Introduction
An estimated 1.8 million new cases of lung cancer
were diagnosed in 2012, which accounted for ~13% of total cancer
diagnoses. In addition, lung cancer was the most frequently
diagnosed cancer and the leading cause of cancer-associated
mortality among men in 2012. Among females, lung cancer was the
leading cause of cancer-associated mortality in more developed
countries, and the second leading cause of cancer-associated
mortality in less developed countries (1). Despite improvements being made in the
diagnosis and treatment of lung cancer in recent decades, the
5-year survival rate of patients with lung cancer is still
relatively low (2–4). Tumor metastasis is one of the major
causes of lung cancer-associated motility. Therefore, in order to
improve the treatment of lung cancer, the mechanism underlying lung
cancer metastasis should be fully understood to facilitate the
establishment of methods that suppress tumor metastasis.
Stromal interaction molecule 1 (STIM1) was initially
identified as a novel human gene, which is mapped to a region of
chromosome 11p15.5 (5). The STIM1
protein mediates Ca2+ influx, following depletion of
intracellular Ca2+ stores, via gating of store-operated
Ca2+ influx channels. STIM1 is critical for the
development and functioning of numerous cell types, including
lymphocytes, skeletal and smooth muscle myoblasts, adipocytes and
neurons, and can interact with various signaling proteins and
pathways in a cell- and tissue-type specific manner (6). STIM1 was initially identified as an
antimetastatic gene in melanoma cells by Suyama et al
(7); however, recent studies have
reported that STIM1 exhibits a metastatic function. Yang et
al (8) observed that STIM1
silencing inhibited the migration and metastasis of breast cancer
cells. Chen et al (9)
revealed that a significantly poorer clinical outcome in primary
tumors was associated with STIM1 upregulation; STIM1 overexpression
markedly enhanced local spread and angiogenesis, and promoted
cancer cell migration and invasion. Additional studies have
indicated that knocking down STIM1 may result in reduced cancer
metastasis in hepatocellular carcinoma (10), melanoma (11,12)
and colorectal cancer (13).
The potential role of STIM1 in lung cancer
metastasis remains to be elucidated. To the best of our knowledge,
one previously published paper reported that STIM1 expression was
increased in non-small cell lung cancer (NSCLC) tissues compared
with in adjacent non-neoplastic lung tissues (14). To further understand the potential
role of STIM1 in lung cancer metastasis, the present study analyzed
the expression of STIM1 in metastatic lung cancer tissues and
non-metastatic lung cancer tissues. In addition, the effects of
STIM1 silencing on A549 cell migration and invasion in
vitro, and on metastasis in vivo, were investigated.
Materials and methods
Cell culture
STIM1 short hairpin (sh)RNA (h) lentiviral particles
(sc-76589-V) and control shRNA lentiviral particles-A (sc-108080)
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). A549 cells (15) were
obtained from American Type Culture Collection (Manassas, VA, USA)
and infected with lentiviral particles according to the
manufacturer's protocol (Santa Cruz Biotechnology, Inc.). The
infected A549 cells were selected with 10 µg/ml puromycin (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The stable clone
infected with STIM1 shRNA (h) lentiviral particles was termed
A549-shSTIM1 and the stable clone infected with control shRNA
lentiviral particles was termed A549-shcon. All cell lines were
cultured in RPMI-1640 medium (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) supplemented with penicillin
(100 U/ml), streptomycin (100 µg/ml), 10% fetal bovine serum (FBS)
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) and 2 mM
L-glutamine at 37°C in an atmosphere containing 5%
CO2.
Biological samples
A total of 49 lung cancer tissue samples were
obtained from patients between January and December 2014, following
surgical resection at the First Hospital Affiliated to Zhengzhou
University (Zhengzhou, China). None of the patients received
radiation therapy or chemotherapy prior to surgery. Histological
type and cell differentiation grade were determined according to
the World Health Organization criteria (16). Written informed consent for
participation in the present study was obtained from all patients
prior to surgery. The present study was approved by the Ethics
Committee of the First Hospital Affiliated to Zhengzhou University,
and the associated methods were conducted in accordance with the
approved guidelines.
Wound healing assay
A wound healing assay was performed according to the
method described in our previous study (17). Briefly, monolayer cells were
wounded by scratching the surface of each well in a 6-well plate as
uniformly as possible with a 200 µl pipette tip. The wells were
then rinsed with phosphate-buffered saline (PBS) three times and
were incubated at 37°C for 48 h. Images of the initial wound, and
the movement of cells into the scratched area, were captured using
a Leica DM IL LED inverted microscope equipped with a digital
imaging system (Leica Microsystems GmbH, Wetzlar, Germany) at 0 and
48 h. The wound width of 5 random views was measured. The healing
width was calculated as wound width at 0 h minus wound width at 48
h, and was normalized to A549-shcon cells (17).
Transwell invasion assay
The cell invasion assay was performed using a
24-well Transwell chamber (Corning Incorporation, Corning, NY,
USA). A549-shSTIM1 and A549-shcon cells were seeded at a density of
4×104 cells into the upper chamber (pore size, 8 µm),
which was precoated with Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA). The lower chamber was filled with 600 µl RPMI-1640
containing 10% FBS. Following a 24 h incubation at 37°C, cells on
the upper-side of the membrane were removed using clean swabs, and
cells on the underside were viewed and counted under a Leica DM IL
LED inverted microscope (Leica Microsystems GmbH). The number of
invaded cells was counted in 5 randomly selected fields (17).
Tail vein metastatic assay
All experimental procedures involving animals were
performed in accordance with the Guide for the Care and Use of
Laboratory Animals (18,19), and the study was approved by the
Ethics Committee of Henan Center for Disease Control and Prevention
(Zhengzhou, China). Female BALB/c nude mice (age, 4 weeks; weight,
20±2 g) were purchased from Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China), were housed in a specific
pathogen-free environment at a controlled temperature of 22±2°C and
50–60% relative humidity, under 12-h light/dark cycles and were
provided with free access to food and water. A549-shSTIM1 cells and
A549-shcon cells were harvested, washed twice with PBS, and
suspended in PBS. A total of 5 mice per group received
5×106 cells in 150 µl PBS by tail vein injection. The
mice were sacrificed at 12 weeks post-injection. Subsequently, the
lung tissues were histologically examined for metastases under a
Zeiss Axioskop 40 microscope (Zeiss AG, Oberkochen, Germany)
(13).
