Introduction
Cancer poses serious threat to life, and millions
suffer from the incidence of various types of tumor. Osteosarcoma
(OS) denotes a primary and epidemic type of bone malignancies
(1). In OS, immature bone tissues
are generated and normal bones are jeopardized (2). Long bones are more prone to the
occurrence of OS, in particular the knees (3). OS is one of the most common tumors
with an incidence rate of ~4 per million. In total, >50% of
patients with OS are children and adolescents (aged <24) while
~25% of cases occur in mid-age (4). It has also been reported that males
incur higher incidence rates than females (5). Due to long lasting developments in
health care, the survival rates for OS patients have increased
(1). However, poor diagnosis and
prognosis reduces the survival rates of patients with OS, and the
expected survival rates are always low (6). As a result, the identification of
novel targets for therapeutic intervention to treat OS is
required.
MicroRNAs (miRNAs or miRs) are a class of short
length, noncoding RNAs that decrease gene expression by base
pairing to the 3′-untranslated regions (3′-UTR) (7,8). The
pairing to the 3′-UTR can therefore either result in the
degradation of target mRNAs or the inhibition of their translation
(9). It has been demonstrated that
miRNAs can occur either individually or as part of a cluster within
the whole genome. Meanwhile, miRNAs also reside in coding and
non-coding regions of genes (10).
To date, >2,000 miRNAs have been identified, and these mediate
>1/3 total mRNAs in the human genome (11). Numerous tumors are associated with
aberrant mRNA expression, and diagnosis targeting miRNAs has been
proven promising (12). Therefore,
elucidating the mechanisms of miRNA regulation is important. miRNAs
are positively or negatively associated with cancer progression,
depending on the diversity in targets (13). For example, He et al
(14) demonstrated that miR-34
inhibits OS development in a p53-dependent manner. Another report
by Zhao et al (15) further
demonstrated that miR-34a mediates tumor suppression via targeting
CD44. In addition, miR-145 also inhibits OS via the downregulation
of Rho associated coiled-coil containing protein kinase 1 (14). On the contrary, miR-20a promotes
the metastasis of OS, with previous detailed mechanistic study
suggesting that miR-20a exerts this function via targeting Fas
expression (15). Meanwhile,
miR-21 is also involved in the progression and metastasis of OS
(16). However, the involvement of
miR-336 in OS remains largely unknown.
In the present study, miR-336 was demonstrated to be
decreased in OS tissues and inversely associated with sex
determining region Y-box 2 (Sox-2) levels. Overexpression of
miR-336 effectively inhibited the invasion and proliferation of OS
cells via inducing apoptosis. Two selected cell lines, HOS and
MG-63, were investigated further and it was demonstrated the
inhibitory effect of miR-336 is reversed by overexpressing Sox-2,
which was later confirmed to be a direct target of miR-336. These
findings suggested novel functions for miR-336, and shed light on
its potential as a target for diagnosis and therapeutic
intervention in OS.
Materials and methods
Cancer cell lines and human
samples
A total of 6 OS cell lines, U-2 OS, SW-1353, ZK-58,
HOS, Saos-2 and MG-63, were used in the present study. All were
commercially purchased from the American Type Culture Collection
(ATCC; Manassas, MA, USA). A control human fetal osteoblast (hFOB)
cell line was also purchased from the ATCC. All cell lines were
cultured in RPMI-1640 medium (Qiagen China Co., Ltd., Shanghai,
China). The RPMI-1640 medium was supplemented with 5% fetal bovine
serum (FBS; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in
humidified 5% CO2 at room temperature. The OS samples
were obtained from surgical archives and were taken from patients
registered at Xiangya Hospital, Central South University (Changsha,
China) between May 2014 and July 2015. A total of 68 patients were
included in this study (47–79 years; 38 male, 30 female). All
patients provided informed, written consent. The protocols of
experimental procedures using human specimens were approved by the
Human Research Ethics Committee of Xiangya Hospital, Central South
University (Changsha, China; approval no. 2014F0027).
MicroRNA-336 transfection
In the present study, a lentiviral system was used
to ectopically overexpress miR-336 in the OS cell lines HOS and
MG-63. The pcDNA3.1 plasmids containing miR-336 mimic
(3′-AGUACGUCAAGGCUCA-5′) or negative control were synthesized by
and purchased from Tiangen Biotech Co., Ltd. (Beijing, China). The
Sox-2 plasmid was synthesized by Tiangen Biotech Co., Ltd and
inserted into a pcDNA3.1 plasmid. An empty pcDNA3.1 vector served
as the negative control. Viral transfection into OS cell lines HOS
and MG-63 were performed using a Lipofectamine 2000 system
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. Following transfection
for 36 h, the culture medium was replenished with fresh medium. All
plasmids were experimentally verified by sequencing.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was harvested from OS cell lines and human
specimens with TRIzol reagent (Thermo Fisher Scientific, Inc.). The
cDNA (a total of 10 µg) generated by reverse transcription was
obtained using the miScript II RT kit (Qiagen GmbH, Hilden,
Germany) according to the manufacturer's protocol. A
well-established TaqMan microRNA RT-qPCR kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used to monitor miR-336
expression. To quantify Sox-2 mRNA expression levels, the
SYBR-Green PCR Master kit (Takara Bio, Inc.) was used. The
thermocycling conditions used were as follows: 50°C for 2 min, 95°C
for 10 min followed by 30 cycles of 95°C for 15 sec and 60°C for 1
min. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as
an internal control. Reactions were performed with the ABI
PRISM® 7000 Sequence Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Expression levels of miR-336 and Sox-2
were determined by the 2−ΔΔCq method (17). The experiments were performed with
≥3 replicates.
The primer sequences used were as follows:
miR-336, forward 5′-GATGCGACGTGAGTAAG-3′ and reverse
5′-CTGAGCCGGGTCCGAGGT-3′; GAPDH, forward
5′-CTCGATTGCATCGACATATCGT-3′ and reverse
5′-ACGCTTCGCGATCGTGCGTGAT-3′; Sox-2, forward
5′-GCTACAATAGCTCACCCTGAT-3′ and reverse
5′-CAATCTCCTGCCTACGAGAG-3′.
