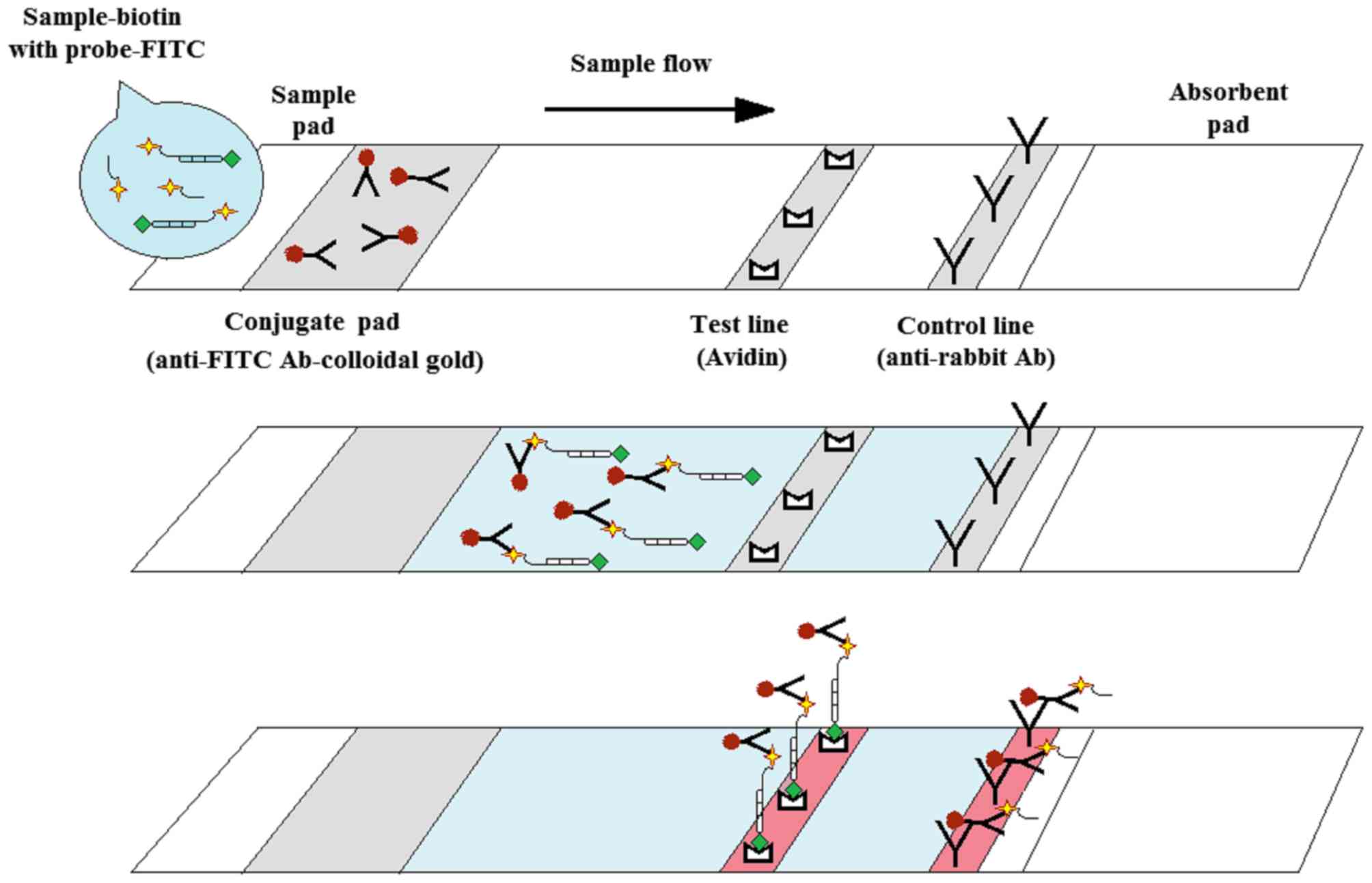
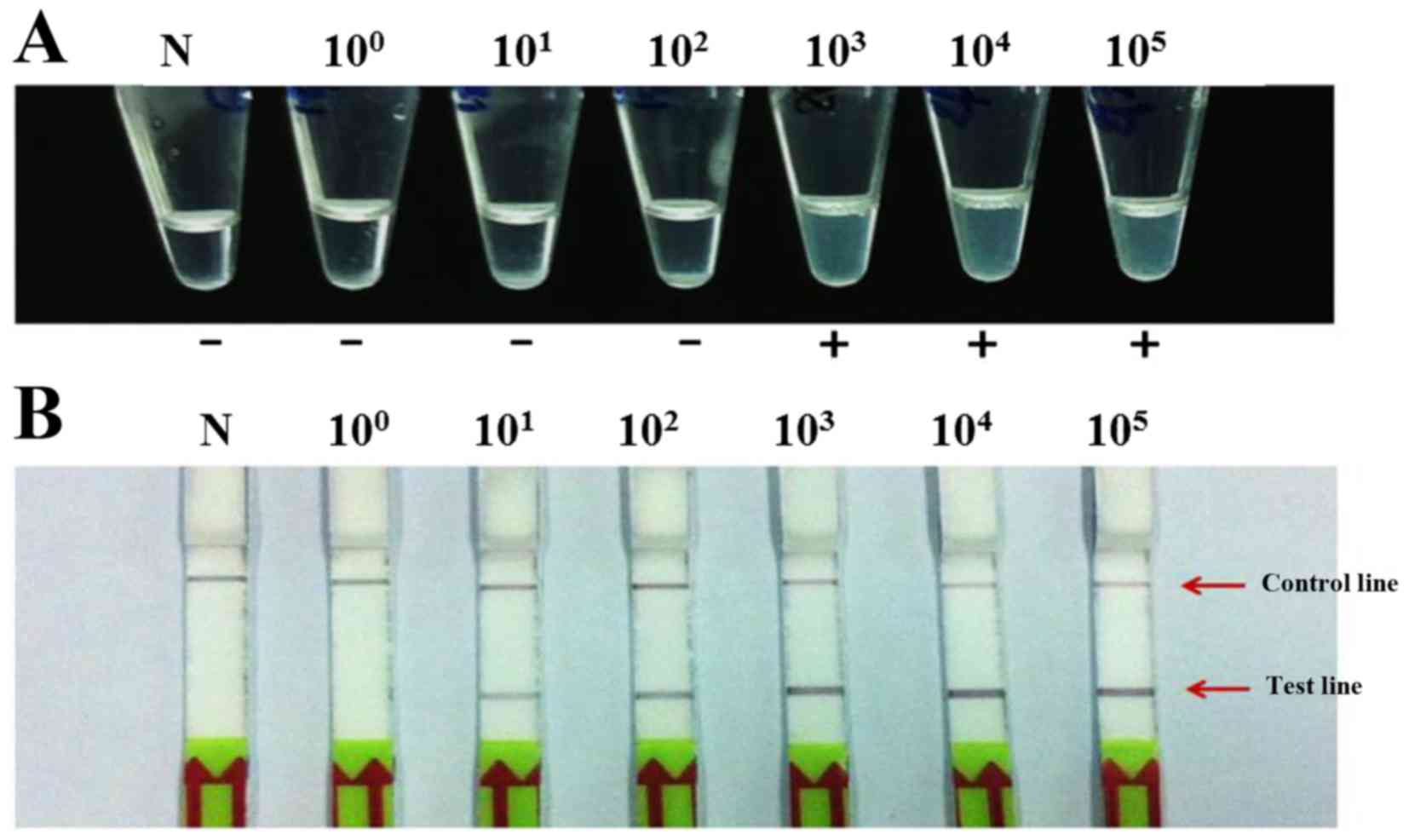
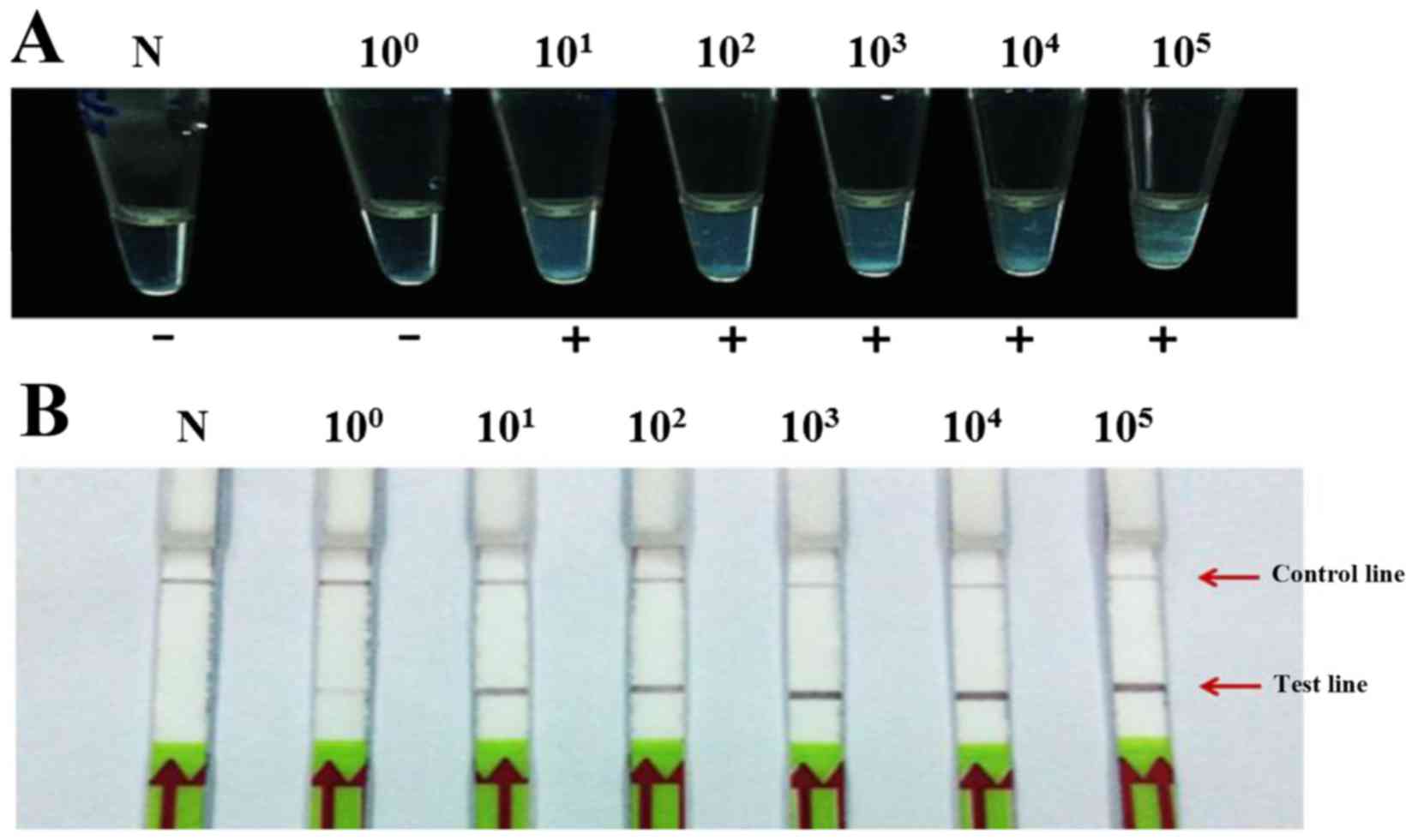
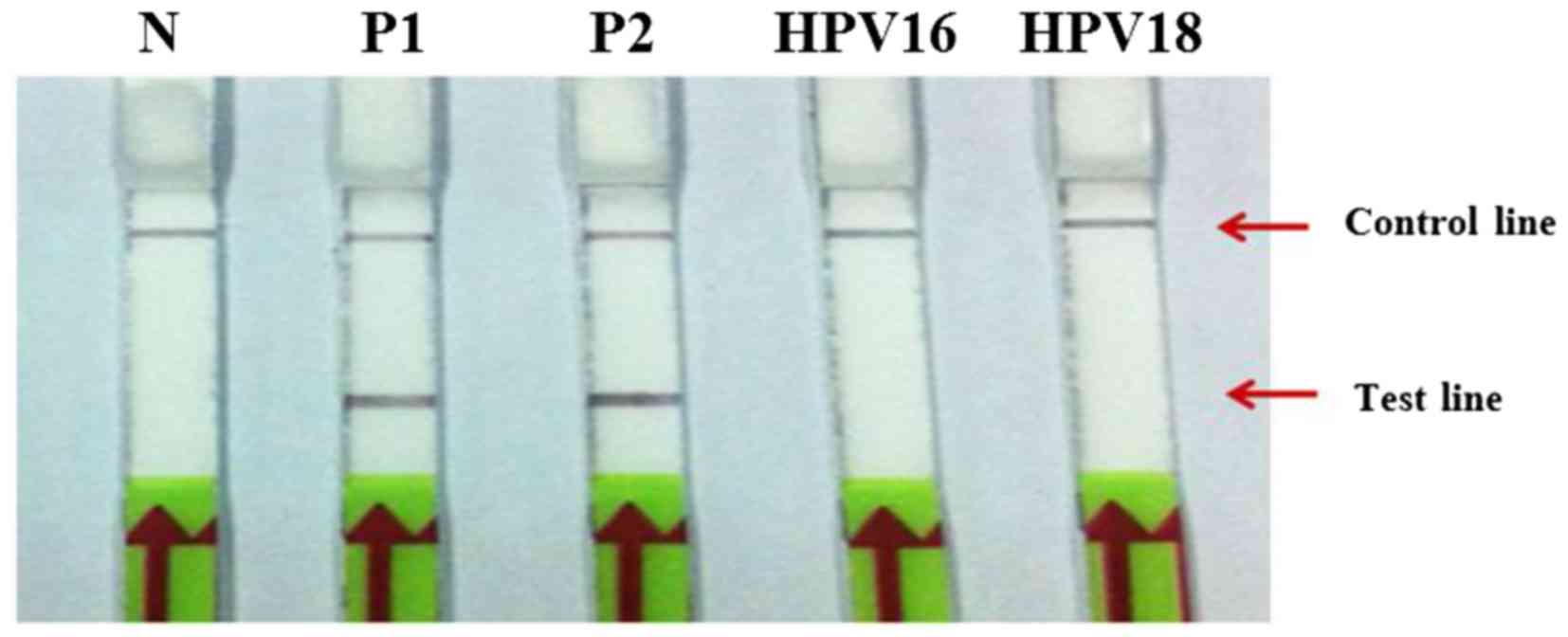
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chansaenroj J, Junyangdikul P, Chinchai T,
Swangvaree S, Karalak A, Gemma N and Poovorawan Y: Large scale
study of HPV genotypes in cervical cancer and different cytological
cervical specimens in Thailand. J Med Virol. 86:601–607. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suthipintawong C, Siriaunkgul S,