Western blot analysis
Total protein was extracted from cultured cells with
lysis buffer solution supplemented with protease and phosphatase
inhibitors (Pierce; Thermo Fisher Scientific, Inc.), and protein
concentration was quantified using a bicinchoninic acid protein
assay (Pierce; Thermo Fisher Scientific, Inc.). Each protein sample
(30 µg) was separated by 10% SDS-PAGE and was then transferred to
nitrocellulose membranes (Pall Life Sciences, Port Washington, NY,
USA). After blocking with 5% bovine serum albumin (Beyotime
Institute of Biotechnology, Haimen, China) in Tris-buffered saline
containing 0.1% Tween-20 at room temperature for 1 h, the membrane
was incubated with primary antibodies at a 1:1,000 dilution
overnight at 4°C. STIM1 (sc-68897), matrix metalloproteinase (MMP)2
(sc-10736), MMP9 (sc-10737), N-cadherin (sc-7939) and E-cadherin
(sc-7870) antibodies were purchased from Santa Cruz Biotechnology,
Inc. Snail1 (#3879S) and Vimentin (#5741S) antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
After incubation with horseradish peroxidase (HRP)-coupled
anti-rabbit-immunglobulin G (ZB-2301; 1:5,000; ZSGB-BIO, Beijing,
China) and HRP-coupled anti-mouse-immunglobulin G (ZB-2305;
1:5,000; ZSGB-BIO) at room temperature for 1 h, the protein bands
were visualized using Bio-Rad Clarity™ western enhanced
chemiluminescence substrate (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and the ChemiDoc™ XRS+ Imaging system (Bio-Rad
Laboratories, Inc.). Anti-β-actin (1:1,000; sc-47778; Santa Cruz
Biotechnology, Inc.) was used as a loading control.
Immunohistochemistry
Immunohistochemistry of human lung cancer tissue
samples was performed using a streptavidin rabbit & mouse HRP
kit (#CW2069; Beijing ComWin Biotech Co., Ltd., Beijing, China)
according to standard procedures. Briefly, following
deparaffinization, antigen retrieval was performed with sodium
citrate buffer and boiling for 15 min. Slides were blocked with
endogenous peroxidase blocking buffer and normal goat serum
(supplied in the kit) at room temperature for 10 min each. Slides
were washed with PBS and incubated with anti-STIM1 primary antibody
