Introduction
Mitochondria are the powerhouse organelles of
mammalian cells, producing >90% of adenosine triphosphate (ATP)
which is used to maintain cellular functions. However, mitochondria
are also known to be major participants in free radical production
and calcium (Ca2+) buffering. During the
pathophysiological process of secondary traumatic spinal cord
injury (SCI), dysfunction of oxidative phosphorylation leads to an
accumulation of excessive reactive oxygen species (ROS) within the
mitochondria (1,2). Meanwhile, the excess Ca2+
ions are transported to the mitochondrial matrix in order to
sustain the homeostatic content of Ca2+ within the
cytosol (3,4). However, an excess of ROS and
Ca2+ induces the opening of mitochondrial permeability
transition pores (MPTPs), which non-selectively allow any
micromolecule with a molecular weight of ≤1.5 kDa to pass through
the mitochondrial inner membrane (5,6). The
subsequent influx of water attenuates the osmotic pressure of the
mitochondrial matrix, leading to mitochondrial swelling. The
outward expansion leads to the disappearance of inner membrane
cristae and, eventually, rupture of the outer membrane (7). Mitochondrial rupture causes the
release of ROS, Ca2+ and pro-apoptotic factors,
including the electron carrier cytochrome c (cyt c),
apoptosis-inducing factor (AIF) and others, into the cytosol.
Collectively, these substances activate several forms of programmed
cell death, including necroptosis, apoptosis and autophagy
(8–10). Therefore, maintaining the proper
function of oxidative phosphorylation and inhibiting MPTP opening
are important therapeutic approaches to alleviate secondary
traumatic SCI.
Spermine is a biological organic aliphatic
polycation with four primary amino groups. It is ubiquitously
present in all living cells, including bacterial, plant and animal
cells. In humans, spermine was initially identified in sperm cells,
where it is involved in proliferation and differentiation.
Naturally occurring spermine is synthesized in the cytosol and then
transported to the mitochondrial matrix. A specific spermine
uniporter is present in the mitochondrial outer and inner
membranes, and has a low affinity and high capacity for spermine
storage (11). The spermine,
accumulated in the uniporter, can be released into the matrix
according to a shift in electric transmembrane potential (12). In the mitochondrial matrix,
spermine acts on the pyruvate dehydrogenase complex and respiratory
chain complexes to regulate oxidative phosphorylation activity
(13). Furthermore, it has been
demonstrated that spermine is involved in scavenging ROS and
inhibiting Ca2+-induced MPTP opening, depending on its
concentration in the matrix (14,15).
However, the mechanisms by which spermine protects mitochondrial
functions are not fully understood.
Accumulating evidence has indicated that tyrosine
phosphorylation has profound effects on mitochondrial functions;
dozens of proteins distributed in mitochondrial subcomponents are
the targeted substrates of tyrosine kinases and phosphatases,
including certain subunits of respiratory chain complexes, cyt
c and the pyruvate dehydrogenase complex (16). Furthermore, it is increasingly
clear that several essential components of the MPTP contain
modulation sites of tyrosine phosphorylation; this includes
hexokinase type І in the cytosol, voltage-dependent anion channel
(VDAC) in the outer membrane, adenine nucleotide translocator
(AdNT) in the inner membrane, and subunit γ of ATP synthase in the
matrix (17,18). Thus, tyrosine kinases and
phosphatases can substantially adjust the activity of the MPTP by
reversible phosphorylation and dephosphorylation. Src family
kinases (SFKs), non-receptor tyrosine kinases that are stably and
abundantly present within mitochondria, have been shown to be
crucial participants in the process of reducing ROS levels,
particularly in rat brain mitochondria (19). In contrast to SFKs, tyrosine
phosphatases, such as SHP-2 and PTP1B, attenuate this scavenging
function. Notably, spermine and SFKs appear to have similar effects
on the regulation of oxidative phosphorylation and inhibition of
MPTP opening. Nevertheless, to the best of our knowledge, no
definitive evidence has been presented of an association between
spermine and SFKs in spinal cord mitochondria.
The present study demonstrates that spermine
increases the process of oxidative phosphorylation and inhibits
MPTP opening in mitochondria isolated from the spinal cord. These
effects are reduced in the presence of an SFK inhibitor and are
enhanced by a phosphatase inhibitor. These findings illustrates
that spermine activates SFKs, which subsequently maintains
mitochondrial bioenergetics.
Materials and methods
Animals and reagents
Female Sprague-Dawley rats weighing 220±20 g were
obtained from Experimental Animal Center of Harbin Medical
University (Harbin, China). The rats were used in experimental
procedures in accordance with the Guide for Care and Use of
Laboratory Animals approved by the China National Institutes of
Health. Spermine and cyclosporin A (CsA) were purchased from
Sigma-Aldrich (Merck Millipore, St. Louis, MO, USA).
Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine(PP2)
was also purchased from Sigma-Aldrich and dissolved in DMSO.