Luciferase reporter assay
HOS and MG-63 cells were plated in a 96-well plate
for 48 h (104 cells/well) in RPMI-1640 medium (Qiagen
China Co., Ltd.) supplemented with 5% FBS (Sigma-Aldrich; Merck
KGaA). The miR-336 and negative control plasmids were transfected
with luciferase reporter plasmids into the indicated OS cells,
using the aforementioned procedure. The 3′-UTR for Sox-2 with
binding site for miR-336 (5′ UGAUACUAUUUGAGCUAUU3′) was cloned into
the Xbal site downstream of the Renilla luciferase reporter plasmid
phRL-TK (Promega Corporation, Madison, WI, USA) leading to
wild-type Sox-2 luciferase plasmids (Sox-2 3′-UTR WT). The mutated
Sox-2 3′-UTR (5′ UGAUACUAUUUGUUUGCAU 3′) was similarly integrated
into phRL-TK to generate a mutant control plasmid (Sox-2 3′-UTR
mut). The luciferase activities were monitored with a
Dual-Luciferase reporter system (Promega Corporation) as relative
luciferase units according to the manufacturer's protocol.
Matrigel invasion assay
The upper chamber of the Transwell plate was coated
with Matrigel (Invitrogen; Thermo Fisher Scientific, Inc.)
overnight. HOS and MG-63 cells were resuspended 24 h following
transfection and loaded into the upper chamber (104
cells/well) in RPMI-1640 medium. The lower chambers were
supplemented with RPMI-1640 medium with additional 3% FBS.
Following 24 h incubation, the upper chambers were removed and
cells migrating into the lower chambers were fixed with 5%
polytetrafluoroethylene (PFA) and stained with crystal violet. The
Transwell assay results were assessed under a Leica fluorescent
microscope (DM-IRB; Leica Microsystems GmbH, Wetzlar, Germany). A
total of five fields were assessed and the experiments were
performed in triplicate. Invasion was evaluated and normalized to
the number of cells counted under control conditions.
Transwell migration assay
A similar protocol was performed as stated for the
invasion assay, in the absence of Matrigel. The final concentration
of cells was ~104 cells in each Transwell chamber. The
lower chamber was loaded with RPMI-1640 medium supplemented with 5%
FBS. Following incubation for 24 h, the cells were loaded into 5%
PFA and stained with crystal violet.
Proliferation assay
The Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used to measure the
proliferation of HOS and MG-63 cells. Following transfection for 36
h, as stated above, HOS and MG-63 cells were re-suspended and
seeded into a 96-well plate (104 cells/well) for 120 h.
25 µl MTT solution was added into the culture with final
concentration of 10 mg/ml for 4 h. The solution was shaken for 5
min, leading to complete solubilization. The proliferation was
evaluated once a day, for a total of 5 days. Crystalline formazan
was dissolved in 200 µl 10% sodium dodecyl sulfate (SDS) solution
for 24 h and the optical density at 490 nm was evaluated using the
Spectramax M5 microplate monitor (Molecular Devices, LLC,
Sunnyvale, CA, USA) following the manufacturer's protocol.
Flow cytometry for apoptosis
detection
HOS and MG-63 cells were loaded into a 12-well plate
(104 cells/well, BD Biosciences, Franklin Lakes, NJ,
USA) with Dulbecco's modified Eagle's medium supplemented with 5%
FBS (Sigma-Aldrich; Merck KGaA) and washed with cold PBS solution
48 h post transfection. The apoptosis of tumor cells was quantified
using a double staining (Annexin V-fluorescein
isothiocyanate/propidium iodide) apoptosis detection toolkit
(Sigma-Aldrich, Merck KGaA), following the manufacturer's protocol.
A Becton Dickinson FACSCalibur (BD Biosciences) cytometer was used
to analyze the data. Samples were processed with CellQuest software
version 3.3 (BD Biosciences).
Western blot
The human OS cell lines HOS and MG-63 were harvested
using lysis buffer (15% glycerol and 3% NP-40) obtained from Qiagen
China Co., Ltd. Samples were centrifuged at 15,000 × g at 4°C for
20 min. The protein extracts (100 µg in each lane) were
electrophorised using 10% SDS-PAGE and migrated to nitrocellulose
membranes (Bio-Rad Laboratories, Inc., Hercules CA, USA). The blot
was blocked with 4% fat-free milk for 1 h at 20°C. The membrane was
coated with rabbit anti-PAX7 antibody (1:1,000; catalog no.
AV32393; Qiagen China Co., Ltd.) and anti-GAPDH antibody (catalog
no. G9545; Qiagen China Co., Ltd.) at 4°C overnight. The
horseradish peroxidase-conjugated secondary antibodies (mouse
anti-human, 1:1,000; catalog no. SAB5201369; Sigma-Aldrich; Merck
KGaA) were then added and incubated at 20°C for 2 h. Immunoblot
signals were visualized using an ImageQuant™ LAS 4000
mini-biomolecular imager (Fujifilm, Tokyo, Japan). The blots were
quantified automatically using ImageJ software version 1.48
(National Institutes of Health, Bethesda, MD, USA). The experiment
was performed in triplicate.
