Introduction
Methamphetamine (MA) is an addictive drug that is
abused globally (1). MA abuse
significantly increases the risk of developing pulmonary arterial
hypertension (PAH) (2,3). Oxidative stress, which occurs in
diseased lungs and is associated with PAH, is thought to be
responsible for the progression of cardiopulmonary changes
(4,5). Reactive oxygen species (ROS) may
serve as signaling molecules to mediate multiple cell functions,
and have been implicated in the pathogenesis of many diseases
(6,7). ROS formation additionally controls
inducible expression of chemokines and other inflammatory genes in
response to infection, implying a causal relationship between
increased ROS production and lung injury (8,9). MA
abuse has previously been demonstrated to induce pulmonary toxicity
in rats (10). Therefore, it has
been hypothesized that methamphetamine may have the capacity to
generate oxidative damage in lung tissues by inducing the formation
of free radicals, including ROS.
Oxidative stress interferes with the expression of
genes and transcriptional factors, including nuclear factor
erythroid-2-related factor 2 (Nrf2)-dependent antioxidant response
element (ARE) (11). It has been
reported that low levels of ROS may induce Nrf2 activation
(12). Nrf2 belongs to a small
family of transcription factors containing a unique
basic-leucine-zipper motif, the cap-n-collar (CNC) family (13). Nrf2 is a transcription factor that
is involved in cellular defense against oxidative stress and
electrophilic insults (14). Nrf2
is located in the cytoplasm as an inactive form associated with its
repressor protein, Kelch-like ECH associating protein 1 (Keap1)
(15). Oxidation of
redox-sensitive cysteines in Keap1 during oxidative stress allows
Nrf2 to dissociate from Keap1 and translocate to the nucleus, where
it heterodimerizes with other proteins and binds to ARE, an
enhancer sequence that regulates the transcription of
cytoprotective enzymes including glutamate-cysteine ligase
catalytic subunit C (GCLC), glutathione S-transferase (GST) and
heme oxygenase-1 (HO-1) (15,16).
Nrf2 induces the expression of several
oxidant-signaling proteins that affect particular cellular
functions, including autophagy, inflammation, apoptosis and
mitochondrial biogenesis, by controlling the transcription of
several drug metabolizing enzymes, transporters, cellular reducing
equivalents including reduced glutathione (GSH) and nicotinamide
adenine dinucleotide phosphate, and proteasomes (17). Multiple enzymes regulated by Nrf2
are essential in the pathogenesis of cardiovascular diseases,
including atherosclerosis, ischemia-reperfusion injury and
hypertension (18). However,
little evidence has been provided concerning whether Nrf2 and its
regulated enzymes are related to MA-induced lung injury. The
present study was designed to investigate the function of Nrf2 and
whether the suppression of Nrf2-mediated antioxidative defense is
associated with lung injury induced by chronic exposure to MA in
rats.
Materials and methods
Drug
MA was obtained from China Criminal Police
University (Shenyang, China). The identity and purity of MA were
determined using a Bio-Rad REMEDi HS system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and confirmed by liquid
chromatography-mass spectrometry-mass spectrometry (Department of
Drug Control, China Criminal Police University). MA was dissolved
in 0.9% sterile saline at 4 mg/ml for drug administration.
Animals and procedures
All procedures were approved by the Institutional
Animal Care and Use Committee of China Medical University
(Shenyang, China). Male Wistar rats (n=30; age, 7 weeks; weight,
180±10 g) from the Animal Resource Center, China Medical University
(Shenyang, China; certificate no. Liaoning 034) were divided into 3
groups (n=10/group): 0.9% saline (control), 5 mg/kg MA (M5), and 10
mg/kg MA (M10). Rats were administrated twice daily for 5 weeks.
They were maintained in standard conditions (at 18–22°C, 50%
humidity) throughout the experimental period and were given ad
libitum access to food and water in an alternating 12-h light/dark
cycle over a period of 5 weeks. The procedure of establishment of
the rat chronic lung injury models was in accordance with previous
studies (10,19).
Doppler ultrasonic detection of
physiological indexes
The abdomen hair of rats was thoroughly clipped and
shaved immediately following intraperitoneal anesthesia with 3%
pentobarbital sodium (45 mg/kg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) to achieve full contact of the probe with the
skin in the region concerned. The rats were examined in the supine
position. Acoustic gel (Skintact; Leonard Lang GmbH, Innsbruck,
Austria) was applied to hold the animal dander andimprove the
resolution of the ultrasound. A Philips IE33 cardiovascular
ultrasound (Philips Healthcare, Andover, MA, USA) with a linear
transducer probe S5-1 was used. The frequency of 4.5 MHz and one
focal zone set at a depth of 0.5–2 cm were used to achieve detailed
imaging of the rats.
Lung wet-to-dry weight (W/D)
ratio
In the present study, lung W/D weight ratio was
calculated to assess tissue edema. Rats were euthanized 5 weeks
following MA administration. The left upper lobes were isolated,
blotted dry and weighed to obtain the ‘wet’ weight. Following this,
the left upper lobes were placed into an oven at 80°C for 48 h to
obtain the ‘dry’ weight. The ratio of the wet lung to dry lung was
calculated to quantify the magnitude of pulmonary edema.
Histological evaluation
The left lower lung lobes were isolated and fixed
with 4% paraformaldehyde for 24 h. Following washing 3 times with
0.1 M PBS (pH 7.2), the fixed tissues were prepared for paraffin
embedding and then cut into 5 µm thick sections. To examine the
inflammatory aspects of the lung, the sections were processed for
hematoxylin and eosin staining. The stained sections were imaged
under a Olympus BX51 fluorescence microscope (Tokyo, Japan) at an
original magnification of ×200. Six sections from each sample were
evaluated and scored independently by two members of the laboratory
trained in histological assessment and use of the scoring system.
Different lobes were examined for the following features:
Interstitial edema, hemorrhage and inflammatory cell infiltration.
Each feature received a score of 0, no injury; 1, minimal injury;
2, moderate injury; or 3, severe injury. This was totaled for a
given lobe's score, and 3 lobes per rat (2–3 rats per group) were
averaged to generate a score, giving a minimum score of 0 and a
maximum of 9 (20).
Western blot assay
The right lungs were stored in liquid nitrogen at
−80°C until analysis. Lung tissues were homogenized in
radioimmunoprecipitation lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology, Haimen, China) containing protease
inhibitor phenylmethylsulfonyl fluoride (cat. no. ST506; Beyotime
Institute of Biotechnology) on ice, and protein concentrations were
determined using Bradford reagent (Bio-Rad Laboratories, Inc.).
