Introduction
The n-3 and n-6 fatty acids (FAs) are essential and
important to human health. However, there is a disproportionally
high proportion of n-6 FAs and low proportion of n-3 FAs in our
current dietary structure. This results in a high ratio of n-6/n-3,
which is associated with cardiovascular disease, inflammation and
cancer (1–3). The n-3 FAs are long-chain
polyunsaturated FAs. Eicosapentaenoic acid (EPA; 20:5n-3) and
docosahexaenoic acid (DHA; 22:6n-3) are two important n-3 FAs. The
primary dietary source of these is oily fish (4). Investigations in human populations
have revealed that a high consumption of fish or fish oils can
decrease the risk of breast, prostate and colon cancer (5,6).
Previous laboratory experiments have shown that n-3 FAs can inhibit
the initiation and development of breast cancer in vivo
(7,8), although others have failed to
identify a significant association.
The n-3 FAs have a number of biological effects, and
effects of n-3 FAs on cancer suppression have been suggested
(9,10), with n-3 FAs affecting the
apoptosis, proliferation, invasion and metastasis of cancer cells.
There are several signaling pathways involved in the effects of n-3
FAs, including protein kinase C, extracellular signal-regulated
kinase (ERK) 1/2, ras and nuclear factor (NF)-κB (11–14).
However, the effect of n-3 FAs on the death receptor-mediated
pathways and its molecular mechanisms remain to be fully
elucidated.
In the present study, the antitumor effect of n-3
FAs was investigated, with a focus on the apoptosis-inducing
effects of DHA on MCF-7 human breast cancer cells in culture. An
improved understanding of the mechanism underlying the antitumor
effects of n-3 FAs may assist in developing novel tumor treatment
strategies involving the use of fish oil as a dietary
supplement.
Materials and methods
Chemicals and reagents
DHA, EPA, linoleic acid (18:2n-6) and arachidonic
acid (20:4n-6) in fetal bovine serum (FBS) were purchased from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). Anti-β-actin
antibody and horseradish peroxidase (HRP)-conjugated anti-rabbit,
anti-mouse and anti-goat IgG were obtained from Amersham; GE
Healthcare Life Sciences (Chalfont, UK; 600567, RPN4301, RPN4201
and PA42002). Antibodies against cytochrome c and tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) were
obtained from BD Pharmingen (Franklin Lake, NJ, USA; 558700 and
556468). The antibodies against cleaved caspase-3, cleaved
caspase-8 and cleaved caspase-9 were obtained from Cell Signaling
Technology, Inc. (Beverly, MA, USA; 9661S, 9496S and 9501S).
Antibodies against death receptor (DR) 4 and 5 were purchased from
Imgenex; Novus Biologicals LLC (Littleton, CO, USA; NB100-56747 and
NB-100-55744). Antibodies against B-cell lymphoma 2 (Bcl-2),
Bcl-2-associated X protein (Bax), Fas, Fas ligand (FasL) and
Smac/Diablo were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA; sc-509, sc-7480, sc-8009, sc-33716 and
sc-393118).
Cell culture
The MCF-7 breast cancer cells were purchased from
Shanghai Life Science of Chinese Academy of Sciences (Shanghai,
China). The MCF-7 cells were cultured in Roswell Park Memorial
Institute 1640 medium (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA), supplemented with 10% heat-inactivated FBS (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), in an incubator at 37°C
with 5% CO2 and 98% relative humidity. The MCF-7 cells
were seeded in 6-well plates in routine cultivation. Cells in the
exponential growth phrase were used in the subsequent
experiments.
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT) assay
The MTT (Roche Diagnostics, Basel, Switzerland)
assay was performed as previously described (15). The MCF-7 cells (1×104
cells per well) were seeded in triplicate on a 96-well plate and
cultured overnight prior to treatment with 25, 50 and 100 µM DHA or
EPA for 24 or 72 h. Following incubation, MTT dye was added and the
mixture was incubated for 4 h at 37°C. The supernatant was removed
and 150 µl DMSO was added to each well to dissolve the crystals
completely, following which an ELISA reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) was used to determine the absorbance at
490 nm. The results are expressed as the percentage of inhibition,
which led to a reduction in absorbance by DHA, compared with that
in the control group.