Tumor cell implantation
A total of 106 HOS or MG-63 cells
expressing miR-336 or negative controls were injected submucosally
into BALB/c nude mice. The animal research was approved by the
Ethics Committee of Xiangya Hospital, Central South University. A
total of 12 mice (4–6 weeks; average weight, 15.5 g; 6 male and 6
female) were used in the study. Mice were housed at 20–22°C, 55–60%
humidity with a light/dark cycle of 10–12 h. Ad libitum
access to food and water was provided. The tumor volume was
measured each week for a total of 5 weeks (the tumor volume ranged
from ~500 to 1,600 mm3 at 5 weeks). At the end of the
implantation study, all mice were sacrificed by overdose of sodium
pentobarbital (5%, 300 mg/kg via intraperitoneal injection;
catalogue no. 1507002; Sigma-Aldrich; Merck KGaA) and Ki-67
immunostaining was performed. Briefly, tumor samples were fixed in
formalin, parraffin-embedded and cut into 5 µm sections with a
microtome. Following deparaffinization and rehydration, antigens
were retrieved with 1x Cytomation target retrieval solution
(DakoCytomation GmbH, Hamburg, Germany) in a decloaker chamber at
95°C for 15 min and then at 20°C for 10 min. Slides were then
incubated with hydrogen peroxide for 2 min. Following rinsing twice
with TBS-0.2% Tween 20, slides were then analyzed using the Ki-67
ELISA kit (catalog no. Ek-M21211; Huayun Biotech, Guangzhou, China
Co., Ltd.).
Statistical analysis
All statistical results were analyzed by SPSS 16.0
(SPSS, Inc., Chicago, IL, USA) using the Student's t-test.
Pearson's correlation coefficient was used to assess the
significance of any correlation between variables. All experiments
were performed in at least triplicate. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-336 mediates the regulation of
Sox-2 in OS
As previously reported, Sox-2 expression levels are
increased in OS cells (18). To
confirm this, the expression level of Sox-2 protein was measured by
western blot. The results revealed that, compared with hFOB cells,
the expression of Sox-2 was visibly elevated in all OS cell lines
(Fig. 1A). HOS and MG-63 cells
were selected for further study because the Sox-2 levels were the
highest (Fig. 1A). To further
identify novel microRNAs involved in OS cell regulation, the online
databases MIRDB (www.mirdb.org) (19), PicTar (pictar.mdc-berlin.de) and
TargetScan (release 6.2, www.targetscan.org) (20) were used to predict the potential
targets (Fig. 1B). miR-429 and
miR-336 were predicted to be plausible candidates by means of
overlapping results. miR-429 has been demonstrated to antagonize
Sox-2 in colorectal cancer previously (21). However, few studies have identified
the involvement of miR-336 in OS. Therefore, miR-336 was selected
for further investigation. A significant negative correlation
between miR-336 expression and Sox-2 mRNA expression levels in
human OS samples was identified (R=−0.4996, P=0.0004; Fig. 1C). The negative correlation was
also significant at the protein level of Sox-2 in human OS samples
(R=−0.5845, P=0.0002; Fig. 1D).
miR-336 expression levels were downregulated in human OS specimens
compared with normal controls, whereas opposite expression patterns
were observed in Sox-2 (Fig. 1C and
D). A dual-luciferase reporter assay was also prepared with
either wild type Sox-2 3′-UTR or a mutated miR-336 binding site
(Fig. 1E). The results revealed
that miR-336 significantly downregulated luciferase activities in
both HOS and MG-63 cells transfected with wild type Sox-2 3′-UTR
compared with the control (P<0.01; Fig. 1F and G, the control denotes HOS and
MG-63 cells transfected with the empty pcDNA3.1 vector). However,
miR-336 had no significant effect if HOS and MG-63 cells were
transfected with plasmids containing mutated Sox-2 3′-UTR (Fig. 1F and G, respectively). These
results suggested that miR-336 expression was downregulated in OS
cells and was negatively correlated with Sox-2 expression.
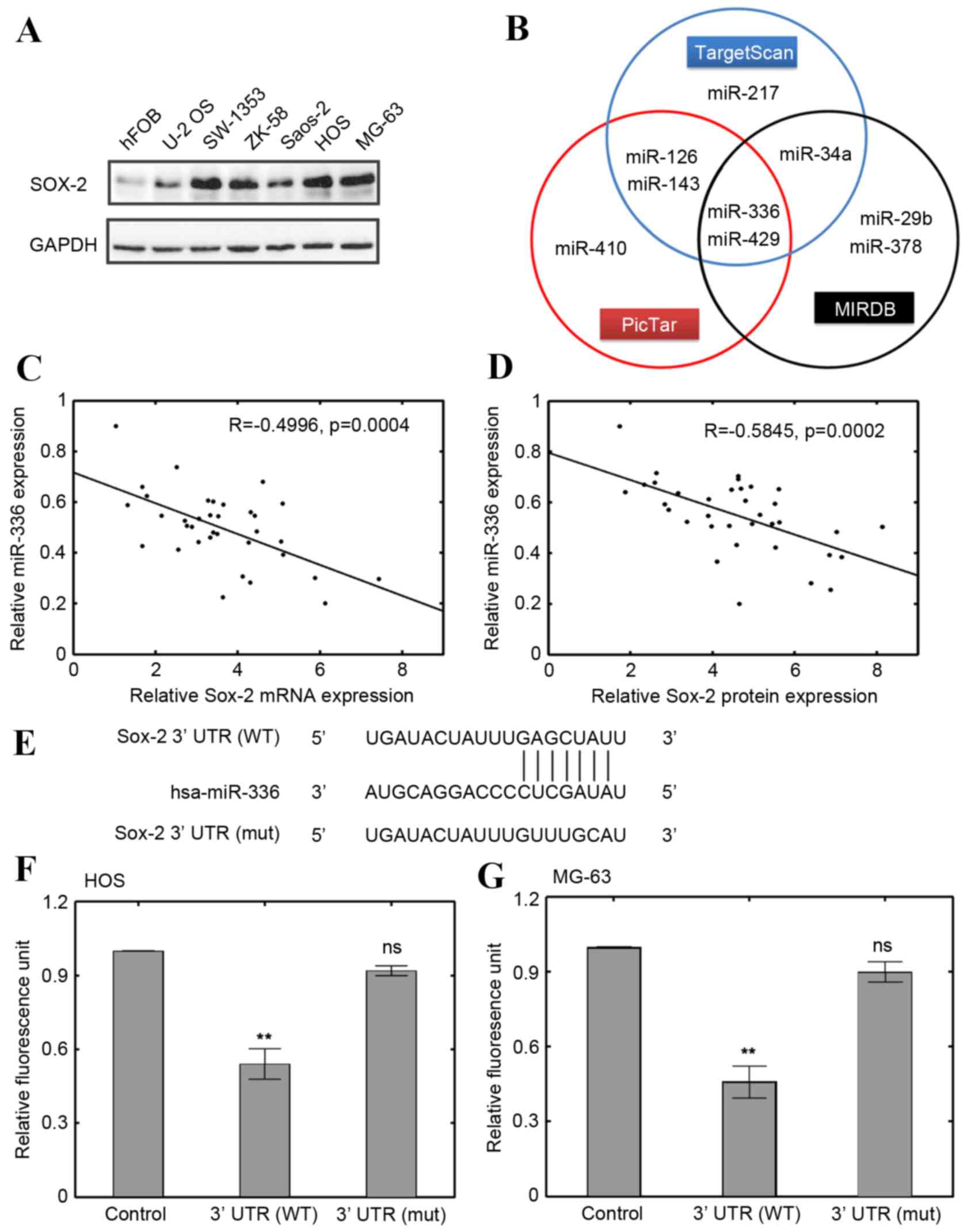 | Figure 1.Correlation between miR-336 and Sox-2.
