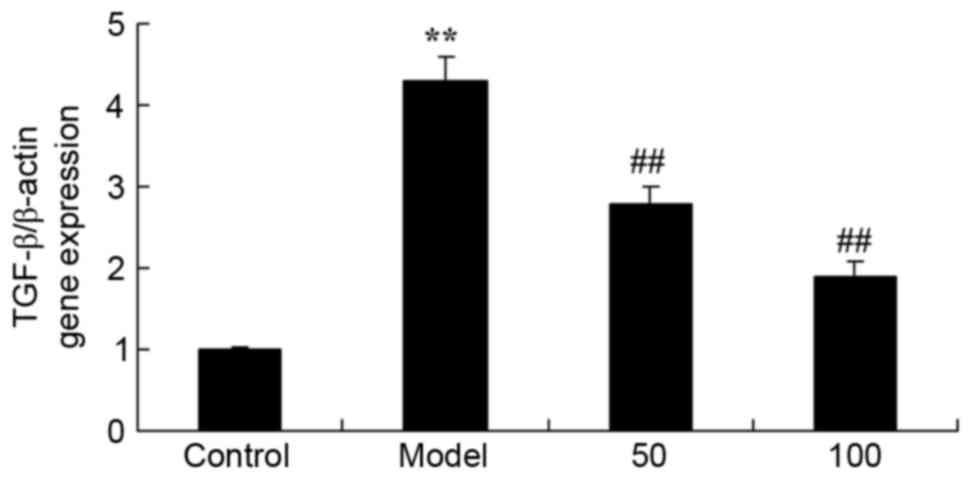
TGF-β gene expression
The effect of D-limonene on TGF-β gene expression in
UC rats is presented in Fig. 9.
TGF-β mRNA expression levels were significantly increased in UC
rats compared with the control group (P=0.0039). However, treatment
with 50 or 100 mg/kg D-limonene significantly reduced TGF-β mRNA
expression levels compared with untreated UC rats (P=0.0052 and
0.0016).
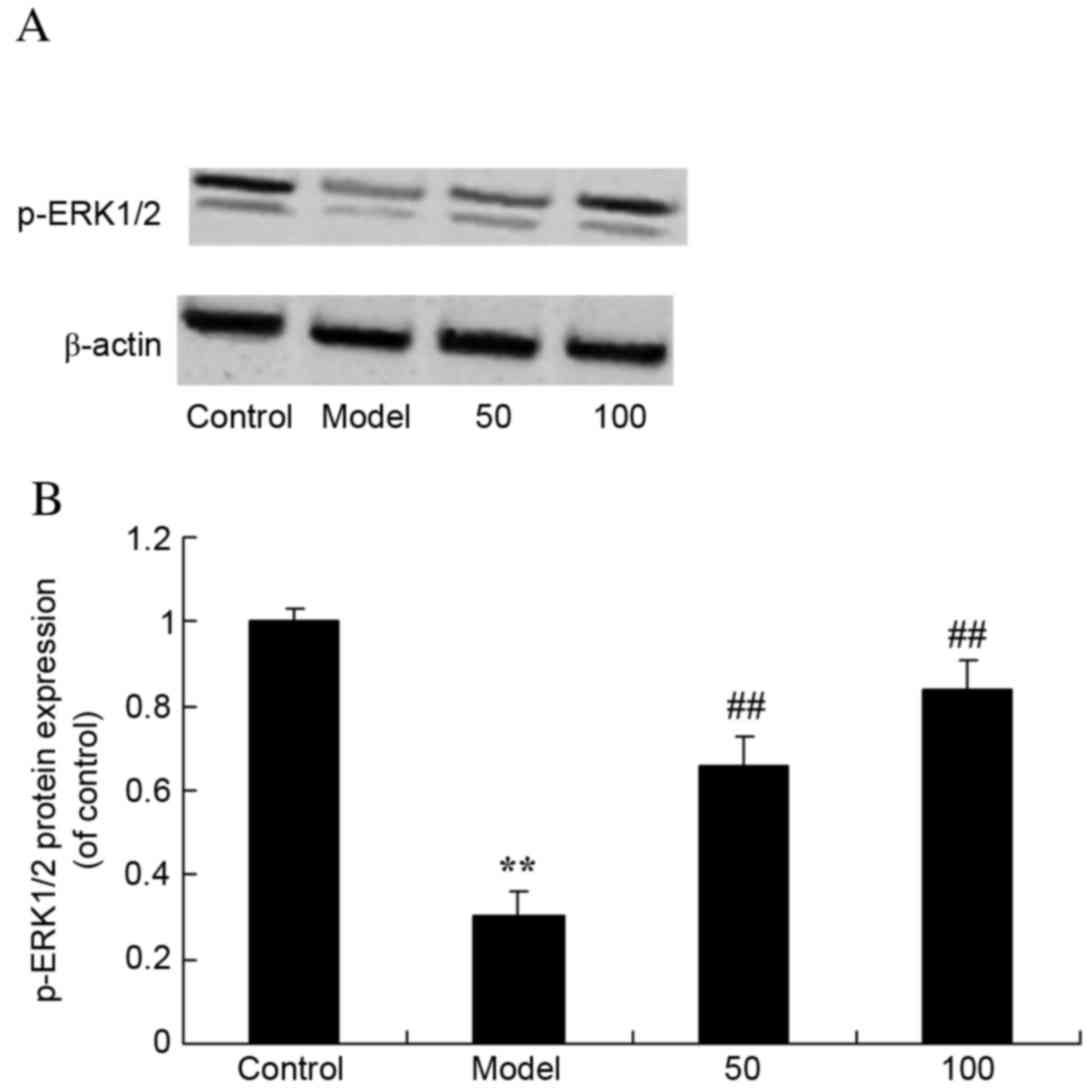
p-ERK1/2 protein expression
levels
To assess the effects of D-limonene on the ERK1/2
signaling pathway, p-ERK1/2 protein expression levels were
measured. Western blot analysis revealed that p-ERK1/2 protein
expression levels were significantly reduced in UC rats compared
with control rats (P=0.0058; Fig.
10). However, treatment with 50 or 100 mg/kg D-limonene
significantly increased p-ERK1/2 protein expression levels compared
with untreated UC rats (P=0.0028 and 0.0006).
Discussion
UC is a type of inflammatory bowel disease. It is
hypothesized that the pathogenesis of UC involves the activation of
the immune system by various microbial antigens, based on genetic
material and environmental factors. This results in an imbalance of
cytokines, which activates a variety of inflammatory cells and
recruits these cells to the site of inflammation, releasing further
inflammatory cytokines and thus leading to chronic inflammation of
the colon (20,21). The present study demonstrated that