(sc-68897; 1:100; Santa Cruz Biotechnology, Inc.) at 4°C overnight,
and then incubated with biotinylated secondary antibody and
HRP-labeled streptavidin (supplied in the kit) at room temperature
for 10 min each. Staining was performed with 100 µl
diaminobenzidine at room temperature for 1 min. The intensity of
STIM1 staining was scored as 0 (no signal), 1 (weak), 2 (moderate)
and 3 (marked). Scores of the percentage of positive cells were
assigned as 0 (<5%), 1 (5–25%), 2 (26–50%) and 3 (>51%). The
scores of each view were multiplied to give a final score of 0–9,
and the final score of one sample was the mean of 5 microscopic
fields. Tumors were finally determined as low-expression (score:
0–1) and high-expression (score: 2–9) (20).
Statistical analysis
Data are presented as the mean ± standard deviation
of 3 independent experiments. Statistical significance was
estimated using SPSS version 13.0 software (SPSS, Inc., Chicago,
IL, USA). Data were analyzed by Student's t-test and χ2
test; all tests were two-sided. P<0.05 was considered to
indicate a statistically significant difference.
Results
STIM1 expression in human lung cancer
tissues
The clinicopathological characteristics of the
patients with lung cancer are presented in Table I. To determine the difference in
STIM1 protein expression between metastatic lung cancer tissues and
non-metastatic lung cancer tissues, immunohistochemistry was
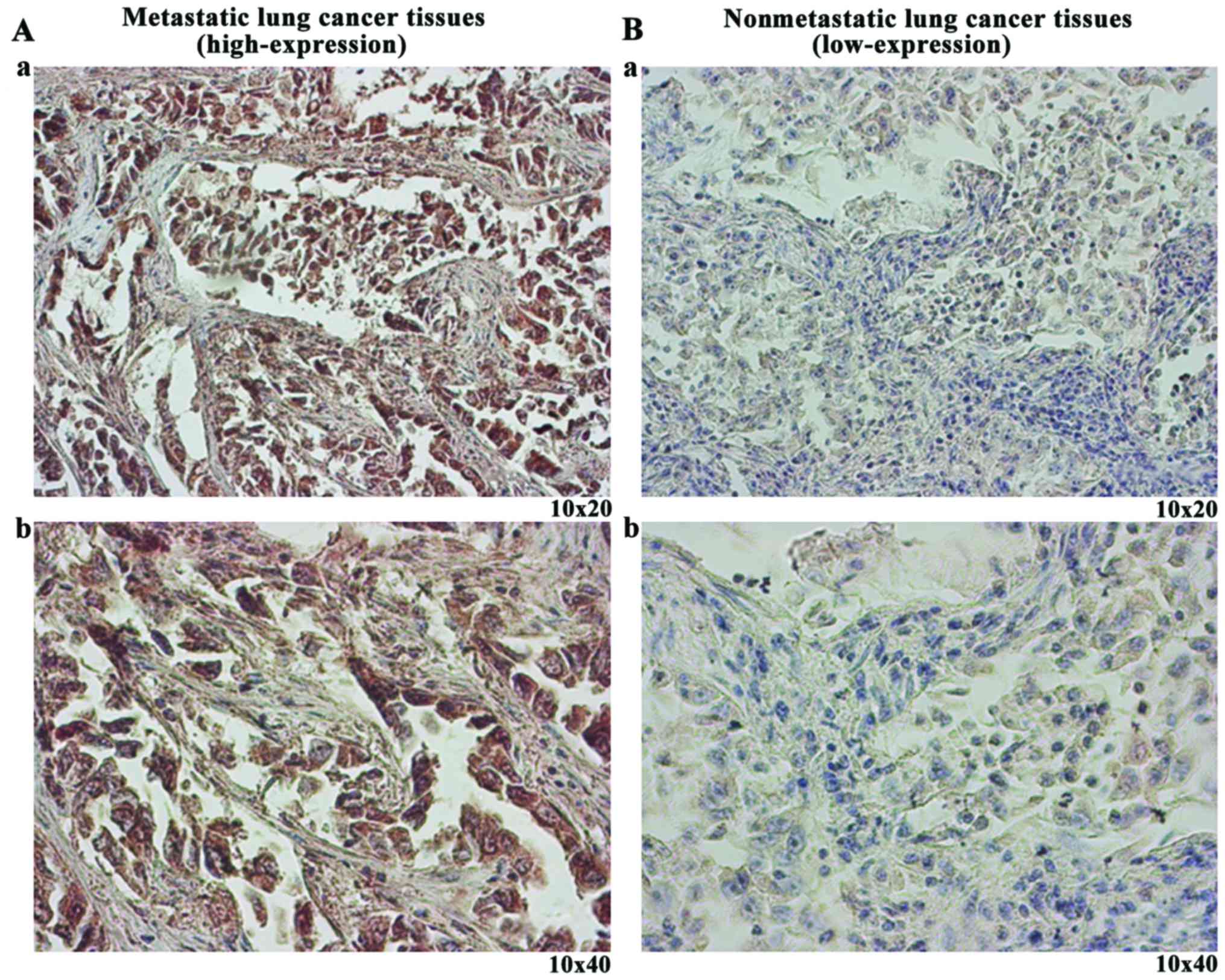
conducted. The results demonstrated that the frequency of STIM1
high-expression was 72.2% (13/18) in metastatic lung cancer
tissues, which was significantly higher than that in non-metastatic
lung cancer tissues (P=0.013; Table
II). Representative images (Fig.
1) exhibit the high-expression (Fig. 1A) and low-expression (Fig. 1B) of STIM1 in lung cancer
tissues.
 | Table I.Clinicopathological characteristics
of patients with lung cancer. |
Table I.
Clinicopathological characteristics
of patients with lung cancer.
| Characteristic | Number |
|---|
| Male | 39 |
| ≥60
years | 26 |
| <60
years | 13 |
| Female | 10 |
| ≥60
years | 6 |
