Introduction
Macrophage colony-stimulating factor (M-CSF), or
colony-stimulating factor-1 (CSF-1), is a member of the
haematopoietic growth factor family (1), and is a primary cytokine regulating
the proliferation, survival and differentiation of macrophages via
interaction with the tyrosine kinase transmembrane M-CSF receptor
(M-CSFR). It has previously been demonstrated that M-CSF affects
various types of cells, including reproductive cells. In 1997, Witt
and Pollard (2) first reported
that the concentration of M-CSF was significantly greater in
follicular fluid (FF), and the M-CSFR mRNA was expressed in
FF-derived cells. To define the role of M-CSF regulated macrophages
in reproduction, Cohen et al (3) studied mice homozygous for a null
mutation [Csfm (op)] and observed that female Csfm (op)/Csfm (op)
mice had fertility defects, including extended oestrous cycles and
poor ovulation rates. Nishimura et al (4) observed that gonadotropins led to a
gradual increase in the serum concentration of M-CSF throughout
ovarian stimulation and that high concentrations of M-CSF were
associated with successful oocyte retrieval. Salmassi et al
(5) revealed that the serum
concentration of M-CSF increased with the growth of follicles and
that a greater serum concentration of M-CSF was associated with
increased in vitro fertilization (IVF) success. Takasaki
et al (6) reported that
during a controlled ovarian hyperstimulation cycle, follicular
development was improved by concomitant administration of M-CSF and
human menopausal gonadotrophin (hMG). All of these findings
suggested that M-CSF is involved in the process of follicular
development and ovulation.
Granulosa cells (GCs) lining the vesicular ovarian
follicle are the largest cell mass in the follicle and are
important in the development and maturation of oocytes, in addition
to their role in the regulation of the ovarian cycle via the
production and secretion of various sex hormones and cytokines. Our
previous study demonstrated that M-CSF secretion of GCs was
enhanced by follicle-stimulating hormone (FSH) and estradiol
(E2) in vitro in a dose-dependent manner;
however, was unaffected by progesterone (P) (7). Conversely, M-CSF elicited the
production of E2 and P by GCs in a dose-dependent
manner, in the presence or absence of FSH (8).
One of the important signaling pathways in cells is
the mitogen activated protein kinase (MAPK) pathway, which involves
extracellular-regulated protein kinase (ERK1/2), c-Jun N-terminal
protein kinase (JNK) and p38 kinase (p38). A previous study
revealed that M-CSF induced vascular endothelial growth factor
(VEGF) in vivo via activation of the MAPK/ERK signaling
pathway, and that inhibition of ERK suppressed M-CSF-induced VEGF
at the mRNA and protein levels (9). A further study revealed that M-CSF
increased scavenger receptor-A expression and function in
macrophages, which requires the specific activation of p38 MAPK;
however, not ERK1/2 or JNK. Further studies are required in order
to elucidate if M-CSF activates GCs via the MAPK signaling pathway
(10).
The present study evaluated the interaction of M-CSF
with FSH during follicular development prior to ovulation by using
human luteinized granulosa cells, and the signaling pathway of
M-CSF was analyzed using the COV434 primary human granulosa cell
tumor cell line.
Materials and methods
Patients
A total of 30 infertile women who had tubal
(excluding hydrosalpinges) or male factor infertility from the
Women's Hospital School Of Medicine, Zhejiang University (Hangzhou,
China) took part in the study. The ethics committee of the Women's
Hospital School Of Medicine, Zhejiang University, approved the
study. Written informed consent was obtained from each study
participant. Patients with endometriosis or polycystic ovary
syndrome were excluded from this study. The age of the patients
ranged from 26 to 33 years (median, 30.8 years). The patients
undergoing intracytoplasmic sperm injection were stimulated with
recombinant human (rh) FSH (EMD Serono; Merck KGaA, Darmstadt,
Germany) with an initial dose of 75–300 U/d, administered by
intramuscular injection (im), followed by downregulation with 1.25
mg injected Diphereline, a gonadotropin-releasing hormone agonist
(Ipsen Pharma Biotech, Signes, France), in the mid luteal phase.
Follicles were aspirated 36 h after 5,000–10,000 IU im of human
chorionic gonadotropin (Shanghai Livzon Pharmaceutical Co., Ltd.,
Shanghai, China), and FF was obtained from oocyte-bearing
follicles.
GC collection and culture
GCs were isolated from the FF of each patient. All
samples were obtained with the informed consent of the patients.
The experiments were performed 3 times, and during each experiment,
follicles were collected from 10 individual patients. The
follicular fluids, which were obtained at the time of oocyte
retrieval, were immediately centrifuged at 1,000 × g for 10 min at
room temperature and the supernatants were removed. The top layer
of white cells was withdrawn and the cells were dispersed with type
I collagenase (0.1%; Sigma-Aldrich, Merck KGaA) for 30 min in a
water bath at 37°C. The cell suspension was subsequently overlaid
slowly and carefully on isopyknic 50% Percoll (GE Healthcare Life
Sciences, Uppsala, Sweden) and centrifuged at 2,000 × g for 10 min
at room temperature. The cells were aspirated from the Percoll
interphase, pooled and washed twice with PBS. The viable cells were
determined by the trypan blue exclusion method (11). The cell density was adjusted to
2×105 cells/ml with medium. The 1 ml GC suspensions were
inoculated in triplicate into 6-well culture plates. Finally, the
cells were cultured in Dulbecco's modified Eagle's medium
(DMEM)/F12 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Invitrogen; Thermo Fisher Scientific, Inc.) and incubated in a
humidified incubator at 37°C with 95% air and 5% CO2.