Vanadate was obtained from Chemservice SA (Grevenmacher,
Luxembourg). The JC-1 fluorescence probe and BCA Protein Assay kit
were obtained from Beyotime Institute of Biotechnology (Shanghai,
China). Primary antibodies against Src, phospho-Y419, phosphor-Y530
and COX IV were purchased from Abcam (Cambridge, MA, USA), and the
alkaline phosphatase (AP)-linked IgG secondary antibody was
purchased from ZSGB Biotech, Co., Ltd. (Beijing, China). Calcium
chloride (CaCl2) was acquired from Sunshine Biotech,
Co., Ltd. (Nanjing, China).
Isolation of spinal cord
mitochondria
Rat spinal cord mitochondria were isolated by a
conventional differential centrifugation method, as modified from a
previously described procedure (20). Briefly, animals were decapitated
after anesthetizing with 10% chloral hydrate (3 ml/kg,
intraperitoneally). The spinal cord segments from T8 to L1 were
rapidly dissected and homogenized in isolation buffer A [320 mM
sucrose, 5 mM HEPES, 0.5 mM EDTA, 0.1% bovine serum albumin (BSA)
(pH 7.4)] with Dounce tissue grinders. The homogenate was subjected
to centrifugation at 1,000 × g for 10 min and supernatant was then
centrifuged at 13,000 × g for 10 min. The mitochondrial pellet was
subsequently suspended in isolation buffer B (210 mM mannitol, 70
mM sucrose, 10 mM succinate, 10 mM HEPES, 0.1% BSA, pH 7.4) for use
in later steps. The freshly prepared mitochondria were used within
4 h, and all procedures were performed at 4°C.
Standard incubation procedures
The mitochondrial suspension was centrifuged prior
to incubation. The pellet was resuspended with respiration medium
containing 230 mM mannitol, 70 mM sucrose, 5 mM succinate, 3 mM
HEPES and 2 mM Tris-phosphate (pH 7.4). Spermine (1 mM) was added
to the isolated mitochondria (1 mg of protein), except for the
control group. PP2 (10 µM) or vanadate (1 mM), inhibitors of SFK or
phosphatase, respectively, was then added to regulate tyrosine
phosphorylation. Incubation was conducted for 30 min at 20°C.
Mitochondrial protein concentrations were determined using the BCA
Protein Assay kit.
Mitochondrial respiration control
ratio (RCR)
Mitochondrial RCR was assessed with Oxygraph
Clark-Type electrodes (Hansatech Instruments, Norfolk, England) as
described previously (21). In a
sealed chamber, the incubated mitochondria were added to the
respiration buffer (125 mM KCl, 2.5 mM
KH2PO4, 2 mM MgCl2, 20 mM HEPES,
0.1% BSA, pH 7.2) to yield a final protein concentration of 500
µg/ml. Measurement of mitochondrial respiration (oxygen
consumption) was initiated by addition of pyruvate (5 mM) and
malate (2.5 mM), which are the oxidative substrates of complex І,
designated as state II respiration. This was followed by the
addition of adenosine diphosphate (ADP; 1 mM) to induce state III
respiration, and then addition of oligomycin (1 µM) as state IV
respiration. RCR was calculated as the ratio of the slope of the
state III respiration curve to the slope of the state IV
respiration curve, which is considered to be a sensitive measure of
mitochondrial function, indicating the integrity of the inner
membrane and the capacity to perform oxidative phosphorylation.
Determination of mitochondrial
membrane potential (∆Ψm)
To measure ∆Ψm, JC-1, a lipophilic cation-susceptive
fluorescent probe, was added to the mitochondria according to
manufacturer's instructions. Briefly, 0.9 ml JC-1 dye working
solution (0.2X) was added to 0.1 ml mitochondria (0.1 mg of
protein) at the beginning of standard incubation (as described
above). After mixing with other reagents, 0.2 ml suspension was
added into each well of a 96-well microplate. Fluorescence
intensity was instantly measured by time scan using a
fluorospectro-photometer (Shimadzu, Kyoto, Japan) with red and
green channels. JC-1 aggregates were formed in the presence of a
higher ∆Ψm, emitting red fluorescence (excitation 525 nm, emission
590 nm), whereas monomers were formed in the presence of a lower
∆Ψm, indicated by green fluorescence (excitation 490 nm, emission
530 nm). The emissions ratio of aggregates to monomers was used to
determine ∆Ψm levels. In addition, carbonyl cyanide
m-chlorophenyl hydrazone (CCCP), an uncoupler of oxidative
phosphorylation, was added as positive control for inner membrane
depolarization.
Ca2+-induced MPTP
opening
MPTP opening was evaluated through Ca2+
overload in vitro, as previously described (22). Following incubation with various
reagents, isolated mitochondria were added into a 96-well
microplate with 0.2 ml of respiration medium. CaCl2 (100
µM) was added to each well in order to abruptly increase the
extramitochondrial Ca2+ concentration. As a
Ca2+ buffering organelle, excess Ca2+ was
taken up by mitochondria, resulting in an elevation of the
Ca2+ concentration in the mitochondrial matrix.
Accumulated Ca2+ induced MPTP opening, facilitating an
influx of H2O into the mitochondrial matrix according to
the change of osmotic pressure. Water influx led to mitochondrial
swelling, accompanied by an increase in volume and attenuation of
absorbance at 540 nm; the decreasing degree of absorbance was used
as an indicator of the sensitivity of MPTP to Ca2+
overload. The 540 nm absorbance of the mitochondrial suspension was
monitored with a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) every two min. CsA (1 µM), an inhibitor of MPTP,
was used as positive control.
