Introduction
Renal cell carcinoma (RCC) is the most common
neoplasm of the kidney in adults, accounting for ~3% of adult
malignancies (1). Surgical
resection remains the only definitive treatment for RCC (2). However, following surgery, 20–40% of
patients will eventually relapse due to developing resistance to
other treatment regimens, for example chemotherapy and radiotherapy
(3). Therefore, there is an
ongoing requirement for novel therapeutic strategies and a greater
understanding of the underlying mechanisms involved in the
development of RCC.
Ras homology gene family, member A (RhoA) is a
member of the Ras-superfamily of small guanosine triphosphatases
(4) and is a pivotal control point
by which cells sense alterations in extracellular matrix and
cytoskeletal organization. RhoA translates these signals to
downstream effectors to mediate cell proliferation and motility
(5,6). Active guanosine triphosphate
(GTP)-bound RhoA causes the formation of stress fibers by
activation of downstream Rho-associated kinases (7), which enhance the formation of
actin/myosin microfilaments (MF) to facilitate cell motility
(8). A previous report suggested
that the localization of RhoA and its effector, mammalian
diaphanous-related formin-1, control microtubule (MT) dynamics, the
actin network and adhesion site formation in migrating cells
(9). Our previous studies revealed
that the formation of an MT ring structure supported apoptotic cell
morphology (10–12). However, little is known about the
role of RhoA in MT polymerization.
The present study used Taxol, a common MT stabilizer
that promotes tubulin polymerization (13), to investigate the association
between RhoA and MT arrangement.
Materials and methods
Cell culture
The RCC cell line OS-RC-2, was obtained from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in RPMI 1640 medium, supplemented with 10%
heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100
µg/ml streptomycin (all from HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), in a humidified incubator at 37°C and 5%
CO2 (14). Following
culture for 24 h, OS-RC-2 cells were treated with 1 µM Taxol
(Shanghai Hualian Pharmaceutical Co., Ltd., Shanghai, China) for 0,
12, 24, 36 and 48 h. Alternatively, following culture for 24 h, the
cells were pretreated with 10 µM MT depolymerizing agent Colchicine
(Col; cat. no. C8190; Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) or 2 µg/ml RhoA inhibitor C3 transferase
(cat. no. CT03-A; Cytoskeleton, Inc., Denver, CO, USA) for 1 h,
which was followed by treatment with 1 µM Taxol for 24 h 37°C and
5% CO2. Untreated cells served as the control.
Immunofluorescent staining of MF, MT
and RhoA
Cells were stained for MF, MT and RhoA as previously
described (15,16). Coverslips were washed twice with
phosphate-buffered saline (PBS) and examined under a confocal
scanning microscope (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Transfection experiments
The pCI-neo empty vector were provided by Professor
Ningsheng Liu, Nanjing Medical University (Nanjing, China). The
control vector was produced by inserting the RhoA coding DNA
sequence into the EcoRI-Sal I site of pCI-neo vector. The
dominant-negative mutants vector RhoAT19N was constructed using Asp
instead of Thr188 (mutations 849 A→T and 850 T→G) using the
site-directed mutagenesis kit (cat. no. 200517; Agilent
Technologies, Inc., Santa Clara, CA, USA). Cells were plated in
25-cm2 culture flasks at a density of 5×105
cells/flask. Following culture for 24 h, cells were transiently
transfected with the control vector and RhoAT19N vector.
Transfection was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in
accordance with the manufacturer's protocol. The concentration of
the DNA plasmid used was determined by the size of culture dishes,
also according to the manufacturer's instructions. Cells were
analyzed at 40 h following transfection (17).