| <60
years | 4 |
| Histological
type |
|
|
Adenocarcinoma | 20 |
|
Squamous cell carcinoma | 26 |
| Small
cell carcinoma | 3 |
| Grade |
|
| G1 | 10 |
| G2 | 29 |
| G3 | 10 |
| Metastasis |
|
|
Yes | 18 |
| No | 31 |
 | Table II.Frequency of stromal interaction
molecule 1 high-expression and low-expression in metastatic and
non-metastatic lung cancer tissues. |
Table II.
Frequency of stromal interaction
molecule 1 high-expression and low-expression in metastatic and
non-metastatic lung cancer tissues.
| Expression | Metastatic lung
cancer | Non-metastatic lung
cancer |
|---|
|
High-expression | 13 | 11 |
| Low-expression | 5 | 20 |
Effects of STIM1 silencing on A549
cell migration and invasion in vitro
To evaluate the effects of STIM1 silencing on the
migration of A549 cells, a wound healing assay was used to
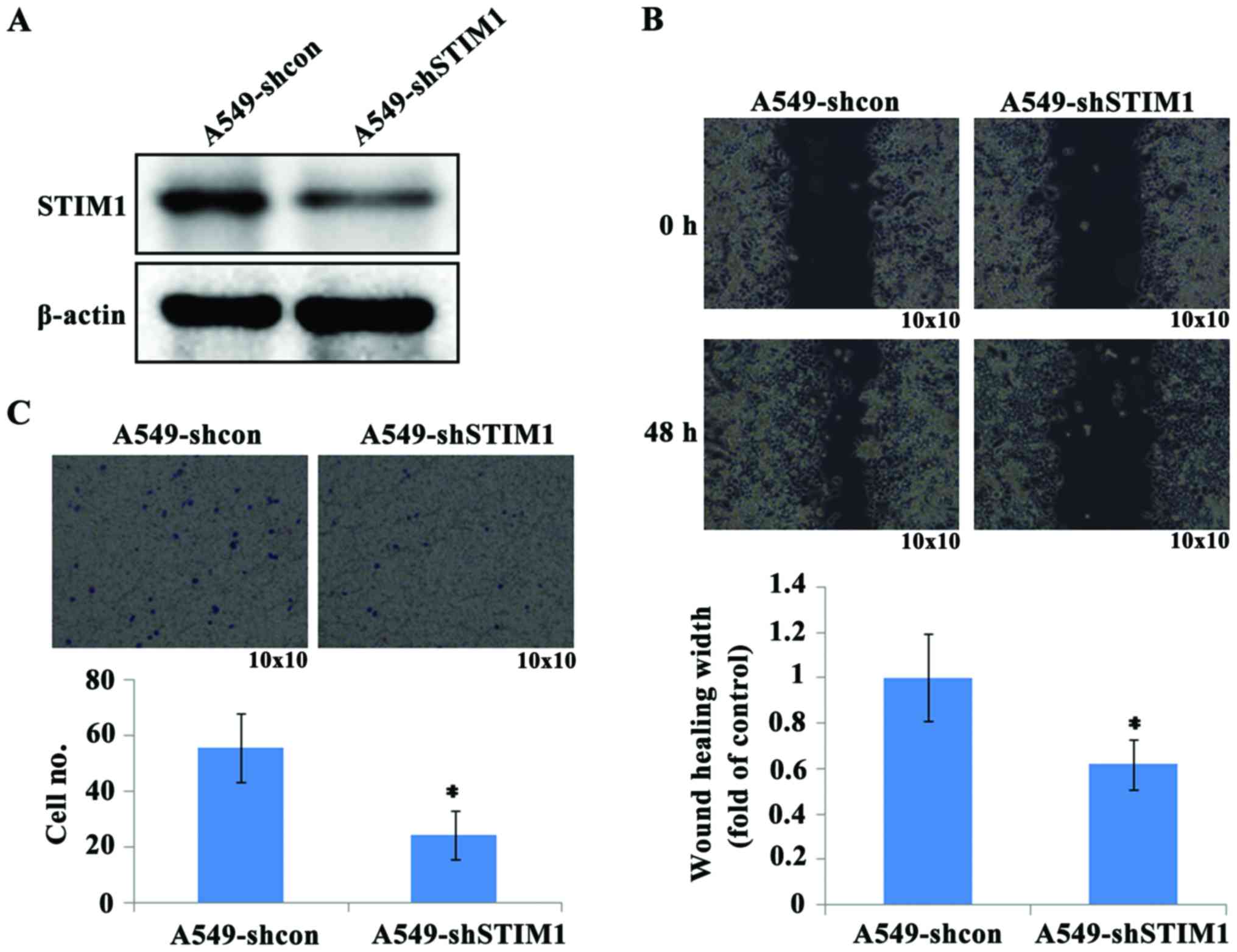
determine the migratory ability of A549 cells. Knockdown of STIM1
expression was confirmed by western blot analysis (Fig. 2A). The results of the wound healing
assay indicated that the cells migrated more slowly to close the
scratched wounds in the A549-shSTIM1 group compared with the
A549-shcon group (Fig. 2B).
Furthermore, a Transwell invasion assay was conducted to evaluate
the effects of STIM1 silencing on the invasive abilities of A549
cells. The results demonstrated that the ability of A549 cells to
invade through the Matrigel matrix was significantly decreased in
the A549-shSTIM1 group compared with the A549-shcon group (Fig. 2C). Taken together, these results
suggested that STIM1 silencing may inhibit the migratory and
invasive abilities of A549 cells in vitro.
Effects of STIM1 silencing on A549
metastasis in vivo
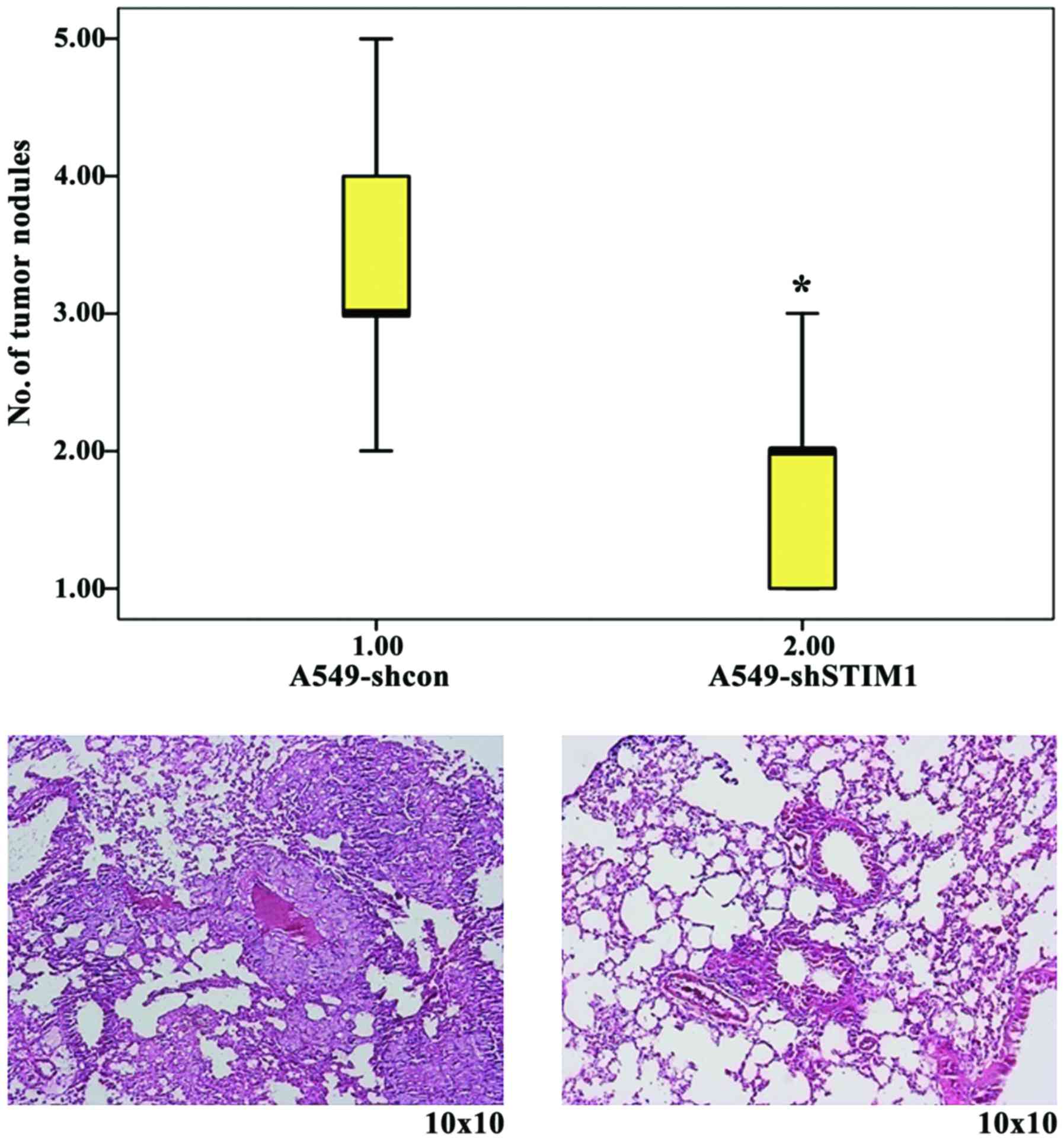
Since STIM1 silencing was revealed to inhibit A549
cell migration and invasion in vitro, the present study then
injected A549-shSTIM1 and A549-shcon cells into nude mice through
the lateral tail vein. The results of a metastatic assay indicated
that the number of metastatic lung nodules was significantly
decreased in the A549-shSTIM1 group compared with the A549-shcon
group (Fig. 3).