treatment with D-limonene significantly suppressed the DAI and
CMDI, and inhibited TNF-α, IL-1β, IL-6 and NF-κB expression levels,
in UC rats. Hirota et al (22) identified that D-limonene reduces
allergic airway inflammation via inhibition of the expression
levels of IL-5, IL-13, eotaxin, monocyte chemoattractant protein-1
and TGF-β1 in Dermatophagoides farinae-treated mice.
Therefore, D-limonene may be a novel therapeutic agent for the
treatment of UC.
During the process of oxidation, a variety of highly
chemically reactive oxygen species may be generated, which leads to
intestinal tissue damage and ulceration. The oxygen free radical
scavenging capacity of UC patients is decreased, therefore
exacerbating disease (23). SOD is
an important enzyme involved in the scavenging of oxygen free
radicals and therefore preventing tissue damage; however, excessive
levels of nitrous oxide reduces SOD levels, thus reducing its
ability to scavenge oxygen free radicals (24,25).
A build-up of free radicals induces a series of chain reactions,
leading to biofilm lipid peroxidation, and thus continuously
disrupts the normal structure and function of the enzyme (26). The present study demonstrated that
D-limonene treatment markedly increased SOD and GSH activities in
UC rats. Furthermore, Rizk et al (27) reported that D-limonene suppressed
SOD and GSH activities in Schistosoma mansoni-infected mice.
