Introduction
Esophageal cancer is one of the most common types of
cancer worldwide and has a high incidence of mortality. It is
estimated that 455,800 new esophageal cancer cases and 400,200
deaths occurred in 2012 (1). The
incidence of esophageal cancer varies depending on location; the
highest rates occur in Eastern Asia and Eastern and Southern
Africa. Esophageal squamous cell carcinoma (ESCC) accounts for
>90% of esophageal cancer cases in China compared with
approximately 26% in the United States. Despite progress in
clinical diagnosis and treatment modalities, ESCC remains
associated with a poor prognosis, and has a 5-year survival rate of
<15% (2–6). Traditional methods of characterizing
tumors depend on visual information, including the size of the
tumor, degree of infiltration and histological features of the
tumor, which form the basis of the tumor-node-metastasis (TNM)
staging system. Although these parameters provide a way to
distinguish between tumor subtypes with distinct biological
characteristics, it does not provide sufficient information for the
establishment of heterogeneous groupings of tumors and patients for
clinical treatment. Therefore, the identification of effective
biomarkers that associate with the biological characteristics of
ESCC patients is important to predict their prognosis and to
improve therapeutic strategies.
Prognostic biomarkers should be chosen based on
distinguishing features between benign and malignant tumors or the
differentiation/pathological staging status of tumors, which may
influence the mode of treatment. Studies have focused on
identifying novel ESCC biomarkers and therapeutic targets for
adjuvant drug treatments for the enhancement of current surgery,
radiotherapy and chemotherapy modalities (7–11).
Although research into the pathogenesis and targeted therapy of
lung, breast, and colorectal cancer has progressed, there remains a
paucity of data on esophageal cancer.
Paired box (PAX) 9 is a member of the paired box
gene family, which is composed of nine transcription factors in
humans (PAX1-9). This family of genes serves key roles in embryonic
development and organogenesis by regulating the expression of genes
involved in cell proliferation, apoptotic resistance and cell
migration (12,13). PAX genes are usually described as
cell lineage-specific regulators of tissues where their expression
profiles are finely tuned both spatially and temporally, and are
recognized as potentially important factors in cancer progression
(14–17). PAX2 is frequently expressed in
primary human cancers, including those derived from breast and
ovaries, and chromosomal translocations involving PAX3,
PAX5, PAX7 and PAX8 genes are present in
alveolar rhabdomyosarcoma, B-lymphoid malignancies and thyroid
cancer (18). PAX3 is required for
the survival of melanoma cell lines and is expressed in breast
cancer (19). In addition, PAX6 is
highly expressed in cancer cell lines, including those derived from
brain and breast tumors (20).
Expression of PAX2 and PAX8 has been observed in kidney tissue
(21). PAX5 is considered to be a
tumor suppressor and is involved in hepatocellular carcinoma
carcinogenesis via direct regulation of the tumor protein p53
signaling pathway (22).
Human PAX9 is located within the 14q12-q13
chromosomal region and contains a 128-amino acid DNA binding paired
domain, which makes sequence-specific contacts with DNA. Due to the
G quadruplex-forming region located near exon 1, which is present
in all the known sequenced placental mammals, PAX9 intron 1 serves
a key role in splicing efficiency (23,24).
As a transcription factor expressed in the tooth mesenchyme during
tooth morphogenesis, heterozygous mutations in PAX9 have been
associated with non-syndromic tooth agenesis, predominantly in the
molars (25–28). In the last decade, the abnormal
expression of the PAX9 gene, which is associated with the
tumorigenesis, development, invasion and metastasis of many types
of cancer, was observed in a variety of malignant human tumors
(29,30). However, the expression level and
prognostic value of PAX9 in malignant tumor cells remain
inconclusive. An experiment comparing genomic abnormalities in a
large cohort of patients with esophageal adenocarcinoma (EAC) and
ESCC using single nucleotide polymorphism arrays demonstrated that
ESCC exhibited greater amplification frequencies in PAX9 (35%) than
EAC (4%) (31). Therefore, PAX9
may be an oncogene specifically involved in ESCC.
Prior to the present study, the prognostic value of
PAX9 had not been investigated in ESCC. In the present study, the
expression of the PAX9 protein in patients with ESCC treated by
curative resection was investigated using immunohistochemical
staining. In addition, the overall survival (OS) of the ESCC
patients was analyzed. The aims of the present study were to
investigate the prognostic significance of PAX9 in resectable ESCC
and to assess its value in predicting radiation sensitivity by
subgroup analysis.
Materials and methods
Patients
A total of 229 ESCC patients who received radical
surgery at Qilu Hospital at Shandong University between 1st January
2008 and 31st December 2009 were included in the present study.
Patients with distant metastases received neoadjuvant treatment or
palliative surgery, and those who were not available for follow-up
or lacked sufficient tumor tissue for analysis were excluded. No
patients received chemotherapy or radiotherapy prior to surgery. A
total of 119 patients received adjuvant treatment with 5,000 cGy/25
fractions radiotherapy and 4–6 cycles of docetaxel and cisplatin.
Of these patients, 35 received synchronous radiochemotherapy.
The histological features of the specimens were
independently evaluated by two pathologists according to the
classification criteria of the World Health Organization. Cohen's
Kappa coefficient revealed a strong association between the two
pathologists (k=0.86). Patient demographic and clinical data,
including age, gender, smoking and alcohol history, tumor location,
histological grade, tumor length, T stage, lymph node status, TNM
stage and adjuvant treatment, were available. The pathological
stages of esophageal cancer were determined according to the
pathological (p) TNM staging system (defined by the American Joint
Committee on Cancer Staging Manual, 7th edition, 2010) (32). The histological grade was
classified according to the degree of differentiation of the tumor
by histological examination using hematoxylin and eosin staining.
Follow-up visits were performed every 3 months for the first 2
years and every 6 months until death or until the end of the study.
Data were censored at the last follow-up visit (December 2014) for
patients without recurrence or death. At each visit, a clinical
history was collected and a physical examination was performed.
Routine diagnostic imaging methods, including upper
gastrointestinal barium meal fluoroscopy and computer tomography,
were performed. Disease-free survival (DFS) and OS were defined as
the interval between the surgery date to the date of recurrence or
death, respectively.
Procedures were performed in accordance with The
Declaration of Helsinki. Ethical approval was obtained from the
Institutional Ethics Committee of Qilu Hospital at Shandong
University, which is accredited by the National Council on Ethics
in Human Research. The participants provided written informed
consent.
Immunohistochemistry
Surgical tissue samples were fixed in 10% neutral
buffered formalin at room temperature overnight and then embedded
in paraffin. For antigen retrieval, 4 µm thick paraffin sections
were deparaffinized in a series of alcohols and microwaved in
citrate buffer (pH 6.0). Endogenous peroxidases were blocked with
3% hydrogen peroxide for 20 min at room temperature. Subsequently,
tissues were blocked with goat serum (ZLI-9021; ZSGB-Bio, Beijing,
China) for 30 min at room temperature. Sections were incubated at
−4°C overnight with a rat monoclonal anti-PAX9 antibody
(SAB4200083; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) diluted
at 1:200 according to the manufacturer's protocol, and probed with
HRP-labeled rabbit anti rat secondary antibody at 37°C for one hour
(Histostain®-Plus kit; Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China). The PAX9 protein was
visualized using a substrate solution containing diaminobenzidine
and hydrogen peroxide (ZLI-9034; 1:1,000; ZSGB-Bio) at room
temperature. The presence of viable tumor was confirmed by
hematoxylin and eosin staining. Tissue sections stained in a
similar manner with PBS instead of the primary antibody were
utilized as negative controls. A total of forty healthy esophageal
mucous membranes from esophageal tissue and esophageal cancer
tissue microarrays (BN02014; Alenabio, Xi'an, China) were utilized
as positive controls.