Following incubation for 24 h, the medium in each well was
replaced. The cells were subsequently cultured with DMEM/F12 medium
plus various concentrations of rhM-CSF (0, 10, 25, 50 or 100 ng/ml;
R&D Systems, Inc., Minneapolis, MN, USA), rhFSH (0, 10, 25, 50
or 100 IU/ml; R&D Systems, Inc.), rhM-CSF plus Letrozole
(10−6 mol/l; Tianjin Meilun Medical Products Group, Co.,
Ltd., Tianjin, China) or rhFSH plus Letrozole (10−6
mol/l). The media was collected following a 24 h incubatory
period.
E2 assay
The E2 concentration of the supernatant
was measured using an estradiol ELISA test kit (CEA461Ge;
Cloud-Clone Corp. Houston, TX, USA). The testing procedures were
performed according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The GC cells were lysed in 1 ml RNAiso Plus (Takara
Biotechnology Co., Ltd., Dalian, China) and the RNA was extracted
according to the manufacturer's protocol. A total of 1 µg total RNA
was reverse-transcribed using the PrimeScript™ RT-PCR
kit (Takara Biotechnology Co., Ltd.). The synthesized cDNA was
amplified via RT-qPCR with SYBR Premix Ex Taq™ (Perfect
Real Time; Takara Biotechnology Co., Ltd.). The oligonucleotide
primer sequences were as follows: Forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′ for β-actin; forward,
5′-CATCATCGGATCTGTCACTGCTCTA-3′ and reverse,
5′-CTCGAAGCTTGGTGAGGACAAAC-3′ for FSH receptor; forward,
5′-CCAAGTTCATTCAGAGCCAGGACTA-3′ and reverse,
5′-TCTGCAGGCACCAGTGTCAAG-3′ for M-CSF receptor. PCR cycling was
performed with the 7500 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific. Inc.). The PCR amplification started at
95°C for 2 min and was followed by 39 cycles at 95°C for 10 sec and
60°C for 30 sec. The comparative parameter threshold cycle (Cq)
normalized to β-actin was used to quantify the mRNA expression
level (12).
Measurement of the proliferation of
COV434 cells
COV434 cells are immortalized GCs derived from human
granulosa cell tumors. They possess numerous characteristics of
normal GCs. COV434 cells were purchased from Beinachuanglian
Biological Technology Co. (Beijing, China). They were cultured in
DMEM/F12 (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS and incubated at 37°C in a humidified incubator with
95% air and 5% CO2.
To assess cell proliferation, COV434 cells in the
exponential growth phase were diluted to 1×105/ml and
seeded into a 96-well plate (2×103 cells/well).
Following 24 h, the medium was replaced, and the cells were
incubated with fresh medium containing rhM-CSF (0, 10, 25, 50 or
100 ng/ml; n=6 per treatment) for a further 24 h. Following this,
20 µl CellTiter 96 AQueousOne Solution (Promega Corporation,
Madison, WI, USA) was added to each well for 4 h at 37°C. The
absorbance was measured at a wavelength of 490 nm using a
microplate reader (SP-Max 1800 L, Shanghai China).
Western blot analysis
Total protein of the cells in each group was
extracted using radioimmunoprecipitation assay lysis buffer (Wuhan
Boster Biological Technology, Ltd., Wuhan, China), and the protein
concentrations were quantified using a Bicinchoninic Acid Protein
Assay kit (Applygen Technologies, Inc., Beijing, China). Total
proteins (50 µg) were separated by 10% SDS-PAGE and electrically
transferred to a polyvinylidene difluoride membrane (EMD Millipore,
Billerica, MA, USA). Following blocking of the membrane in 5%
non-fat milk at room temperature for 1 h, diluted primary
antibodies (mouse anti-human p-JNK (1:1,000; 9255s), rabbit
anti-human p-ERK1/2 (1:1,000; 4370s) and anti-phospho-p38
monoclonal antibody (1:1,000; 4511s) (all from CST Biological
Reagents Company Limited- Shanghai, China) were added, and the
membrane was incubated at 4°C overnight. Subsequently, horseradish
peroxidase (HRP)-conjugated secondary antibody [Goat anti-mouse
(1:5,000; BA1050) or sheep anti-rabbit (1:5,000; BA1054) (both from
Beyotime Institute of Biotechnology, Haimen, China)] was added to
the membrane and incubated at room temperature for 45 min. Blots
were visualized with Enhanced Chemiluminescence substrate
(Immobilon™ Western Chemiluminescent HRP Substrate; Merck KGaA).