Tungsinmunkong K, Pientong C, Ekalaksananan T, Karalak A, Kleebkaow
P, Vinyuvat S, Triratanachat S, Khunamornpong S and Chongsuwanich
T: Human papilloma virus prevalence, genotype distribution, and
pattern of infection in Thai women. Asian Pac J Cancer Prev.
12:853–856. 2011.PubMed/NCBI
|
|
4
|
Saslow D, Solomon D, Lawson HW, Killackey
M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur
DC, et al: American cancer society, American society for colposcopy
and cervical pathology and American society for clinical pathology
screening guidelines for the prevention and early detection of
cervical cancer. CA Cancer J Clin. 62:147–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arney A and Bennett KM: Molecular
diagnostics of human papillomavirus. Labmedicine. 41:523–530.
2010.
|
|
6
|
Zaravinos A, Mammas IN, Sourvinos G and
Spandidos DA: Molecular detection methods of human papillomavirus
(HPV). Int J Biol Marker. 24:215–222. 2009. View Article : Google Scholar
|
|
7
|
Notomi T, Okayama H, Masubuchi H, Yonekawa
T, Watanabe K, Amino N and Hase T: Loop-mediated isothermal
amplification of DNA. Nucleic Acids Res. 28:E632000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saetiew C, Limpaiboon T, Jearanaikoon P,
Daduang S, Pientong C, Kerdsin A and Daduang J: Rapid detection of
the most common high-risk human papillomaviruses by loop-mediated
isothermal amplification. J Virol Methods. 178:22–30. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fischbach J, Xander NC, Frohme M and
Glokler JF: Shining a light on LAMP assays-a comparison of LAMP
visualization methods including the novel use of berberine.