Analysis of apoptosis using flow
cytometry
An Annexin V-FITC apoptosis detection kit was used
for the analysis of apoptosis (Invitrogen; Thermo Fisher
Scientific, Inc.). DHA concentrations of 25, 50 and 100 µM were
used to treat the MCF-7 cells (1×106 cells/ml),
respectively, for 48 h at 37°C. The cells were digested in trypsin,
washed twice with PBS and resuspended in 500 µl binding buffer. The
cell suspensions were then treated with 5 µl of Annexin V-FITC and
5 µl propidium iodide (PI). Following treatment for 10 min at room
temperature, the apoptosis of the cells were determined immediately
using flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Analysis of proteins using western
blot analysis
The MCF-7 cells were grown on 6-well plates
(1×106 cells/ml), to which 25, 50 and 100 µM DHA were
added and incubated for 48 h. The cells were lysed in 0.5 ml lysis
buffer (PBS containing 1% Triton X-100 and 1 mM PMSF) at 4°C for 10
min. The concentrations of protein were then measured using a BCA
assay. For western blot analysis, cell lysates (5 µg) from the
cancer cell culture were subjected to 12% SDS-PAGE, transferred to
polyvinylidene difluoride membranes, and blocked with 5% nonfat
milk in TBS-Tween buffer (TBST; 20-mM Tris-HCl, 120 mMNaCl, 0.1%
Tween-20) for 1 has described previously (16). Membranes were incubated with
primary antibodies against cleaved caspase-3, -8 −9 (dilution,
1:500), Bcl-2, Bax, Fas, FasL (dilution, 1:1,000), Smac (dilution,
1:400), DR4 and DR5 (dilution, 1:200) at 4°C overnight. Following 3
washes (5 min each) with TBST, the membranes were incubated with an
appropriate secondary antibody for 2 h at room temperature.
Membranes were washed 3 times (5 min each) with TBST, and protein
expression was quantified using a Gel EDAS analysis system (Cold
Spring USA Corporation, Cherry Hill, NJ, USA) and Gel-Pro Analyzer
3.1 software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Statistical analyses were performed using one-way
analysis of variance with SPSS 11.0 software (SPSS, Inc., Chicago,
IL, USA). Comparison between groups was performed using Duncan's
test. All data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Inhibitory effect of DHA on the growth
of breast cancer cells
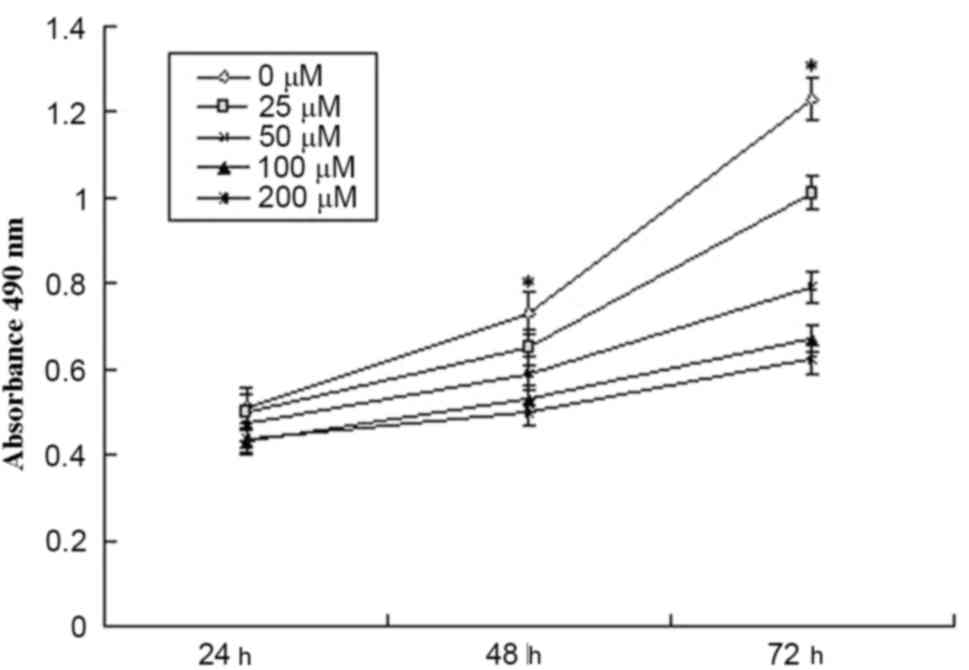
The effect of DHA on the viability of MCF-7 cells
was initially determined using an MTT assay. As shown in Fig. 1, DHA treatment decreased the
viability of the MCF-7 cells (P<0.05). Following treatment with
100 µM DHA for 72 h, the proliferation of the MCF-7 cells was
inhibited by 45.5%.
DHA induces apoptosis of breast cancer
cells
The apoptosis of cells was estimated using flow
cytometry. The apoptotic rates were higher in the DHA-treated
cells, compared with that in the control (Fig. 2). Following treatment with DHA (25,
50 and 100 µM) for 48 h, the proportions of early apoptotic MCF-7
cells were 26.7, 38.1 and 50.6%, respectively (P<0.05).