Western blot analysis
Western blot analysis was used to detect the
phosphorylation level of residue Y419, the activation site, and
residue Y530, the inhibition site of Src kinases. Mitochondria were
incubated at 20°C with spermine in the presence of PP2 or vanadate
and, after 30 min, the reaction was stopped by the addition of
lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 2
mM EGTA, 0.2% Triton X-100, 0.3% NP-40, 1 mM DTT and 100 µM
phenylmethylsufonyl fluoride. Following incubation on ice for 30
min, the mixtures were centrifuged at 12,000 × g for 15 min to
remove mitochondrial membrane fragments. The protein concentration
was also determined by BCA Protein Assay kit. Equal amounts of
protein from groups incubated with different agents were loaded and
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis, and the samples were then transferred to
polyvinylidene difluoride membranes using a Semi-Dry Transfer Cell
(Bio-Rad Laboratories, Inc.). The membrane was blocked in TBST
solution with 5% skimmed milk at room temperature for 2 h.
Subsequently, the membrane was incubated overnight at 4°C with
primary antibodies against Src (dilution, 1:5,000) and phospho-Y419
(dilution, 1:5,000) and phospho-Y530 (dilution, 1:500) diluted in
TBST. Following three washes, the membrane was incubated with the
AP-IgG secondary antibody (dilution, 1:5,000) for 1 h at room
temperature. The protein bands were detected using a FluorChem E
imaging analysis system and AlphaView software 3.4.0.0 (Cell
Biosciences, Inc., Santa Clara, CA, USA). Src was used as the
loading control for semi-quantitative analysis.
Statistical analysis
All data are presented as the mean ± standard error.
Data from RCR and western blotting were analyzed by one-way
analysis of variance (ANOVA) among the groups using SPSS 18.0
software package (SPSS, Inc., Chicago, IL, USA). When appropriate,
a post-hoc test was performed using least significant differences
(LSD) test. Data analysis from the detection of
Ca2+-induced MPTP opening and ∆Ψm was performed by a
repeated-measures ANOVA and LSD post-hoc test over the experimental
period. The differences among the groups were considered
significant at P<0.05 (two-tailed).
Results
Spermine increases the RCR of spinal
cord mitochondria
The effects of spermine on mitochondrial respiration
(state III, state IV and RCR) were examined following incubation
with spermine, PP2 or vanadate, as well as in the control group
(Fig. 1), indicating that spermine
markedly altered the mitochondrial bioenergetics of oxidative
phosphorylation. Typical curves representing mitochondrial
respiration in the spermine and control group are shown in Fig. 1A, illustrating the successful
isolation of the mitochondria by a procedure with high functional
quality. Treatment with spermine led to an apparent increase in the
slope of ADP phosphorylation (state III). While the sole PP2, an
inhibitor of Src kinases, marginally decreased the slope of state
III. Based on the post-hoc analysis of total data, the magnitude of
state III respiration following spermine treatment was increased by
16% compared with the control (P<0.05; Fig. 1C). However, no significant changes
were detectable in state IV respiration between the spermine and
control groups (P>0.05; Fig. 1A and
C). Consequently, there was a significant increase in the RCR
of the spermine group compared with the control (P<0.05;
Fig. 1D). Representative
respiration curves following the addition of spermine with PP2 are
shown in Fig. 1B; in the spermine
with PP2 group, state III respiration was observed to be decreased
by 40% compared with the spermine group and by 22% compared with
the control group (P<0.05; Fig.
1C), and state III respiration had no significant difference
compared with the sole PP2 group (P>0.05; Fig. 1C). Due to the absence of
significant changes in state IV respiration, the RCR of spermine
with PP2 was decreased by 47% compared with the spermine group
(P<0.01), suggesting that Src kinases were the major regulators
of ADP phosphorylation (Fig. 1D).
Following the addition of vanadate, the curves of state III
respiration, state IV respiration and RCR exhibited similar
tendencies to those in the spermine group, as shown in Fig. 1B, which further demonstrated that
tyrosine phosphorylation was modulated by spermine.