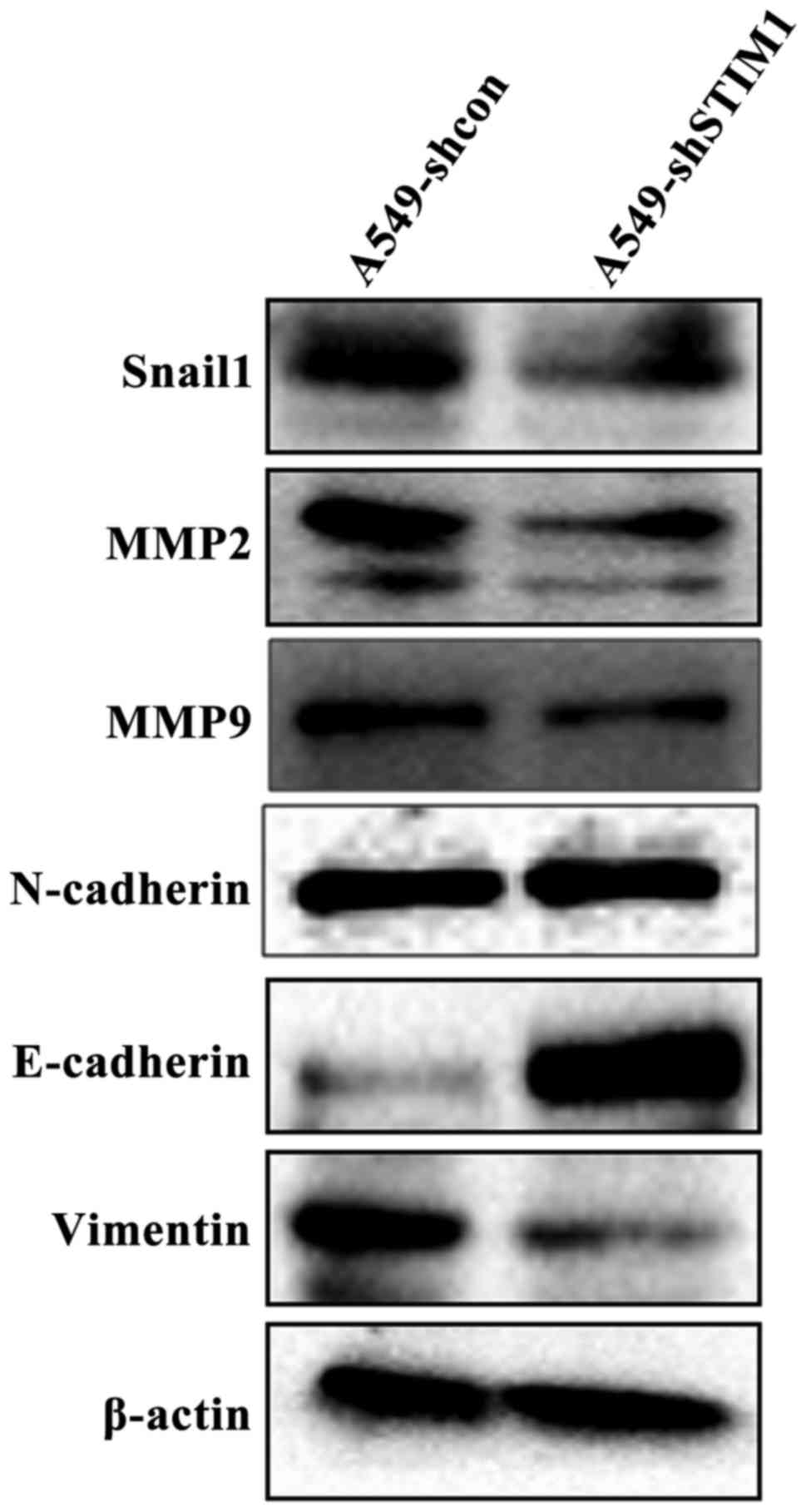
STIM1 silencing downregulates the
protein expression levels of Snail1, MMP2, MMP9 and Vimentin, and
upregulates the protein expression levels of E-cadherin
Since specific proteins, such as Snail1, MMP2, MMP9,
N-cadherin, Vimentin and E-cadherin, are associated with the
progression and metastasis of lung cancer (21–25),
the present study aimed to determine whether STIM1 silencing could
affect the expression levels of these proteins. As shown in
Fig. 4, western blot analysis
indicated that the protein expression levels of Snail1, MMP2, MMP9
and Vimentin were markedly decreased in A549-shSTIM1 cells compared
with in A549-shcon cells. However, the protein expression levels of
E-cadherin were markedly increased in A549-shSTIM1 cells compared
with in A549-shcon cells. There were no obvious alterations in the
protein expression levels of N-cadherin between A549-shSTIM1 cells
and A549-shcon cells.