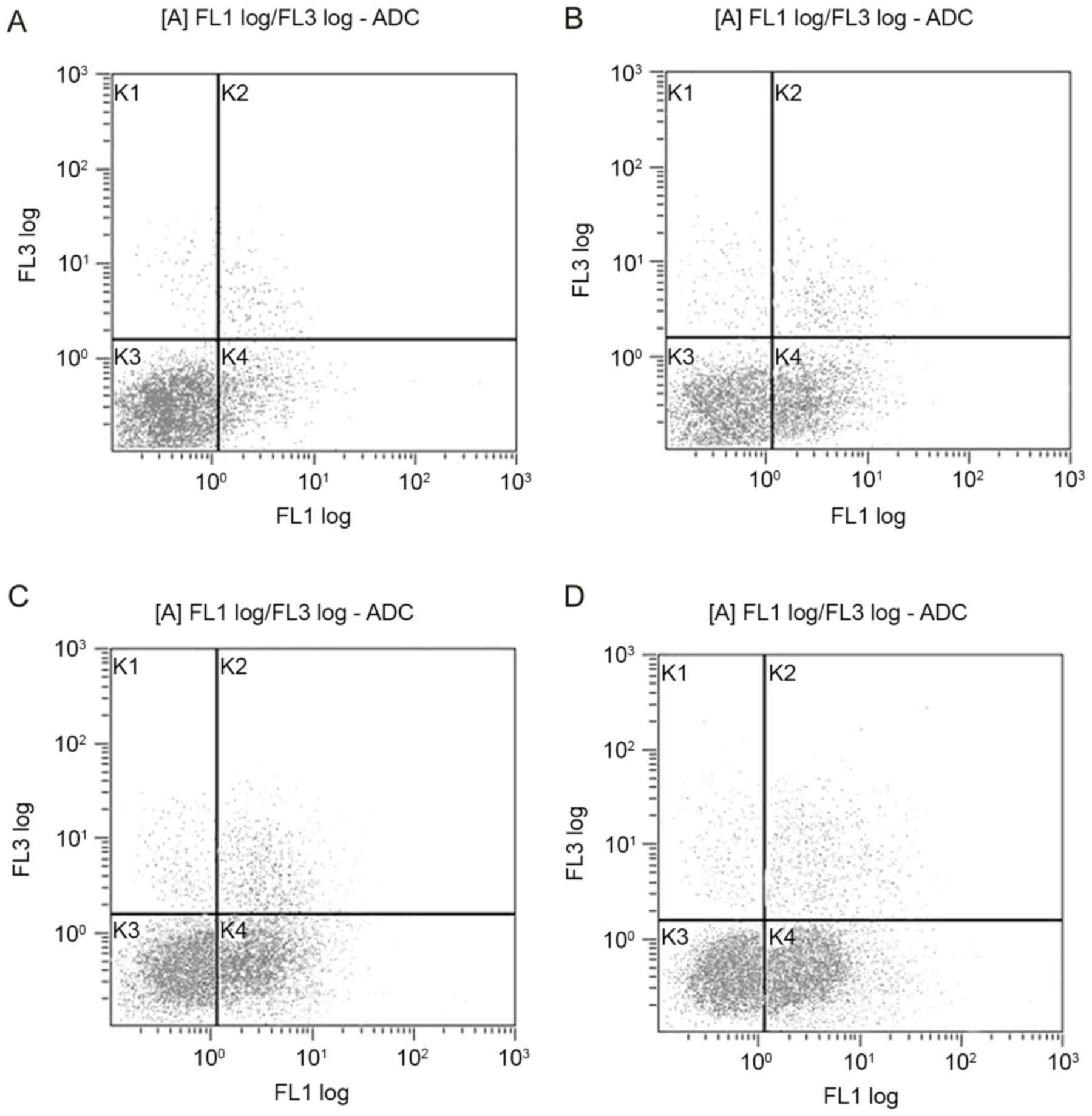 | Figure 2.Flow cytometric analysis of the
apoptosis of MCF-7 cells treated with DHA for 48 h. (A) Control;
(B) 25 µM DHA; (C) 50 µM DHA; (D) 100 µM DHA. MCF-7 cells were
stained with Annexin V-FITC and propidium iodide. Following
treatment with DHA, the early apoptotic rates of the MCF-7 cells
were 26.7, 38.1 and 50.6% respectively, and were significantly
increased, compared with that in the untreated cells (P<0.05).