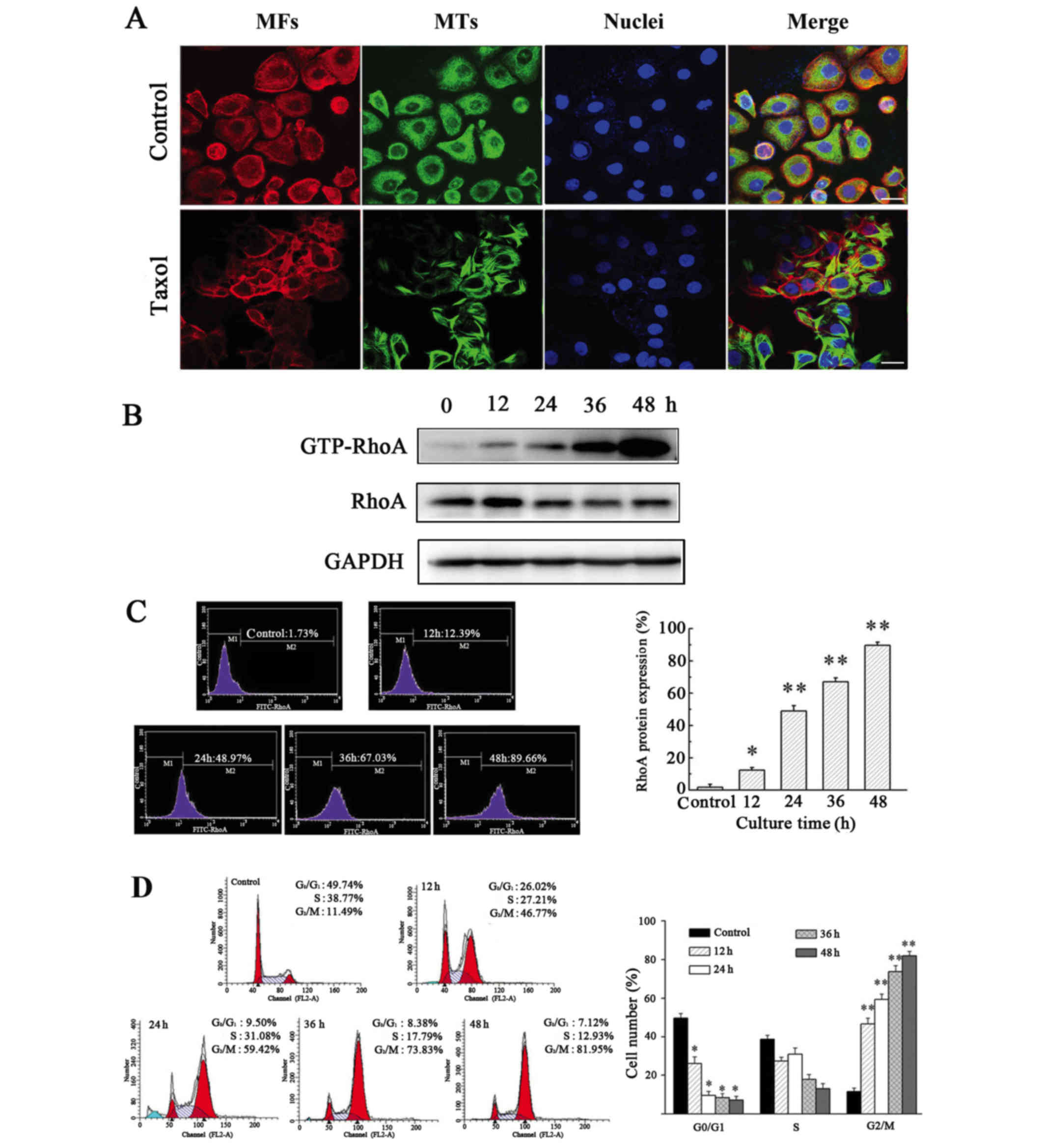
Cell cycle measurements
For flow cytometric analysis of the cell cycle,
1×106 cells were harvested by centrifugation (room
temperature, 184 × g, 5 min), washed with PBS and fixed with
ice-cold 70% ethanol overnight. Fixed cells were treated with 25
µg/ml RNase A at 37°C for 30 min and stained with 50 µg/ml
propidium iodide (Sigma Aldrich; Merck Millipore, Darmstadt,
Germany) for 30 min in the dark. The fluorescence intensity of
individual cells was measured using a flow cytometer (Beckman
Coulter, Inc., Miami, FL, USA). At least 10,000 cells were counted
using CXP Cytometer version 2.2 software (Beckman Coulter, Inc.)
(18).
RhoA protein expression
The expression of RhoA protein was measured by flow
cytometry as previously described (6). Briefly, 1×106 cells were
fixed with 4% (w/v) paraformaldehyde for 30 min at 4°C, and
permeabilized using 0.1% Triton X-100. Nonspecific antibody binding
was blocked by incubation in 0.5% bovine serum albumin (BSA;
Beijing Solarbio Science & Technology Co., Ltd.) for 30 min at
room temperature. Cells were subsequently incubated with primary
RhoA antibodies (dilution, 1:500; cat. no. sc-418; Santa Cruz
Biotechnology, Inc.) in 0.01 M PBS at 4°C overnight. Following
this, the cells were incubated with a FITC-conjugated anti-mouse
secondary antibody (dilution, 1:50; cat. no. BA1101; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for 1 h at room
temperature in the dark. The expression of RhoA was then measured
by flow cytometry (BD Biosciences, San Jose, CA, USA).