Immunostaining evaluation
Tissue sections were analyzed with an Olympus
IX71S1F-3 Inverted Microscope (Olympus Corporation, Tokyo, Japan)
by two independent operators using the blinding method (33). At least five fields of view were
observed in each tissue section, and at least 100 cells were
counted in each field at a high magnification (×200). Cells that
exhibited brown granules in the cytoplasm were considered as
positive. Using semi-quantitative analysis, the criteria for
scoring the stained sections were: 1, 0–25%; 2, 26–50%; 3, 51–75%;
and 4, 76–100%. According to the intensity of PAX9 staining,
tissues were scored as follows: 0, negative; 1, weakly positive; 2,
strongly positive. Final scores were calculated by multiplying the
percentage of cells stained by the staining intensity of the
tissues, and were categorized as PAX9-negative (final score 0–3) or
PAX9-positive (final score 4–8) (34). In addition, tumor-adjacent tissues
were obtained from all patients, and PAX9 expression in these
tissues was determined.
Statistical analysis
Statistical analyses were performed using SPSS
software version 19.0 (IBM SPSS, Armonk, NY, USA). Statistical
differences between tissue groups were evaluated using the
Chi-square test. Kaplan-Meier curves were utilized to analyze the
distribution of 5-year DFS and OS, and the log-rank test was
performed to compare differences between the survival curves. The
same methods were adopted to perform the stratified analysis in
which subgroups were divided according to different adjuvant
treatment. Variables were subjected to univariate analysis and
multivariate survival analysis using the Cox proportional hazard
regression model. The statistical tests were two-sided, and
P-<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 229 patients were included in this study.
The median age was 60 years (range, 32–84 years), and 80.8% of the
patients were male. Tumor locations included the following:
cervical, 7 cases; upper thoracic, 16 cases; middle thoracic, 130
cases; and lower thoracic, 76 cases. The tumors ranged from 0.5 cm
to 10.0 cm in length, with an average of 4.17 cm. The
histopathological stage was well-differentiated in 55 cases,
moderately-differentiated in 99 cases, and poorly-differentiated in
75 cases. A total of 88 patients (38.4%) presented with T1/T2
tumors and 141 patients (61.6%) presented with T3/T4 tumors, and
109 patients (47.6%) presented with positive lymph nodes.
Twenty-six patients were in pathological stage I, 101 patients were
in stage II and 102 patients were in stage III. A total of 110
patients (48.0%) were treated with surgery alone, 53 (23.1%)
patients were treated with postoperative chemotherapy, 101 (44.1%)
patients were treated with postoperative radiotherapy, and 35
(15.3%) patients were treated with postoperative chemoradiation.
Two hundred and thirteen patients (93.0%) experienced tumor
recurrence, and 182 (79.5%) patients died during the follow-up
period. The estimated 1-, 3-, and 5-year DFS and OS rates were
74.7, 41.9 and 15.3% and 88.6, 51.5 and 31.4%, respectively. The
DFSs ranged from 1.0 to 81.4 months (median, 25.9 months), and the
OS ranged from 1.7 to 83.2 months (median, 37.0 months).
Immunohistochemical analysis
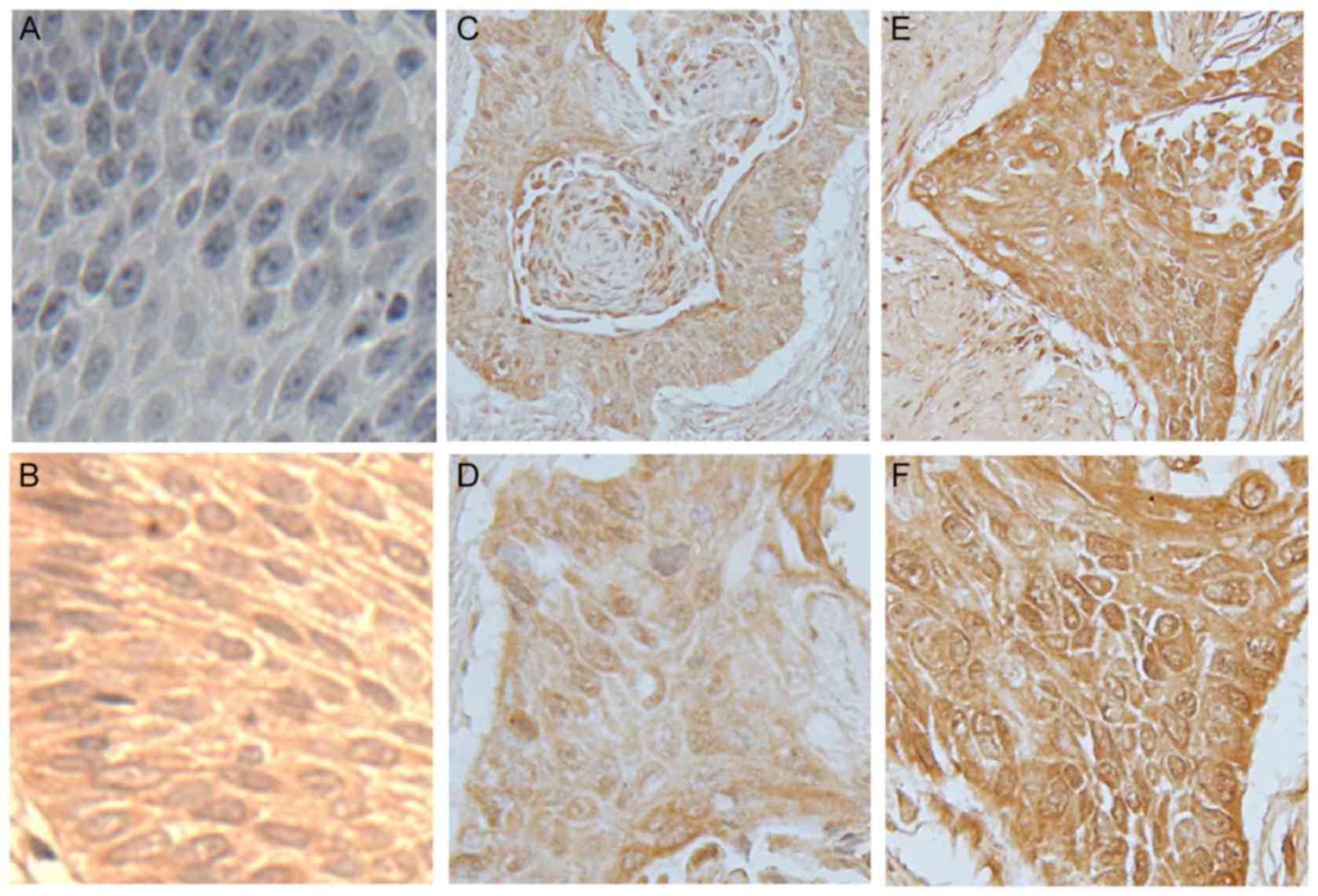
Examples of PAX9-negative and PAX9-positive tissue
staining are shown in Fig. 1.