The gated quadrants were as follows: K1, Annexin V-/PI+, necrosis;
K2, Annexin V+/PI+, late apoptosis and necrosis; K3, Annexin
V-/PI-, normal cells without apoptosis or necrosis; K4, Annexin
V+/PI-, early apoptosis. DHA, docosahexaenoic acid. |
Effect of DHA on the expression of the
Bcl-2 family proteins
The increased expression of Bax is reported as an
apoptosis-signaling pathway in breast cancer cells and proteins of
the Bcl-2 family are important factors in apoptosis regulation
(17–19). In the present study, the expression
levels of Bcl-2 and Bax were examined in MCF-7 cells following
treatment with DHA. The expression of Bcl-2 was reduced and the
expression of Bax was increased following DHA treatment. Therefore,
the ratio of Bcl-2/Bax was decreased (Fig. 3).
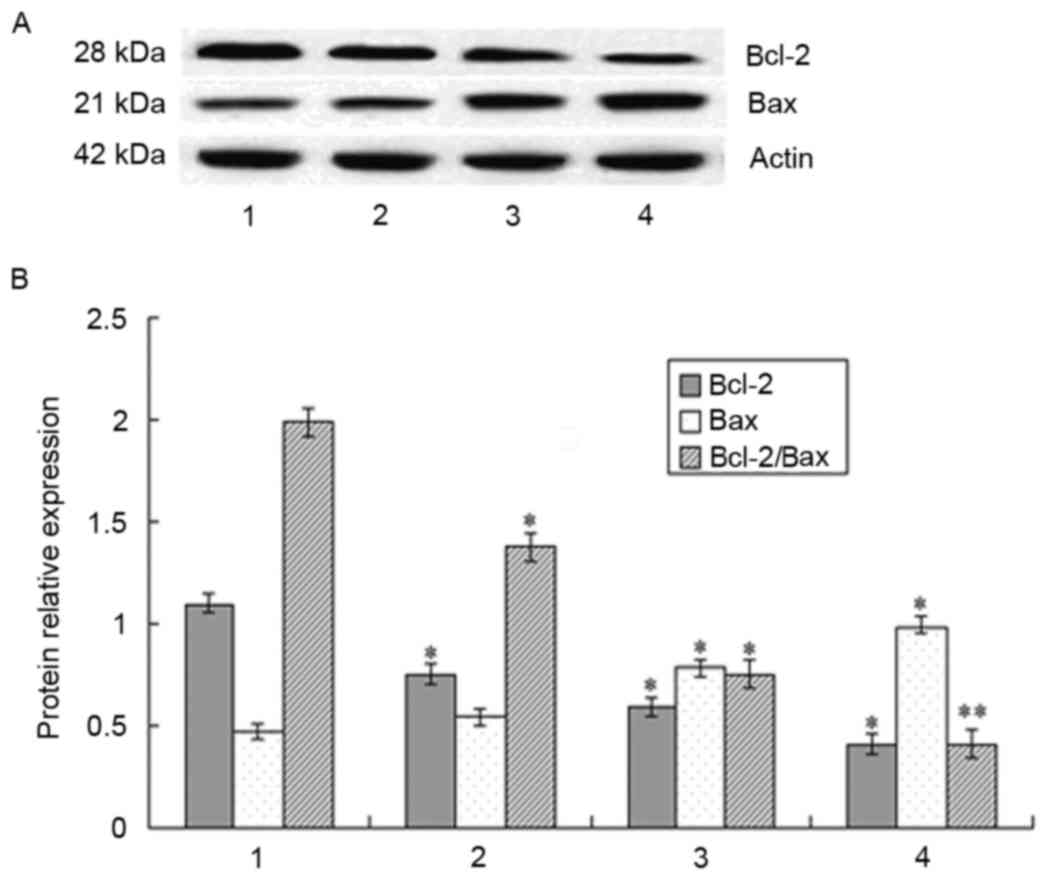 | Figure 3.Expression of Bcl-2 and Bax in MCF-7
cells following treatment with DHA for 48 h, detected using western
blot analysis. (A) Protein levels of Bcl-2 and Bax were measured
using western blot analysis. Expression of β-actin was used as the
internal control. (B) Relative expression levels of Bcl-2 and Bax
and the expression ratio of the two are shown. DHA reduced the
level of Bcl-2, increased the level of Bax and reduced the
Bcl-2/Bax ratio. Lane 1, control cells; lanes 2–4, MCF-7 cells
treated with 50, 100 and 200 µM DHA, respectively. *P<0.05 and
**P<0.01, vs. control. Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; DHA, docosahexaenoic acid. |
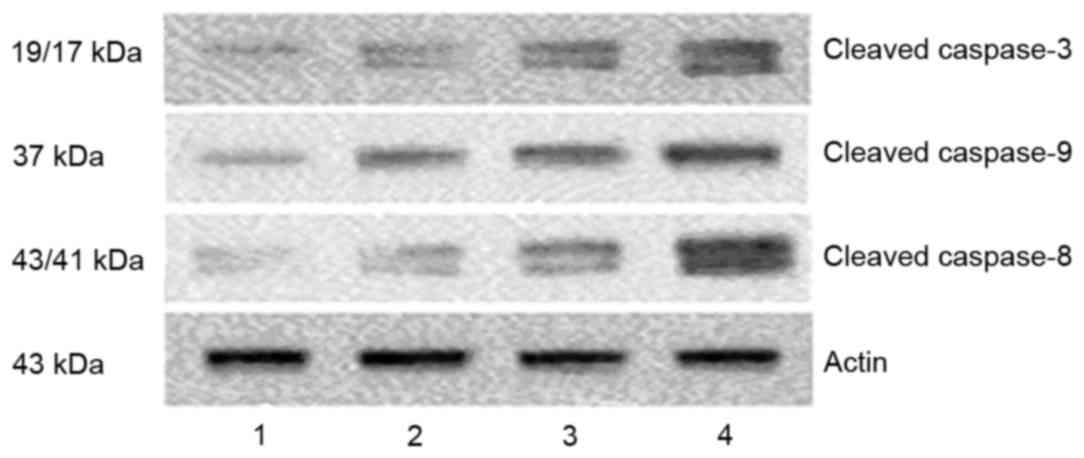
DHA increases the activation of
caspases
Caspases are central effectors in apoptosis. In
order to identify the mechanisms responsible for DHA-induced
apoptosis, the present study detected the levels of activated
caspases using western blot analysis to identify the mechanism