(A) Sox-2 protein expression levels in 6 OS cell lines and an hFOB
cell line. (B) Prediction of candidate miRNAs using PicTar,
TargetScan and MIRDB. (C) Correlation between miR-336 expression
and Sox-2 mRNA expression levels in OS specimens (n=35; R=−0.4496).
(D) Correlation between miR-336 expression and Sox-2 protein
expression levels in OS specimens (n=35; R=−0.5845). (E) Identified
binding sequences of miR-336 to Sox-2 3′-UTR. Vectors with WT Sox-2
and mut Sox-2 were either transfected alone or with miR-336 vector
in (F) HOS and (G) MG-63 cells. **P<0.01 and
nsP>0.05 vs. control. miR-336, microRNA-336; Sox-2,
sex determining region Y-box 2; OS, osteosarcoma; hFOB, human fetal
osteoblasts; miRNA, microRNA; 3′-UTR, 3′-untranslated region; WT,
wild type; mut, mutant; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; ns, not significant. |
Overexpressing miR-336 inhibits OS
progression by inducing apoptosis
To further establish the functional link between
Sox-2 and miR-336, miR-336 was ectopically overexpressed using an
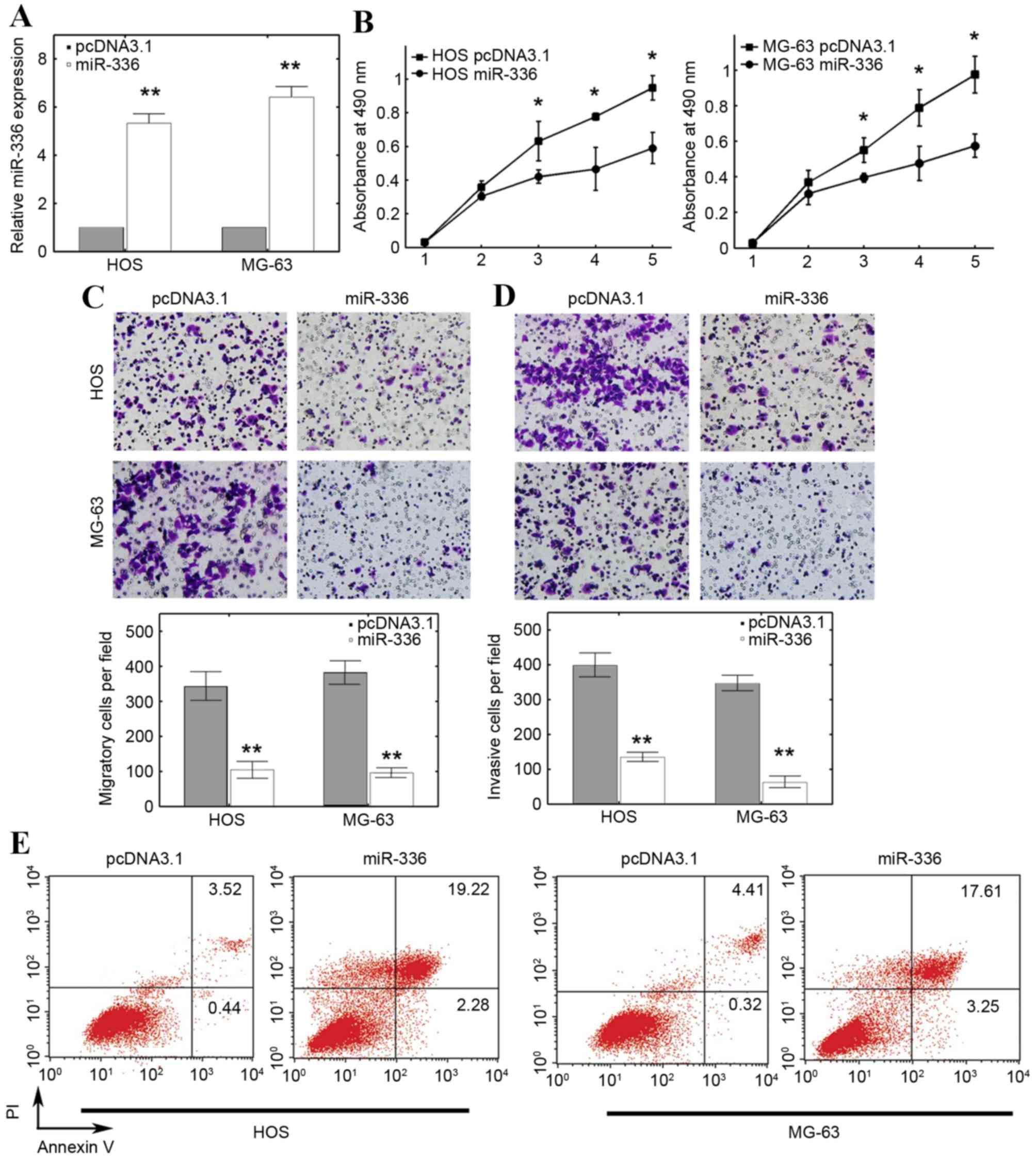
miR-336 mimic in HOS and MG-63 cells. The RT-qPCR results
demonstrated that miR-336 expression was significantly increased in
cells transfected with miR-336 mimic compared with cells
transfected with the empty pcDNA3.1 control vector (P<0.01;
Fig. 2A). The results of the 5 day