Discussion
STIM1 was initially identified as an antimetastatic
gene, since STIM1 silencing resulted in the accelerated mobility of
melanoma cells in an in vitro scratch-wound assay (7); however, subsequent studies reported
conflicting results. Umemura et al (11) reported that STIM1 knockdown
inhibited cell migration in metastatic cell lines and decreased the
number of metastatic colonies in mouse lung tissues. Yang et
al (8) demonstrated that
silencing STIM1 inhibited the migration and metastasis of breast
cancer cells by suppressing focal adhesion turnover. In addition,
Chen et al (9) reported
that the expression levels of STIM1 were significantly associated
with the risk of cervical cancer metastasis and survival. Silencing
STIM1 attenuated the endogenous migration of cervical cancer cells,
whereas STIM1 overexpression enhanced cervical cancer cell
migration and invasion. Zhang et al (13) reported that the enhanced expression
of STIM1 promoted colorectal cancer cell metastasis in vitro
and in vivo, whereas silencing STIM1 with small interfering
RNA reduced metastasis. Furthermore, ectopic expression of STIM1 in
colorectal cancer cells induced epithelial to mesenchymal
transition (EMT), whereas silencing STIM1 exerted discordant
effects (13). Taken together, the
potential role of STIM1 in tumor metastasis is inconsistent among
different tumor types; the detailed reasons why have yet to be
elucidated. One of the main causes of lung cancer-associated
mortality is tumor metastasis; however, it has yet to be determined
whether STIM1 serves an important role in lung cancer metastasis.
Therefore, the present study aimed to determine the role of STIM1
in lung cancer metastasis.
The present study demonstrated that the frequency of
STIM1 high-expression was increased in metastatic lung cancer
tissues compared with in non-metastatic lung cancer tissues, thus
suggesting that STIM1 overexpression may promote lung cancer
metastasis. To confirm the findings demonstrated in human samples,
the effects of STIM1 silencing on the invasive and migratory
abilities of A549 cells were investigated in vitro. The
results indicated that STIM1 silencing inhibited the migratory and
invasive capabilities of A549 cells, as determined using wound
healing and Transwell invasion assays. These findings supported the
aforementioned observations to a certain extent. In an animal
model, the present study demonstrated that STIM1 silencing
inhibited the metastasis of A549 cells in vivo, which
further verified the role of STIM1 in lung cancer metastasis.
The present study indicated that STIM1 was expressed
not only in cancer cells but also in stromal cells. Previous
studies (26–28) have reported that the tumor stroma
serves a crucial role in tumorigenesis. The tumor stroma contains
increased amounts of inflammatory infiltrates, an increased
microvessel density with dysfunctional lymphatics and blood
vessels, and a denser extracellular matrix with reactive
fibroblasts (29). Throughout the
entire process of cancer etiology, progression and metastasis, the
microenvironment of the local host tissue may be considered an
active participant. Invasion occurs within the tumor-host
microecology, where stroma and tumor cells exchange enzymes and
cytokines that modify the local extracellular matrix, stimulate
migration, and promote proliferation and survival (30). However, it remains unclear how
STIM1 expression is regulated in cancer tissues, which should be
explored in future studies.
E-cadherin is a calcium-dependent, epithelial cell
adhesion molecule, the reduced expression of which has been
associated with increased lymph node metastasis in NSCLC (31–33).
Transfection of E-cadherin cDNA in human lung tumor cells has
previously been reported to reduce the invasive potential of tumors
(34), and overexpression of
E-cadherin in NSCLC cell lines may inhibit cell migration (35). Vimentin is an intermediate filament
protein whose expression is correlated with increased metastatic
disease, reduced patient survival and poor prognosis in lung cancer
(36,37). Vimentin expression is also
associated with prognosis via alteration of the invasive ability of
NSCLC cells (38). Vimentin
depletion inhibited lung cancer cell migration and invasion in
vitro, and metastasis in vivo (39). In the present study, STIM1
silencing downregulated Vimentin protein expression and upregulated
E-cadherin protein expression. A previous study demonstrated that
STIM1 silencing downregulated Vimentin expression and upregulated
E-cadherin expression in colorectal cancer cells; however,
overexpression of STIM1 had the opposite effect (13). Taken together, these findings
supported the hypothesis that STIM1 may regulate the expression of
Vimentin and E-cadherin in various cell lines. However, there is no
significant difference in cell morphology between A549-shcon cells
and A549-shSTIM1 cells (data not shown). The discrepancy in the
alterations of EMT markers and cell phenotype observed in the
present study should be explored in future studies.
MMPs are a class of proteolytic enzymes that are
closely associated with tumor invasion and metastasis. MMP2 and
MMP9 are the major enzymes that degrade type IV collagen, which
serve important roles in lung cancer metastasis (40–42).
A previous study demonstrated that increased MMP2 expression may
serve as an independent prognosis factor in NSCLC, which is closely
associated with clinical stage, pathological grade, lymphatic
metastasis and prognosis (43).
MicroRNA-129 may regulate MMP9 to control metastasis of NSCLC
(44). In the present study, STIM1
silencing decreased the protein expression levels of MMP2 and MMP9
in A549 cells. A previous study reported that STIM1 knockdown could
reduce the levels of secreted MMP2 in melanoma cells in
vitro (12). Snail1 is a
zinc-finger transcription factor that contains a highly conserved
C-terminal region comprising 4–6 zinc fingers, which serves as the
DNA-binding domain that recognize consensus E-box type elements
(45,46). Snail1 has been reported to serve a
key role in lung cancer metastasis and progression (46–48).
Furthermore, previous studies have indicated that Snail1 may
regulate the expression of E-cadherin, Vimentin, MMP2 and MMP9 in
lung cancer cell lines (48,49).
The present study observed that STIM1 silencing downregulated the
expression of Snail1 protein; however, the molecular mechanism
underlying STIM1-mediated regulation of Snail1 expression remains
unclear, and should be further investigated in future studies.
In conclusion, the present study suggested that
STIM1 silencing inhibited the migration and invasion of A549 cells
in vitro, and lung cancer cell metastasis in vivo.