Thus, D-limonene may have antioxidative effects in UC rats.
COX-2 is expressed at low levels in healthy mucosa
and during UC remission; however, its expression levels are
significantly increased in active UC. It is primarily expressed in
epithelial, endothelial and inflammatory cells (28). The enhanced expression levels of
COX-2 are a protective response in the recovery process, which
improves the protection of intestinal mucosal cells, promotes the
hyperplasia of intestinal epithelial cells and intestinal blood
flow, and promotes the repair of epithelial cells. COX-2 may
inhibit the apoptosis of epithelial cells by reducing arachidonic
acid (AA) and regulating B-cell lymphoma 2, and is additionally a
key enzyme for the synthesis of PGs (29). The membrane phospholipids release
AA products, and produce a variety of PGs and leukotrienes by COX
(30). These inflammatory
mediators cause symptoms including redness, swelling, heat, pain,
edema and inflammatory cell infiltration, which may affect bowel
transport, bowel activity and immune regulation, thus aggravating
the existing inflammation (29).
The present study demonstrated that D-limonene treatment
significantly reduced MMP-2 and −9mRNA expression levels, and iNOS
and COX-2 protein expression levels, in UC rats. Wilson et
al (13) identified that
D-limonene may suppress MMP-2 and −9. Rehman et al (15) demonstrated that D-limonene inhibits
doxorubicin-induced oxidative stress and inflammation via COX-2 and
iNOS signaling pathways in the kidneys of Wistar rats.
PGE2, a type of PG, is a metabolite of the 20-carbon
unsaturated fatty acid AA (31).
AA exists in the cell membrane phospholipid bilayer, and upon
exposure to external stimuli, is hydrolyzed by activated
phospholipase A2 and C, and is synthesized to PG. This process is
mediated by COX and a series of synthetases (31). PGE2 is an important inflammatory
factor and a previous study (32)
demonstrated that it may increase vascular permeability and cause
edema, inducing leukocytechemotaxis, leading to inflammatory cell
infiltration, and thus resulting in colonic mucosal inflammation,
tissue damage and ulceration. An additional study (33) demonstrated that mucosal PGE2
content in UC patients is significantly increased, and is
associated with the degree of mucosal inflammation. The present
study revealed that treatment with D-limonene significantly
decreased PGE2 production in UC rats. Yoon et al (16) suggested that D-limonene reduces
lipopolysaccharide-induced production of iNOS and PGE2 in RAW 334.7
macrophages.
TGF-β is a cytokine with a variety of physiological
functions. Mothers against decapentaplegic (SMAD) proteins are
signaling molecules within cells that may be activated by the
compound generated by TGF-β and its receptor, which additionally
transmits the signals into the nucleus (34). The TGF-β1/SMAD3 signaling pathway
contributes to the regulation of the immune response, induces the
synthesis of the extracellular matrix components, collagen and
mucin, inhibits the release of extracellular collagen proteolytic
enzymes, promotes fibrosis, and facilitates repair of damaged
tissue. A previous study (35)
demonstrated that compared with healthy individuals, the expression
levels of TGF-β1 and -β2 protein and mRNA in active or non-active
UC patients were significantly increased. In the present study,
D-limonene significantly inhibited TGF-βmRNA expression levels in
UC rats.
ERK is an important member of the mitogen-activated
protein kinase (MAPK) system, which serves important roles in the
mediation of inflammatory responses and the regulation of
inflammatory cytokine production, the promotion of epithelial cell
proliferation and differentiation, and the inhibition of intestinal
epithelium apoptosis (36).
p-ERK1/2 translocates from the cytoplasm to the nucleus and is thus
involved in a variety of cellular biological reactions (37). The MAPK signaling pathway is
important for the biological effect of TGF-β1 (38,39).
It has been reported (38) that
the relative protein expression levels of p-ERK1/2 and p-MAPK
kinase 1/2 in the colonic mucosa of UC rats are increased compared
with healthy rats. Rufino et al (40) demonstrated that D-limonene may have
anti-inflammatory, anticatabolic and proanabolic effects in a cell
model of osteoarthritis via increasing ERK1/2 activation. The
results of the present study revealed that D-limonene significantly
activated the ERK1/2 signaling pathway in UC rats.