PAX9-positive expression was observed in the nucleus and cytoplasm
of tumor and normal esophageal mucosa tissues. In healthy
esophageal mucous membranes and tumor-adjacent tissues, the
positive expression rates of PAX9 were 99.1% and 97.8%,
respectively. In ESCC tissues, 52.8% stained positive for PAX9
(121/229). In ESCC tissues, no significant correlation was
identified between the expression of PAX9 and clinicopathological
features of the patients, including gender, age, smoking history,
drinking history, tumor location, tumor length, differentiation, T
stage, N stage, pTNM stage or adjuvant treatment (Table I).
 | Table I.Association of clinicopathological
variables of ESCC patients and PAX9 expression in tumor tissue
samples. |
Table I.
Association of clinicopathological
variables of ESCC patients and PAX9 expression in tumor tissue
samples.
|
|
| PAX9 |
|---|
|
|
|
|
|---|
| Clinicopathological
characteristics | Total patients
(n=229) | Negative (n=108)
(%) | Positive (n=121)
(%) | P-value |
|---|
| Gender |
|
|
| 0.801 |
|
Male | 185 | 88 (47.6 | 97 (52.4 |
|
|
Female | 44 | 20 (45.5 | 24 (54.5 |
|
| Age |
|
|
| 0.383 |
|
≤60 | 116 | 58 (50.0 | 58 (50.0 |
|
|
>60 | 113 | 50 (44.2 | 63 (55.8 |
|
| Smoking
history |
|
|
| 0.179 |
| Never
or light | 108 | 56 (51.9 | 52 (48.1 |
|
|
Heavy | 121 | 52 (43.0 | 69 (57.0 |
|
| Drinking
history |
|
|
| 0.052 |
| Never
or light | 118 | 63 (53.3 | 55 (46.7 |
|
|
Heavy | 111 | 45 (40.5 | 66 (59.5 |
|
| Site of tumor |
|
|
| 0.287 |
|
Cervical | 7 | 2 (28.6 | 5 (71.4 |
|
| Upper
thoracic | 16 | 9 (56.3 | 7 (43.7 |
|
| Middle
thoracic | 130 | 56 (43.1 | 74 (56.9 |
|
| Lower
thoracic | 76 | 41 (53.9 | 35 (46.1 |
|
| Tumor length |
|
|
| 0.941 |
| <4
cm | 102 | 46 (45.1 | 56 (54.9 |
|
| ≥4
cm | 127 | 62 (48.8 | 65 (51.2 |
|
|
Differentiation |
|
|
| 0.893 |
|
Well | 55 | 25 (45.5 | 30 (54.5 |
|
|
Moderate | 99 | 46 (46.5 | 53 (53.5 |
|
|
Poor | 75 | 37 (49.3 | 38 (50.7 |
|
| T stage |
|
|
| 0.150 |
| T1 | 23 | 6 (26.1 | 17 (73.9 |
|
| T2 | 65 | 31 (47.7 | 34 (52.3 |
|
| T3 | 126 | 62 (49.2 | 64 (50.8 |
|
| T4 | 15 | 9 (60.0 | 6 (40.0 |
|
| N stage |
|
|
| 0.162 |
| N0 | 120 | 51 (42.5 | 69 (57.5 |
|
| N1 | 87 | 45 (51.7 | 42 (48.3 |
|
| N2 | 19 | 9 (47.4 | 10 (52.6 |
|
| N3 | 3 | 3 (100.0 | 0 (0.00 |
|
| pTNM stage |
|
|
| 0.105 |
| I | 26 | 10 (38.5 | 16 (61.5 |
|
| II | 101 | 42 (41.6 | 59 (58.4 |
|
|
III | 102 | 56 (54.9 | 46 (45.1 |
|
| Adjuvant
treatment |
|
|
| 0.060 |
|
None | 110 | 55 (50.0 | 55 (50.0 |
|
|
Radiotherapy | 66 | 24 (36.4 | 42 (63.6 |
|
|
Chemotherapy | 18 | 7 (38.9 | 11 (61.1 |
|
|
CRT | 35 | 22 (62.9 | 13 (37.1 |
|
The prognostic value of PAX9 and other variables in
ESCC tumors was investigated, as demonstrated in Table II. In PAX9-negative tumors, the
1-, 3-, and 5-year DFS and OS rates were 72.2, 35.2 and 5.6%, and
86.1, 44.4, and 23.1%, respectively. By contrast, in PAX9-positive
tumors, the 1-, 3-, and 5-year DFS and OS rates were 76.9, 47.9,
and 24.0%, and 90.9, 57.9, and 38.8%, respectively. The median OS
for the patients in the PAX9-negative group was 29.4 months (range,
1.7–73.1 months) compared with 42.0 months (range, 3.5–83.2 months)
for patients in the PAX9-positive group.
 | Table II.Univariate analysis of survival of
ESCC patients treated with curative surgery. |
Table II.
Univariate analysis of survival of
ESCC patients treated with curative surgery.
|
|
| DFS | OS |
|---|
|
|
|
|
|
|---|
|
Characteristics | Total patients | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Gender |
|
|
|
|
|
|
|
|
Male | 185 |
| 1.000 | Ref. |
| 1.000 | Ref. |
|
Female | 44 | 0.695 | 0.933 | 0.695–1.321 | 0.705 | 0.930 | 0.637–1.356 |
| Age |
|
|
|
|