The expression level of each protein was estimated using β-actin as
the internal reference.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(IBM SPSS, Armonk, NY, USA). Data were expressed as the mean ±
standard deviation of three independent experiments. Multiple
comparisons were performed by one-way analysis of variance and
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Production of E2 by GCs
increases with stimulation from FSH, M-CSF and decreases in
presence of oestrogen synthesis inhibitor Letrozole
Following the addition of various concentrations of
rhM-CSF (0, 10, 25, 50 or 100 ng/ml) or rhFSH (0, 10, 25, 50 or 100
IU/ml) to the cultured GCs, the E2 levels increased in a
dose-dependent manner (P<0.05; Tables I and II). The E2 levels following
rhM-CSF treatment ranged from a 1.33–2.02 fold increase and levels
following rhFSH treatment ranged from a 1.61–2.6 -fold increase,
compared with baseline. Following the combined treatments with the
oestrogen synthesis inhibitor Letrozole (10−6mmol/l),
the concentrations of E2 decreased, and there was no
significant difference between the two groups (Tables I and II).
 | Table I.Effect of M-CSF and Letrozole on the
production of E2 by GCs. |
Table I.
Effect of M-CSF and Letrozole on the
production of E2 by GCs.
|
| rhM-CSF (ng/ml) |
|---|
|
|
|
|---|
| Letrozole
(mol/l) | 0 | 10 | 25 | 50 | 100 |
|---|
|
| E2 (pg/ml) |
|
|
|
| 0 |
356.44±44.33 |
475.32±42.77* |
580.16±41.81a |
686.48±37.49a |
720.55±31.65a |
| 10–6 |
55.07±11.47 |
53.67±9.77 |
58.74±13.26 |
62.44±11.23 |
64.54±12.76 |
 | Table II.Effect of FSH and Letrozole on the
production of E2 by GCs. |
Table II.
Effect of FSH and Letrozole on the
production of E2 by GCs.
|
| rhFSH (IU/ml) |
|
|
|
| Letrozole
(mol/l) | 0 | 10 | 25 | 50 | 100 |
|---|
|
| E2 (pg/ml) |
|
|
|
| 0 |
443.42±34.36 |
715.29±21.92a |
815.82±55.33a |
963.32±74.06a |
1154.24±70.5a |
| 10–6 |
65.53±13.15 |
67.01±11.68 |
63.28±13.01 |
69.36±12.00 |
74.18±11.31 |
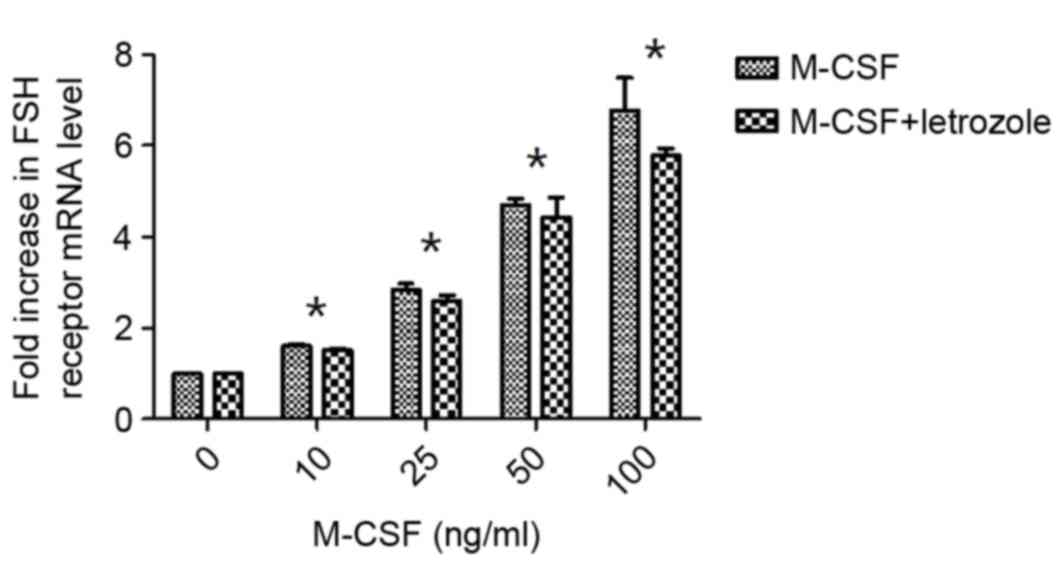
Effects of M-CSF on FSH receptor
expression differ following inhibition of E2 synthesis
by Letrozole
The expression levels of the FSH receptor were
demonstrated to increase with addition of M-CSF in a dose-dependent
manner, in the presence and absence of Letrozole. However, with or
without the addition of Letrozole, no significant differences were
observed following treatment with rhM-CSF. The expression of the
FSH receptor was slightly reduced when Letrozole was added to the
culture medium; however, there were no significant differences
compared with the absence of Letrozole (P>0.05). Increases in
FSH receptor production by M-CSF with or without Letrozole became
significant compared with the control at >10 ng/ml, (P<0.05;
Fig. 1).