Western blot analysis
The OS-RC-2 cells were lyzed in ice-cold
radioimmunoprecipitation buffer (cat. no. R0010; Beijing Solarbio
Science & Technology Co., Ltd.) containing PMSF protease
inhibitor (cat. no. Amresco0754; Beijing Solarbio Science &
Technology Co., Ltd.) and phosphatase inhibitors (cat. no. C500017;
Sangon Biotech Co., Ltd., Shanghai, China). Equal quantities of
protein (40 µg) were separated by 12% SDS-PAGE, prior to
transferring proteins to polyvinylidene difluoride membranes (0.4
µm; EMD Millipore, Billerica, MA, USA). The membranes were blocked
in 5% BSA in TBST (0.1% Tween-20) with 0.2% sodium azide and
incubated with primary antibodies: RhoA (dilution, 1:500; cat. no.
sc-418; Santa Cruz Biotechnology, Inc.), GTP-RhoA (dilution, 1:500;
cat. no. 80601; Neweast Biosciences, King of Prussia, PA, USA) or
GAPDH (dilution, 1:1,000; cat. no. 2118S; Cell Signaling
Technology, Danvers, MA, USA) overnight at 4ºC. Following washing,
membranes were incubated with a horseradish peroxidase-conjugated
goat anti-mouse/rabbit IgG secondary antibody (dilution, 1:5000;
cat. no. BA1050/BA1054; Wuhan Boster Biological Technology, Ltd.)
at room temperature for 2 h, and chemiluminescence was performed
using an Enhanced Chemiluminescence Detection kit (Shanghai
Biyuntian Biological Co., Ltd., Shanghai, China), according to the
manufacturer's protocol, with Tanon MP version 4.1.2 software
(Tanon Science and Technology Co., Ltd., Shanghai, China) (19).
Statistical analysis
All experiments were repeated three times. Data are
expressed as the mean ± standard deviation. One-way analysis of the
variance and Fisher's least significant difference post hoc tests
were performed to evaluate the differences among groups by SPSS