underlying the induction of apoptosis by DHA. Following treatment
of the cells with DHA, the levels of cleavedcaspases-8, -9 and -3
were increased (Fig. 4).
DHA induces the mitochondria to
release cytochrome c and Smac/Diablo
In apoptosis, mitochondria release cytosolic
cytochrome c and Smac/Diablo, which then activate caspase-9
(20). As DHA increased the
activation of caspase-9, the levels of cytochrome c and
Smac/Diablo in the cytoplasm were determined in the present study.
As shown in Fig. 5, DHA treatment
markedly increased the levels of cytoplasmic cytochrome c
and Smac/Diablo. This suggested that DHA induced the mitochondria
to release cytochrome c and Smac/Diablo.
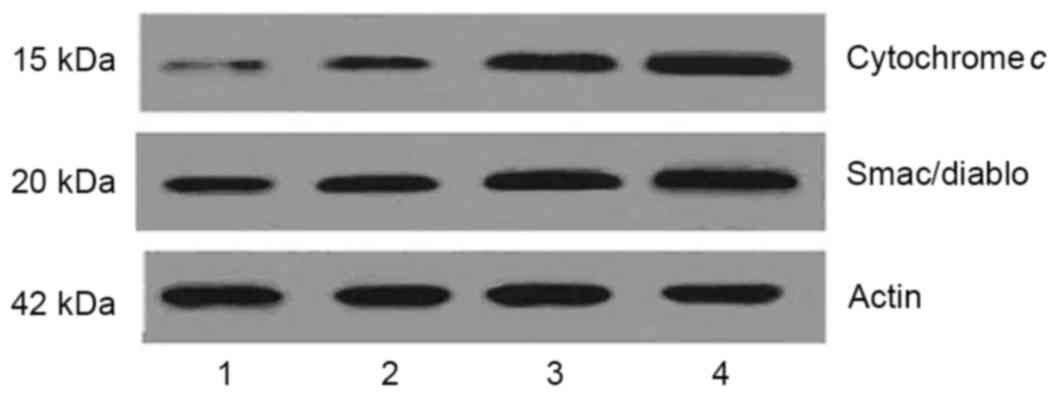 | Figure 5.DHA increases the release of
cytochrome c and Smac/Diablo from the mitochondria in MCF-7
cells. Cells were treated with 50, 100 and 200 µM DHA,
respectively, for 48 h, then treated with subcellular
fractionation. The levels of cytochrome c and Smac/Diablo in
the resultant cytosolic fractions were detected using western blot
analysis. Following treatment with DHA, the levels of cytochrome
c and Smac/Diablo in the cytoplasm were increased. Lane 1,
control cells; lanes 2–4, MCF-7 cells treated with 50, 100 and 200
µM DHA, respectively. DHA, docosahexaenoic acid. |
DHA increases the expression of death
receptors
Caspase-8 is involved in the death receptor-mediated
apoptotic pathway. In the present study, DHA treatment increased
the level of activated caspase-8; therefore, the levels of death
receptors and membrane-bound death receptor ligands were
determined. Following DHA intervention, the expression levels of
Fas, DR4, and TRAIL were increased, whereas no significant changes
in the expression levels of FasL or DR5were observed (Fig. 6).