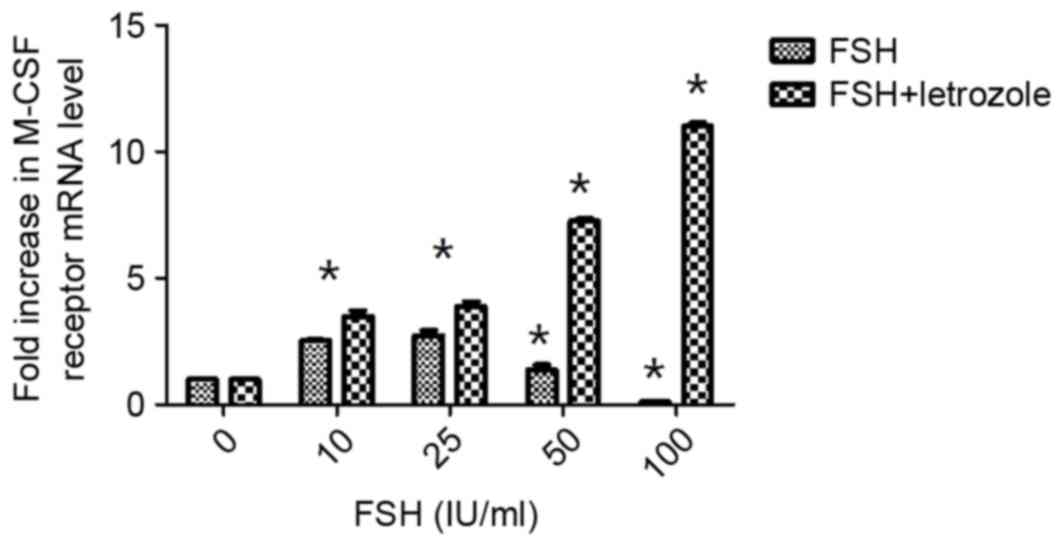
Effects of FSH on M-CSF receptor
expression following inhibition of E2 synthesis by
Letrozole
As presented in Fig.
2, when rhFSH was added to the culture medium at different
concentrations, M-CSFR mRNA expression levels increased. There were
significant differences in M-CSFR concentrations compared with the
control when the rhFSH concentration was 10 to 25 IU/ml
(P<0.05). However, this effect diminished with increasing
concentrations of rhFSH and even reversed at the greatest
concentration (Fig. 2). Therefore,
FSH may regulate the production of M-CSF in the follicular phase.
However, rhFSH administered in combination with Letrozole increased
M-CSFR expression levels in a dose-dependent manner.
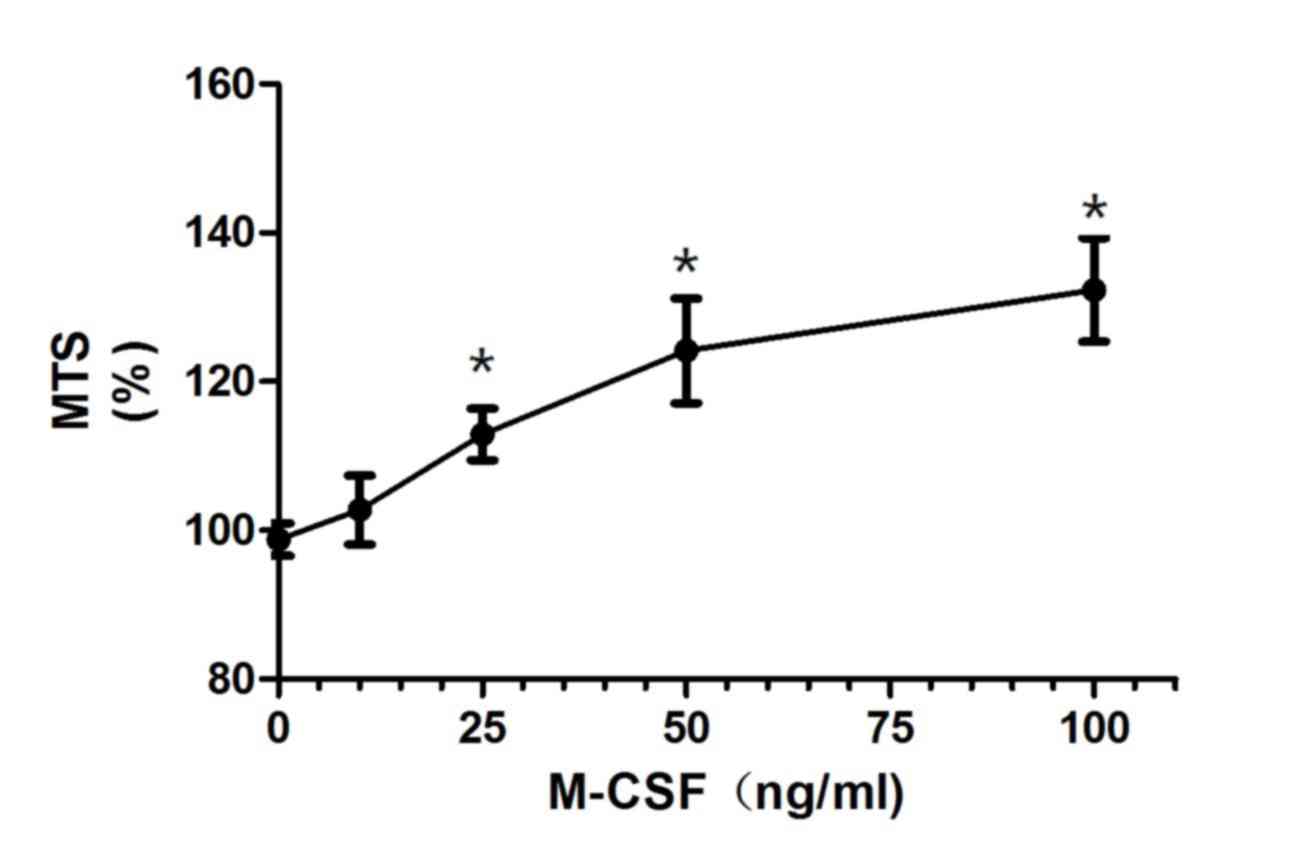
M-CSF promotes the proliferation of
granulosa cells
The COV434 cell line, which was established from a
primary human granulosa cell tumor, was used to test the effect of
M-CSF on the proliferation of granulosa cells. At 25 ng/ml rhM-CSF,
the proliferation of COV434 cells increased significantly compared
with the control (112.89%; P<0.05; Fig. 3). An increase in the rhM-CSF
concentration to 50 ng/ml resulted in the proliferation of
granulosa cells increasing to 124.14% and then to 132.32% at 100
ng/ml, which indicated that M-CSF promoted the proliferation of
granulosa cells.