|
|
|
|
≤60 | 116 |
| 1.000 | Ref. |
| 1.000 | Ref. |
|
>60 | 113 | 0.608 | 0.932 | 0.712–1.220 | 0.217 | 0.832 | 0.622–1.114 |
| Smoking
history |
|
|
|
|
|
|
|
| Never
or light | 108 |
| 1.000 | Ref. |
| 1.000 | Ref. |
|
Heavy | 121 | 0.960 | 1.007 | 0.769–1.319 | 0.982 | 0.997 | 0.745–1.334 |
| Drinking
history |
|
|
|
|
|
|
|
| Never
or light | 118 |
| 1.000 | Ref. |
| 1.000 | Ref. |
|
Heavy | 111 | 0.545 | 0.920 | 0.702–1.205 | 0.436 | 0.890 | 0.664–1.193 |
| Tumor site |
|
|
|
|
|
|
|
|
Cervical | 7 |
| 1.000 | Ref. |
| 1.000 | Ref. |
| Upper
thoracic | 16 | 0.879 | 1.077 | 0.413–2.813 | 0.858 | 1.098 | 0.394–3.060 |
| Middle
thoracic | 130 | 0.996 | 0.998 | 0.437–2.278 | 0.760 | 0.869 | 0.353–2.139 |
| Lower
thoracic | 76 | 0.688 | 1.187 | 0.514–2.744 | 0.956 | 0.974 | 0.392–2.425 |
| Tumor length |
|
|
|
|
|
|
|
| <4
cm | 102 |
| 1.000 | Ref. |
| 1.000 | Ref. |
| ≥4
cm | 127 | 0.428 | 1.117 | 0.850–1.467 | 0.616 | 1.078 | 0.803–1.447 |
|
Differentiation |
|
|
|
|
|
|
|
|
Well | 55 |
| 1.000 | Ref. |
| 1.000 | Ref. |
|
Moderate | 99 | 0.199 | 1.254 | 0.888–1.772 | 0.058 | 1.444 | 0.988–2.111 |
|
Poor | 75 | 0.006 | 1.679 | 1.163–2.422 | 0.004 | 1.813 | 1.213–2.711 |
| T stage |
|
|
|
|
|
|
|
| T1 | 23 |
| 1.000 | Ref. |
| 1.000 | Ref. |
| T2 | 65 | 0.025 | 1.874 | 1.084–3.241 | 0.094 | 1.638 | 0.920–2.917 |
| T3 | 126 | 0.003 | 2.198 | 1.307–3.697 | 0.035 | 1.796 | 1.042–3.097 |
| T4 | 15 | <0.001 | 4.343 | 2.123–8.888 | 0.002 | 3.279 | 1.571–6.843 |
| N stage |
|
|
|
|
|
|
|
| N0 | 120 |
| 1.000 | Ref. |
| 1.000 | Ref. |
| N1 | 87 | <0.001 | 2.522 | 1.876–3.389 | <0.001 | 3.102 | 2.247–4.821 |
| N2 | 19 | <0.001 | 5.288 | 3.167–8.831 | <0.001 | 4.839 | 2.873–8.151 |
| N3 | 3 | 0.001 | 7.625 | 2.385–24.376 | <0.001 | 9.859 | 3.042–31.953 |
| pTNM stage |
|
|
|
|
|
|
|
| I | 26 |
| 1.000 | Ref. |
| 1.000 | Ref. |
| II | 101 | 0.056 | 1.633 | 0.988–2.697 | 0.202 | 1.427 | 0.826–2.467 |
|
III | 102 | <0.001 | 4.783 | 2.860–7.999 | <0.001 | 4.946 | 2.862–8.548 |
| Adjuvant
treatment |
|
|
|
|
|
|
|
|
None | 110 |
| 1.000 | Ref. |
| 1.000 | Ref. |
|
Radiotherapy | 66 | 0.120 | 0.774 | 0.560–1.069 | 0.152 | 0.774 | 0.545–1.099 |
|
Chemotherapy | 18 | 0.181 | 1.410 | 0.853–2.330 | 0.846 | 1.056 | 0.610–0.827 |
|
CRT | 35 | 0.631 | 1.101 | 0.743–1.631 | 0.765 | 1.067 | 0.698–1.632 |
| PAX9 |
|
|
|
|
|
|
|
|
Negative | 108 |
| 1.000 | Ref. |
| 1.000 | Ref. |
|
Positive | 121 | <0.001 | 0.577 | 0.436–0.764 | 0.001 | 0.605 | 0.450–0.813 |
According to univariate analysis, which deals with
one predictor variable, PAX9, differentiation, T stage, N stage and
pTNM stage were statistically associated with DFS and OS (Table II). To further exclude confounding
factors and obtain more reliable results, multivariate analysis was
then performed, which deals with multiple predictor variables. In a
multivariate analysis of DFS and OS, neither differentiation or T
stage were independent prognostic factors (Table III). According to the Cox
proportional hazard regression model, PAX9 was an independent
predictor for DFS (HR=0.641, 95% CI: 0.472–0.869, P=0.004) and OS
(HR=0.673, 95% CI: 0.491–0.922, P=0.014; Table III). Lymph node metastasis (N
stage) was associated with decreased DFS (N1: HR=1.988, 95% CI:
1.118–3.534, P=0.019; N2: HR=3.357, 95% CI: 1.612–6.993, P=0.001;
N3: HR=4.029, 95% CI: 1.001–16.224, P=0.049), and decreased OS (N1:
HR=1.988, 95% CI: 1.118–3.534, P=0.019; N2: HR=3.357, 95% CI:
1.612–6.993, P=0.001; N3: HR=4.029, 95% CI: 1.001–16.224, P=0.049).
In addition, pTNM stage III was an independent prognostic factor
for DFS (HR=2.552, 95% CI: 1.290–7.319, P=0.031) and OS (HR=2.537,
95% CI: 1.274–7.365, P=0.037), respectively (Table III). Therefore, the results