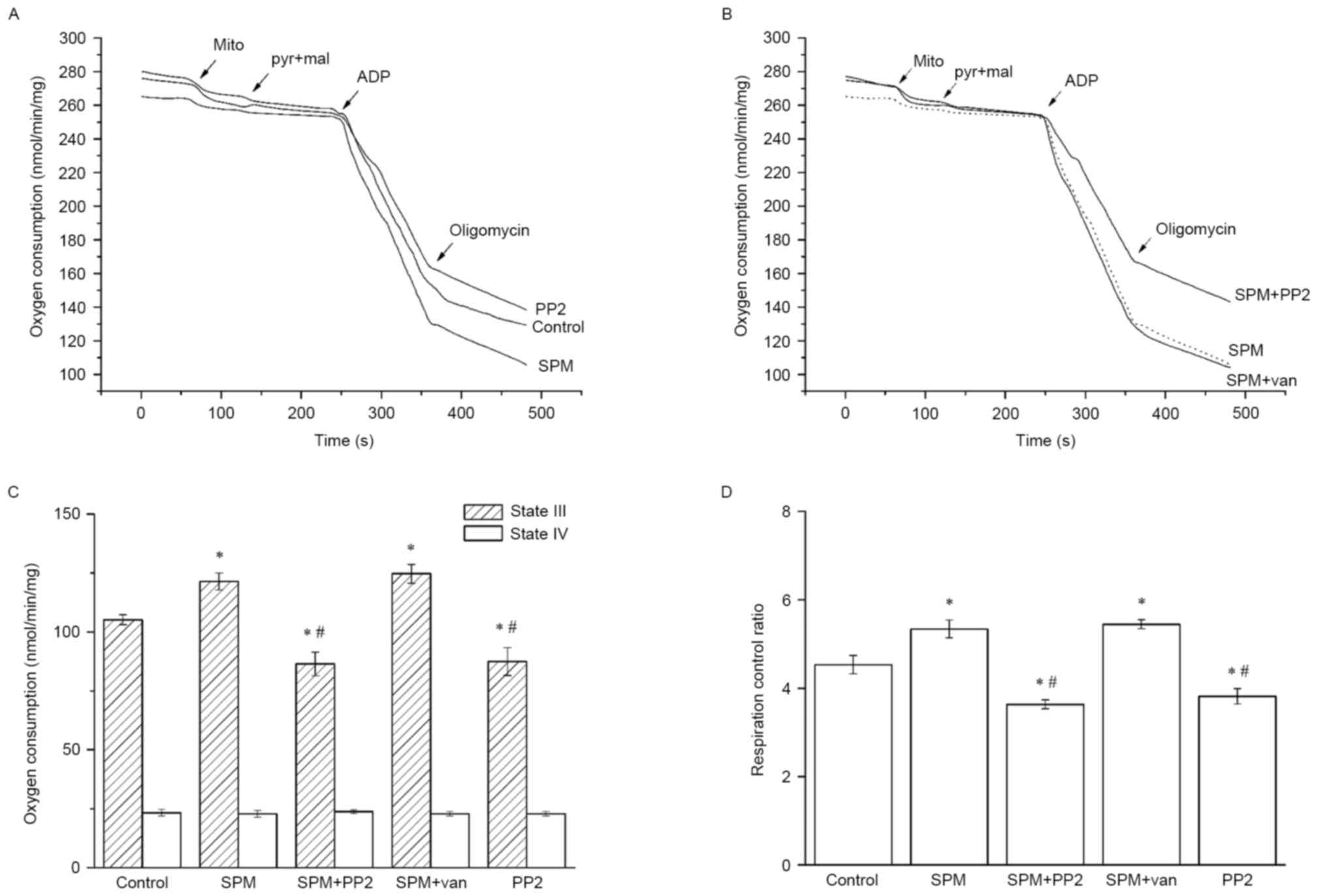 | Figure 1.Effects of spermine on mitochondrial
oxidative phosphorylation. Isolated mitochondria (500 µg of
protein) were incubated in respiration medium including 230 mM
mannitol, 70 mM sucrose, 5 mM succinate, 3 mM HEPES and 2 mM
Tris-phosphate (pH 7.4) for 30 min before oxygen consumption
measurements using Clark-type electrode. (A) Respiration curves of
incubated mitochondria. Respiration medium was in the presence of
spermine (1 mM) or sole PP2 (10 µM). Addition times of
mitochondria, pyruvate (5 mM) and malate (2.5 mM), ADP (1 mM),
oligomycin (1 µM, an inhibitor of ATP Synthase) were labeled in the
figure by arrows. (B) Respiration curves of mitochondria with
spermine and inhibitors. Mitochondria were subjected to spermine
addition PP2 (10 µM) or vanadate (1 mM). The slopes represented the
velocities of oxygen consumption. (C) Oxygen consumption of state
III and state IV respiration. State III respiration was induced by
addition of ADP and state IV respiration was began with oligomycin.