v13.0 software (SPSS, Inc., Chicago, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Taxol induces MT polymerization, cell
cycle arrest and GTP-RhoA protein expression in OS-RC-2 cells
Taxol is an effective chemotherapeutic used to treat
cancer patients; it binds to and stabilizes MT, causing abnormal MT
aggregation (20).
Immunofluorescent staining revealed that in the absence of Taxol,
tubulin was widely distributed throughout the cytoplasm and
accumulated in the perinuclear regions of cells. However, in cells
treated with Taxol, a distinct rearrangement of MT was observed,
including the formation of rigid MT bundles in the cytoplasm and
the area surrounding the nucleus (Fig.
1A).
To determine the effect of Taxol-induced MT
polymerization on the expression of GTP-RhoA and the cell cycle,
western blotting and flow cytometric analysis were performed.
Expression of GTP-RhoA was significantly upregulated following
Taxol treatment in a time-dependent manner (Figs. 1B and C). Additionally, Taxol
treatment caused an increase in GTP-RhoA protein expression in a
dose-dependent manner (data not shown). The proportion of cells in
G2/M phase increased >4-fold in cells treated with Taxol for 12
h, compared with the control (Fig.
1D). Therefore, these results suggested that Taxol-induced MT
polymerization is accompanied by an increase in GTP-RhoA protein
expression levels and cell cycle arrest.
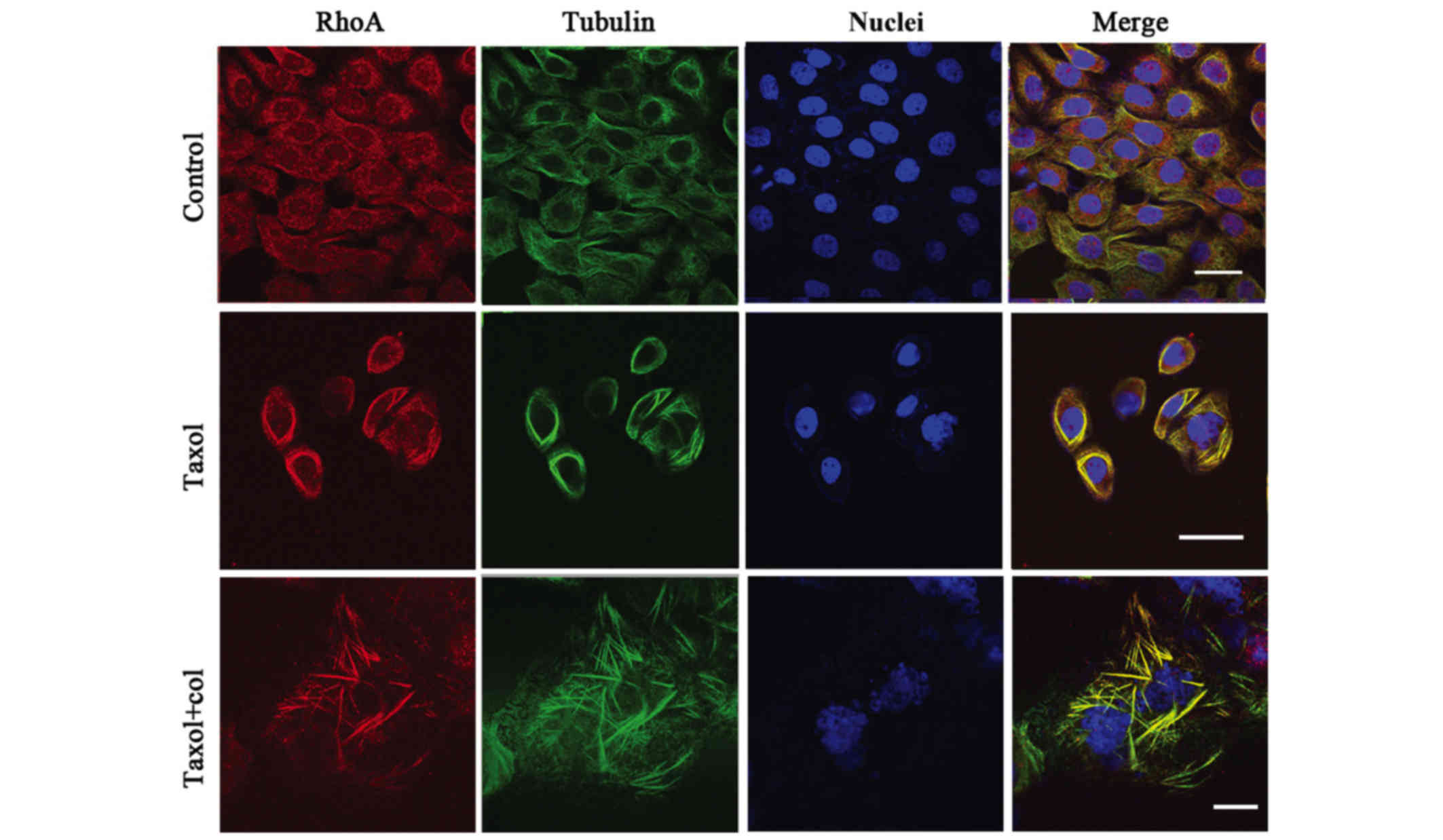
Taxol treatment causes concurrent
redistribution of MT and GTP-RhoA
To investigate the potential association between
GTP-RhoA and MT arrangement, their cellular localization was
determined via immunofluorescent staining. The results revealed a
consistent co-localization of GTP-RhoA and MT structure (yellow