showed that PAX9, N stage and pTNM stage III were prognostic
factors of survival in ESCC.
 | Table III.Multivariate analyses of prognostic
variables of ESCC treated with curative surgery. |
Table III.
Multivariate analyses of prognostic
variables of ESCC treated with curative surgery.
|
| DFS | OS |
|---|
|
|
|
|
|---|
|
Characteristics | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Gender |
|
|
|
|
|
Male | 1.000 | Ref. |
|
| 1.000 | Ref. |
|
Female | 0.715 | 0.933 | 0.641–1.356 | 0.458 | 0.852 | 0.559–1.299 |
| Age |
|
|
|
|
|
≤60 | 1.000 | Ref. |
|
| 1.000 | Ref. |
|
>60 | 0.747 | 1.048 | 0.789–1.392 | 0.786 | 0.958 | 0.704–1.304 |
| Smoking
history |
|
|
|
|
| Never
or light | 1.000 | Ref. |
|
| 1.000 | Ref. |
|
Heavy | 0.155 | 1.251 | 0.919–1.703 | 0.409 | 1.150 | 0.825–1.604 |
| Drinking
history |
|
|
|
|
| Never
or light | 1.000 | Ref. |
|
| 1.000 | Ref. |
|
Heavy | 0.917 | 1.016 | 0.751–1.375 | 0.946 | 0.989 | 0.713–1.371 |
| Tumor site |
|
|
|
|
|
Cervical | 1.000 | Ref. |
|
| 1.000 | Ref. |
| Upper
thoracic | 0.389 | 0.638 | 0.229–1.774 | 0.234 | 0.509 | 0.167–1.549 |
| Middle
thoracic | 0.118 | 0.489 | 0.200–1.200 | 0.101 | 0.442 | 0.167–1.172 |
| Lower
thoracic | 0.366 | 0.658 | 0.266–1.629 | 0.244 | 0.557 | 0.208–1.492 |
| Tumor length |
|
|
|
|
| <4
cm | 1.000 | Ref. |
|
| 1.000 | Ref. |
| ≥4
cm | 0.646 | 1.071 | 0.799–1.437 | 0.413 | 1.145 | 0.828–1.582 |
|
Differentiation |
|
|
|
|
|
Well | 1.000 | Ref. |
|
| 1.000 | Ref. |
|
Moderate | 0.462 | 0.861 | 0.577–1.284 | 0.780 | 1.063 | 0.691–1.636 |
|
Poor | 0.741 | 1.077 | 0.694–1.670 | 0.335 | 1.261 | 0.786–2.024 |
| T stage |
|
|
|
|
| T1 | 1.000 | Ref. |
|
| 1.000 | Ref. |
| T2 | 0.701 | 1.157 | 0.550–2.435 | 0.948 | 0.976 | 0.469–2.030 |
| T3 | 0.633 | 1.214 | 0.548–2.688 | 0.999 | 1.001 | 0.458–2.185 |
| T4 | 0.301 | 1.766 | 0.601–5.194 | 0.607 | 1.332 | 0.446–3.978 |
| N stage |
|
|
|
|
| N0 | 1.000 | Ref. |
|
| 1.000 | Ref. |
| N1 | 0.019 | 1.988 | 1.118–3.534 | 0.004 | 2.407 | 1.315–4.405 |
| N2 | 0.001 | 3.357 | 1.612–6.993 | 0.009 | 2.824 | 1.298–6.147 |
| N3 | 0.049 | 4.029 | 1.001–16.224 | 0.029 | 4.642 | 1.169–18.432 |
| pTNM stage |
|
|
|
|
| I | 1.000 | Ref. |
|
| 1.000 | Ref. |
| II | 0.321 | 1.491 | 0.677–3.286 | 0.399 | 1.415 | 0.632–3.171 |
|
III | 0.031 | 2.552 | 1.290–7.319 | 0.037 | 2.537 | 1.274–7.365 |
| Adjuvant
treatment |
|
|
|
|
|
None | 1.000 | Ref. |
|
| 1.000 | Ref. |
|
Radiotherapy | 0.064 | 0.646 | 0.455–1.016 | 0.072 | 0.641 | 0.438–1.038 |
|
Chemotherapy | 0.563 | 1.183 | 0.669–2.092 | 0.437 | 0.776 | 0.409–1.472 |
|
CRT | 0.081 | 0.658 | 0.411–1.052 | 0.075 | 0.584 | 0.354–1.062 |
| PAX9 |
|
|
|
|
|
Negative | 1.000 | Ref. |
|
| 1.000 | Ref. |
|
Positive | 0.004 | 0.641 | 0.472–0.869 | 0.014 | 0.673 | 0.491–0.922 |
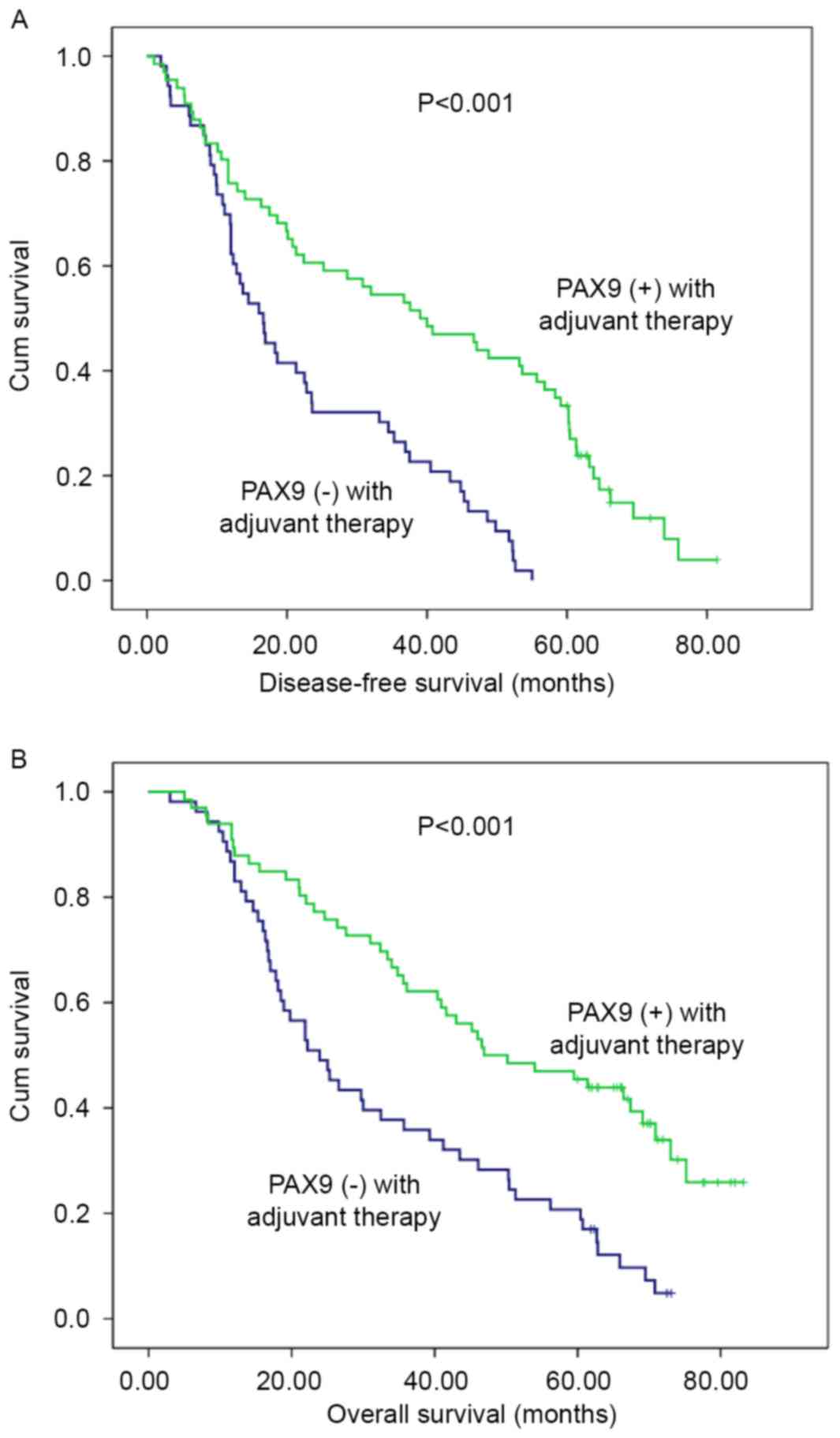
Considering adjuvant therapy associations, different
stratified analyses were further performed to compare the survival
curves between the PAX9-negative and PAX9-positive groups. For
patients that did not receive adjuvant therapy, no significant
differences in the 5-year DFS (P=0.494) and OS (P=0.663) were
observed. However, there was a significant difference in the 5-year
DFS and OS (P<0.001) in patients that received adjuvant therapy
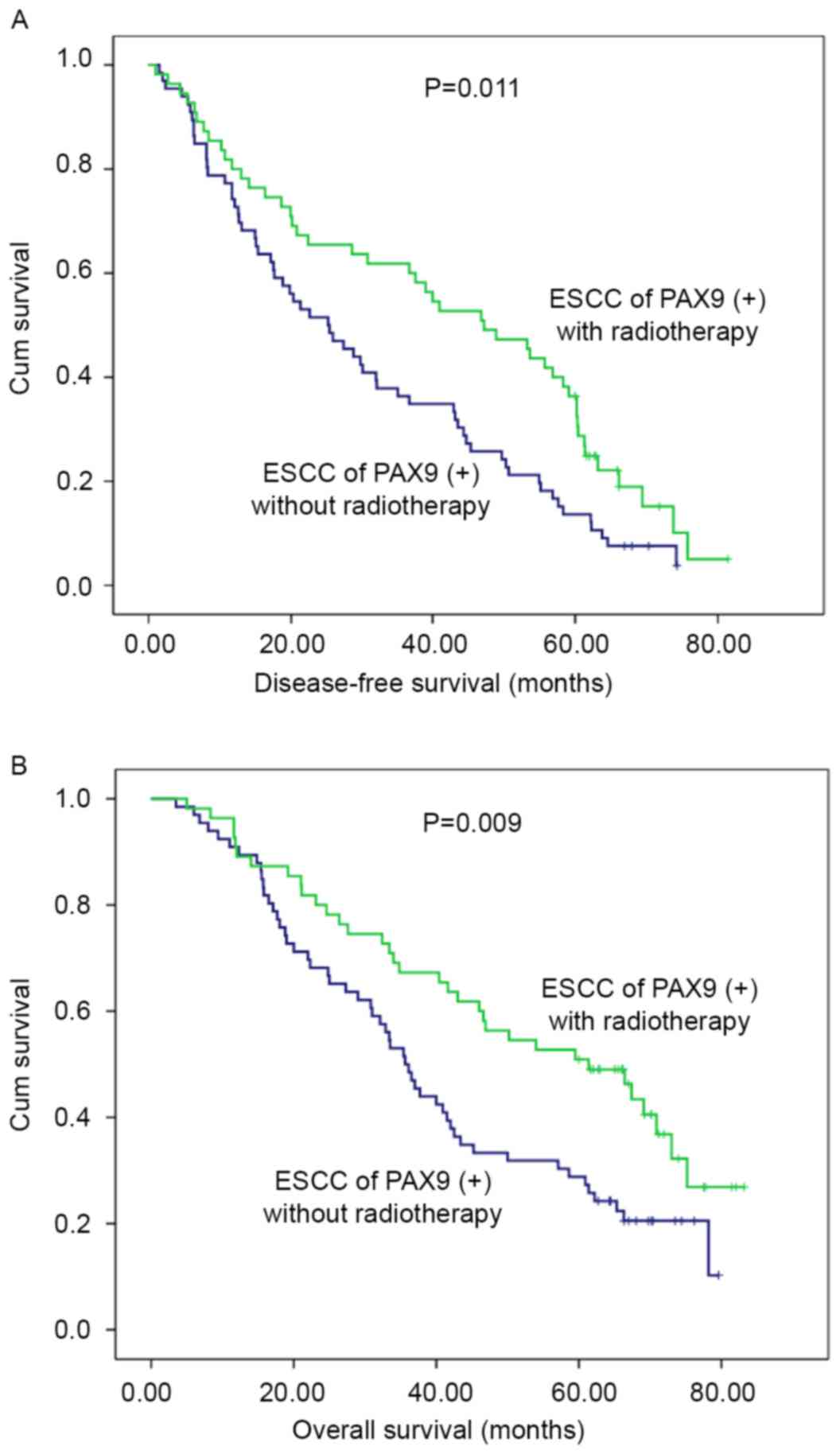
(Fig. 3). In addition, subgroup
analysis was performed according to PAX9 positivity. In the
PAX9-positive group, the patients who received adjuvant
radiotherapy had an improved prognosis compared with patients who
did not receive adjuvant radiotherapy, as demonstrated by
significant differences in the 5-year DFS (P=0.011) and OS
(P=0.009) (Fig. 4).