To the state III respiration, spermine significantly increased the
oxygen consumption compared with the control, whereas this effect
was partially inhibited by sole or additional PP2. However, there
was no significant difference between the groups of state IV
respiration. (D) Mitochondrial RCR. The RCR is the slopes ratio of
state III to state IV which reflected the mitochondrial
bioenergetics and oxidative phosphorylation. The RCR was
significantly increased by addition of spermine compared with the
control, however, sole or additional PP2 both attenuated the RCR
compared with the spermine group. SPM spermine, van vanadate, mito
mitochondria, pyr pyruvate, mal malate. Results shown are mean ± SD
with n=6 for each group. *P<0.05 vs. control group;
#P<0.05 vs. spermine group. PP2,
amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; ADP,
adenosine diphosphate; ATP, adenosine triphosphate; RCR,
respiratory control ratio. |
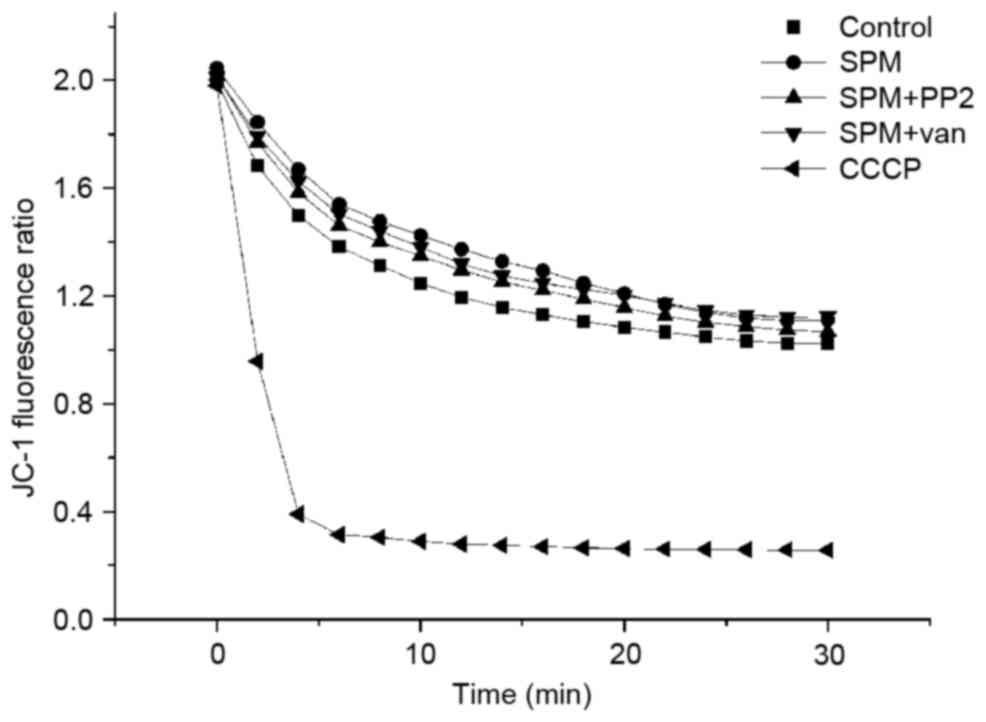
Spermine maintains ∆Ψm
independently of tyrosine phosphorylation
In energized mitochondria, aggregates of JC-1
accumulate in the mitochondrial matrix emitting red fluorescence
due to the potential difference across the inner membranes.
Following membrane depolarization, aggregates dissociate into
monomers with green fluorescence. Thus, the fluorescence intensity
ratio of aggregates to monomers reflects variations in ∆Ψm. As
shown in Fig. 2, the addition of
spermine marginally increased the ∆Ψm ratio compared with the
control group (P<0.05). However, this observation opposed
results previously obtained in liver mitochondria, in which the
positive charges of the mitochondria neutralized the negative
potential in the matrix, leading to a decrease in ∆Ψm (23). The different findings may be
explained by the fatty acid contents of membrane phospholipids; as
abundant fatty acids within the central nervous system
mitochondrial membranes in the spinal cord have a physiologically
high proton permeability, which respond rapidly to a slight change
of ∆Ψm (19). In the presence of
positive charges, spermine partially neutralized the anionic groups
of membrane phospholipids, resulting in an increase of ∆Ψm value.
Also, in this case, ∆Ψm was not influenced by the addition of PP2
or vanadate compared with the spermine group (P>0.05). The
uncoupling reagent CCCP (used as a positive control) caused an
instant dissipation of ∆Ψm due to the opening of the membrane
proton leak channel.
Spermine inhibits MPTP opening in
isolated spinal cord mitochondria
A reduction in absorbance may be observed in
response to the swelling of mitochondria, reflecting the opening of
ion channels and membrane pores. To clarify whether the
Ca2+-activated channels or membrane pores were MPTPs,
CsA, a specific inhibitor of MPTP, was added to the buffer solution
as positive control. The change in relative absorbance at 540 nm
(ΔA540) over the initial 20 min was assessed to evaluate the
functions of spermine. As shown in Fig. 3A, CaCl2 induced rapid
swelling of energized mitochondria in the standard respiration
medium; however, this effect was greatly inhibited by CsA,
indicating that MPTPs were only Ca2+-activated channel
or pores. In the presence of spermine (1 mM), ΔA540 was attenuated
by 40% compared with the control group (P<0.01), suggesting that
spermine may incompletely inhibit MPTP opening.