colorization). GTP-RhoA and MT were widely distributed in control
cells. The GTP-RhoA protein was located adjacent to the MT bundles
in cells treated with Taxol. Following treatment with the MT
depolymerizing agent (Col), the MT bundles were partially disrupted
and the GTP-RhoA distribution dispersed. The results demonstrated a
concurrent redistribution of MT arrangement and GTP-RhoA in Taxol
treated cells (Fig. 2).
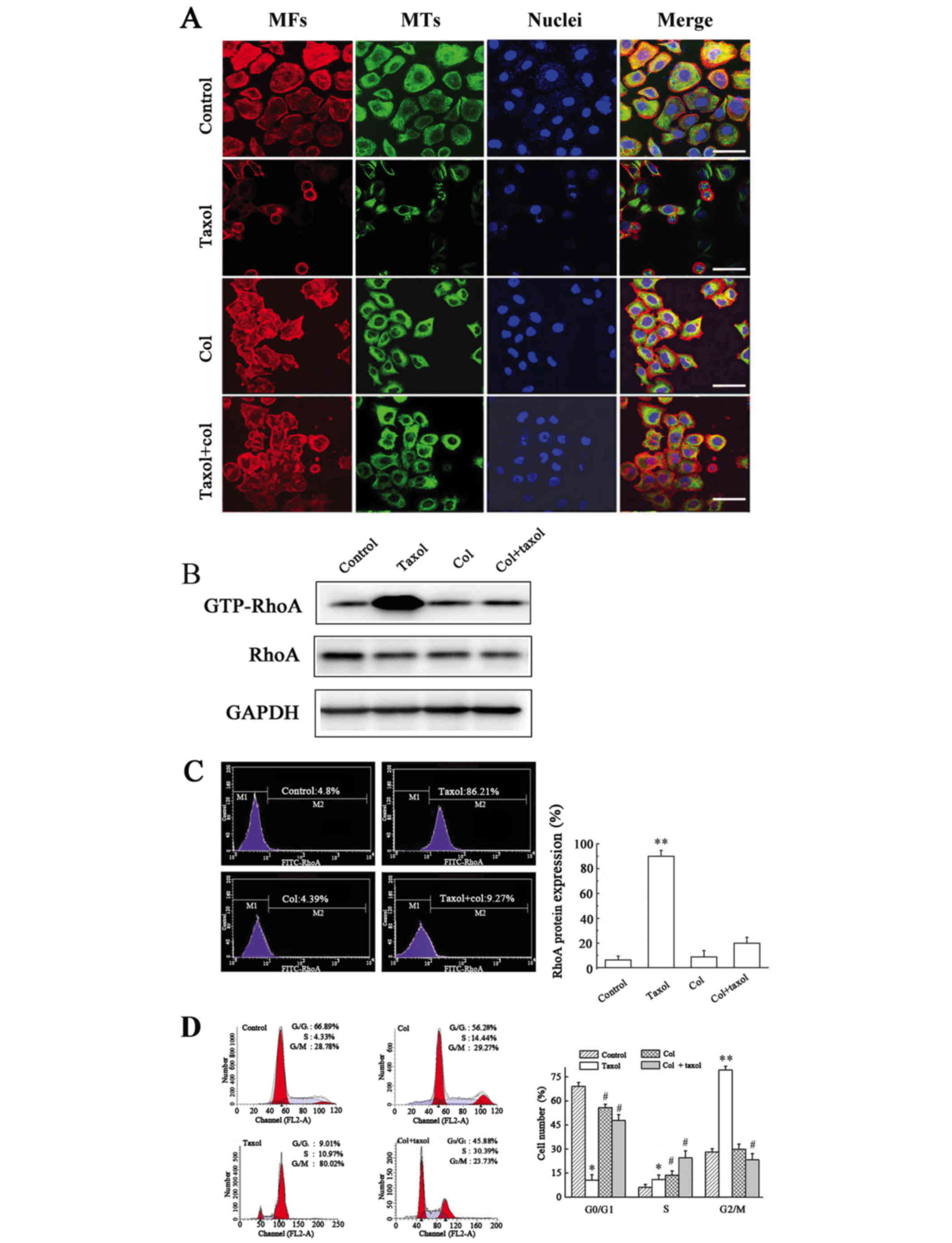
Disruption of Taxol-induced MT
polymerization decreases GTP-RhoA protein expression and reverses
cell cycle arrest
Next, the association between MT polymerization and
GTP-RhoA protein expression was investigated. Combined treatment
with Col and Taxol disrupted Taxol-induced MT bundling, however,
little effect on MF arrangement was detected (Fig. 3A). The combination of Col and Taxol
abolished Taxol-induced GTP-RhoA protein expression levels to the
levels of the untreated control, as determined by western blotting
(Fig. 3B) and flow cytometric
analysis (Fig. 3C), and the
Taxol-induced increase in the proportion of cells in G2/M phase was
significantly reduced (Fig. 3D).
These results suggested that MT arrangement regulated the protein
expression levels of GTP-RhoA and cell cycle arrest.
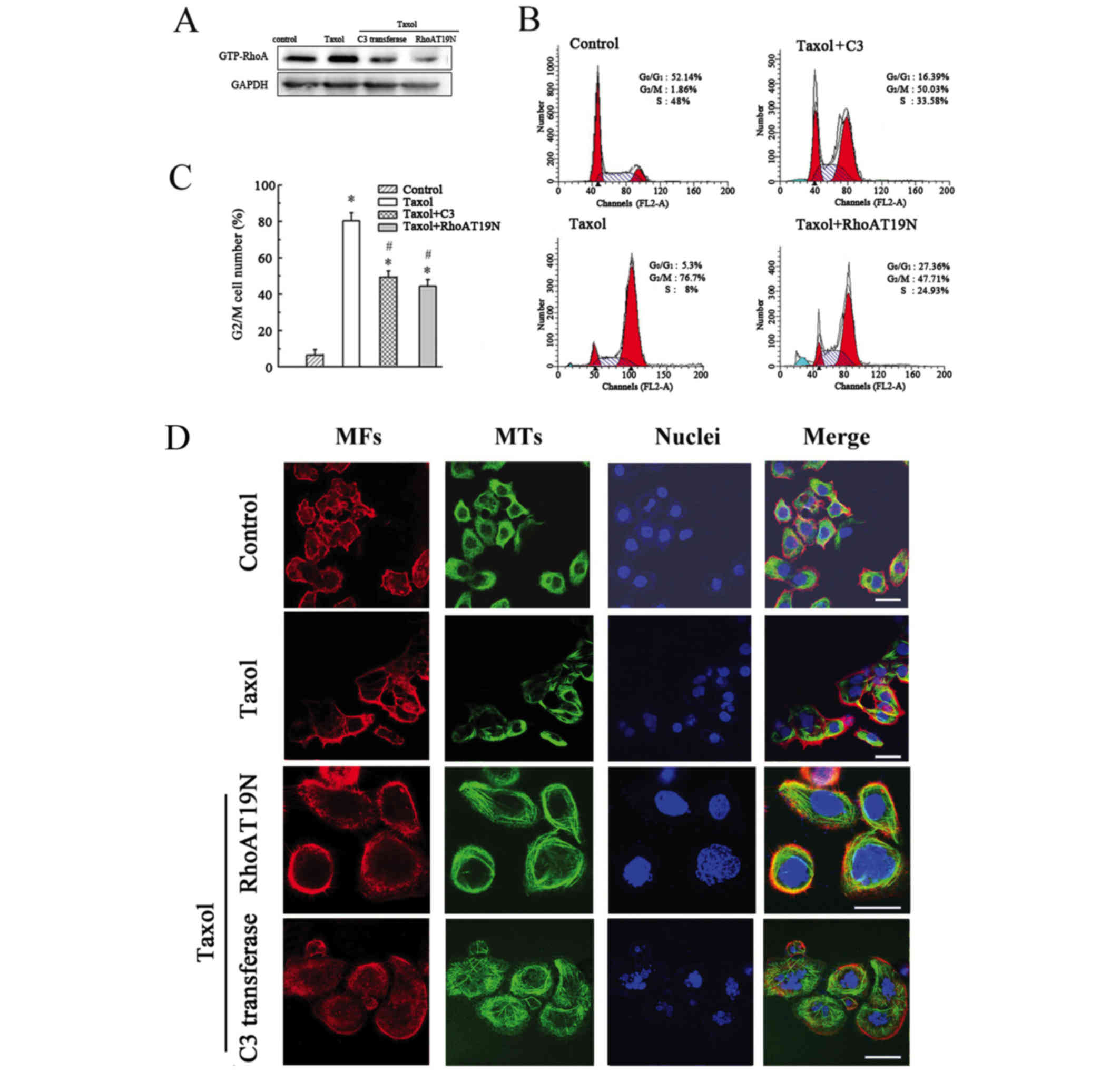
Inhibition of GTP-RhoA has no effect
on Taxol-induced MT bundling but inhibits the Taxol-induced
increase in G2/M cell number
To investigate the effect of GTP-RhoA on
cytoskeleton arrangement and cell cycle, cells were pretreated with
a RhoA inhibitor (C3 transferase) for 1 h or transfected with a
RhoAT19N mutant to decrease GTP-RhoA expression (Fig. 4A). The inhibition of GTP-RhoA
expression partially reduced the proportion of cells in G2/M