Biotechniques. 58:189–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Posthuma-Trumpie GA, Korf J and van
Amerongen A: Lateral flow (immuno)assay: Its strengths, weaknesses,
opportunities and threats. A literature survey. Anal Bioanal Chem.
393:569–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yetisen AK, Akram MS and Lowe CR:
Paper-based microfluidic point-of-care diagnostic devices. Lab
Chip. 13:2210–2251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sotlar K, Diemer D, Dethleffs A, Hack Y,
Stubner A, Vollmer N, Menton S, Menton M, Dietz K, Wallwiener D, et
al: Detection and typing of human papillomavirus by e6 nested
multiplex PCR. J Clin Microbiol. 42:3176–3184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Villiers EM: Papillomavirus and HPV
typing. Clin Dermatol. 15:199–206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kiatpathomchai W, Jaroenram W, Arunrut N,
Jitrapakdee S and Flegel TW: Shrimp Taura syndrome virus detection
by reverse transcription loop-mediated isothermal amplification
combined with a lateral flow dipstick. J Virol Methods.
153:214–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumvongpin R, Jearanaikool P, Wilailuckana
C, Sae-ung N, Prasongdee P, Daduang S, Wongsena M, Boonsiri P,
Kiatpathomchai W, Swangvaree SS, et al: High sensitivity,
loop-mediated isothermal amplification combined with colorimetric
gold-nanoparticle probes for visual detection of high risk human
papillomavirus genotypes 16 and 18. J Virol Methods. 234:90–95.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peitsaro P, Johansson B and Syrjänen S:
Integrated human papillomavirus type 16 is frequently found in
cervical cancer precursors as demonstrated by a novel quantitative
real-time PCR technique. J Clin Microbiol. 40:886–891. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Damay A, Didelot-Rousseau MN, Costes V,
Konate I, Ouedraogo A, Nagot N, Foulongne V, Van de Perre P, Mayaud
P and Segondy M: Viral load and physical status of human
papillomavirus (HPV) 18 in cervical samples from female sex workers
infected with HPV 18 in Burkina Faso. J Med Virol. 81:1786–1791.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wanga X, Tenga D, Guana Q, Tiana F and
Wang J: Detection of roundup ready soybean by loop-mediated
isothermal amplification combined with a lateral-flow dipstick.
Food Cont. 29:213–220. 2013. View Article : Google Scholar
|
|
19
|
Notomi T, Mori Y, Tomita N and Kanda H:
Loop-mediated isothermal amplification (LAMP): Principle, features,
and future prospects. J Microbiol. 53:1–5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mori Y, Hirano T and Notomi T: Sequence
specific visual detection of LAMP reactions by addition of cationic
polymers. BMC Biotechnol. 6:32006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaewphinit T, Arunrut N, Kiatpathomchai W,
Santiwatanakul S, Jaratsing P and Chansiri K: Detection of
Mycobacterium tuberculosis by using loop-mediated isothermal
amplification combined with a lateral flow dipstick in clinical
samples. Biomed Res Int. 2013:9262302013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khunthong S, Jaroenram W, Arunrut N,
Suebsing R, Mungsantisuk I and Kiatpathomchai W: Rapid and
sensitive detection of shrimp yellow head virus by loop-mediated
isothermal amplification combined with a lateral flow dipstick. J
Virol Methods. 188:51–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kil EJ, Kim S, Lee YJ, Kang EH, Lee M, Cho
SH, Kim MK, Lee KY, Heo NY, Choi HS, et al: Advanced loop-mediated
isothermal amplification method for sensitive and specific
detection of Tomato chlorosis virus using a uracil DNA glycosylase
to control carry-over contamination. J Virol Methods. 213:68–74.
2015. View Article : Google Scholar : PubMed/NCBI
|