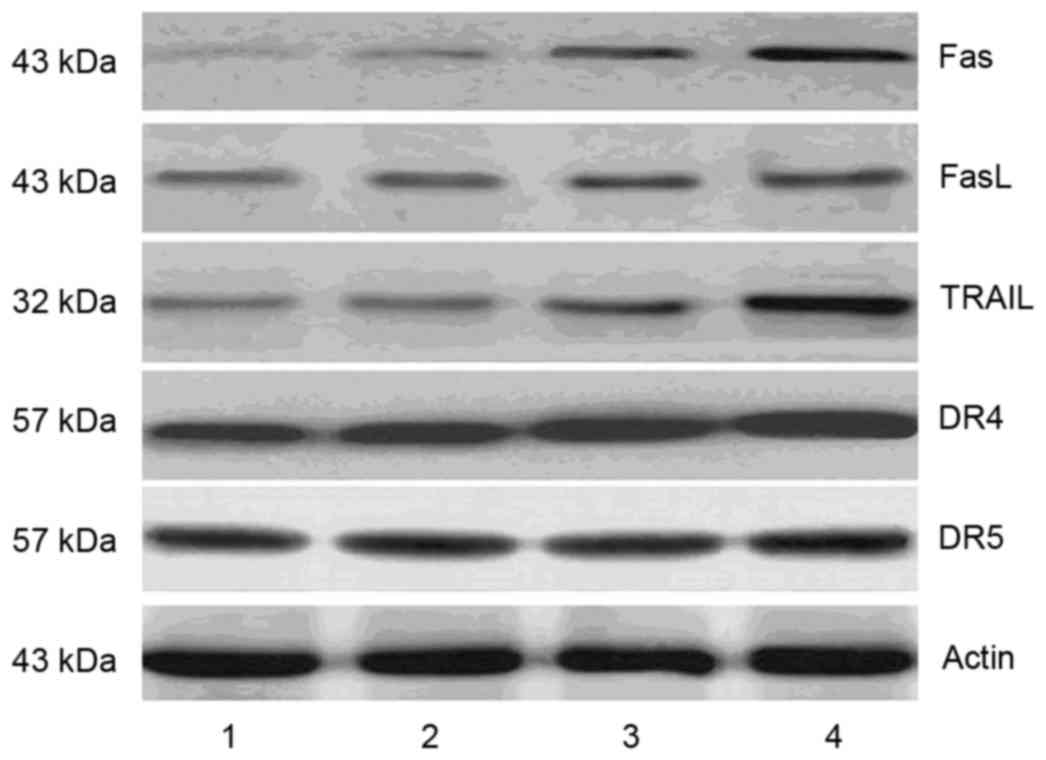 | Figure 6.DHA increases the expression of cell
death receptors and their ligands in MCF-7 cells. Following
treatment with different concentrations of DHA, the protein levels
of Fas, FasL, DR4, DR5 and TRAIL were measured using western blot
analysis. The results showed that DHA treatment increased the
expression levels of Fas, DR4, and TRAIL, whereas the expression
levels of FasL and DR5 remained unchanged. Lane 1, control cells;
lanes 2–4, MCF-7 cells treated with 50, 100 and 200 µM DHA,
respectively. DHA, docosahexaenoic acid; FasL, Fas ligand; DR,
death receptor; TRAIL, tumor necrosis factor-related
apoptosis-inducing ligand. |
Discussion
In the present study, it was found that DHA, an
n-3FA, resulted in the inhibition of cell viability and the
induction of cell apoptosis. These results confirmed and extended
earlier observations (21–23) on the antitumor effect of n-3
FAs.
The results of previous studies revealed that the
antitumor effect of DHA is due to the induction of apoptosis
(21–23). There are also reports showing that
EPA treatment of HL-60 cells induces the activation of caspase-3,
-6, -8 and -9, and the release of cytochrome c from
mitochondria (24). In the present
study, the results of the flow cytometry confirmed that DHA
treatment induced apoptosis of the breast cancer cells.
The exact role of n-3 FAs in the development,
progression and prevention of breast cancer remains to be fully
elucidated. The n-3 FAs are involved in the development and
progression of tumors via multiple mechanisms. Several signaling
pathways are associated with carcinogenesis and tumor progression
by n-3 polyunsaturated FAs. For example, n-3FAscan downregulate and
inactivate cellular signaling mediators, including protein kinase
C, ras, ERK1/2, and NF-κB (11–14).