The present study extends the knowledge regarding lung cancer
progression and suggests that STIM1 may be a potential therapeutic
target for the treatment of human lung cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. U1404815)
and the Henan Collaborative Innovation Center of Molecular
Diagnosis and Laboratory Medicine (grant no. XTCX-2015-PY7).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cui T, Srivastava AK, Han C, Yang L, Zhao
R, Zou N, Qu M, Duan W, Zhang X and Wang QE: XPC inhibits NSCLC
cell proliferation and migration by enhancing E-Cadherin
expression. Oncotarget. 6:10060–10072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osarogiagbon RU, Lin CC, Smeltzer MP and
Jemal A: Prevalence, prognostic implications, and survival
modulators of incompletely resected non-small cell lung cancer in
the U.S. national cancer data base. J Thorac Oncol. 11:e5–e16.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin JJ, Cardarella S, Lydon CA, Dahlberg
SE, Jackman DM, Jänne PA and Johnson BE: Five-year survival in
EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs.
J Thorac Oncol. 11:556–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parker NJ, Begley CG, Smith PJ and Fox RM:
Molecular cloning of a novel human gene (D11S4896E) at chromosomal
region 11p15.5. Genomics. 37:253–256. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnstone LS, Graham SJ and Dziadek MA:
STIM proteins: Integrators of signalling pathways in development,
differentiation and disease. J Cell Mol Med. 14:1890–1903. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suyama E, Wadhwa R, Kaur K, Miyagishi M,
Kaul SC, Kawasaki H and Taira K: Identification of
metastasis-related genes in a mouse model using a library of
randomized ribozymes. J Biol Chem. 279:38083–38086. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang S, Zhang JJ and Huang XY: Orai1 and
STIM1 are critical for breast tumor cell migration and metastasis.
Cancer Cell. 15:124–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YF, Chiu WT, Chen YT, Lin PY, Huang
HJ, Chou CY, Chang HC, Tang MJ and Shen MR: Calcium store sensor
stromal-interaction molecule 1-dependent signaling plays an
important role in cervical cancer growth, migration, and
angiogenesis. Proc Natl Acad Sci USA. 108:15225–15230. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang N, Tang Y, Wang F, Zhang H, Xu D,
Shen Y, Sun S and Yang G: Blockade of store-operated Ca(2+) entry
inhibits hepatocarcinoma cell migration and invasion by regulating
focal adhesion turnover. Cancer Lett. 330:163–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Umemura M, Baljinnyam E, Feske S, De
Lorenzo MS, Xie LH, Feng X, Oda K, Makino A, Fujita T, Yokoyama U,
et al: Store-operated Ca2+ entry (SOCE) regulates
melanoma proliferation and cell migration. PLoS One. 9:e892922014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun J, Lu F, He H, Shen J, Messina J,
Mathew R, Wang D, Sarnaik AA, Chang WC, Kim M, et al: STIM1- and
Orai1-mediated Ca(2+) oscillation orchestrates invadopodium
formation and melanoma invasion. J Cell Biol. 207:535–548. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z, Liu X, Feng B, Liu N, Wu Q, Han
Y, Nie Y, Wu K, Shi Y and Fan D: STIM1, a direct target of
microRNA-185, promotes tumor metastasis and is associated with poor
prognosis in colorectal cancer. Oncogene. 34:4808–4820. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Zhang M, Xu L, Lin D, Cai S and Zou
F: The apoptosis of non-small cell lung cancer induced by cisplatin
through modulation of STIM1. Exp Toxicol Pathol. 65:1073–1081.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Pan T, Wang H, Li L, Li J, Zhang C
and Yang H: Silencing of TGIF attenuates the tumorigenicity of A549
cells in vitro and in vivo. Tumour Biol. 37:12725–12730. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Travis W, Brambilla E, Muller-Hermelink H
and Harris C: World Health Organization classification of
tumoursPathology and Genetics Tumours of the Lung, Pleura, Thymus
and Heart. Lyon: IARC Press; 2004
|
|
17
|
Wang Y, Zhai W, Wang H, Xia X and Zhang C:
Benzo(a)pyrene promotes A549 cell migration and invasion through
up-regulating Twist. Arch Toxicol. 89:451–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American physiological
society. Physiologist. 39:199208. –211. 1996.PubMed/NCBI
|
|
19
|
Bratcher NA and Reinhard GR: Creative
implementation of 3Rs principles within industry programs: Beyond
regulations and guidelines. J Am Assoc Lab Anim Sci. 54:133–138.
2015.PubMed/NCBI
|
|
20
|
Hui L, Zhang S, Dong X, Tian D, Cui Z and
Qiu X: Prognostic significance of twist and N-cadherin expression
in NSCLC. PLoS One. 8:e621712013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bae GY, Choi SJ, Lee JS, Jo J, Lee J, Kim
J and Cha HJ: Loss of E-cadherin activates EGFR-MEK/ERK signaling,
which promotes invasion via the ZEB1/MMP2 axis in non-small cell
lung cancer. Oncotarget. 4:2512–2522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gong L, Wu D, Zou J, Chen J, Chen L, Chen
Y, Ni C and Yuan H: Prognostic impact of serum and tissue MMP-9 in
non-small cell lung cancer: A systematic review and meta-analysis.
Oncotarget. 7:18458–18468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang X, Han M, Han H, Wang B, Li S, Zhang
Z and Zhao W: Silencing snail suppresses tumor cell proliferation
and invasion by reversing epithelial-to-mesenchymal transition and
arresting G2/M phase in non-small cell lung cancer. Int J Oncol.
50:1251–1260. 2017.PubMed/NCBI
|
|
24
|
Ye Z, Zhang X, Luo Y, Li S, Huang L, Li Z,
Li P and Chen G: Prognostic values of Vimentin expression and its
clinicopathological significance in non-small cell lung cancer: A
meta-analysis of observational studies with 4118 cases. PLoS One.
11:e01631622016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong Z, Xia Y, Wang P, Liu B and Chen Y:
Low expression of microRNA-30c promotes invasion by inducing
epithelial mesenchymal transition in non-small cell lung cancer.