In conclusion, the present study demonstrated the
that D-limonene suppresses MMP-2 and −9 mRNA expression levels via
regulation of the iNOS, COX-2, PGE2, TGF-β and ERK1/2 signaling
pathways in a UC rat model, indicating its potential antioxidant
and anti-inflammatory properties. The current study indicates that
D-limonene may be a novel potential target for the therapeutic
effects of UC.
References
|
1
|
Brandse JF, van den Brink GR, Wildenberg
ME, van der Kleij D, Rispens T, Jansen JM, Mathôt RA, Ponsioen CY,
Löwenberg M and D'Haens GR: Loss of infliximab into feces is
associated with lack of response to therapy in patients with severe
ulcerative colitis. Gastroenterology. 149:350–355.e2. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boschetti G, Nancey S, Moussata D,
Stefanescu C, Roblin X, Chauvenet M, Stroeymeyt K, Bouhnik Y and
Flourié B: Tacrolimus induction followed by maintenance monotherapy
is useful in selected patients with moderate-to-severe ulcerative
colitis refractory to prior treatment. Dig Liver Dis. 46:875–880.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okuyama Y, Andoh A, Nishishita M, Fukunaga
K, Kamikozuru K, Yokoyama Y, Ueno Y, Tanaka S, Kuge H, Yoshikawa S,
et al: Multicenter prospective study for clinical and endoscopic
efficacies of leukocytapheresis therapy in patients with ulcerative
colitis. Scand J Gastroenterol. 48:412–418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cleynen I, Boucher G, Jostins L, Schumm
LP, Zeissig S, Ahmad T, Andersen V, Andrews JM, Annese V, Brand S,
et al: Inherited determinants of Crohn's disease and ulcerative
colitis phenotypes: A genetic association study. Lancet.
387:156–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heikens JT, De Vries J, De Jong DJ, den
Oudsten BL, Hopman W, Groenewoud JM, van der Kolk MB, Gooszen HG
and van Laarhoven CJ: Evaluation of long-term function,
complications, quality of life and health status after restorative
proctocolectomy with ileo neo rectal and with ileal pouch anal
anastomosis for ulcerative colitis. Colorectal Dis. 15:e323–e329.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adedokun OJ, Xu Z, Padgett L, Blank M,
Johanns J, Griffiths A, Ford J, Zhou H, Guzzo C, Davis HM and Hyams
J: Pharmacokinetics of infliximab in children with
moderate-to-severe ulcerative colitis: Results from a randomized,
multicenter, open-label, phase 3 study. Inflamm Bowel Dis.
19:2753–2762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guardiola J, Lobatón T, Rodríguez-Alonso
L, Ruiz-Cerulla A, Arajol C, Loayza C, Sanjuan X, Sánchez E and
Rodríguez-Moranta F: Fecal level of calprotectin identifies
histologic inflammation in patients with ulcerative colitis in
clinical and endoscopic remission. Clin Gastroenterol Hepatol.
12:1865–1870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeda Y, Nakase H, Namba K, Inoue S, Ueno
S, Uza N and Chiba T: Upregulation of T-bet and tight junction
molecules by Bifidobactrium longum improves colonic inflammation of
ulcerative colitis. Inflamm Bowel Dis. 15:1617–1618. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laake KO, Line PD, Aabakken L, Løtveit T,
Bakka A, Eide J, Roseth A, Grzyb K, Bjørneklett A and Vatn MH:
Assessment of mucosal inflammation and circulation in response to
probiotics in patients operated with ileal pouch anal anastomosis
for ulcerative colitis. Scand J Gastroenterol. 38:409–414. 2003.
View Article : Google Scholar
|
|
10
|
Yang SK, Jung HY, Kang GH, Kim YM, Myung
SJ, Shim KN, Hong WS and Min YI: Appendiceal orifice inflammation
as a skip lesion in ulcerative colitis: An analysis in relation to
medical therapy and disease extent. Gastrointest Endosc.