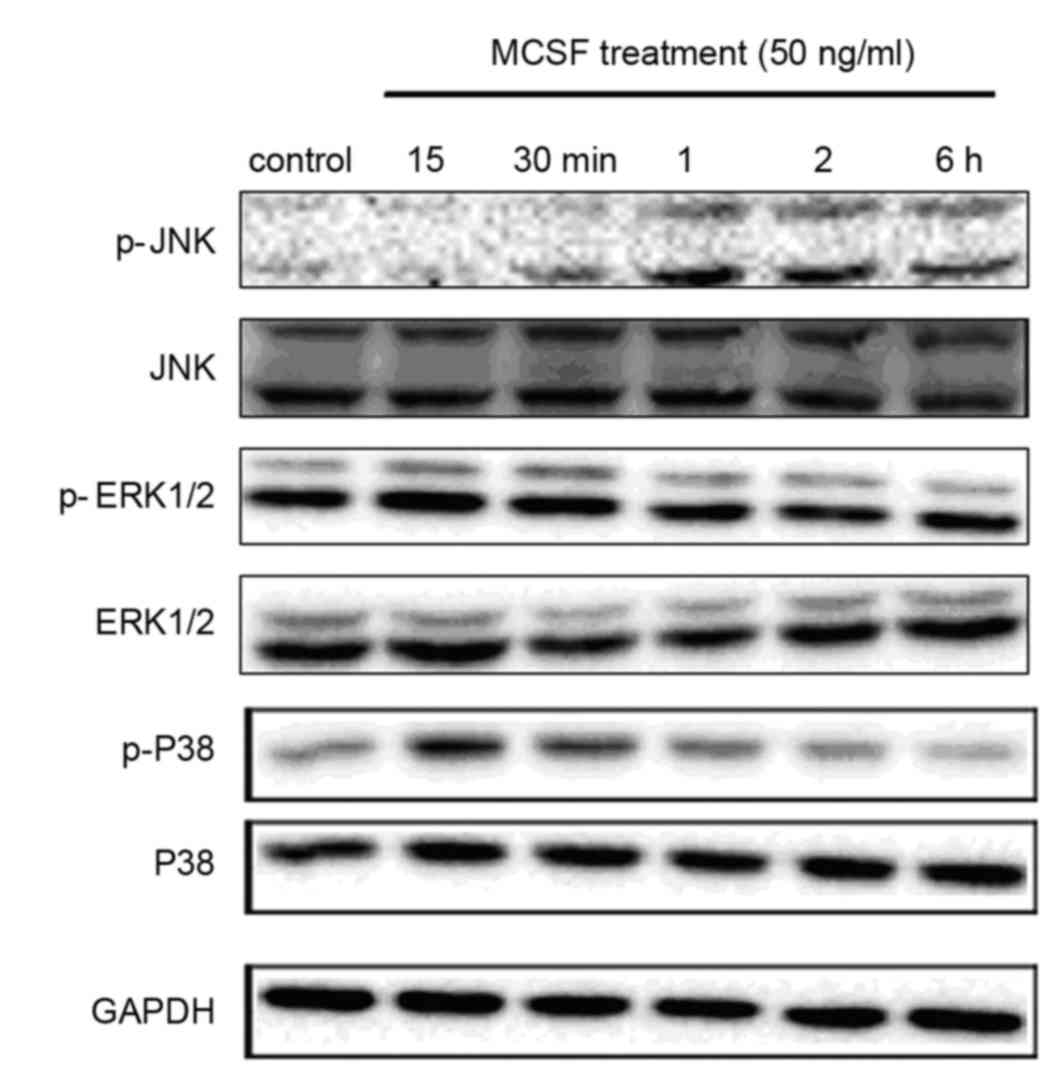
Activation of the biomarkers in the
MAPK signaling pathway following M-CSF exposure
The MAPK signaling pathways are important for cells
to respond to numerous extracellular signals. To investigate the
underlying mechanisms involved in the effect of M-CSF on GCs, the
present study examined the phosphorylation levels of various MAPK
family proteins (ERK, p38 and JNK) in COV434 cells via western
blotting. Cells were treated with 50 ng/ml rhM-CSF for 0, 15 or 30
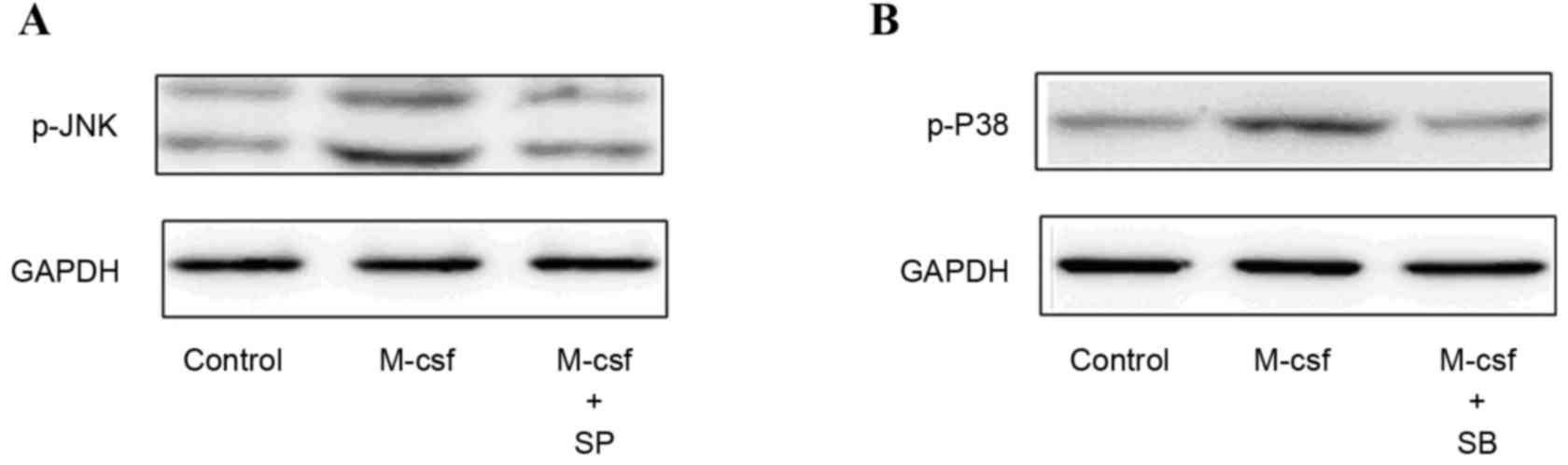
min, or 1, 2 or 6 h. Phosphorylation of the MAPKs p38 and JNK was
stimulated by rhM-CSF in COV434 cells in a time-dependent manner;
however, there was no marked activation of the ERK1/2 pathway.
Phosphorylation of JNK peaked at 1 h and that of p38 peaked at 15
min, then declined (Fig. 4).
Pretreating the cells with the p38 MAPK inhibitor SB203580 (10 µM)
or the JNK MAPK inhibitor SP600125 (20 µM) blocked the activation
of the corresponding MAPKs (Fig.
5). However, compared with the control group, the differences
in oestrogen levels in the control, M-CSF+SB and M-CSF+SP groups
were not statistically significant (418.7±60.2, 406.28±58.3 and
420.23±54.84 pg/ml, respectively; P>0.05).
Discussion
It is traditionally believed that ovarian functions
are primarily controlled by gonadotropins secreted from the
pituitary gland. This explanation has recently been challenged by
novel findings that suggest the products generated from the ovary
itself, including growth factors and cytokines, are able to
modulate follicular development (13). A growing body of evidence suggests