MTT assay revealed that miR-336 significantly inhibited the
viability of HOS and MG-63 cells at 3, 4 and 5 days compared with
the cells transfected with the empty pcDNA3.1 control vector
(P<0.05; Fig. 2B). Further
investigation revealed that ectopic miR-336 expression
significantly suppressed migration in HOS and MG-63 cells compared
with the cells transfected with the empty pcDNA3.1 control vector
(P<0.01; Fig. 2C). In addition,
the invasive capacity was also reduced in HOS and MG-63 cells
transfected with a miR-336 mimic compared with the cells
transfected with the empty pcDNA3.1 control vector (P<0.01;
Fig. 2D). The apoptosis rate of OS
cells was also demonstrated to be affected by miR-336
overexpression: miR-336 overexpression increased the overall
apoptosis rate in HOS and MG-63 cells compared with cells
transfected with the empty pcDNA3.1 control vector (Fig. 2E). These results suggested that
overexpression of miR-336 inhibited tumor progression, potentially
through inducing apoptosis.
Restoring Sox-2 expression reverses
the effect of miR-336 in OS cells
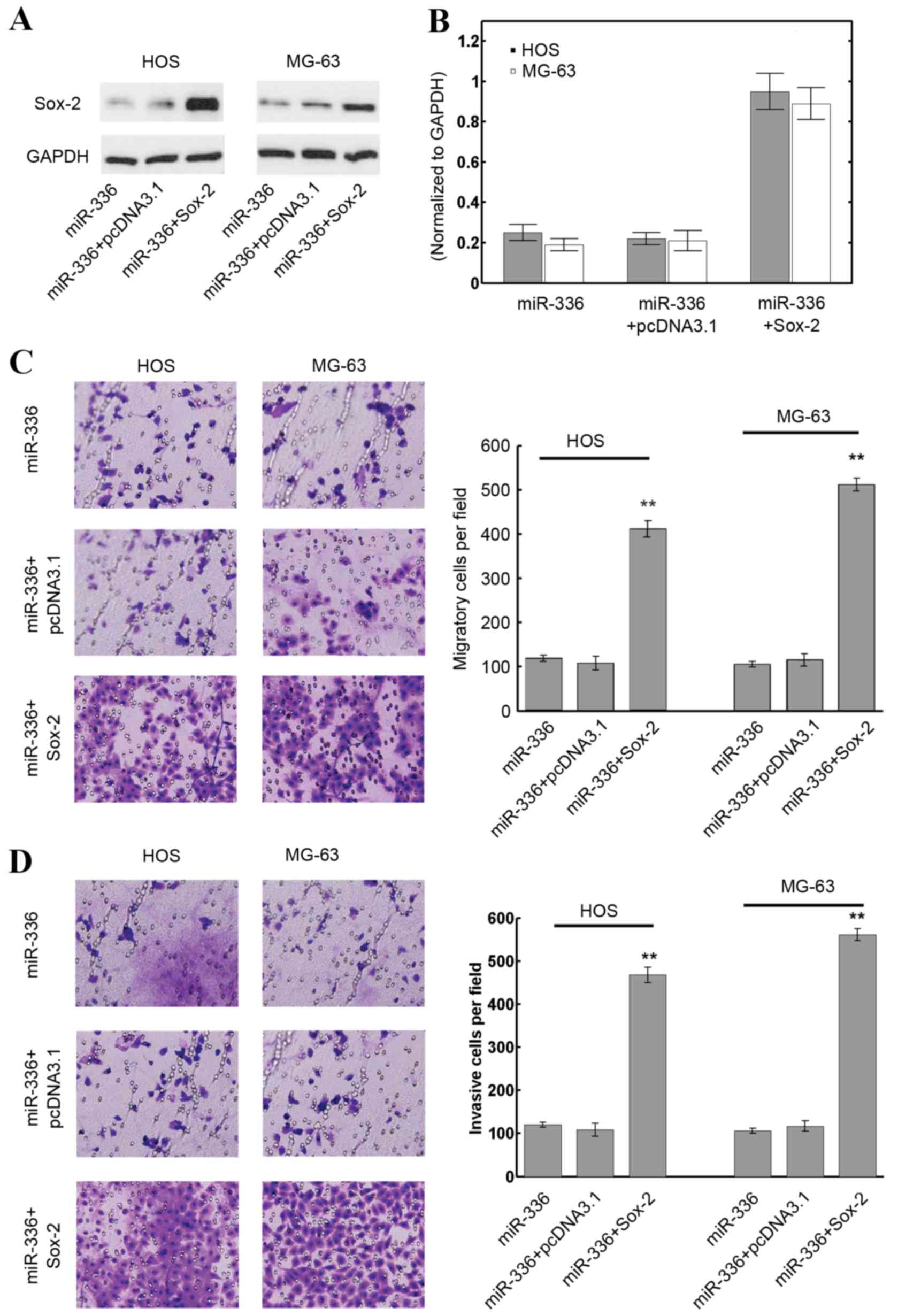
As miR-336 was confirmed to inhibit OS cell
malignancy through targeting Sox-2, overexpression of Sox-2 was
assessed to determine whether it would reverse the adverse
phenotype of OS cells. HOS and MG-63 cells were transfected with
miR-336 plasmids, miR-336 plasmids+pcDNA3.1 or miR-336+Sox-2.