compared with Taxol treatment alone (Fig. 4B and C). However, no marked effect
on MT arrangement was observed in cells treated with the
combination of C3 transferase or RhoAT19N mutant and Taxol
(Fig. 4D). Thus, these findings
confirmed that the cell cycle was affected by alterations in RhoA
expression; however, GTP-RhoA expression did not influence MT
arrangement.
Discussion
Taxol is a member of the taxane class of
antineoplastic microtubule damaging agents and is widely used to
treat a variety of human malignancies, including breast, ovarian,
lung, prostate and bladder cancers. Taxol may stabilize
microtubules and subsequently cause cell death by arresting the
cell cycle at G2/M (21,22). The present study demonstrated that
Taxol-induced MT polymerization is accompanied by an increase in
GTP-RhoA protein expression levels and cell cycle arrest in RCC
cells. A previous report revealed that disruption of MT arrangement
causes enhanced GTP-RhoA activity via release of an MT-associated
Rho activator, guanine nucleotide exchange factor (GEF) -H1
(GEF-H1) (23). Downstream of
RhoA, Rho-associated protein kinase (ROCK) signaling serves a
pivotal role in the rescue of the mutant huntingtin-expressing
cells from cell death caused by MT depolymerizing agents (23). Furthermore, a recent report has
discussed a similar feedback loop between the Rho/ROCK signaling
pathway and MT dynamics in an acute T lymphoblastic leukemia cell
line (24,25). The mechanism underlying MT
rearrangement-associated alteration of GTP-RhoA expression remains
to be fully understood. Therefore, the present study investigated
the regulatory association between the MT cytoskeleton and cell
cycle progression, in particular, the involvement of GTP-RhoA.