Nuclear and cytoplasmic fractions were extracted using aprotein
extraction reagent (cat. no. P0027; Beyotime Institute of
Biotechnology). The protein concentrations were determined using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology) prior to storage at −80°C. Protein samples (80 µg)
were separated by 10% SDS-PAGE and subsequently transferred onto a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). Following blocking of the nonspecific site with 5% non-fat
dry milk for 1 h at room temperature, the membrane was incubated
with primary rabbit anti-Nrf2 (1:600 dilution; cat. no. 16396-1-AP;
Proteintech Group, Inc., Chicago, IL, USA), anti-GCLC (1:200
dilution; cat. no. bs-8402R; Beijing Biosynthesis Biotechnology
Co., Ltd., Beijing, China), anti-HO-1 (1:200 dilution; cat. no.
bs-2075R; Beijing Biosynthesis Biotechnology Co., Ltd.), primary
mouse anti-β-actin (1:3,000 dilution; cat. no. sc-130300; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and anti-α-tubulin
(1:3,000 dilution; cat. no. 66031, ProteinTech Group, Inc.)
overnight at 4°C. The membrane was then incubated for an additional
2 h at room temperature with goat anti-mouse secondary antibody
(1:4,000 dilution; cat. no. ZB-2305; Zhongshan Golden Bridge
Biotechnology Co Ltd., Beijing, China) for β-actin and α-tubulin,
or goat anti-rabbit secondary antibody (1:2,000 dilution; cat. no.
SA00001-2; ProteinTech Group, Inc.) for other proteins. The
immunoreactive proteins were detected using an enhanced
chemiluminescence western blotting detection kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The relative protein
expression was quantified with Molecular Dynamics Image Quant
software (GE Healthcare Life Sciences, Chalfont, UK). Expression of
nuclear Nrf2 was normalized to α-tubulin, while expression of the
other proteins was normalized to β-actin.
Measurement of GSH and oxidized
glutathione (GSSG) in rat lungs
Lung tissues (n=6) were homogenized with 10 ml
ice-cold lysis buffer (50 mM phosphate buffer containing 1 mM EDTA)
per gram of tissue. The homogenate was centrifuged at 10,000 × g
for 15 min at 4°C, and then deproteinated with 1.25 M
metaphosphoric acid (cat. no. 04103, Sigma-Aldrich; Merck KGaA) and
stored at −20°C. Total glutathione and oxidized glutathione levels
were determined using a glutathione assay kit (Cayman Chemical
Company, Ann Arbor, MI, USA), according to the manufacturer's
protocol.
Measurement of lung ROS levels
Samples of lung tissue from rats in each group (n=6)
were homogenized using a Polytron homogenizer (Kinematica, Lucerne,
Switzerland) to extract protein. The homogenate was centrifuged at
15,000 × g for 30 min at 4°C and the supernatant was collected and
stored at −80°C for enzyme-linked immunosorbent assay (ELISA). ROS
concentration in the lung tissues was measured using a ROS ELISA
kit (cat. no. MAB7475; R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer's protocol. The absorbance was
detected using a Tecan Sunrise microplate reader (Tecan Group,
Ltd., Männedorf, Switzerland) at a wave length of 450 nm and the
corresponding concentration was determined from the standard
curve.
Statistical analysis
All data are presented as mean ± standard deviation.
Statistical analysis was performed using one-way analysis of
variance followed by the least significant difference (LSD) test
with SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Linear
regression analysis was used to evaluate the correlation of lung
ROS level with MA-induced lung injury. P<0.05 was considered to
indicate a statistically significant difference.
Results
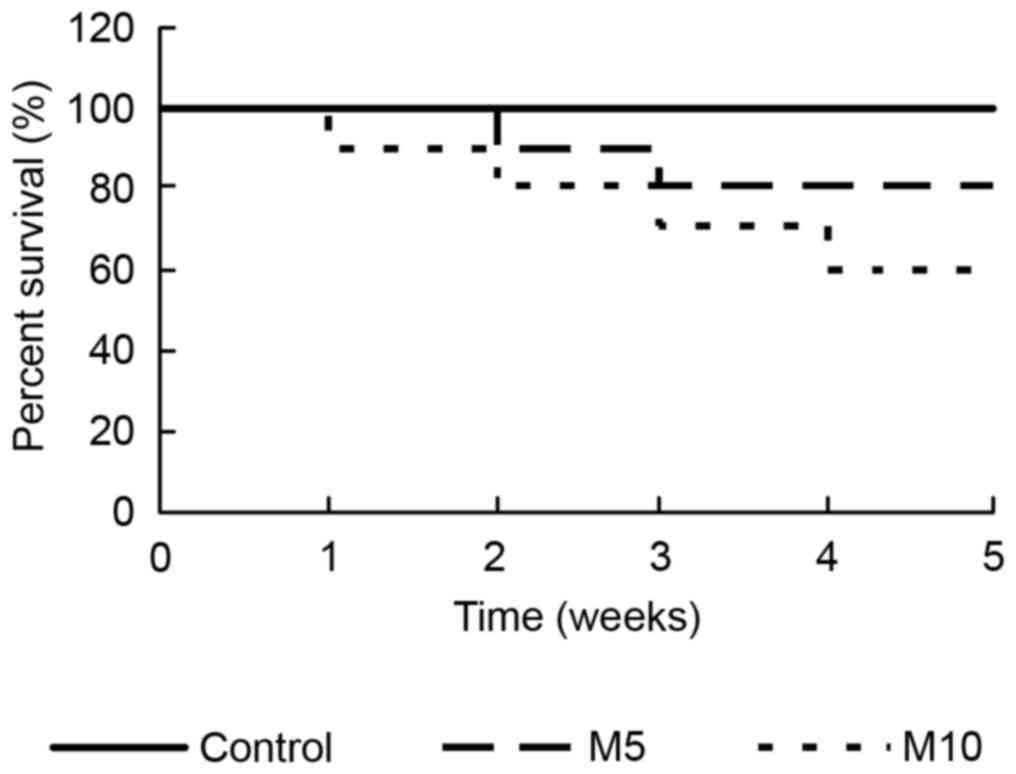
Comparison of the survival rate of
rats in different groups
In the present experiment, 10 rats in different
groups were administrated with 0.9% saline, 5 mg/kg MA and 10 mg/kg
MA, respectively. The survival rate was assessed at the end of
every week. During the time of the model establishment, there was
no mortality in the control group, but the survival rate reduced
from 100% in the M5 group and 90% in the M10 group at the end of
the 1st week, to 80% in the M5 group and 60% in the M10 group at
the end of the last week. Compared with the control group, the
survival rate was significantly reduced in the M10 group
(P<0.05; Fig. 1). The survival
curves were compared using the log-rank test.