Mol Med Rep. 10:2575–2579. 2014.PubMed/NCBI
|
|
26
|
Nitsche U, Stangel D, Pan Z, Schlitter AM,
Esposito I, Regel I, Raulefs S, Friess H, Kleeff J and Erkan M:
Periostin and tumor-stroma interactions in non-small cell lung
cancer. Oncol Lett. 12:3804–3810. 2016.PubMed/NCBI
|
|
27
|
Moss LA Shuman and Stetler-Stevenson WG:
Influence of stromal components on lung cancer carcinogenesis. J
Carcinog Mutagen. 13:2013.doi: 10.4172/2157-2518.S13-008.
|
|
28
|
Zhang T, Xu J, Shen H, Dong W, Ni Y and Du
J: Tumor-stroma ratio is an independent predictor for survival in
NSCLC. Int J Clin Exp Pathol. 8:11348–11355. 2015.PubMed/NCBI
|
|
29
|
Donnem T, Al-Saad S, Al-Shibli K,
Delghandi MP, Persson M, Nilsen MN, Busund LT and Bremnes RM:
Inverse prognostic impact of angiogenic marker expression in tumor
cells versus stromal cells in non small cell lung cancer. Clin
Cancer Res. 13:6649–6657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sulzer MA, Leers MP, van Noord JA, Bollen
EC and Theunissen PH: Reduced E-cadherin expression is associated
with increased lymph node metastasis and unfavorable prognosis in
non-small cell lung cancer. Am J Respir Crit Care Med.
157:1319–1323. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shibanuma H, Hirano T, Tsuji K, Wu Q,
Shrestha B, Konaka C, Ebihara Y and Kato H: Influence of E-cadherin
dysfunction upon local invasion and metastasis in non-small cell
lung cancer. Lung Cancer. 22:85–95. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu D, Huang C, Kameyama K, Hayashi E,
Yamauchi A, Kobayashi S and Yokomise H: E-cadherin expression
associated with differentiation and prognosis in patients with
non-small cell lung cancer. Ann Thorac Surg. 71:949–955. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moersig W, Horn S, Hilker M, Mayer E and
Oelert H: Transfection of E-cadherin cDNA in human lung tumor cells
reduces invasive potential of tumors. Thorac Cardiovasc Surg.
50:45–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Asnaghi L, Vass WC, Quadri R, Day PM, Qian
X, Braverman R, Papageorge AG and Lowy DR: E-cadherin negatively
regulates neoplastic growth in non-small cell lung cancer: Role of
Rho GTPases. Oncogene. 29:2760–2771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi Y, Wu H, Zhang M, Ding L, Meng F and
Fan X: Expression of the epithelial-mesenchymal transition-related
proteins and their clinical significance in lung adenocarcinoma.
Diagn Pathol. 8:892013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dauphin M, Barbe C, Lemaire S,
Nawrocki-Raby B, Lagonotte E, Delepine G, Birembaut P, Gilles C and
Polette M: Vimentin expression predicts the occurrence of
metastases in non small cell lung carcinomas. Lung Cancer.
81:117–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tadokoro A, Kanaji N, Liu D, Yokomise H,
Haba R, Ishii T, Takagi T, Watanabe N, Kita N, Kadowaki N and
Bandoh S: Vimentin regulates invasiveness and is a poor prognostic
marker in non-small cell lung cancer. Anticancer Res. 36:1545–1551.
2016.PubMed/NCBI
|
|
39
|
Havel LS, Kline ER, Salgueiro AM and
Marcus AI: Vimentin regulates lung cancer cell adhesion through a
VAV2-Rac1 pathway to control focal adhesion kinase activity.
Oncogene. 34:1979–1990. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rojiani MV, Alidina J, Esposito N and
Rojiani AM: Expression of MMP-2 correlates with increased
angiogenesis in CNS metastasis of lung carcinoma. Int J Clin Exp
Pathol. 3:775–781. 2010.PubMed/NCBI
|
|
41
|
Qian Z, Zhao X, Jiang M, Jia W, Zhang C,
Wang Y, Li B and Yue W: Downregulation of cyclophilin A by siRNA
diminishes non-small cell lung cancer cell growth and metastasis
via the regulation of matrix metallopeptidase 9. BMC Cancer:.
12:4422012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He H, Zheng L, Sun YP, Zhang GW and Yue
ZG: Steroidal saponins from Paris polyphylla suppress adhesion,
migration and invasion of human lung cancer A549 cells via
down-regulating MMP-2 and MMP-9. Asian Pac J Cancer Prev.
15:10911–10916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qian Q, Wang Q, Zhan P, Peng L, Wei SZ,
Shi Y and Song Y: The role of matrix metalloproteinase 2 on the
survival of patients with non-small cell lung cancer: A systematic
review with meta-analysis. Cancer Invest. 28:661–669. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li J, Wang H, Ke H and Ni S: MiR-129
regulates MMP9 to control metastasis of non-small cell lung cancer.
Tumour Biol. 36:5785–5790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yanagawa J, Walser TC, Zhu LX, Hong L,
Fishbein MC, Mah V, Chia D, Goodglick L, Elashoff DA, Luo J, et al:
Snail promotes CXCR2 ligand-dependent tumor progression in
non-small cell lung carcinoma. Clin Cancer Res. 15:6820–6829. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu J, Hu G, Chen D, Gong AY, Soori GS,
Dobleman TJ and Chen XM: Suppression of SCARA5 by Snail1 is
essential for EMT-associated cell migration of A549 cells.
Oncogenesis. 2:e732013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu CW, Li CH, Peng YJ, Cheng YW, Chen HW,
Liao PL, Kang JJ and Yeng MH: Snail regulates Nanog status during
the epithelial-mesenchymal transition via the Smad1/Akt/GSK3β
signaling pathway in non-small-cell lung cancer. Oncotarget.
5:3880–3894. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Merikallio H, Turpeenniemi-Hujanen T,
Pääkkö P, Mäkitaro R, Riitta K, Salo S, Salo T, Harju T and Soini
Y: Snail promotes an invasive phenotype in lung carcinoma. Respir
Res. 13:1042012. View Article : Google Scholar : PubMed/NCBI
|