that the ovary is a site of inflammatory reaction (14). In addition, numerous cells in the
ovary produce cytokines independently of the presence of
leukocytes, thus ovaries are sites of cytokine action and
production. There is substantial evidence that cytokines are
involved in the ovarian control of follicular development, and they
are considered to be important regulators of steroidogenesis and
gamete production. The importance of cytokines in reproduction is
receiving increasing attention (15).
M-CSF is an important cytokine that may be produced
by a variety of cells. In addition to regulating proliferation
(16), differentiation and
migration of tissue macrophages, M-CSF is important for the
maintenance of ovarian function and the promotion of sex hormone
secretion (17). Various
investigators have demonstrated that M-CSF may promote early
embryonic development (18). In
numerous tissues of osteopetrotic
(Csfmop/Csfmop) mice, which have a naturally
occurring null mutation in the M-CSF gene, macrophages are severely
depleted (19). The
M-CSF-deficient mice have extended oestrous cycles (14 days vs. 4–5
days in wild-type mice) and significantly reduced ovulation rates
compared with their wild-type counterparts. However, the extended
cycles returned to normal and the number of ovulated ova markedly
increased following daily M-CSF administration. The number of
antral and mature follicles in the pro-estrous ovary was
substantially reduced in op/op mice than in control mice and
increased following daily M-CSF administration (20,21).