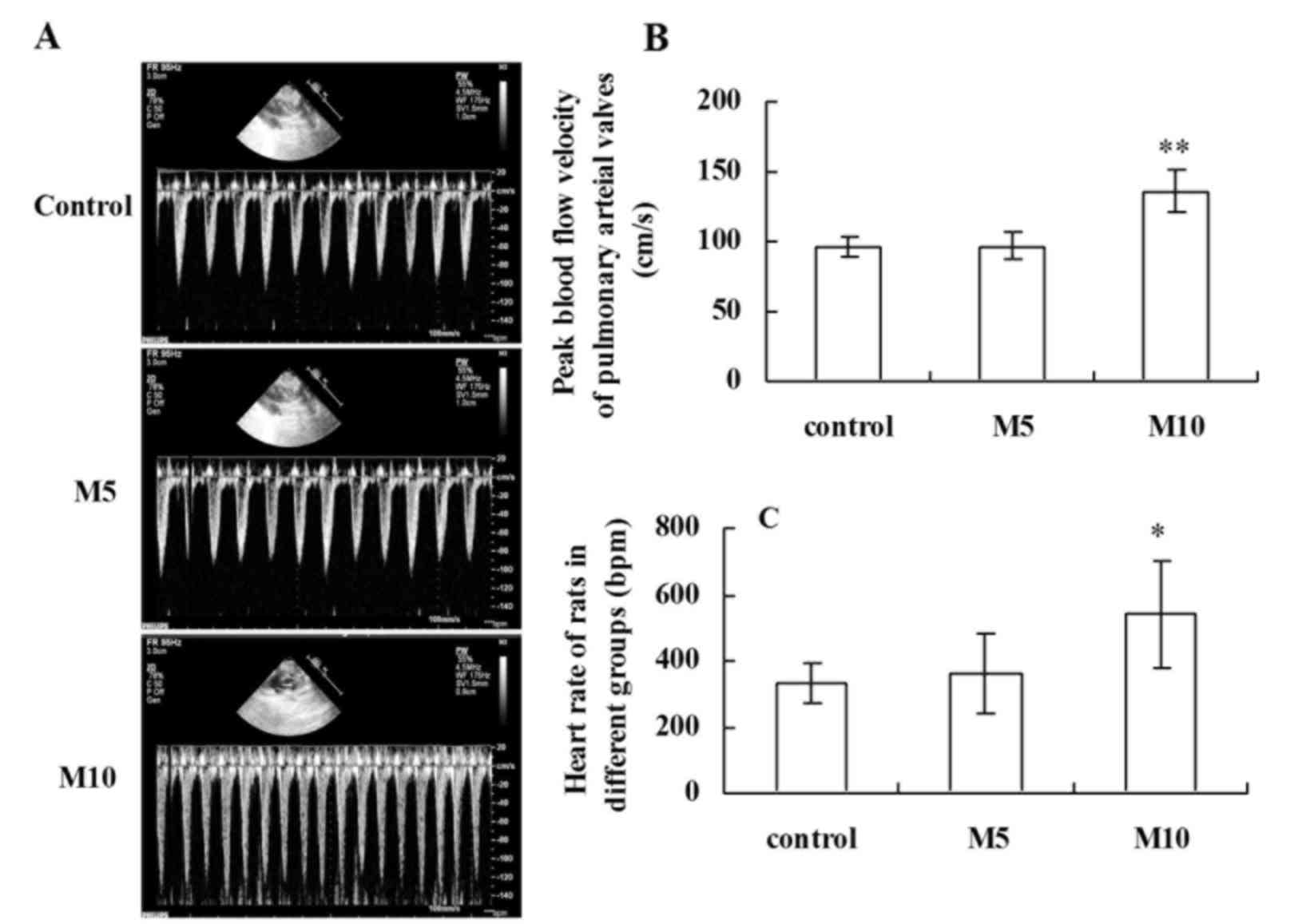
Effect of MA on heart rate (HR) and
peak blood flow velocity of pulmonary arterial valves (PAV)
Blood flow velocity alters with respiration
(21). The fluctuation of
respiratory blood flow velocity is helpful to evaluate the degree
of damage of lung tissue in patients (21,22).
Ultrasonic imaging (Fig. 2A)
revealed that 10 mg/kg MA significantly increased mean blood flow
velocity (Fig. 2B) and HR
(Fig. 2C) compared with the
control.
HR was ~330±60 bpm in the control group, and
following treatment with 5 mg/kg MA the.
PAV was ~96±7.4 cm/s in the control group, and
following 5 mg/kg MA treatment, PAV did not significantly differ
between the control group and M5 group (P>0.05; Fig. 2B). However, 10 mg/kg MA treatment
significantly increased PAV to 136±14 cm/s compared with the
control group (P<0.01; Fig.
2B).
HR was elevated to 360±120 bpm, but no significant
differences were observed compared with the control group
(P>0.05). However, 10 mg/kg MA treatment significantly increased
HR to 540±180 bpm compared with the control group (P<0.05;
Fig. 2C).
Histopathological analysis of lung
injury induced by MA
To assess lung injury response to MA,
histopathological analysis was performed on sections stained with
hematoxylin and eosin. Following MA administration for 5 weeks,
visible differences in the level of lung injury were observed in
the M5 and M10 groups compared with the control group. Perivascular
exudates, thickened alveolar septa, slight hemorrhage, airspace
edema and inflammatory cell infiltration were observed in rats
treated with MA (Fig. 3A). Only
inflammatory cell infiltration was significantly increased in the
M5 group compared with the control (P<0.05; Fig. 3B). However, edema, hemorrhage,
inflammatory cell infiltration and total lung injury scores were
significantly increased in the M10 group compared with the control
group (P<0.05, P<0.05, P<0.01 and P<0.01, respectively;
Fig. 3B and C, respectively).
Consistent with this injury pattern, the lung W/D weight ratios of
rats were significantly increased in the M5 and M10 groups compared
with the control (P<0.05 and P<0.01, respectively; Fig. 3D).