Discussion
The morbidity and mortality rates for ESCC increase
annually; however, advances in therapeutic strategies that
significantly alter patient outcome remains to be achieved. This
may be due to the complexity of the oncogenic process, which
involves somatic acquisition of large numbers of mutations that
gives rise to complex, heterogenic genetic profiles among patients,
making it difficult to characterize and treat effectively. Although
previous reports focus on PAX9 expression in different tumors
(35–37), there is a paucity of data
describing PAX9 expression in esophageal cancer. PAX9 may be a key
gene involved in ESCC. In the present study, the correlation
between PAX9 expression and survival in 229 ESCC patients who
received curative surgery was investigated. In addition,
associations between PAX9 and ESCC radiosensitivity was
determined.
A greater tumor length, advanced T stage and poor
differentiation were associated with low DFS and OS rates, as
determined by univariate analysis; however, these factors were not
found to be independent predictors of prognosis by multivariate
analysis, which is more important in the analysis of prognostic
factors. In addition, lymph node metastasis and advanced pTNM stage
were independent prognostic factors, which concurred with previous
studies (7,38). The results of the present study
demonstrated that negative PAX9 expression is significantly
associated with poor prognosis in ESCC patients. In addition, in a
stratified analysis, the patients were divided into two subgroups
according to whether postoperative adjuvant therapy was given in
the PAX9 positive or negative groups, respectively. PAX9-positive
patients in the adjuvant group exhibited a significantly prolonged
prognosis compared with PAX9-negative patients; however, this was
not the case in the surgery group. It was hypothesized that PAX9
expression may best predict survival in patients that received
postoperative treatment. Adjuvant radiotherapy benefited patients
in the PAX9-positive group; however, this was not the case in the
PAX9-negative group, compared with patients who did not receive
adjuvant radiotherapy. To the best of our knowledge, similar
studies have not been conducted in ESCC. Abnormal expression levels
of PAX9 suggested that it may be involved in the development of
ESCC. In the present study, the PAX9 protein was expressed in the
majority of healthy epithelia and the mucosa adjacent to esophageal
tumors. In malignant squamous cells, loss or decreased expression
levels of PAX9 were observed. These results are consistent with
previous findings (39). However,
the results of the present study did not demonstrate statistical
differences between PAX9 expression and the degree of cell
differentiation, which contradicts findings reported in the
literature (39). This may be due
to differences in the case numbers, enrollment criteria and ESCC
TNM staging standards. Knockdown of PAX9 resulted in the loss or
disorganization of squamous epithelium and downregulation of the
differentiation markers, Krt4 and Krt5, in zebrafish esophagus
(40). PAX9 expression levels may
vary in different tumors. Decreased PAX9 expression serves a role
in congenital tooth agenesis and as a high risk factor for
epithelial ovarian cancer (35).
PAX9 is significantly highly expressed in malignant melanomas and
nodular melanomas (41).
At present, no effective predictive biomarkers have
been identified that accurately estimate sensitivity to radiation
therapy in ESCC tumors. The present study demonstrated that
adjuvant radiotherapy may benefit patients with PAX9-positive
tumors and suggests that PAX9 may be a predictor of radiation
sensitivity in ESCC patients. The ability to identify ESCC patients
with a poor prognosis, which was observed in patients with
PAX9-positive tumors who did not receive radiotherapy after
surgery, suggested that radiotherapy, the standard of care for
unresectable patients, may be beneficial in PAX9-positive patients.
Therefore, the present study revealed the clinical significance and
predictive value of PAX9 in ESCC. In the clinic, ESCC patients
frequently receive radiation therapy or concurrent chemoradiation,
generally at high doses of 50.4–64.8 Gy, which is administered in
fractions of 1.8–2.0 Gy per day (42,43).
However, the curative effects vary largely in patients with the
same pathological diagnosis and radiotherapy dose, and this remains
to be elucidated. Tumor-associated factors that contribute to the
efficacy of radiotherapy include oxygen status, proliferative
potential and capacity to repair radiation damage. However, there
remains a lack of understanding of the differences in ESCC patient
responses to radiotherapy. The findings of the present study may
additionally aid the generation of a rational strategy for targeted
therapy in PAX9-positive ESCC patients.
Biomarkers have been utilized for predicting
tumorigenesis, progression and prognosis (44,45).
In ESCC, PAX9 downregulation may influence the cell cycle via by
maintaining tumor cells in the G0 stage and that may
cause cells to be more resistant to cellular radiosensitivity.
Cells in the G2 and M phases of the cell cycle are
sensitive to ionizing radiation, whereas those in the G0
phase are resistant (46). Studies
on PAX9 expression in other tumor types have been published over
the last 10 years. For example, Lee et al revealed that PAX9
inhibition causes the induction of apoptosis with enhanced cleavage
of caspase-3 and poly ADP-ribose polymerase, increased expression
levels of Bax and reduced expression levels of Bcl-2 in oral
squamous cell carcinoma. In addition, cells transfected with PAX9
siRNA and cells infected with adenovirus-mediated expression of
dominant-negative c-myb have been reported to display cell cycle
arrest at the G0 phase (30). The findings of the present study
may support this mechanism.
PAX9 has been demonstrated to be involved in
numerous signaling pathways, including the Janus kinase/signal
transducers and activators of transcription (JAK/STAT),
wingless-type MMTV integration site family (Wnt), bone
morphogenetic protein (BMP) and phosphatidylinositol
3-kinase/protein kinase B (PI3K/Akt) pathways. However, the
involvement of PAX9 in carcinogenesis is not likely to be limited
to these signaling pathways. PAX9 may serve different roles via
different mechanisms in various tumor tissues. In the mothers
against DPP homolog (Smad) 3 and Smad4 signaling pathways, PAX9
serves an important role in the suppression of microRNA-450b-5p by
transforming growth factor-β1 (36). In a study conducted by Kendall
et al (29), PAX9 was
identified as a lung cell lineage addiction oncogene, representing
a fundamental tumor survival mechanism with important therapeutic
implications; in addition to thyroid transcription factor 1 (TTF1)
and NK2 homeobox 8 (NKX2-8), PAX9 serves a role in the maintenance
of squamous cell carcinoma tumor cells that exhibit the 14q13.3
amplification, which is a recurrent amplicon in lung cancer. The
amplification of these genes frequently results in the promotion of
lung cancer and cell proliferation. Hsu et al demonstrated
that this three-gene signature is associated with genes and
pathways involved in embryonal tissue development (JAK/STAT, Wnt,
BMP and Hedgehog) and lung development (mitogen activated protein
kinase, PI3 K and JAK/STAT) (47).