It was previously demonstrated that the proliferation capacity of
GCs in antral follicles was reduced in op/op mice; however, was
elevated following daily M-CSF administration. The numbers of GCs
and macrophages in the antral follicles were significantly
decreased in op/op mice and were increased following M-CSF
treatment (22). These results
indicated that M-CSF is extensively involved in the process of
folliculogenesis and ovulation.
Our previous study demonstrated that GCs produced
M-CSF, and that M-CSF induced a significant increase of
E2 and P secretion from luteinized GCs in a
dose-dependent manner (23). The
present study verified these conclusions and furthermore,
investigated the interactions among M-CSF, FSH and E2.
The results demonstrated that low concentrations of FSH increased
the expression of M-CSFR; however, this was reversed at high
concentrations. To eliminate the effect of high oestrogen levels in
the culture medium, the oestrogen synthesis inhibitor Letrozole was
added. The results revealed that with increasing concentrations of
FSH, M-CSF receptor mRNA expression increased, suggesting that FSH
promoted the expression of M-CSFR, whereas high levels of oestrogen
inhibited this effect. However, the combination of M-CSF with the
receptor may produce biological effects; therefore, it was
hypothesized that FSH accelerates the production of M-CSF to
enhance follicular development in the early phase; however, in the
late phase, this effect is inhibited with the increase of the
oestrogen concentration, to avoid excessive production of M-CSF
when the follicle has reached a certain stage. Based on the
characteristics of M-CSF secretion and its important role in
follicular development and ovulation, it has been proposed that the
M-CSF level in infertile patients may be used as a predictor of the
success of assisted reproductive therapy (5).
Follicular development is regulated by
gonadotropins; a high concentration of which may recruit more
follicles. The present study revealed that M-CSF had a role in
promoting the production of FSHR in GCs, and this effect appeared
to be dose-dependent. FSH is the most important promotor in the
development of the ovarian follicle prior to ovulation. This
finding indicated that M-CSF may sensitize GCs to the stimulatory
effect of FSH and assist in follicular growth. Chakraborty et
al (24) suggested that FSHR
is expressed in the foetal hamster ovary to account for FSH-induced
primordial follicle formation and cyclic AMP production. It was
previously demonstrated that M-CSF facilitated the production of
E2. To investigate if the alteration of FSHR is directly
affected by M-CSF, Letrozole was added to inhibit the effect of
oestrogen, and the same results were observed. The present study
suggested that M-CSF may act directly on FSHR.
Takasaki et al (6) studied the benefit of M-CSF adjuvant
therapy for poor responders during a controlled ovarian
hyperstimulation cycle and suggested that concomitant
administration of M-CSF and hMG improved follicle development,
particularly in patients with low serum CSF-1 levels in the early
follicular phase. This demonstrated the complementary effect
between gonadotropin and M-CSF, and verified the feasibility of
M-CSF in clinical treatment.
M-CSFR is the primary receptor for M-CSF. The
binding of M-CSF to M-CSFR leads to the activation of cell
signaling pathways, including the Janus kinase/signal transducer
and activator of transcription, phosphoinositide 3-kinase, MAPK,
diacylglycerol/protein kinase C and S-locus receptor kinase
signaling pathways (25,26). The present study revealed that
M-CSF induced phosphorylation of JNK and p38. Furthermore, the
oestrogen level in COV434 cells treated with M-CSF was not reduced
when the cells were pretreated with the JNK inhibitor SP600125 or
the P38 inhibitor SB203580. These results implied that JNK and p38
signaling is involved in the function of M-CSF. However, other
signaling pathways may be involved, suggesting that further
investigation is required to fully elucidate the underlying
mechanism.
In conclusion, M-CSF is important in regulating the
response of GCs to gonadotropin, which may have a promotive effect
in the early phase of follicular development. Its functional effect
may occur in part due to activation of the JNK and p38 signaling
pathways. Further studies are required in order to fully elucidate
the association between M-CSF and GCs in animal models and to
identify if M-CSF may potentially be used as a novel follicular
development regulator in the future.
Acknowledgements
The present study was supported by the Science
Foundation of Zhejiang Province (grant no. Y2090796) and the
National Natural Science Foundation of China (grant no.
31470078).
References
|
1
|
Clark SC and Kamen R: The human
hematopoietic colony-stimulating factors. J Science. 236:1229–1237.
1987. View Article : Google Scholar
|
|
2
|
Witt BR and Pollard JW: Colony stimulating
factor-1 in human follicular fluid. J Fertil Steril. 68:259–264.
1997. View Article : Google Scholar
|
|
3
|
Cohen PE, Zhu L, Nishimura K and Pollard
JW: Colony-stimulating factor 1 regulation of neuroendocrine
pathways that control gonadal function in mice. J Endocrinology.
143:1413–1422. 2002. View Article : Google Scholar
|
|
4
|
Nishimura K, Tanaka N, Kawano T, Matsuura
K and Okamura H: Change in macrophage colony-stimulating factor
concentration in serum and follicular fluid in in-vitro
fertilization and embryo transfer cycles. J Fertil Steril.