Transfection of the empty pcDNA3.1 vector was used as a control for
the Sox-2 plasmid transfection group. Transfection with an empty
pcDNA3.1 vector control with a miR-336 mimic did not affect the
expression of Sox-2 with miR-336 precursor cotransfection (Fig. 3A). However, transfection with Sox-2
visibly increased intrinsic Sox-2 levels in HOS and MG-63 cells,
even with miR-336 cotransfection, compared with the miR-336 and
miR-336 + pcDNA3.1 groups (Fig.
3A). The quantification results revealed that Sox-2
transfection increased Sox-2 protein expression levels by ~5 fold
compared with the miR-336 and miR-336 + pcDNA3.1 groups (P<0.01;
Fig. 3B). The migration and
invasion of OS cell lines when Sox-2 levels were restored was then
investigated. The results revealed that Sox-2 induction
significantly promoted migration in HOS and MG-63 cells compared
with the miR-336 and miR-336 + pcDNA3.1 groups (P<0.01; Fig. 3C). The invasion of HOS and MG-63
cells transfected with Sox-2 plasmids was also significantly
increased compared with the miR-336 and miR-336 + pcDNA3.1 groups
(P<0.01; Fig. 3D). These
results suggested that restoring Sox-2 expression counteracted the
effect of miR-336 in OS cell lines.
The miR-336 suppresses tumor growth in
vivo
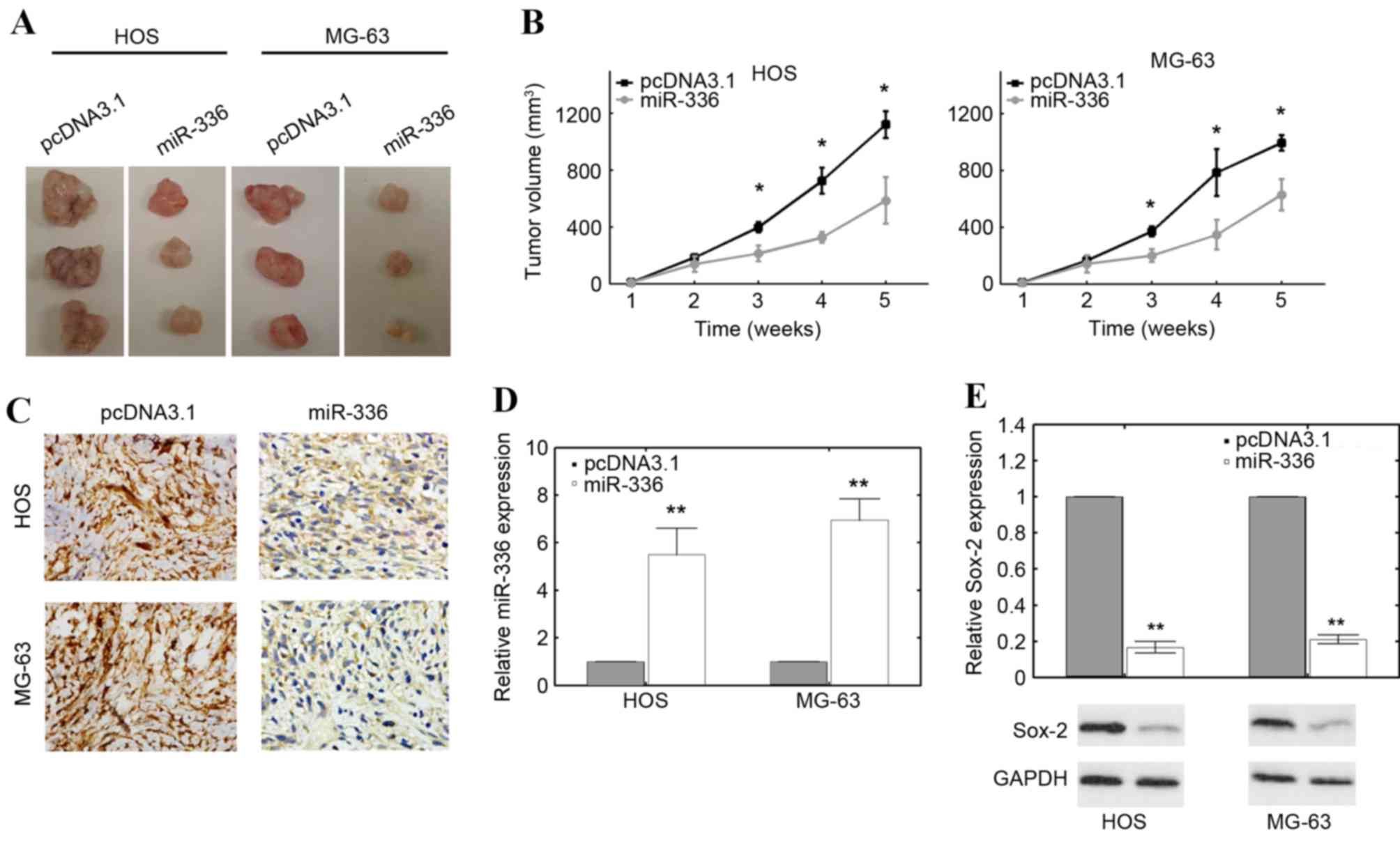
The aforementioned results demonstrated the in
vitro effect of miR-336 in OS cell lines. However, whether
miR-336 has a similar tumor suppressive function in vivo
remains unclear. HOS and MG-63 cells stably transfected with an
miR-336 mimic or the empty pcDNA3.1 vector were subcutaneously
injected into nude mice. All mice were sacrificed according to
formal protocols 5 weeks following implantation, and the volume of
solid tumors was measured. miR-336-transfected HOS and MG-63 cells
transfection produced smaller solid tumors compared with empty
pcDNA3.1 vector controls (Fig.