The majority of the adenocarcinoma samples exhibited a higher
probability of PAX9 and TTF1 activation relative to SCC
cells. In addition, the specific patterns of coactivation of
developmental transcription factors (PAX9, TTF-1 and NKX2-8) were
independent of KRAS or epidermal growth factor mutational events.
PAX9 activation was associated with cisplatin sensitivity.
However, the specific mechanism of action of PAX9 requires further
investigation.
ESCC has been widely considered a genetic disease
resulting from synergistic action of tumor suppressor
genes/oncogenes, and there is interconnectivity between multiple
signaling pathways. High-throughput technologies, including cDNA
microarrays, proteomics, transcriptomics, genome-wide association
studies and microRNA arrays, have allowed access to a host of
cancer genomes, thereby increasing understanding of
cancer-associated pathobiology and signaling networks. Combined
analysis of the activity spectra and proteomics data revealed that
the primary signaling pathways involved in ESCC were
mitogen-activated protein kinase, Wnt and Akt pathways, which led
to the identification of regulatory networks, cross-transcription
factors, microRNAs and target genes (44,48,49).
Driven by heritable and somatic alterations in DNA, ESCC displays
genetic and epigenetic alterations, including DNA methylation,
histone deacetylation, chromatin remodeling, gene imprinting and
noncoding RNA regulation, which underpin carcinogenesis,
progression and metastasis. Protein-based biomarkers are possibly
more appropriate biomarkers than DNA- or RNA-based markers.
Therefore, proteomic markers are closer and more relevant to
disease state initiation and progression (50).
The present study has some limitations. The number
of cases assessed was limited. Despite its preliminary nature, the
present study suggests that PAX9 is an independent prognostic
factor for ESCC. More studies involving a larger number of samples
are required to further confirm these results. In addition, the
patients selected for the present study were post-operative. The
selection of non-surgery patients with observable and measurable
tumors may be beneficial to investigate the association between
PAX9 expression and the short-term curative effects of
radiotherapy. In vitro and in vivo experiments are
required to ascertain the predictive value of PAX9 in radiation
sensitivity in ESCC.
In the present study, low expression levels of PAX9
were significantly associated with poor survival in ESCC patients
following surgery. PAX9 may be an independent prognostic factor for
ESCC patient survival. Additionally, PAX9 may serve as a possible
predictor of radiation sensitivity, which may assist clinicians in
developing a rational approach to personalized treatment for ESCC
patients. However, the role of PAX9 in ESCC requires further
investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572958) and Ji'nan
Municipal Science and Technology Bureau Fund (grant no.
26010105081212).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dutton SJ, Ferry DR, Blazeby JM, Abbas H,
Dahle-Smith A, Mansoor W, Thompson J, Harrison M, Chatterjee A,
Falk S, et al: Gefitinib for oesophageal cancer progressing after
chemotherapy (COG): A phase 3, multicentre, double-blind,
placebo-controlled randomised trial. Lancet Oncol. 15:894–904.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Conroy T, Galais MP, Raoul JL, Bouché O,
Gourgou-Bourgade S, Douillard JY, Etienne PL, Boige V, Martel-Lafay
I, Michel P, et al: Definitive chemoradiotherapy with FOLFOX versus
fluorouracil and cisplatin in patients with oesophageal cancer
(PRODIGE5/ACCORD17): Final results of a randomised, phase 2/3
trial. Lancet Oncol. 15:305–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu HD, Guo JH, Mao AW, Lv WF, Ji JS, Wang
WH, Lv B, Yang RM, Wu W, Ni CF, et al: Conventional stents versus
stents loaded with (125)iodine seeds for the treatment of
unresectable oesophageal cancer: A multicentre, randomised phase 3
trial. Lancet Oncol. 15:612–619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graf D, Vallböhmer D, Knoefel WT, Budach W
and Häussinger D: Multimodal treatment of esophageal carcinoma.
Dtsch Med Wochenschr. 139:2141–2147. 2014.PubMed/NCBI
|
|
6
|
Paul S and Altorki N: Outcomes in the
management of esophageal cancer. J Surg Oncol. 110:599–610. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang N, Liu F, Cao F, Jia Y, Wang J, Ma W,
Tan B, Wang K, Song Q and Cheng Y: RACK1 predicts poor prognosis
and regulates progression of esophageal squamous cell carcinoma
through its epithelial-mesenchymal transition. Cancer Biol Ther.
16:528–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Urakawa N, Utsunomiya S, Nishio M,
Shigeoka M, Takase N, Arai N, Kakeji Y, Koma Y and Yokozaki H:
GDF15 derived from both tumor-associated macrophages and esophageal
squamous cell carcinomas contributes to tumor progression via Akt
and Erk pathways. Lab Inves. 95:491–503. 2015. View Article : Google Scholar
|
|
9
|
Nishi T, Takeuchi H, Matsuda S, Ogura M,
Kawakubo H, Fukuda K, Nakamura R, Takahashi T, Wada N, Saikawa Y,
et al: CXCR2 expression and postoperative complications affect
long-term survival in patients with esophageal cancer. World J Surg
Oncol. 13:2322015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Usui A, Hoshino I, Akutsu Y, Sakata H,
Nishimori T, Murakami K, Kano M, Shuto K and Matsubara H: The
molecular role of Fra-1 and its prognostic significance in human
esophageal squamous cell carcinoma. Cancer. 118:3387–3396. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forghanifard MM, Khales Ardalan S,
Javdani-Mallak A, Rad A, Farshchian M and Abbaszadegan MR: Stemness
state regulators SALL4 and SOX2 are involved in progression and
invasiveness of esophageal squamous cell carcinoma. Med Oncol.
31:9222014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paixão-Côrtes VR, Salzano FM and Bortolini
MC: Evolutionary history of chordate PAX genes: Dynamics of change
in a complex gene family. PLoS One. 8:e735602013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dressler GR: Patterning and early cell
lineage decisions in the developing kidney: The role of Pax genes.
Pediatr Nephrol. 26:1387–1394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li CG and Eccles MR: PAX Genes in Cancer;
Friends or Foes? Front Genet. 3:62012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robson EJ, He SJ and Eccles MR: A PANorama
of PAX genes in cancer and development. Nat Rev Cancer. 6:52–62.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Fang WH, Krupinski J, Kumar S,
Slevin M and Kumar P: Pax genes in embryogenesis and oncogenesis. J
Cell Mol Med. 12:2281–2294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lang D, Powell SK, Plummer RS, Young KP
and Ruggeri BA: PAX genes: Roles in development, pathophysiology,
and cancer. Biochem Pharmacol. 73:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muratovska A, Zhou C, He S, Goodyer P and
Eccles MR: Paired-Box genes are frequently expressed in cancer and
often required for cancer cell survival. Oncogene. 22:7989–7997.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubic JD, Little EC, Lui JW, Iizuka T and
Lang D: PAX3 and ETS1 synergistically activate MET expression in
melanoma cells. Oncogene. 34:4964–4974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Curto GG, Gard C and Ribes V: Structures
and properties of PAX linked regulatory networks architecting and
pacing the emergence of neuronal diversity. Semin cell Dev Biol.