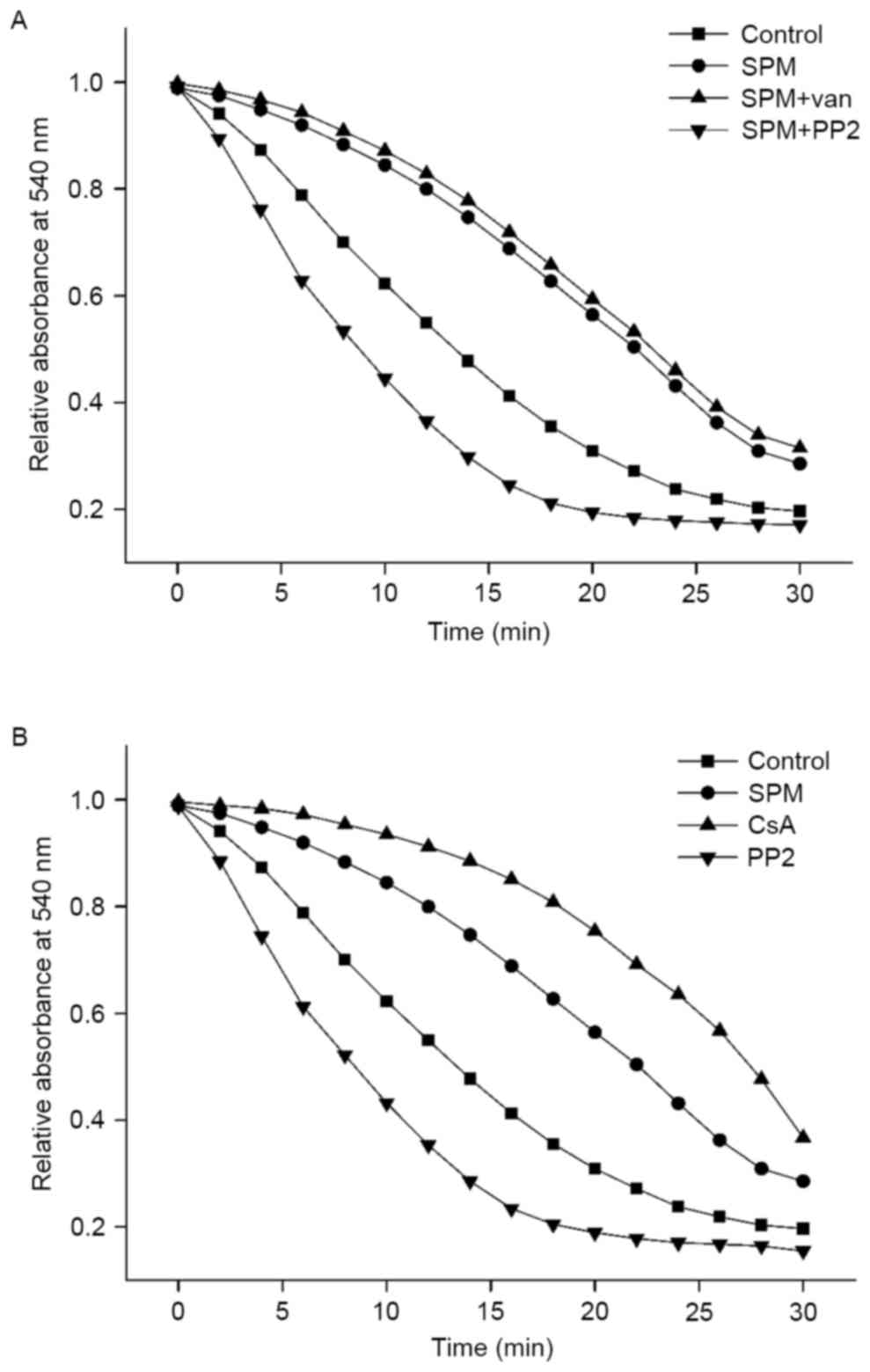 | Figure 3.Effects of spermine on
Ca2+-induced MPTP opening. Mitochondria (1 mg of
protein) were incubated in respiration medium as Fig. 1 described with spermine (1 mM)
except the control, sole PP2 and CsA curves. (A) The MPTP opening
curves of mitochondria with spermine and inhibitors. PP2 (10 µM) or
vanadate (1 mM), inhibitor of SFK or phosphatase, was added to
spermine for regulation tyrosine phosphorylation. (B) The MPTP
opening curves of mitochondria with control reagents. CsA (1 µM)
was used to prevent the MPTP opening as positive control. PP2 (10
µM) without spermine was used to inhibit tyrosine phosphorylation.
Absorbance at 540 nm was monitored in the presence of
CaCl2 (100 µM). SPM spermine, van vanadate, CsA
cyclosporine A. Each plot is representative of six independent
measurements. MPTP, mitochondrial permeability transition pores;
PP2, amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine;
CsA, cyclosporin A; SFK, Src family kinases. |
To identify the regulatory effect of spermine on
tyrosine phosphorylation, PP2 (10 µM), with or without spermine,
was added into the respiration medium, both resulting in
accelerated swelling of mitochondria and significantly increased
rate of ΔA540. The curves of spermine with PP2 group and
sole PP2 had an approximate tendency, and the relative absorbance
both decreased sharply, reaching its plateau after ~14 min, which
was a shorter time interval than that in other group. These
findings implied that tyrosine dephosphorylation may increase the
sensitivity of MPTP opening. As tyrosine phosphorylation is
regulated by kinases and phosphatases, vanadate (1 mM), an
inhibitor of phosphatases, was used to enhance phosphorylation;
this slightly attenuated the ΔA540 compared with the
spermine group, indicating that vanadate and spermine had
homologous effects on the regulation of MPTP opening.