49:743–747. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mawdsley JE, Jenkins DG, Macey MG,
Langmead L and Rampton DS: The effect of hypnosis on systemic and
rectal mucosal measures of inflammation in ulcerative colitis. Am J
Gastroenterol. 103:1460–1469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mannon PJ, Hornung RL, Yang Z, Yi C,
Groden C, Friend J, Yao M, Strober W and Fuss IJ: Suppression of
inflammation in ulcerative colitis by interferon-b-1a is
accompanied by inhibition of IL-13 production. Gut. 60:449–455.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilson MJ, Lindgren BR and Sinha AA: The
effect of dietary supplementation with limonene or myo-inositol on
the induction of neoplasia and matrix metalloproteinase and
plasminogen activator activities in accessory sex organs of male
Lobund-Wistar rats. Exp Mol Pathol. 85:83–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marmulla R and Harder J: Microbial
monoterpene transformations-a review. Front Microbiol. 5:3462014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rehman MU, Tahir M, Khan AQ, Khan R,
Hamiza Oday-O, Lateef A, Hassan SK, Rashid S, Ali N, Zeeshan M and
Sultana S: D-limonene suppresses doxorubicin-induced oxidative
stress and inflammation via repression of COX-2, iNOS, and NFκB in
kidneys of Wistar rats. Exp Biol Med (Maywood). 239:465–476. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoon WJ, Lee NH and Hyun CG: Limonene
suppresses lipopolysaccharide-induced production of nitric oxide,
prostaglandin E2, and pro-inflammatory cytokines in RAW 2647
macrophages. J Oleo Sci. 59:415–421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaudhary SC, Siddiqui MS, Athar M and
Alam MS: D-Limonene modulates inflammation, oxidative stress and
Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum Exp
Toxicol. 31:798–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao JW, He XM, Tang HY and Wang YD:
Protective role of metalloproteinase inhibitor (AE-941) on
ulcerative colitis in rats. World J Gastroenterol. 18:7063–7069.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Assche G, Manguso F, Zibellini M,
Cabriada N, uño JL, Goldis A, Tkachenko E, Varoli G, Kleczkowski D,
Annese V, D'Heygere F, et al: Oral prolonged release beclomethasone
dipropionate and prednisone in the treatment of active ulcerative
colitis: Results from a double-blind, randomized, parallel group
study. Am J Gastroenterol. 110:708–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trifan A, Stanciu C, Stoica O, Girleanu I
and Cojocariu C: Impact of Clostridium difficile infection on
inflammatory bowel disease outcome: A review. World J
Gastroenterol. 20:11736–11742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirota R, Nakamura H, Bhatti SA, Ngatu NR,
Muzembo BA, Dumavibhat N, Eitoku M, Sawamura M and Suganuma N:
Limonene inhalation reduces allergic airway inflammation in
Dermatophagoides farinae-treated mice. Inhal Toxicol. 24:373–381.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jorgensen JR and Mortensen PB: Influence
of feces from patients with ulcerative colitis on butyrate
oxidation in rat colonocytes. Dig Dis Sci. 44:2099–2109. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alagozlu H, Gorgul A, Bilgihan A, Tuncer C
and Unal S: Increased plasma levels of advanced oxidation protein
products (AOPP) as a marker for oxidative stress in patients with
active ulcerative colitis. Clin Res Hepatol Gastroenterol.
37:80–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keshavarzian A, Banan A, Farhadi A,
Komanduri S, Mutlu E, Zhang Y and Fields JZ: Increases in free
radicals and cytoskeletal protein oxidation and nitration in the
colon of patients with inflammatory bowel disease. Gut. 52:720–728.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roediger WE: Review article: Nitric oxide
from dysbiotic bacterial respiration of nitrate in the pathogenesis
and as a target for therapy of ulcerative colitis. Aliment
Pharmacol Ther. 27:531–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rizk M, Ibrahim N and El-Rigal N:
Comparative in vivo antioxidant levels in Schistosoma mansoni
infected mice treated with praziquantel or the essential oil of
Melaleuca armillaris leaves. Pak J Biol Sci. 15:971–978. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang CX, Guo LK and Guo XF: Interaction
between the polymorphisms of cyclooxygenase-2-1195G/A,
MnSOD9Ala/val genes and the high-fat diets and its correlation with
ulcerative colitis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 37:37–43.