44:75–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mentrikoski MJ, Wendroth SM and Wick MR:
Immunohistochemical distinction of renal cell carcinoma from other
carcinomas with clear-cell histomorphology: Utility of CD10 and
CA-125 in addition to PAX-2, PAX-8, RCCma, and adipophilin. Appl
Immunohistochem Mol Morphol. 22:635–641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu W, Li X, Chu ES, Go MY, Xu L, Zhao G,
Li L, Dai N, Si J, Tao Q, et al: Paired box gene 5 is a novel tumor
suppressor in hepatocellular carcinoma through interaction with p53
signaling pathway. Hepatology. 53:843–853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ribeiro MM, Teixeira GS, Martins L,
Marques MR, de Souza AP and Line SR: G-quadruplex formation
enhances splicing efficiency of PAX9 intron 1. Hum Genet.
134:37–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Narasimhan K, Hilbig A, Udayasuryan B,
Jayabal S, Kolatkar PR and Jauch R: Crystallization and preliminary
X-ray diffraction analysis of the Pax9 paired domain bound to a DC5
enhancer DNA element. Acta crystallogr F Struct Biol Commun.
70:1357–1361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitsui SN, Yasue A, Masuda K, Watanabe K,
Horiuchi S, Imoto I and Tanaka E: Novel PAX9 mutations cause
non-syndromic tooth agenesis. J Dent Res. 93:245–249. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boeira BR Jr and Echeverrigaray S: Novel
missense mutation in PAX9 gene associated with familial tooth
agenesis. J Oral Pathol Med. 42:99–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu J, Yang X, Zhang C, Ge L and Zheng S:
A novel nonsense mutation in PAX9 is associated with sporadic
hypodontia. Mutagenesis. 27:313–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang J, Song G, Li Q and Bian Z: Novel
missense mutations in PAX9 causing oligodontia. Arch Oral Biol.
57:784–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kendall J, Liu Q, Bakleh A, Krasnitz A,
Nguyen KC, Lakshmi B, Gerald WL, Powers S and Mu D: Oncogenic
cooperation and coamplification of developmental transcription
factor genes in lung cancer. Proc Natl Acad Sci USA.
104:16663–16668. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JC, Sharma M, Lee YH, Lee NH, Kim SY,
Yun JS, Nam SY, Hwang PH, Jhee EC and Yi HK: Pax9 mediated cell
survival in oral squamous carcinoma cell enhanced by c-myb. Cell
Biochem Funct. 26:892–899. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bandla S, Pennathur A, Luketich JD, Beer
DG, Lin L, Bass AJ, Godfrey TE and Litle VR: Comparative genomics
of esophageal adenocarcinoma and squamous cell carcinoma. Ann
Thorac Surg. 93:1101–1106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manualand the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeidan AM, Salem MR, Mazoit JX, Abdullah
MA, Ghattas T and Crystal GJ: The effectiveness of cricoid pressure
for occluding the esophageal entrance in anesthetized and paralyzed
patients: An experimental and observational glidescope study.
Anesth Analq. 118:580–586. 2014. View Article : Google Scholar
|
|
34
|
Prasad B, Kashyap B, Babu GS, Kumar GR and
Manyam R: Expression of podoplanin in different grades of oral
squamous cell carcinoma. Ann Med Health Sci Res. 5:299–304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bonds J, Pollan-White S, Xiang L, Mues G
and D'Souza R: Is there a link between ovarian cancer and tooth
agenesis? Eur J Med Genet. 57:235–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun MM, Li JF, Guo LL, Xiao HT, Dong L,
Wang F, Huang FB, Cao D, Qin T, Yin XH, et al: TGF-beta1
suppression of microRNA-450b-5p expression: A novel mechanism for
blocking myogenic differentiation of rhabdomyosarcoma. Oncogene.
33:2075–2086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Harris T, Pan Q, Sironi J, Lutz D, Tian J,
Sapkar J, Perez-Soler R, Keller S and Locker J: Both gene
amplification and allelic loss occur at 14q13.3 in lung cancer.
Clin Cancer Res. 17:690–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang WP, Wang Z and Jia Y: CEP55
overexpression predicts poor prognosis in patients with locally
advanced esophageal squamous cell carcinoma. Oncol Lett.
13:236–242. 2017.PubMed/NCBI
|
|
39
|
Gerber JK, Richter T, Kremmer E, Adamski
J, Höfler H, Balling R and Peters H: Progressive loss of PAX9
expression correlates with increasing malignancy of dysplastic and
cancerous epithelium of the human oesophagus. J Pathol.
197:293–297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen H, Beasley A, Hu Y and Chen X: A
Zebrafish model for studies on esophageal epithelial biology. PLoS
One. 10:e01438782015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roesch A, Mueller AM, Stempfl T, Moehle C,
Landthaler M and Vogt T: RBP2-H1/JARID1B is a transcriptional
regulator with a tumor suppressive potential in melanoma cells. Int
J Cancer. 122:1047–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cooper JS, Guo MD, Herskovic A, Macdonald
JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler
JJ, Spencer S, et al: Chemoradiotherapy of locally advanced
esophageal cancer: Long-term follow-up of a prospective randomized
trial (RTOG 85–01). Radiation therapy oncology group. JAMA.
281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky
TM, Martenson J, Komaki R, Okawara G, Rosenthal SA and Kelsen DP:
INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial
of combined-modality therapy for esophageal cancer: High-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu R, Gu J, Jiang P, Zheng Y, Liu X,
Jiang X, Huang E, Xiong S, Xu F, Liu G, et al: DNMT1-microRNA126
epigenetic circuit contributes to esophageal squamous cell
carcinoma growth via ADAM9-EGFR-AKT signaling. Clin Cancer Res.
21:854–863. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee KW, Sung CO, Kim JH, Kang M, Yoo HY,
Kim HH, Um SH and Kim SH: CD10 expression is enhanced by Twist1 and
associated with poor prognosis in esophageal squamous cell
carcinoma with facilitating tumorigenicity in vitro and in vivo.
Int J Cancer. 136:310–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hein AL, Ouellette MM and Yan Y:
Radiation-induced signaling pathways that promote cancer cell
survival (review). Int J Oncol. 45:1813–1819. 2014.PubMed/NCBI
|
|
47
|
Hsu DS, Acharya CR, Balakumaran BS, Riedel
RF, Kim MK, Stevenson M, Tuchman S, Mukherjee S, Barry W, Dressman
HK, et al: Characterizing the developmental pathways TTF-1, NKX2-8,
and PAX9 in lung cancer. Proc Natl Acad Sci USA. 106:5312–5317.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen J, Kwong DL, Cao T, Hu Q, Zhang L,
Ming X, Chen J, Fu L and Guan X: Esophageal squamous cell carcinoma
(ESCC): Advance in genomics and molecular genetics. Dis Esophagus.
28:84–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bu F, Liu X, Li J, Chen S, Tong X, Ma C,
Mao H, Pan F, Li X, Chen B, et al: TGF-β1 induces epigenetic
silence of TIP30 to promote tumor metastasis in esophageal
carcinoma. Oncotarget. 6:2120–2133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mishra A and Verma M: Cancer biomarkers:
Are we ready for the prime time? Cancers (Basel). 2:190–208. 2010.
View Article : Google Scholar : PubMed/NCBI
|