Spermine upregulates the
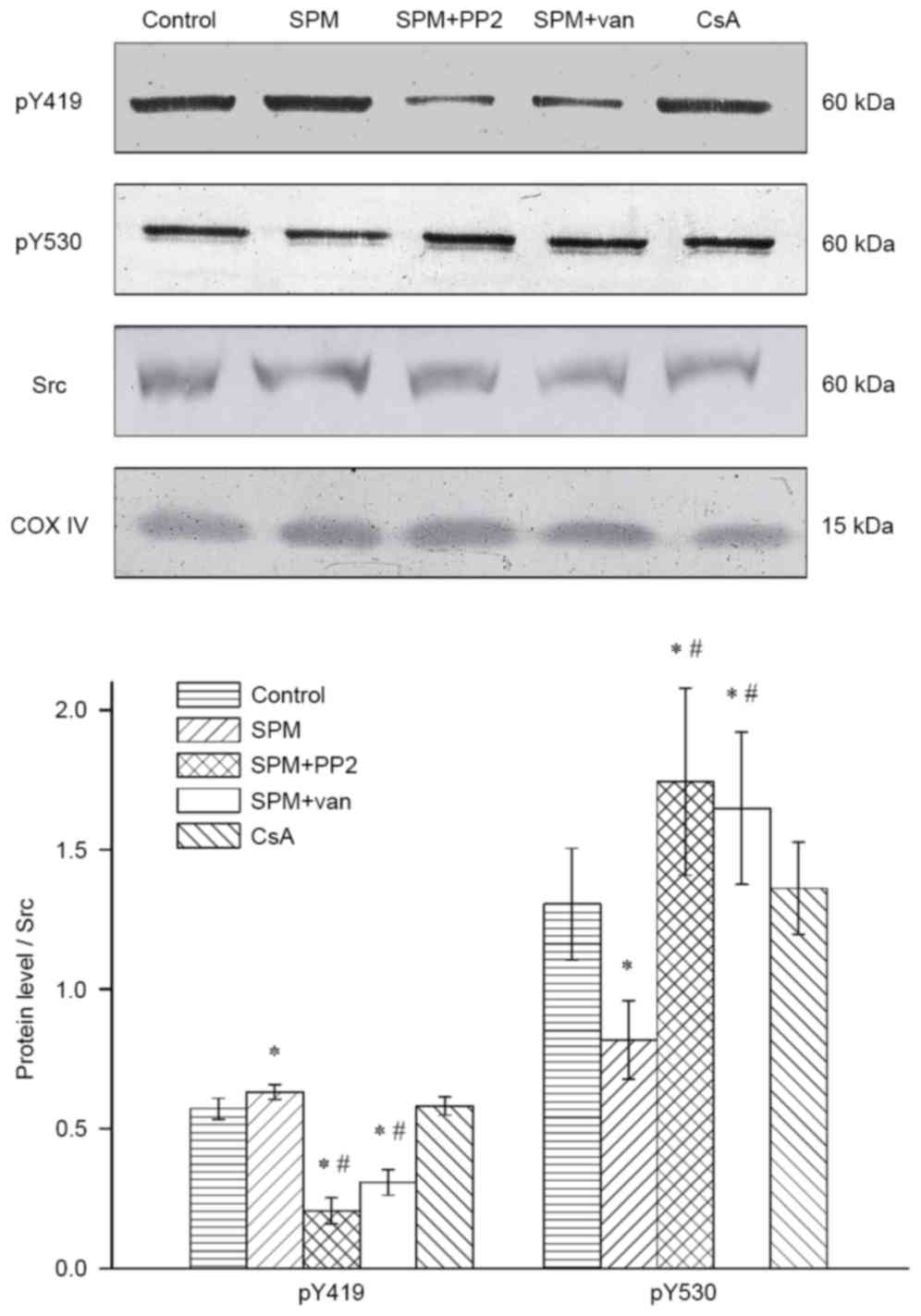
phosphorylation levels of Src kinases within mitochondria
The protein expression levels of Src kinases were
evaluated by immunoblot analysis (Fig.
4). This illustrated that spermine treatment was associated
with a significant upregulation of activated Src kinase
(phospho-Y419) compared with the control group (P<0.05).
Compared with the spermine-treated mitochondria, the addition of
PP2 significantly downregulated the expression level of
phospho-Y419 (P<0.01). Notably, vanadate exhibited a parallel
function: reducing the phosphorylation of residues Y419, as
observed for PP2. This may be related to the modification of Src
kinase activation; the process of activation is initiated by the
dephosphorylation of inhibitory residues Y530, which is performed
by tyrosine phosphatases. As an inhibitor of phosphatases, vanadate
prevented the activation of residues Y419.
To ascertain whether CsA induced tyrosine
phosphorylation of the MPTP subunits, CsA was individually added
into the mitochondria and western blot analysis was subsequently
performed. The results showed that there was no significant
difference compared with the control group (P>0.05). These
findings suggest that spermine may be a crucial regulator of Src
kinases.
Discussion
The present study provides evidence that exogenous
spermine significantly increases oxidative phosphorylation activity
and inhibits the opening of MPTP in mitochondria isolated from the
spinal cord. Furthermore, SFKs play an essential role in modulating
these physiological processes, and are dependent on reversible
phosphorylation and dephosphorylation. These data suggest that
spermine potently preserves mitochondrial bioenergetics by
regulating the activities of SFKs.
Mitochondrial dysfunction can induce a series of
pathophysiological alterations, including a deficiency of ATP
synthesis, increase of ROS production and release of pro-apoptotic
factors, which further cause the necrosis and apoptosis of cells,
as well as edema and inflammation within tissues. These process are
ubiquitous in hepatic injury, myocardial ischemia reperfusion, and
particularly evident in secondary SCI (2). Several previous studies reported that
spermine could improve the mitochondrial functions in various
tissue, whereas the underlying mechanisms were quite different
between central nervous system and other organs (24,25).