4A). The difference in tumor volume became significant as early
as 3 weeks following implantation in mice injected with HOS and
MG-63 cells compared with controls (P<0.05; Fig. 4B). Ki-67 staining, which is
indicative of proliferation in cells, was also visibly decreased
following transfection with an miR-336 mimic compared with empty
pcDNA3.1 vector controls (Fig.
4C). The RT-qPCR results confirmed that miR-336 expression was
significantly upregulated in these solid tumors resulting from HOS
and MG-63 cells transfected with an miR-336 mimic compared with
empty pcDNA3.1 vector controls (P<0.01 and P<0.01,
respectively; Fig. 4D). Sox-2
levels were also significantly downregulated in solid tumors
resulting from HOS and MG-63 cells transfected with an miR-336
mimic compared with empty pcDNA3.1 vector controls (P<0.01 and
P<0.01, respectively; Fig. 4E).
These results suggested that miR-336 functions as a tumor
suppressor in vivo.
Discussion
Previous evidence suggests that dysregulation of
miRNA function does not occur randomly (22). Multiple studies have established a
link between miRNA profiles and tumorigenesis (23). miRNAs are involved in tumor
progression in diverse ways, which potentially depend on the type
and stages of tumors. Therefore, sophisticated knowledge of the
physiological and pathological mechanisms of microRNAs may improve
early diagnosis and therapies for diseases, including cancer.
In the present study, miR-336 was confirmed to
function as tumor suppressor in OS cells. Few previous reports have
focused on the function of miR-336 in OS tumors. A previous study
demonstrated a link between miR-336 and breast cancer, where
miR-336 was deregulated in cancerous tissues (24). A further report by Croset et
al (23) revealed that serum
miR-336 levels are significantly associated with tumor burden and
procollagen I amino-terminal propeptide in advanced lung cancer.
Sukata et al (24) also
demonstrated a weak correlation between miR-336 expression and
incidence of hepatocarcinoma. These reports implied that miR-336
may be involved in tumor progression, including in liver, lung and
breast cancers. Truettner et al (25) also demonstrated that miR-336 may be
involved in hypoxia induced responses in cortical pericytes through
miRNA profiling, although the exact mechanism remains elusive.
However, no previous studies exist regarding the involvement of
miR-336 in osteosarcoma. The present study therefore denotes a
novel representation concerning the functional involvement of
miR-336 and has identified miR-336 as a novel tumor suppressor in
OS. Whether miR-336 functions in a tumorigenic or tumor-suppressive
manner in other types of cancer, together with the exact molecular
mechanisms underlying this function, merit further
investigation.
Sox-2 is an important transcription factor in cancer
stem cells (25). Sox-2 has
previously been demonstrated to maintain self-renewal in OS cells
(26). Knocking down Sox-2
expression reduces the transforming capacity of OS cells (26). The tumorigenic potential and
differentiation of OS cells is associated with Sox-2 gene
expression and the colony formation ability of derived OS cell
lines was diminished when Sox-2 was downregulated (26,27).
The oncogenic potential of Sox-2 may function by antagonizing
active Wnt signaling, although multiple potential mechanisms have
been proposed (28,29). Sox-2 also behaves as an oncogene in
other types of tumor, including squamous cell carcinoma,
hepatocarcinoma and lung cancer (30,31).
Notably, no activating mutations were identified in the Sox-2 gene
through genomic study, further highlighting the importance of Sox-2
in tumorigenesis (26). Therefore,
tumorigenesis may be significantly associated with Sox-2 in
multiple tumors, including OS. Multiple miRNA regulators for Sox-2
have been previously reported (21,32,33).
The present study adds a further layer of complexity in miRNA
mediated regulation of OS, by demonstrating that miR-336 inhibited
tumor progression by targeting Sox-2. Ectopic expression of miR-336
reduced the migratory and invasive potential of OS cell lines in
vitro as well as the growth of solid tumors in vivo.
Taken together, miR-336 serves as a novel tumor suppressor in OS
and effectively downregulates Sox-2 expression.
Overall, the present study reports a novel function
of miR-336, which was demonstrated to regulate the expression of
the oncogenic factor Sox-2 in OS. Increasing miR-336 expression may
abolish OS progression, while restoring Sox-2 expression may
counteract the inhibitory effect of miR-336. This suggests that
Sox-2 may be involved in miR-336 mediated regulation. The intricate
relation between miR-336 and the Sox-2 signaling pathway merits
further investigation to provide critical insights into its
potential for diagnosis and micRNA-targeted therapeutic
intervention in OS.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81171698, 81301542
and 81371956) and the Hunan Provincial Innovation Foundation For
Postgraduates (grant no. CX2015B060).
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
2
|
Morrow JJ and Khanna C: Osteosarcoma
genetics and epigenetics: Emerging biology and candidate therapies.
Crit Rev Oncog. 20:173–197. 2015. View Article : Google Scholar :
|
|
3
|
Admassi D: Osteosarcoma of medial cuniform
bone. Ethiop Med J. 47:305–308. 2009.
|
|
4
|
Miao J, Wu S, Peng Z, Tania M and Zhang C:
MicroRNAs in osteosarcoma: Diagnostic and therapeutic aspects.
Tumour Biol. 34:2093–2098. 2013. View Article : Google Scholar
|
|
5
|
Osborne TS and Khanna C: A review of the
association between osteosarcoma metastasis and protein
translation. J Comp Pathol. 146:132–142. 2012. View Article : Google Scholar :
|
|
6
|
Akiyama T, Dass CR and Choong PF: Novel
therapeutic strategy for osteosarcoma targeting osteoclast
differentiation, bone-resorbing activity, and apoptosis pathway.
Mol Cancer Ther. 7:3461–3469. 2008. View Article : Google Scholar
|
|
7
|
Miska EA: How microRNAs control cell
division, differentiation and death. Curr Opin Genet Dev.