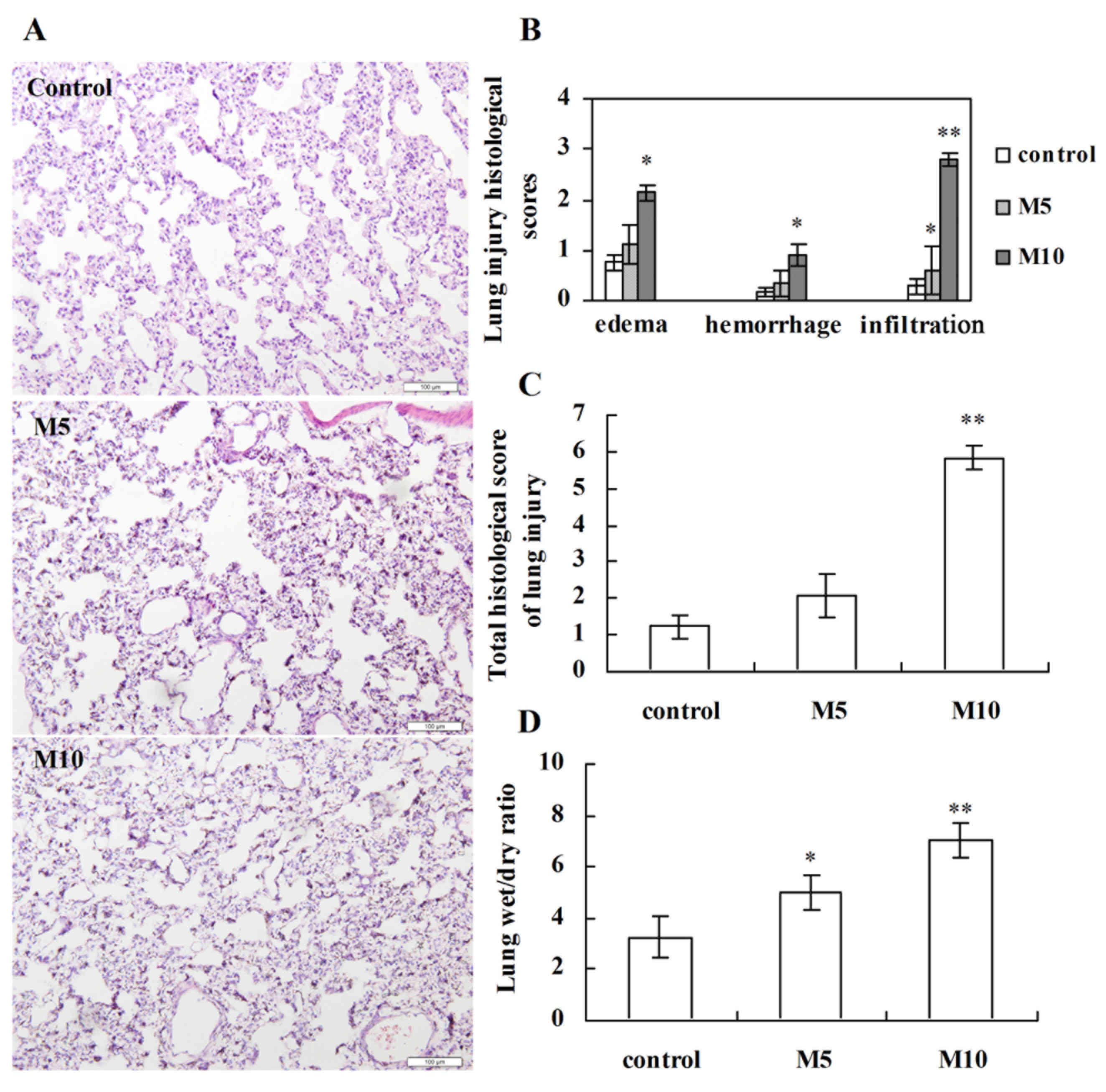 | Figure 3.Histopathological analysis of lung
injury induced by methamphetamine. (A) Representative images of
lung injury following hematoxylin and eosin staining from the
control, M5 and M10 groups (magnification, ×200). (B) Histological
scores for interstitial edema, hemorrhage and inflammatory cell
infiltration. Each feature received a score of 0, no injury; 1,
minimal injury; 2, moderate injury or 3, severe injury. (C) Total
lung injury scores in different groups. The score for each rat was
given as a minimum of 0 and a maximum of 9. (D) Comparison of lung
wet-to-dry weight ratio in different groups. Data are expressed as
the mean ± standard deviation (n=6). *P<0.05, **P<0.01 vs.
control group. M5, 5 mg/kg methamphetamine; M10, 10 mg/kg
methamphetamine. |
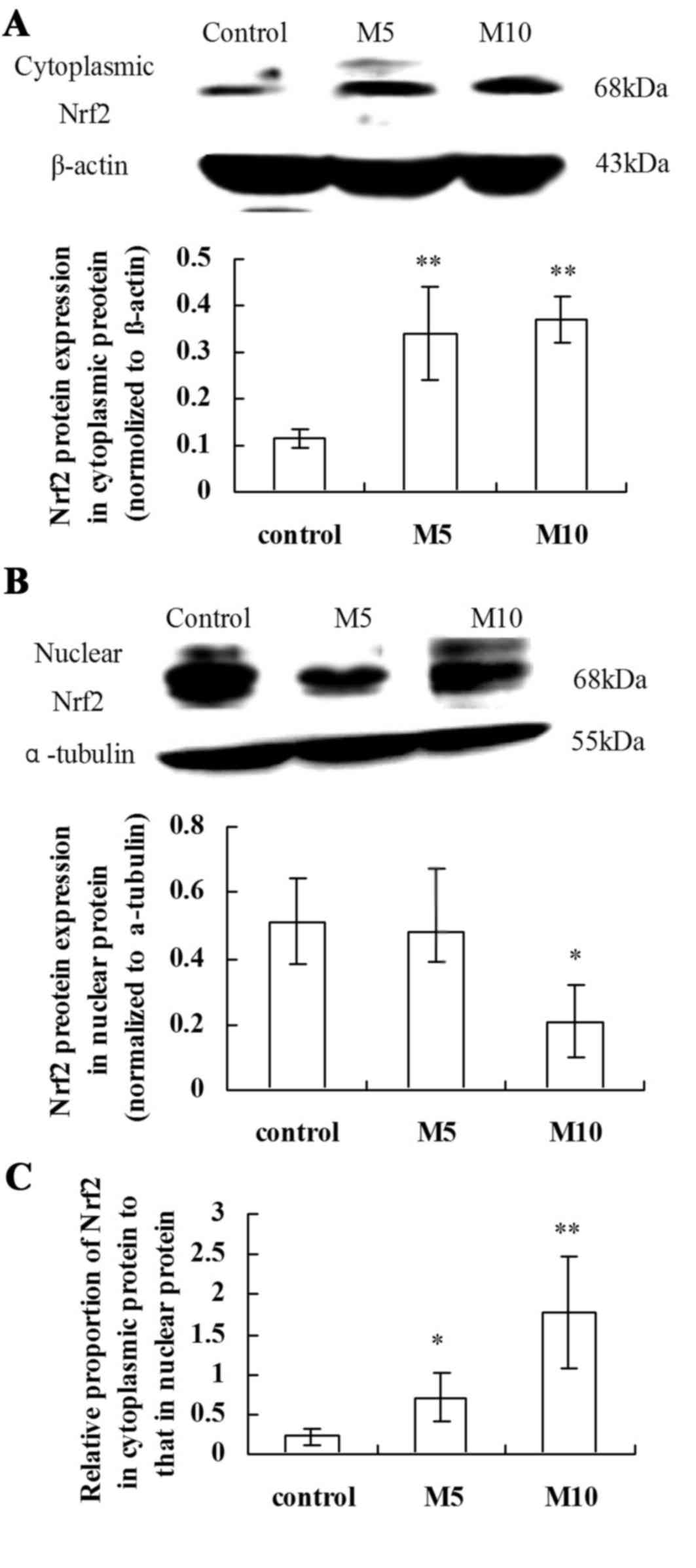
Effect of chronic MA exposure on Nrf2
expression in rat lungs
Cytoplasmic and nuclear extracts were subjected to
immunoblotting against Nrf2. MA significantly increased Nrf2
cytoplasmic protein expression levels in the M5 group compared with
the control group (P<0.01; Fig.
4A). Nrf2 nuclear protein expression levels were significantly
reduced in the M10 group compared with the control group
(P<0.05; Fig. 4B). The relative
proportion of Nrf2 cytoplasmic to nuclear protein was significantly
increased in the M5 and M10 groups compared with the control group
(P<0.05 and P<0.01, respectively; Fig. 4C). Therefore, MA treatment
significantly suppressed the translocation of Nrf2 from the
cytoplasm to nucleus and inhibited the Nrf2-mediated antioxidative
defense in a concentration-dependent manner.