Co-localization between GTP-RhoA and MT in
Taxol-treated cells was observed. GTP-RhoA was widely dispersed in
control cells; following Taxol treatment, a GTP-RhoA bundle and an
MT ring structure emerged. Furthermore, although the combination of
Taxol and col partially destroyed the MT bundle, it was accompanied
by the dispersal of GTP-RhoA in the cytoplasm. RhoA is a key
regulator of cytoskeletal organization that regulates cell
migration and invasion (26),
polarity, division and early embryo development (27). In addition, Meiri et al
(28) suggested that RhoGEF GEF-H1
may be sequestered in an inactive state on polymerized MTs by the
dynein motor light-chain Tctex-1. They also indicated that GEF-H1
activation in response to the G-protein-coupled receptor ligands
lysophosphatidic acid or thrombin is independent of MT
depolymerization. However, the present study revealed that
Taxol-induced cell toxicity is dependent on MT arrangement, as the
disruption in MT bundling decreased GTP-RhoA protein expression
levels and relieved cell cycle arrest. Notably, the present study
demonstrated that decreased expression of GTP-RhoA with a specific
inhibitor or by transfection with the RhoAT19N mutant plasmid had
no marked effect on Taxol-induced specific MT bundling; however, it
did reduce the Taxol-induced increase in the proportion of cells in
G2/M phase. These results suggested that the expression level of
GTP-RhoA had no significant effect on MT arrangement. By contrast,
MT rearrangement increased GTP-RhoA protein expression levels in
Taxol treated cells. In conclusion, to the best of our knowledge,
the results of the present study suggested that Taxol-induced MT
arrangement regulates the expression levels of GTP-RhoA protein and
cell cycle arrest in RCC cells. However, alterations in GTP-RhoA
expression levels did not significantly influence MT
arrangement.
Acknowledgements
The present study was supported by Zhejiang
Provincial Medical Science Foundation of China (grant no.
2015RCB024), the Zhejiang Provincial Natural Science Foundation of
China (grant no. LY13H160038), the National Natural Science
Foundation of China (grant nos. 81372209 and 81071653), and the
Natural Foundation of Ningbo Science and Technology Bureau of China
(grant no. 2010A610047).
References
|
1
|
Cui L, Zhou H, Zhao H, Zhou Y, Xu R, Xu X,
Zheng L, Xue Z, Xia W, Zhang B, et al: MicroRNA-99a induces
G1-phase cell cycle arrest and suppresses tumorigenicity in renal
cell carcinoma. BMC Cancer. 12:5462012. View Article : Google Scholar :
|
|
2
|
van Spronsen DJ, de Weijer KJ, Mulders PF
and De Mulder PH: Novel treatment strategies in clear-cell
metastatic renal cell carcinoma. Anticancer Drugs. 16:709–717.
2005. View Article : Google Scholar
|
|
3
|
Reeves DJ and Liu CY: Treatment of
metastatic renal cell carcinoma. Cancer Chemother Pharmacol.
64:11–25. 2009. View Article : Google Scholar
|
|
4
|
Van Aelst L and D'Souza-Schorey C: Rho
GTPases and signaling networks. Genes Dev. 11:2295–2322. 1997.
View Article : Google Scholar
|
|
5
|
Kang HG, Jenabi JM, Zhang J, Keshelava N,
Shimada H, May WA, Ng T, Reynolds CP, Triche TJ and Sorensen PH:
E-cadherin cell-cell adhesion in ewing tumor cells mediates
suppression of anoikis through activation of the ErbB4 tyrosine
kinase. Cancer Res. 67:3094–3105. 2007. View Article : Google Scholar :
|
|
6
|
Cheng HL, Su SJ, Huang LW, Hsieh BS, Hu
YC, Hung TC and Chang KL: Arecoline induces HA22T/VGH hepatoma
cells to undergo anoikis - involvement of STAT3 and RhoA
activation. Mol Cancer. 9:1262010. View Article : Google Scholar :
|
|
7
|
Vega FM and Ridley AJ: Rho GTPases in
cancer cell biology. FEBS Lett. 582:2093–2101. 2008. View Article : Google Scholar
|
|
8
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar
|
|
9
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.
|
|
10
|
Wang P, Xu S, Zhao K, Xiao B and Guo J:
Increase in cytosolic calcium maintains plasma membrane integrity
through the formation of microtubule ring structure in apoptotic
cervical cancer cells induced by trichosanthin. Cell Biol Int.
33:1149–1154. 2009. View Article : Google Scholar
|
|
11
|
Jiang Q, Bai T, Shen S, Li L, Ding H and
Wang P: Increase of cytosolic calcium induced by trichosanthin
suppresses cAMP/PKC levels through the inhibition of adenylyl
cyclase activity in HeLa cells. Mol Biol Rep. 38:2863–2868. 2011.