15:563–568. 2005. View Article : Google Scholar
|
|
8
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar
|
|
9
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar :
|
|
10
|
Feng B, Zhang K, Wang R and Chen L:
Non-small-cell lung cancer and miRNAs: Novel biomarkers and
promising tools for treatment. Clin Sci (Lond). 128:619–634. 2015.
View Article : Google Scholar
|
|
11
|
van Rooij E: The art of microRNA research.
Circ Res. 108:219–234. 2011. View Article : Google Scholar
|
|
12
|
Liu X, Zhang J, Xie B, Li H, Shen J and
Chen J: MicroRNA-200 family profile: A promising ancillary tool for
accurate cancer diagnosis. Am J Ther. 23:e388–e397. 2016.
View Article : Google Scholar :
|
|
13
|
Oom AL, Humphries BA and Yang C:
MicroRNAs: Novel players in cancer diagnosis and therapies. Biomed
Res Int. 2014:9594612014. View Article : Google Scholar :
|
|
14
|
He C, Xiong J, Xu X, et al: Functional
elucidation of MiR-34 in osteosarcoma cells and primary tumor
samples. Biochem Biophys Res Commun. 388:35–40. 2009. View Article : Google Scholar
|
|
15
|
Zhao H, Ma B, Wang Y, et al: miR-34a
inhibits the metastasis of osteosarcoma cells by repressing the
expression of CD44. Oncol Rep. 29:1027–1036. 2013.
|
|
16
|
Ziyan W, Shuhua Y, Xiufang W and Xiaoyun
L: MicroRNA-21 is involved in osteosarcoma cell invasion and
migration. Med Oncol. 28:1469–1474. 2011. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Yang C, Hou C, Zhang H, Wang D, Ma Y,
Zhang Y, Xu X, Bi Z and Geng S: miR-126 functions as a tumor
suppressor in osteosarcoma by targeting Sox2. Int J Mol Sci.
15:423–437. 2013. View Article : Google Scholar :
|
|
19
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43:D146–D152. 2015. View Article : Google Scholar
|
|
20
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar
|
|
21
|
Li J, Du L, Yang Y, Wang C, Liu H, Wang L,
Zhang X, Li W, Zheng G and Dong Z: MiR-429 is an independent
prognostic factor in colorectal cancer and exerts its
anti-apoptotic function by targeting SOX2. Cancer Lett. 329:84–90.
2013. View Article : Google Scholar
|
|
22
|
Leonardo TR, Schultheisz HL, Loring JF and
Laurent LC: The functions of microRNAs in pluripotency and
reprogramming. Nat Cell Biol. 14:1114–1121. 2012. View Article : Google Scholar
|
|
23
|
Croset M, Santini D, Iuliani M, et al:
MicroRNAs and bone metastasis: a new challenge. Molecules.
19:10115–10128. 2014. View Article : Google Scholar
|
|
24
|
Sukata T, Sumida K, Kushida M, et al:
Circulating microRNAs, possible indicators of progress of rat
hepatocarcinogenesis from early stages. Toxicol Lett. 200:46–52.
2011. View Article : Google Scholar
|
|
25
|
Truettner JS, Katyshev V, Esen-Bilgin N,
Dietrich WD and Dore-Duffy P: Hypoxia alters MicroRNA expression in
rat cortical pericytes. Microrna. 2:32–44. 2013. View Article : Google Scholar :
|
|
26
|
Basu-Roy U, Seo E, Ramanathapuram L, Rapp
TB, Perry JA, Orkin SH, Mansukhani A and Basilico C: Sox2 maintains
self renewal of tumor-initiating cells in osteosarcomas. Oncogene.
31:2270–2282. 2012. View Article : Google Scholar
|
|
27
|
Basu-Roy U, Ambrosetti D, Favaro R,
Nicolis SK, Mansukhani A and Basilico C: The transcription factor
Sox2 is required for osteoblast self-renewal. Cell Death Differ.
17:1345–1353. 2010. View Article : Google Scholar :
|
|
28
|
Mansukhani A, Ambrosetti D, Holmes G,
Cornivelli L and Basilico C: Sox2 induction by FGF and FGFR2
activating mutations inhibits Wnt signaling and osteoblast
differentiation. J Cell Biol. 168:1065–1076. 2005. View Article : Google Scholar :
|
|
29
|
Zhang Y, Yeh LK, Zhang S, Call M, Yuan Y,
Yasunaga M, Kao WW and Liu CY: Wnt/β-catenin signaling modulates
corneal epithelium stratification via inhibition of Bmp4 during
mouse development. Development. 142:3383–3393. 2015. View Article : Google Scholar :
|
|
30
|
Bass AJ, Watanabe H, Mermel CH, Yu S,
Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, et
al: SOX2 is an amplified lineage-survival oncogene in lung and
esophageal squamous cell carcinomas. Nat Genet. 41:1238–1242. 2009.
View Article : Google Scholar :
|
|
31
|
Sun C, Sun L, Li Y, Kang X, Zhang S and
Liu Y: Sox2 expression predicts poor survival of hepatocellular
carcinoma patients and it promotes liver cancer cell invasion by
activating Slug. Med Oncol. 30:5032013. View Article : Google Scholar
|
|
32
|
Deng Z, Du WW, Fang L, Shan SW, Qian J,
Lin J, Qian W, Ma J, Rutnam ZJ and Yang BB: The intermediate
filament vimentin mediates microRNA miR-378 function in cellular
self-renewal by regulating the expression of the Sox2 transcription
factor. J Biol Chem. 288:319–331. 2013. View Article : Google Scholar
|
|
33
|
Zhang Y, Eades G, Yao Y, Li Q and Zhou Q:
Estrogen receptor α signaling regulates breast tumor-initiating
cells by down-regulating miR-140 which targets the transcription
factor SOX2. J Biol Chem. 287:41514–41522. 2012. View Article : Google Scholar :
|