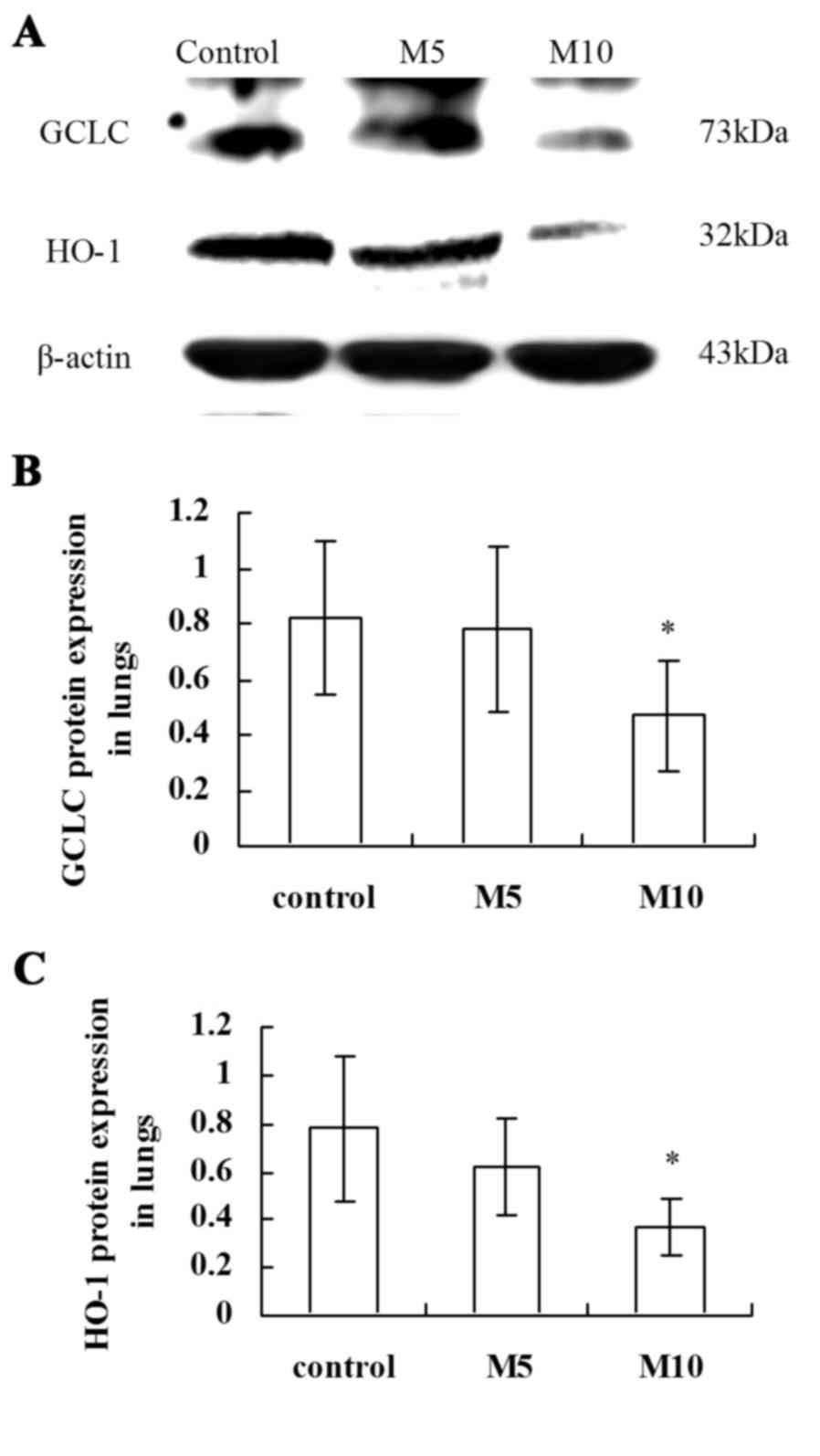
Inhibitory effect of MA on antioxidant
enzymes GCLC and HO-1 in rat lungs
The western blot assay (Fig. 5A) demonstrated that the Nrf2 target
genes GCLC (P<0.05; Fig. 5B)
and HO-1 (P<0.05; Fig. 5C)
protein expression levels were significantly reduced in the M10
group compared with the control group.
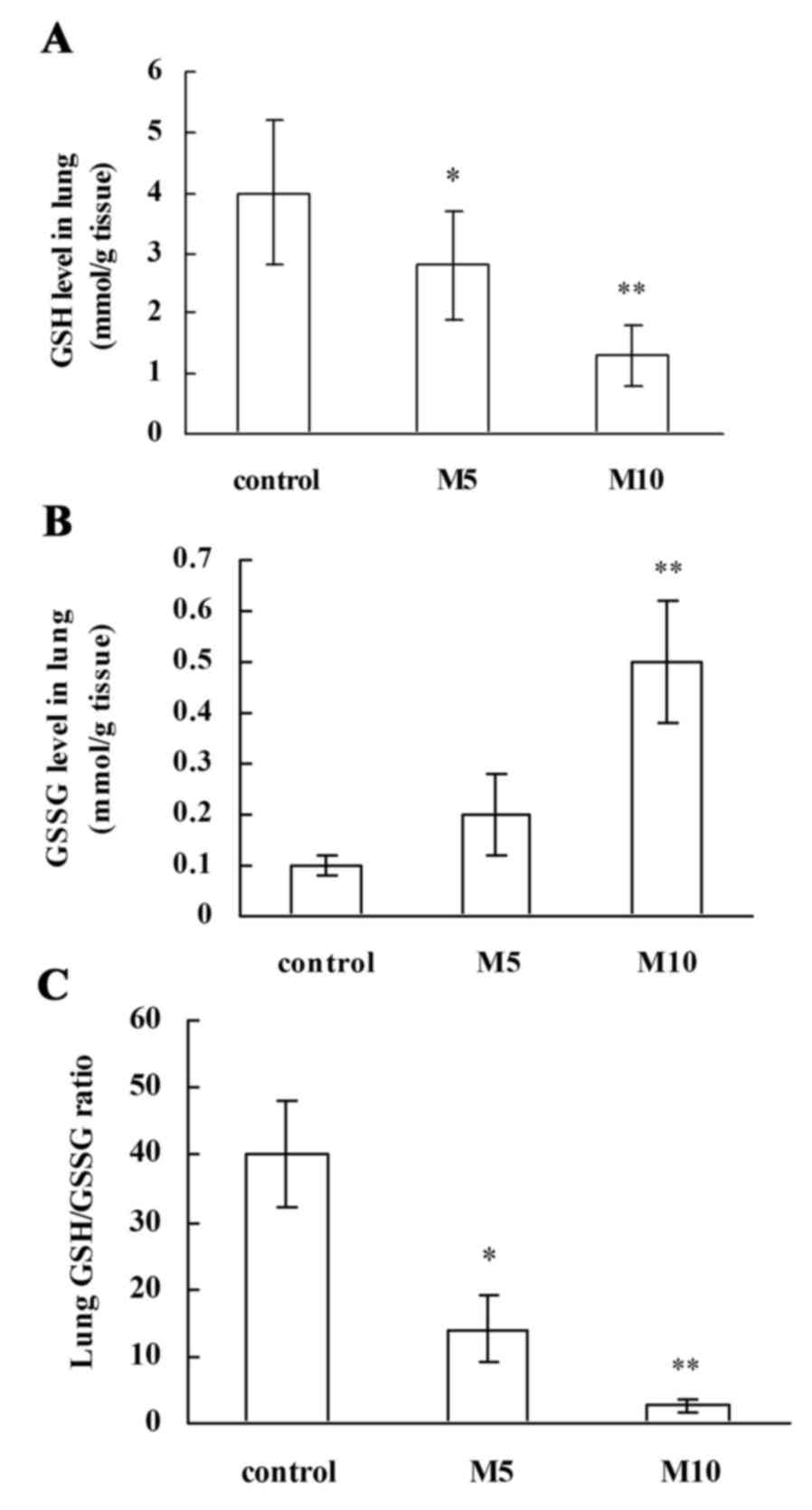
Effect of MA on GSH and GSSG protein
expression levels in lung tissues
The GSH protein level and the ratio of GSH to GSSG
(GSH/GSSG) are involved in antioxidative defense (23). In the present study, MA treatment
significantly and dose-dependently downregulated lung GSH protein
levels compared with the control (M5 group, P<0.05; M10 group,
P<0.01; Fig. 6A), and lung GSSG
protein levels were additionally significantly upregulated in the
M10 group compared with the control group (P<0.01; Fig. 6B). Associated with the decreased
GSH protein level, the GSH/GSSG ratios were dose-dependently
significantly reduced by MA treatment compared with the control (M5
group, P<0.05; M10 group, P<0.01; Fig. 6C). These findings support that MA
suppresses antioxidative defense in rat lungs.