View Article : Google Scholar
|
|
12
|
Wang P and Li JC: Trichosanthin-induced
specific changes of cytoskeleton configuration were associated with
the decreased expression level of actin and tubulin genes in
apoptotic Hela cells. Life Sci. 81:1130–1140. 2007. View Article : Google Scholar
|
|
13
|
Gerdes JM and Katsanis N: Small molecule
intervention in microtubule-associated human disease. Hum Mol
Genet. 14:R291–R300. 2005.[CrossRef]. View Article : Google Scholar
|
|
14
|
Guo Z, Jin X and Jia H: Inhibition of
ADAM-17 more effectively down-regulates the Notch pathway than that
of γ-secretase in renal carcinoma. J Exp Clin Cancer Res.
32:262013. View Article : Google Scholar :
|
|
15
|
Wang P, Huang S, Wang F, Ren Y, Hehir M,
Wang X and Cai J: Cyclic AMP-response element regulated cell cycle
arrests in cancer cells. PLoS One. 8:e656612013. View Article : Google Scholar :
|
|
16
|
Zhao K, Wang W, Guan C, Cai J and Wang P:
Inhibition of gap junction channel attenuates the migration of
breast cancer cells. Mol Biol Rep. 39:2607–2613. 2012. View Article : Google Scholar
|
|
17
|
He M, Cheng Y, Li W, Liu Q, Liu J, Huang J
and Fu X: Vascular endothelial growth factor C promotes cervical
cancer metastasis via up-regulation and activation of
RhoA/ROCK-2/moesin cascade. BMC Cancer. 10:1702010. View Article : Google Scholar :
|
|
18
|
Peng B, Chang Q, Wang L, Hu Q, Wang Y,
Tang J and Liu X: Suppression of human ovarian SKOV-3 cancer cell
growth by Duchesnea phenolic fraction is associated with cell cycle
arrest and apoptosis. Gynecol Oncol. 108:173–181. 2008. View Article : Google Scholar
|
|
19
|
Yang H, Zhou J, Mi J, Ma K, Fan Y, Ning J,
Wang C, Wei X, Zhao H and Li E: HOXD10 acts as a tumor-suppressive
factor via inhibition of the RHOC/AKT/MAPK pathway in human
cholangiocellular carcinoma. Oncol Rep. 34:1681–1691. 2015.
|
|
20
|
Bober BG and Shah SB: Paclitaxel alters
sensory nerve biomechanical properties. J Biomech. 48:3559–3567.
2015. View Article : Google Scholar
|
|
21
|
Luo Y, Wang X, Wang H, Xu Y, Wen Q, Fan S,
Zhao R, Jiang S, Yang J, Liu Y, et al: High bak expression is
associated with a favorable prognosis in breast cancer and
sensitizes breast cancer cells to paclitaxel. PLoS One.
10:e01389552015. View Article : Google Scholar :
|
|
22
|
Khanna C, Rosenberg M and Vail DM: A
review of paclitaxel and novel formulations including those
suitable for use in dogs. J Vet Intern Med. 29:1006–1012. 2015.
View Article : Google Scholar :
|
|
23
|
Varma H, Yamamoto A, Sarantos MR, Hughes
RE and Stockwell BR: Mutant huntingtin alters cell fate in response
to microtubule depolymerization via the GEF-H1-RhoA-ERK pathway. J
Biol Chem. 285:37445–37457. 2010. View Article : Google Scholar :
|
|
24
|
Takesono A, Heasman SJ, Wojciak-Stothard
B, Garg R and Ridley AJ: Microtubules regulate migratory polarity
through Rho/ROCK signaling in T cells. PLoS One. 5:e87742010.
View Article : Google Scholar :
|
|
25
|
Chang YC, Nalbant P, Birkenfeld J, Chang
ZF and Bokoch GM: GEF-H1 couples nocodazole-induced microtubule
disassembly to cell contractility via RhoA. Mol Biol Cell.
19:2147–2153. 2008. View Article : Google Scholar :
|
|
26
|
Tang Z, Zhang N, Di W and Li W: Inhibition
of microtubule-associated protein 1 light chain 3B via
small-interfering RNA or 3-methyladenine impairs hypoxia-induced
HO8910PM and HO8910 epithelial ovarian cancer cell migration and
invasion and is associated with RhoA and alterations of the actin
cytoskeleton. Oncol Rep. 33:1411–1417. 2015.
|
|
27
|
Zhang Y, Duan X, Cao R, Liu HL, Cui XS,
Kim NH, Rui R and Sun SC: Small GTPase RhoA regulates cytoskeleton
dynamics during porcine oocyte maturation and early embryo
development. Cell Cycle. 13:3390–3403. 2014. View Article : Google Scholar :
|
|
28
|
Meiri D, Marshall CB, Mokady D, LaRose J,
Mullin M, Gingras AC, Ikura M and Rottapel R: Mechanistic insight
into GPCR-mediated activation of the microtubule-associated RhoA
exchange factor GEF-H1. Nat Commun. 5:48572014. View Article : Google Scholar
|