In addition, n-3 FAs can affect prostate inflammation and
carcinogenesis via the cyclooxygenase (COX)-2 enzymatic pathway
(25). Kang et al (26) revealed that apoptosis was induced
by DHA treatment in MCF-7 cells, and that DHA promoted the
formation of reactive oxygen species (ROS) and activation of
caspase-8. Previous studies have also suggested that DHA can
improve the prognosis of patients with breast cancer following
chemotherapy through increasing the formation of ROS (27). Dimri et al (28) found that dietary n-3 FAs suppressed
the expression of enhancer of zeste homologue 2 (EZH2), and
downregulated the expression of E-cadherin and insulin-like growth
factor binding protein 3, which are targets of EZH2 in breast
cancer cells. The other possible mechanism was associated with the
inhibitory effect of n-3 FAs on the expression of COX, the p21 gene
and the p53 gene (29). DHA and
EPA have also been reported to downregulate cell surface expression
of C-X-C chemokine receptor type 4 (CXCR4) and markedly decrease
CXCR4-mediated cell migration in breast cells in vitro
(30). The exact molecular
mechanism underlying this signaling remains to be fully elucidated.
Previously, it was reported that the proteins of the Bcl-2 family
may be important in n-3 FA-induced cell death. In the present
study, data revealed that DHA at concentrations of 25–100 µM
increased the expression of Bax but reduced the expression of
Bcl-2, increased the release of cytochrome c and Smac/Diablo
from mitochondria, promoted the activation of caspases, and
increased the levels of Fas, DR4 and TRAIL in MCF-7 breast cancer
cells. These results suggested that DHA activated caspases and
induced apoptosis through the mitochondria-mediated and death
receptor-mediated apoptotic pathways.
The results of the present study indicated that
further investigations are required to determine whether the
supplementation of dietary n-3 FAs has an inhibitory effect on
tumor growth in patients with breast cancer.
Acknowledgements
The present study was completed with the support of
the Youth Science Fund Project of the Natural Science Foundation of
China (grant no. 81502298), the Shandong Provincial Natural Science
Foundation (grant nos. ZR2014JL056 and ZR2010CM010), the
Development Project of Shandong Province Medical Science and
Technology (2013WS0262), Qingdao Postdoctoral Application Research
Project (grant no. 2015165), the Ministry of Education Key
Laboratory Open Foundation of Marine Culture of China Ocean
University (grant no. 2009001), and the Qingdao University Medical
College Young Teachers Cultivation Project (grant no.
600201304).
References
|
1
|
Umemoto N, Ishii H, Kamoi D, Aoyama T,
Sakakibara T, Takahashi H, Tanaka A, Yasuda Y, Suzuki S, Matsubara
T and Murohara T: Reverse association of omega-3/omega-6
polyunsaturated fatty acids ratios with carotid atherosclerosis in
patients on hemodialysis. Atherosclerosis. 249:65–69. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molfino A, Gioia G, Fanelli Rossi F and
Muscaritoli M: The role for dietary omega-3 fatty acids
supplementation in older adults. Nutrients. 6:4058–4073. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gomez-Candela C, Puchalt Roldan MC, Palma
Milla S, Lopez Plaza B and Bermejo L: The role of omega-3 fatty
acids in diets. J Am Coll Nutr. 34:(Suppl 1). S42–S47. 2015.
View Article : Google Scholar
|
|
4
|
Bartram HP, Gostner A, Scheppach W, Reddy
BS, Rao CV, Dusel G, Richter F, Richter A and Kasper H: Effects of
fish oil on rectal cell proliferation, mucosal fatty acids, and
prostaglandin E2 release in healthy subjects. Gastroenterology.
105:1317–1322. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J and Ma DW: The role of n-3
polyunsaturated fatty acids in the prevention and treatment of
breast cancer. Nutrients. 6:5184–5223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laviano A, Rianda S, Molfino A and Fanelli
FR: Omega-3 fatty acids in cancer. Curr Opin Clin Nutr Metab Care.
16:156–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang F and Chen Y, Long J, Dong L, Wang Y
and Chen Y: Effect of n-3 and n-6 polyunsaturated fatty acids on
lipid metabolic genes and estrogen receptor expression in MCF-7
breast cancer cells. Clin Lab. 61:397–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xue M, Wang Q, Zhao J, Dong L, Ge Y, Hou
L, Liu Y and Zheng Z: Docosahexaenoic acid inhibited the
Wnt/β-catenin pathway and suppressed breast cancer cells in vitro
and in vivo. J Nutr Biochem. 25:104–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Larsson SC, Kumlin M, Ingelman-Sundberg M
and Wolk A: Dietary long-chain n-3 fatty acids for the prevention
of cancer: A review of potential mechanisms. Am J Clin Nutr.