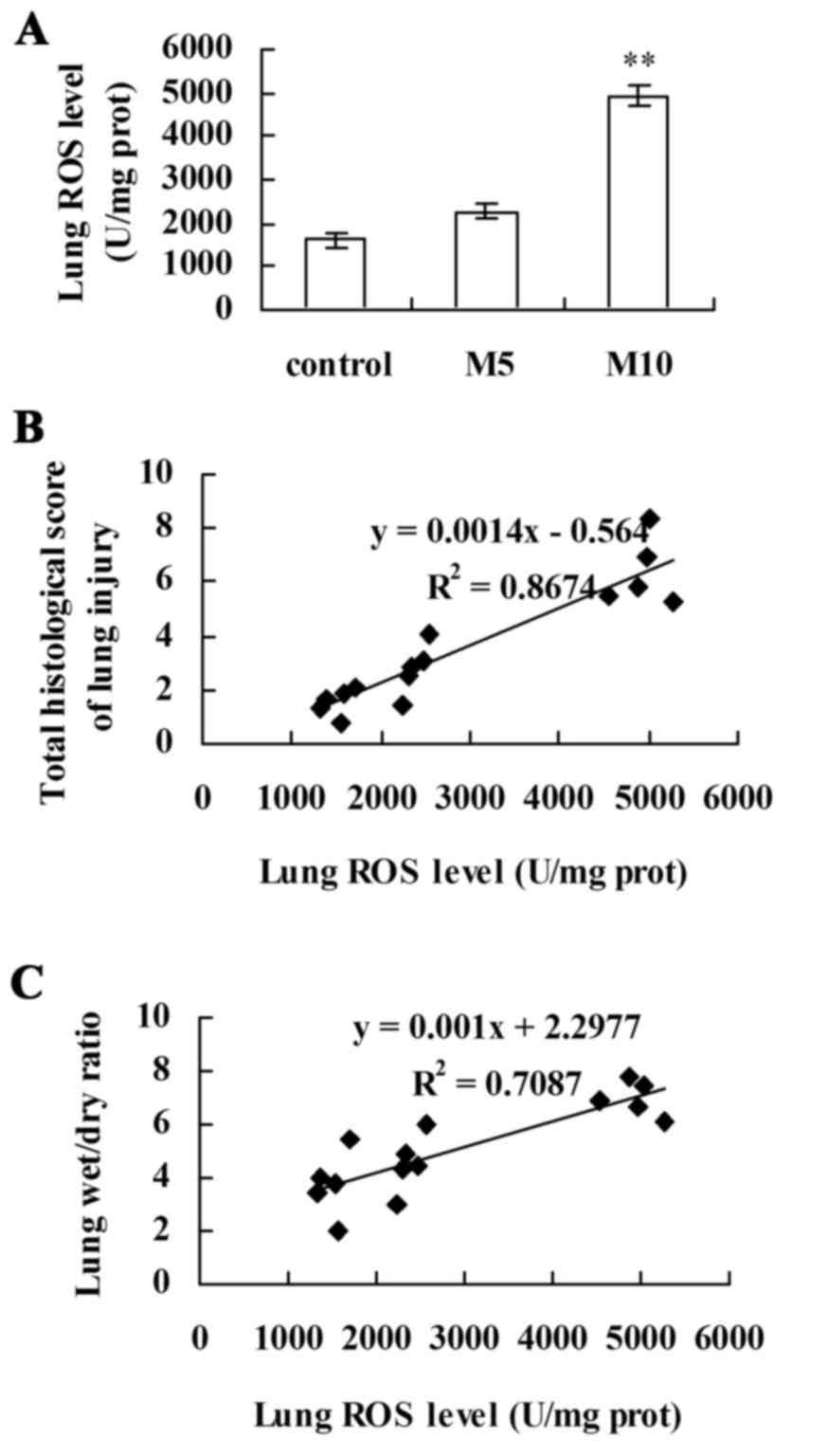
Correlation between lung ROS levels
and the indexes of lung injury
The results of ELISA revealed that, although no
significant differences were observed in ROS level between the
control and M5 groups, ROS levels were significantly increased in
the M10 group compared with the control (P<0.01; Fig. 7A). Linear regression analysis
revealed a significant positive correlation between lung ROS levels
and the indexes of lung injury: Lung injury scores
(R2=0.8,674; P<0.05; Fig. 7B) and W/D weight ratios
(R2=0.7,087; P<0.05; Fig. 7C). These results suggested that
alterations in lung ROS levels coincided with alterations in lung
injury induced by chronic exposure to MA.
Discussion
The results of the present study indicated that
chronic exposure to MA induced an increase in HR and PAV and a
decrease in the survival rate of rats, and led to histopathological
alterations common to lung injury: Perivascular exudates, airspace
edema, slight hemorrhage and inflammatory cell infiltration.
Furthermore, 10 mg/kg MA treatment significantly inhibited the
expression of nuclear Nrf2 protein and Nrf2 target genes (GCLC and
HO-1), and MA dose-dependently reduced GSH protein levels and the
ratio of GSH/GSSG, accompanied by increases in ROS levels in rat
lungs, which implied that MA suppresses Nrf2-mediated antioxidative
properties. Linear regression analysis revealed that there was a
positive correlation between lung ROS level and lung injury
indexes, as determined by histological scores of lung injury and
lung W/D ratio. These findings suggested that suppression of
Nrf2-mediated antioxidative defense is associated with lung injury
induced by chronic exposure to MA in rats.