2015.PubMed/NCBI
|
|
29
|
Hussein I Abdallah Hajj, Freund JN,
Reimund JM, Shams A, Yamine M, Leone A and Jurjus AR:
Enteropathogenic e.coli sustains iodoacetamide-induced ulcerative
colitis-like colitis in rats: Modulation of IL-1β, IL-6, TNF-α,
COX-2, and apoptosisi. J Biol Regul Homeost Agents. 26:515–526.
2012.PubMed/NCBI
|
|
30
|
Fratila OC and Iliaş TI: COX-2 and Ki-67
immunohistochemical markers in the assessment of long-standing
ulcerative colitis associated dysplasia. Rom J Morphol Embryol.
54:143–149. 2013.PubMed/NCBI
|
|
31
|
Roulis M, Nikolaou C, Kotsaki E, Kaffe E,
Karagianni N, Koliaraki V, Salpea K, Ragoussis J, Aidinis V,
Martini E, et al: Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2
pathway links innate sensing to epithelial homeostasis. Proc Natl
Acad Sci USA. 111:E4658–E4667. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tammali R, Ramana KV and Srivastava SK:
Aldose reductase regulates TNF-alpha-induced PGE2 production in
human colon cancer cells. Cancer Lett. 252:299–306. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guan F, Wang H, Shan Y, Chen Y, Wang M,
Wang Q, Yin M, Zhao Y, Feng X and Zhang J: Inhibition of COX-2 and
PGE in LPS-stimulated RAW264.7 cells by lonimacranthoide VI, a
chlorogenic acid ester saponin. Biomed Rep. 2:760–764.
2014.PubMed/NCBI
|
|
34
|
Li C, Iness A, Yoon J, Grider JR, Murthy
KS, Kellum JM and Kuemmerle JF: Noncanonical STAT3 activation
regulates excess TGF-β1 and collagen I expression in muscle of
stricturing Crohn's disease. J Immunol. 194:3422–3431. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fleissner D, Frede A, Knott M, Knuschke T,
Geffers R, Hansen W, Dobos G, Langhorst J, Buer J and Westendorf
AM: Generation and function of immunosuppressive human and murine
CD8+ T cells by transforming growth factor-β and retinoic acid.
Immunology. 134:82–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Setia S, Nehru B and Sanyal SN:
Upregulation of MAPK/Erk and PI3K/Akt pathways in ulcerative
colitis-associated colon cancer. Biomed Pharmacother. 68:1023–1029.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dambacher J, Beigel F, Seiderer J, Haller
D, Göke B, Auernhammer CJ and Brand S: Interleukin 31 mediates MAP
kinase and STAT1/3 activation in intestinal epithelial cells and
its expression is upregulated in inflammatory bowel disease. Gut.
56:1257–1265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv Q, Qiao SM, Xia Y, Shi C, Xia YF, Chou
GX, Wang ZT, Dai Y and Wei ZF: Norisoboldine ameliorates
DSS-induced ulcerative colitis in mice through induction of
regulatory T cells in colons. Int Immunopharmacol. 29:787–797.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rezaie A, Khalaj S, Shabihkhani M, Nikfar
S, Zamani MJ, Mohammadirad A, Daryani NE and Abdollahi M: Study on
the correlations among disease activity index and salivary
transforming growth factor-beta 1 and nitric oxide in ulcerative
colitis patients. Ann N Y Acad Sci. 1095:305–314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rufino AT, Ribeiro M, Sousa C, Judas F,
Salgueiro L, Cavaleiro C and Mendes AF: Evaluation of the
anti-inflammatory, anti-catabolic and pro-anabolic effects of
E-caryophyllene, myrcene and limonene in a cell model of
osteoarthritis. Eur J Pharmacol. 750:141–150. 2015. View Article : Google Scholar : PubMed/NCBI
|