79:935–945. 2004.PubMed/NCBI
|
|
10
|
Chapkin RS, McMurray DN and Lupton JR:
Colon cancer, fatty acids and anti-inflammatory compounds. Curr
Opin Gastroenterol. 23:48–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCarty MF: Fish oil may impede tumour
angiogenesis and invasiveness by down-regulating protein kinase C
and modulating eicosanoid production. Med Hypotheses. 46:107–115.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collett ED, Davidson LA, Fan YY, Lupton JR
and Chapkin RS: N-6 and n-3 polyunsaturated fatty acids
differentially modulate oncogenic Ras activation in colonocytes. Am
J Physiol Cell Physiol. 280:C1066–C1075. 2001.PubMed/NCBI
|
|
13
|
Sauer LA, Blask DE and Dauchy RT: Dietary
factors and growth and metabolism in experimental tumors. J Nutr
Biochem. 18:637–649. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cavazos DA, Price RS, Apte SS and
deGraffenried LA: Docosahexaenoic acid selectively induces human
prostate cancer cell sensitivity to oxidative stress through
modulation of NF-κB. Prostate. 71:1420–1428. 2001. View Article : Google Scholar
|
|
15
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: Assessment of radiosensitivity. Cancer Res.
47:943–946. 1987.PubMed/NCBI
|
|
16
|
Cho HJ, Kim WK, Kim EJ, Jung KC, Park S,
Lee HS, Tyner AL and Park JH: Conjugated linoleic acid inhibits
cell proliferation and ErbB3 signaling in HT-29 human colon cell
line. Am J Physiol Gastrointest Liver Physiol. 284:G996–G1005.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choudhuri T, Pal S, Agwarwal ML, Das T and
Sa G: Curcumin induces apoptosis in human breast cancer cells
through p53-dependent Bax induction. FEBS Lett. 512:334–340. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bruey JM, Bruey-Sedano N, Luciano F, Zhai
D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, et
al: Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation
by interaction with NALP1. Cell. 129:45–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho MY, Park SY, Park S, Lee YR, Han GD
and Kim JA: Geranyl derivative of phloroacetophenone induces cancer
cell-specific apoptosis through Bax-mediated mitochondrial pathway
in MCF-7 human breast cancer cells. Biol Pharm Bull. 35:98–104.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c
and dATP-dependent formation of Apaf-1/caspase-9 complex initiates
an apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun H, Berquin IM, Owens RT, O'Flaherty JT
and Edwards IJ: Peroxisome proliferator-activated receptor
gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids
promotes apoptosis of human breast cancer cells. Cancer Res.
68:2912–2919. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sauer LA, Dauchy RT, Blask DE, Krause JA,
Davidson LK and Dauchy EM: Eicosapentaenoic acid suppresses cell
proliferation in MCF-7 human breast cancer xenografts in nude rats
via a pertussis toxin-sensitive signal transduction pathway. J
Nutr. 135:2124–2129. 2005.PubMed/NCBI
|
|
23
|
Siddiqui RA, Jenski LJ, Neff K, Harvey K,
Kovacs RJ and Stillwell W: Docosahexanoic acid induces apoptosis in
Jurkat cells by a protein phosphatase-mediated process. Biochim
Biophys Acta. 1499:265–275. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arita K, Yamamoto Y, Takehara Y, Utsumi T,
Kanno T, Miyaguchi C, Akiyama J, Yoshioka T and Utsumi K:
Mechanisms of enhanced apoptosis in HL-60 cells by UV-irradiated
n-3 and n-6 polyunsaturated fatty acids. Free Radic Biol Med.
35:189–199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reese AC, Fradet V and Witte JS: Omega-3
fatty acids, genetic variants in COX-2 and prostate cancer. J
Nutrigenet Nutrigenomics. 2:149–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang KS, Wang P, Yamabe N, Fukui M, Jay T
and Zhu BT: Docosahexaenoic acid induces apoptosis in MCF-7 cells
in vitro and in vivo via reactive oxygen species formation and
caspase 8 activation. PLoS One. 5:e102962010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bougnoux P, Hajjaji N, Ferrasson MN,
Giraudeau B, Couet C and Le Floch O: Improving outcome of
chemotherapy of metastatic breast cancer by docosahexaenoic acid: A
phase II trial. Br J Cancer. 101:1978–1985. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dimri M, Bommi PV, Sahasrabuddhe AA,
Khandekar JD and Dimri GP: Dietary omega-3 polyunsaturated fatty
acids suppress expression of EZH2 in breast cancer cells.
Carcinogenesis. 31:489–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rose DP and Connolly JM: Regulation of
tumor angiogenesis by dietary fatty acids and eicosanoids. Nutr
Cancer. 37:119–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Altenburg JD and Siddiqui RA: Omega-3
polyunsaturated fatty acids down-modulate CXCR4 expression and
function in MDA-MB-231 breast cancer cells. Mol Cancer Res.
7:1013–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|