The lungs are the principal organ exposed to MA
abuse (24). Baylor et al
(25) confirmed that inhalation of
MA results in interstitial pulmonary fibrosis and progressive
massive fibrosis in the absence of other causes. Previous studies
have also demonstrated that chronic exposure to MA induces
pulmonary toxicity, including pulmonary vascular remodeling and
pulmonary inflammation (10,19).
Blood flow velocity changes with respiration. In the present study,
10 mg/kg MA significantly increased the heart rate accompanied with
the increasing peak blood flow velocity of pulmonary arterial
valves, which reflected the clinical manifestation: palpitation and
shortness of breath of pulmonary arterial hypertension (2). The fluctuation of blood flow velocity
of pulmonary arterial valves is helpful to indirectly evaluate the
degree of damage of lung tissue in patients (21,22).
In the present study, MA induced perivascular exudates, airspace
edema, slight hemorrhage and inflammatory cell infiltration, which
resulted in a decline in cardiopulmonary function and the survival
rate of rats. These findings indicated that lung injury was one of
the important causes of the mortality that resulted from chronic
exposure to MA.
Oxidative stress is a general term used for
describing pathologies of various diseases at a molecular level,
and reflects an imbalance between free radical production and
antioxidant defense of the biological system (26,27).
ROS activate redox-sensitive transcription factors including Nrf2
during oxidative stress (11,28,29).
Nrf2 is a key molecule in the antioxidant defense system (30). Nrf2 is a transcription factor
responsible for regulating a group of antioxidant enzymes including
GCLC and HO-1 against oxidative stress (31,32).
The present study demonstrated that MA increased cytoplasmic Nrf2
protein expression, but significantly inhibited nuclear Nrf2
protein expression levels. MA also significantly increased the
relative proportion of cytoplasmic Nrf2 compared with nuclear Nrf2,
which confirmed that MA significantly suppressed the translocation
of Nrf2 from the cytoplasm to the nucleus. Furthermore, MA
significantly inhibited the protein expression of Nrf2 target
genes, the antioxidant enzymes GCLC and HO-1, in rat lungs. These
findings suggested that chronic exposure to MA inhibits the
Nrf2-mediated antioxidative defense system.
GSH is an abundant endogenous antioxidant and a
critical regulator of oxidative stress and immune function
(33,34). Cellular GSH homeostasis is
maintained by catalyzing the reduction of GSSG into GSH (34,35).
As a co-factor for various enzymatic reactions, GSH reduces
cellular ROS levels to protect against oxidative stress-induced
injury (35–37). ROS can be produced from multiple
sources, including mechanical ventilation, surgical trauma,
manipulated lung tissue, and hyperoxia in the ventilated lung
(38). ROS have been demonstrated
to regulate inflammation and structural changes in the lungs
(39). In the present study, it
was revealed that MA dose-dependently reduced the GSH level and the
ratio of GSH/GSSG, accompanied by increases in the ROS level in rat
lungs. In addition, there was a positive correlation between lung
ROS levels and histological scores of lung injury and the lung W/D
ratio, which suggested that alterations in lung ROS levels
coincided with the degree of lung injury induced by chronic
exposure to MA. Taken together, this indicated that the
GSH-dependent Nrf2 antioxidant defense was involved in regulating
oxidative stress-induced lung injury by MA.
In conclusion, suppression of Nrf2-mediated
antioxidative defense was demonstrated to be associated with lung
injury induced by chronic exposure to MA in rats. The present study
suggests that Nrf2, as the key factor in the balance between
oxidant load and antioxidative capacity, may be an important
therapeutic target for MA-induced chronic lung toxicity.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81503058) and the Natural
Science Foundation of Liaoning Province (grant no. 2014021065).
References
|
1
|
Patel D, Desai GM, Frases S, Cordero RJ,
DeLeon-Rodriguez CM, Eugenin EA, Nosanchuk JD and Martinez LR:
Methamphetamine enhances Cryptococcus neoformans pulmonary
infection and dissemination to the brain. MBio. 4:pii: e00400. –13.
2013. View Article : Google Scholar
|
|
2
|
Rothman RB and Baumann MH: Methamphetamine
and idiopathic pulmonary arterial hypertension: Role of the
serotonin transporter. Chest. 132:1412–1413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Volkow ND, Fowler JS, Wang GJ, Shumay E,
Telang F, Thanos PK and Alexoff D: Distribution and
pharmacokinetics of methamphetamine in the human body: Clinical
implications. PLoS One. 5:e152692010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eba S, Hoshikawa Y, Moriguchi T, Mitsuishi
Y, Satoh H, Ishida K, Watanabe T, Shimizu T, Shimokawa H, Okada Y,
et al: The nuclear factor erythroid 2-related factor 2 activator
oltipraz attenuates chronic hypoxia-induced cardiopulmonary
alterations in mice. Am J Respir Cell Mol Biol. 49:324–333. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Houten B: Pulmonary arterial
hypertension is associated with oxidative stress-induced genome
instability. Am J Respir Crit Care Med. 192:129–130. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ushio-Fukai M: Localizing NADPH
oxidase-derived ROS. Sci STKE. 2006:re82006.PubMed/NCBI
|
|
7
|
Auten RL and Davis JM: Oxygen toxicity and
reactive oxygen species: The devil is in the details. Pediatr Res.
66:121–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiou SY, Lee YS, Jeng MJ, Tsao PC and
Soong WJ: Moderate hypothermia attenuates oxidative stress injuries
in alveolar epithelial A549 cells. Exp Lung Res. 39:217–228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang CH, Yang ML, Tsai CH, Li YC, Lin YJ
and Kuan YH: Ginkgo biloba leaves extract (EGb 761) attenuates
lipopolysaccharide-induced acute lung injury via inhibition of
oxidative stress and NF-κB-dependent matrix metalloproteinase-9
pathway. Phytomedicine. 20:303–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Liu M, Wang HM, Bai Y, Zhang XH,
Sun YX and Wang HL: Involvement of serotonin mechanism in
methamphetamine-induced chronic pulmonary toxicity in rats. Hum Exp
Toxicol. 32:736–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Montes S, Juárez-Rebollar D, Nava-Ruíz C,
Sánchez-García A, Heras-Romero Y, Rios C and Méndez-Armenta M:
Immunohistochemical study of Nrf2-antioxidant response element as
indicator of oxidative stress induced by cadmium in developing
rats. Oxid Med Cell Longev. 2015:5706502015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Komaravelli N and Casola A: Respiratory
viral infections and subversion of cellular antioxidant defenses. J
Pharmacogenomics Pharmacoproteomics. 5:pii: 1000141.