69:53–57. 1998. View Article : Google Scholar
|
|
5
|
Salmassi A, Mettler L, Jonat W, Buck S,
Koch K and Schmutzler AG: Circulating level of macrophage
colony-stimulating factor can be predictive for human in vitro
fertilization outcome. J Fertil Steril. 93:116–123. 2010.
View Article : Google Scholar
|
|
6
|
Takasaki A, Ohba T, Okamura Y, Honda R,
Seki M, Tanaka N and Okamura H: Clinical use of colony-stimulating
factor-1 in ovulation induction for poor responders. J Fertil
Steril. 90:2287–2290. 2008. View Article : Google Scholar
|
|
7
|
Zhang Z, Salmassi A and Mettler L:
Expression of macrophage colony-stimulating factor in human
luteinized granulosa cells. Zhonghua Fu Chan Ke Za Zhi. 37:472–474.
2002.(In Chinese). PubMed/NCBI
|
|
8
|
Zhang Z, Salmassi A and Mettler L: Effects
of macrophage colony-stimulating factor on production of estradiol
and progesterone by human luteinized granulosa cells in vitro and
detection of macrophage colony-stimulating factor receptor on human
luteinized granulosa cells. Zhonghua Fu Chan Ke Za Zhi. 37:338–341.
2002.(In Chinese). PubMed/NCBI
|
|
9
|
Curry JM, Eubank TD, Roberts RD, Wang Y,
Pore N, Maity A and Marsh CB: M-CSF signals through the MAPK/ERK
pathway via sp1 to induce VEGF production and induces angiogenesis
in vivo. J PLoS One. 3:e34052008. View Article : Google Scholar
|
|
10
|
Nikolic D, Calderon L, Du L and Post SR:
SR-A ligand and M-CSF dynamically regulate SR-A expression and
function in primary macrophage via P-38 MAPK activation. J BMC
Immunol. 12:372011. View Article : Google Scholar
|
|
11
|
Strober W: Trypan Blue exclusion test of
cell viability. Curr Protoc Immunol: Appendix. 3:Appendix 3B. 2001.
View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Field SL, Dasgupta T, Cummings M and Orsi
NM: Cytokines in ovarian folliculogenesis, oocyte maturation and
luteinisation. Mol Reprod Dev. 81:284–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ojeda-Ojeda M, Murri M, Insenser M and
Escobar-Morreale HF: Mediators of low-grade chronic inflammation in
polycystic ovary syndrome (PCOS). Curr Pharm Des. 19:5775–5791.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ianchiĭ RI, Voznesens'ka TIu and Shepel'
OA: Cytokines and their role in reproductive system. Fiziol Zh.
53:82–90. 2007.(in Ukrainian). PubMed/NCBI
|
|
16
|
Luo J, Elwood F, Britschgi M, Villeda S,
Zhang H, Ding Z, Zhu L, Alabsi H, Getachew R, Narasimhan R, et al:
Colony-stimulating factor 1 receptor (CSF1R) signaling in injured
neurons facilitates protection and survival. J Exp Med.
210:157–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Z, Fang Q and Wang J: Involvement of
macrophage colony-stimulating factor (M-CSF) in the function of
follicular GCs. Fertil Steril. 90:749–754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawamura K, Chen Y, Shu Y, Cheng Y, Qiao
J, Behr B, Pera RA and Hsueh AJ: Promotion of human early embryonic
development and blastocyst outgrowth in vitro using
autocrine/paracrine growth factors. PLoS One. 7:e493282012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Araki M, Fukumatsu Y, Katabuchi H, Shultz
LD, Takahashi K and Okamura H: Follicular development and ovulation
in macrophage colony-stimulating factor-deficient mice homozygous
for the osteopetrosis (op) mutation. Biol Reprod. 54:478–484. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sweet MJ and Hume DA: CSF-1 as a regulator
of macrophage activation and immune responses. Arch Immunol Ther
Exp (Warsz). 51:169–177. 2003.PubMed/NCBI
|
|
21
|
Chitu V and Stanley ER: Colony-stimulating
factor-1 in immunity and inflammation. Curr Opin Immunol. 18:39–48.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schönlau F, Schlesiger C, Ehrchen J,
Grabbe S, Sorg C and Sunderkötter C: Monocyte and macrophage
functions in M-CSF-deficient op/op mice during experimental
leishmaniasis. J Leukoc Biol. 73:564–573. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Fang Q and Wang J: Involvement of
macrophage colony-stimulating factor (M-CSF) in the function of
follicular GCs. Ferti lSteril. 90:749–754. 2008. View Article : Google Scholar
|
|
24
|
Chakraborty P and Roy SK: Expression of
FSH receptor in the hamster ovary during perinatal development. Mol
Cell Endocrinol. 400:41–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee AW, Mao Y, Penninger JM and Yu S: Gab2
promotes colony-stimulating factor 1-regulated macrophage expansion
via alternate effectors at different stages of development. Mol
Cell Biol. 31:4563–4581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Popa-Wagner A, Stöcker K, Balseanu AT,
Rogalewski A, Diederich K, Minnerup J, Margaritescu C and Schäbitz
WR: Effects of granulocyte-colony stimulating factor after stroke
in aged rats. Stroke. 41:1027–1031. 2010. View Article : Google Scholar : PubMed/NCBI
|