2014.PubMed/NCBI
|
|
13
|
Kim SG, Lee WH and Kim YW: Nrf2
(NF-E2-Related Factor2)Encyclopedia of Signaling Molecules. Choi S:
1st. Springer; New York, NY: pp. 1262–1268. 2012
|
|
14
|
Kim J, Cha YN and Surh YJ: A protective
role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in
inflammatory disorders. Mutat Res. 690:12–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Atia AE and Abdullah AB: Modulation of
Nrf2/Keap1 pathway by dietary phytochemicals. Int J Res Med Sci.
2:375–381. 2014. View Article : Google Scholar
|
|
16
|
D'Oria V, Petrini S, Travaglini L, Priori
C, Piermarini E, Petrillo S, Carletti B, Bertini E and Piemonte F:
Frataxin deficiency leads to reduced expression and impaired
translocation of NF-E2-related factor (Nrf2) in cultured motor
neurons. Int J Mol Sci. 14:7853–7865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiang HS and Maric M: Lysosomal thiol
reductase negatively regulates autophagy by altering glutathione
synthesis and oxidation. Free Radic Biol Med. 51:688–699. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Howden R: Nrf2 and cardiovascular defense.
Oxid Med Cell Longev. 2013:1043082013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu M, Wang Y, Wang HM, Bai Y, Zhang XH,
Sun YX and Wang HL: Fluoxetine attenuates chronic
methamphetamine-induced pulmonary arterial remodelling: Possible
involvement of serotonin transporter and serotonin 1B receptor.
Basic Clin Pharmacol Toxicol. 112:77–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Z, Tang X, Zhao X, Zhang M, Zhang W,
Hou S, Yuan W, Zhang H, Shi L, Jia H, et al: Tylvalosin exhibits
anti-inflammatory property and attenuates acute lung injury in
different models possibly through suppression of NF-κB activation.
Biochem Pharmacol. 90:73–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kudo K, Terae S, Ishii A, Omatsu T, Asano
T, Tha KK and Miyasaka K: Physiologic change in flow velocity and
direction of dural venous sinuses with respiration: MR venography
and flow analysis. AJNR Am J Neuroradiol. 25:551–557.
2004.PubMed/NCBI
|
|
22
|
Halimi KE, Negadi M, Bouguetof H, Zemour
L, Boumendil D and Mentouri Chentouf Z: Respiratory variations in
aortic blood flow velocity and inferior vena cava diameter as
predictors of fluid responsiveness in mechanically ventilated
children using transthoracic echocardiography in a pediatric PICU.
Critical Care. 19:(Suppl 1). S1812015. View
Article : Google Scholar
|
|
23
|
Qin T, Yin Y, Yu Q and Yang Q: Bursopentin
(BP5) protects dendritic cells from lipopolysaccharide-induced
oxidative stress for immunosuppression. PLoS One. 10:e01174772015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wells SM, Buford MC, Braseth SN, Hutchison
JD and Holian A: Acute inhalation exposure to vaporized
methamphetamine causes lung injury in mice. Inhal Toxicol.
20:829–838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baylor PA, Sobenes JR and Vallyathan V:
Interstitial pulmonary fibrosis and progressive massive fibrosis
related to smoking methamphetamine with talc as filler. Respir
Care. 58:e53–e55. 2013.PubMed/NCBI
|
|
26
|
Antus B, Drozdovszky O, Barta I and
Kelemen K: Comparison of airway and systemic malondialdehyde levels
for assessment of oxidative stress in cystic fibrosis. Lung.
193:597–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sellamuthu PS, Arulselvan P, Kamalraj S,
Fakurazi S and Kandasamy M: Protective nature of mangiferin on
oxidative stress and antioxidant status in tissues of
streptozotocin-induced diabetic rats. ISRN Pharmacol.
2013:7501092013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh S, Vrishni S, Singh BK, Rahman I and
Kakkar P: Nrf2-ARE stress response mechanism: A control point in
oxidative stress-mediated dysfunctions and chronic inflammatory
diseases. Free Radic Res. 44:1267–1288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kovac S, Angelova PR, Holmström KM, Zhang
Y, Dinkova-Kostova AT and Abramov AY: Nrf2 regulates ROS production
by mitochondria and NADPH oxidase. Biochim Biophys Acta.
1850:794–801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Messier EM, Day BJ, Bahmed K, Kleeberger
SR, Tuder RM, Bowler RP, Chu HW, Mason RJ and Kosmider B:
N-acetylcysteine protects murine alveolar type II cells from
cigarette smoke injury in a nuclear erythroid 2-related
factor-2-independent manner. Am J Respir Cell Mol Biol. 48:559–567.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Masuda Y, Vaziri ND, Li S, Le A,
Hajighasemi-Ossareh M, Robles L, Foster CE, Stamos MJ, Al-Abodullah
I, Ricordi C and Ichii H: The effect of Nrf2 pathway activation on
human pancreatic islet cells. PLoS One. 10:e01310122015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishii T, Itoh K, Takahashi S, Sato H,
Yanagawa T, Katoh Y, Bannai S and Yamamoto M: Transcription factor
Nrf2 coordinately regulates a group of oxidative stress-inducible
genes in macrophages. J Biol Chem. 275:16023–16029. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Richie JP Jr, Nichenametla S, Neidig W,
Calcagnotto A, Haley JS, Schell TD and Muscat JE: Randomized
controlled trial of oral glutathione supplementation on body stores
of glutathione. Eur J Nutr. 54:251–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sinha-Hikim I, Shen R, Kovacheva E, Crum
A, Vaziri ND and Norris KC: Inhibition of apoptotic signalling in
spermine-treated vascular smooth muscle cells by a novel
glutathione precursor. Cell Biol Int. 34:503–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SH, Smith AJ, Tan J, Shytle RD and
Giunta B: MSM ameliorates HIV-1 Tat induced neuronal oxidative
stress via rebalance of the glutathione cycle. Am J Transl Res.
7:328–338. 2015.PubMed/NCBI
|
|
36
|
Yang SR, Park JR and Kang KS: Reactive
oxygen species in mesenchymal stem cell aging: Implication to lung
diseases. Oxid Med Cell Longev. 2015:4862632015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jayakumar S, Kunwar A, Sandur SK, Pandey
BN and Chaubey RC: Differential response of DU145 and PC3 prostate
cancer cells to ionizing radiation: Role of reactive oxygen
species, GSH and Nrf2 in radiosensitivity. Biochim Biophys Acta.
1840:485–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia R, Xu J, Yin H, Wu H, Xia Z, Zhou D,
Xia ZY, Zhang L, Li H and Xiao X: Intravenous infusion of
dexmedetomidine combined isoflurane inhalation reduces oxidative
stress and potentiates hypoxia pulmonary vasoconstriction during
one-lung ventilation in patients. Mediators Inflamm.
2015:2380412015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang X, Yao H, Chen Y, Sun L, Li Y, Ma X,
Duan S, Li X, Xiang R, Han J and Duan Y: Inhibition of glutathione
production induces macrophage CD36 expression and enhances
cellular-oxidized low density lipoprotein (oxLDL) uptake. J Biol
Chem. 290:21788–21799. 2015. View Article : Google Scholar : PubMed/NCBI
|