In brain tissue, the transport process of spermine into
mitochondria were regulated by tyrosine phosphorylation, which was
differ from liver mitochondria regulation by peroxides (19). In the present study, it was
confirmed that spermine induces an increase of RCR and suppresses
MPTP opening, which indicates that spermine is involved in
mitochondrial protection in isolated spinal cord. Consistently,
several studies have demonstrated the beneficial effects of
spermine on mitochondria. For example, spermine has been shown to
buffer excess mitochondrial Ca2+ to the equivalent level
as in the cytosol (26). As a free
radical scavenger, spermine can suppress ROS production and retain
the reduced sulfhydryl groups at normal levels, exerting an
antioxidative effect (27,28). It is also reported that exogenous
spermine stabilizes the membrane structures in myocardial
ischemia-reperfusion (29).
Furthermore, spermine appears to inhibit inflammation in microglial
cells (30). However, these
conclusions conflict with other hypotheses that spermine is
detrimental to cellular metabolism, as it may produce ROS and
induce MPTP opening (14). The
opposing functions reported for spermine may be caused by the
following factors: (i) according to the concentration, spermine may
exert dual effects on the mitochondria (31); (ii) depending on the metabolic
state, spermine may generate spermine dialdehyde and hydrogen
peroxide, which are oxidizing and toxic for cells (32); (iii) as a second messenger,
spermine may participate in the signal transduction of various
signaling pathways, which intersect with one another and may even
exert differential targeting effects. To the best of our knowledge,
the definitive mechanism of spermine has not been clarified in
spinal cord mitochondria. Therefore, the present study investigated
the regulatory effect of spermine on SFKs, which are fundamental
signaling components within mitochondria.
Considering that oxidative phosphorylation is the
central target of SFKs (33), our
first hypothesis deals with the possible effect of spermine in
altering mitochondrial respiration, and the results appear to
confirm this effect. In the measurement of respiration, state III
reflects the maximum rate of coupled respiration, and state IV
reflects the capacity of proton reflux (10). Therefore, the RCR represents the
ability to integrate oxidative phosphorylation. As shown in
Fig. 1, the process of oxidative
phosphorylation is enhanced by spermine, as demonstrated by the
increase of RCR and the oxygen consumption of state III. This
effect is not associated with a change in state IV respiration, as
the inner membrane remains intact and the ∆Ψm remains at
physiological levels (Fig. 2).
These results are opposite to that in liver (23). The membrane potential of liver
mitochondria is readily decreased by charge neutralization of
spermine, while abundant fatty acids within spinal cord
mitochondria increase the charge flow across the inner membrane,
and ultimately maintain the ∆Ψm in physiological levels
(34). Furthermore, PP2, with or
without spermine, markedly attenuates the activity of the electron
transport chain, illustrating the activating effect of spermine on
Src kinases. It should be noted that several subunits of the
electron transport chain are regulated by Src kinases in rat brain
mitochondria, including the 39 kDa subunit of complex I,
flavoprotein of complex II, core protein 2 of complex III, subunit
II of complex IV and the α and β subunits of complex V (35). These subunits are activated by the
phosphorylation of a tyrosine residue. Meanwhile, the tyrosine
residues are inactivated by tyrosine phosphatases. In this case,
vanadate inhibits dephosphorylation, increasing RCR and the oxygen
consumption of state III.
Subsequently, we hypothesized that spermine inhibits
MPTP opening by regulating tyrosine kinases. The molecular
constituents of the MPTP remain controversial; however, several
integral proteins have been confirmed to comprise the MPTP,
including VDAC in the mitochondrial outer membrane, AdNT in the
inner membrane and cyclophilin D in the matrix (36). The N-terminal region of VDAC is the
site of tyrosine phosphorylation (37), and AdNT is known to be
phosphorylated on Y190 and Y194 tyrosine residues (17). Therefore the functions of VDAC and
AdNT are conspicuously influenced by tyrosine phosphorylation. As
shown in Fig. 3, 1 mM spermine
inhibits the opening of MPTPs, the effect of which is partially
abolished by PP2, indicating that spermine is involved in
regulating the activity of tyrosine kinases. This effect supports
the role of spermine in the oxidative respiratory chain. This
inhibition on MPTP of spermine is in accordance with that in liver
mitochondria as previous reported (24). However, the mechanism of spermine
in liver is enhancement the inhibitory effects of ADP rather than
the regulation of tyrosine phosphorylation. The degree of opening
of the MPTP is influenced by the mitochondrial physiological
status. Exogenous Ca2+ is primarily absorbed through a
specific uniporter, and this process is dependent on the normal
∆Ψm level (11). As
shown in Fig. 2, spermine and
other reagents marginally alter the ∆Ψm, which maintains
the ∆Ψm at physiological levels. The results of
Ca2+-induced mitochondrial swelling are dramatically
affected by the extent of channel/pore opening in the inner
membranes, other than MPTPs. CsA, as a specific inhibitor, is used
to suppress the opening of the MPTP by binding to cyclophilin D in
the matrix (38). As shown in
Fig. 3, CsA effectively
counteracted the influx of water from the cytoplasm, illustrating
that other channels or pores may be partially opened by
Ca2+.
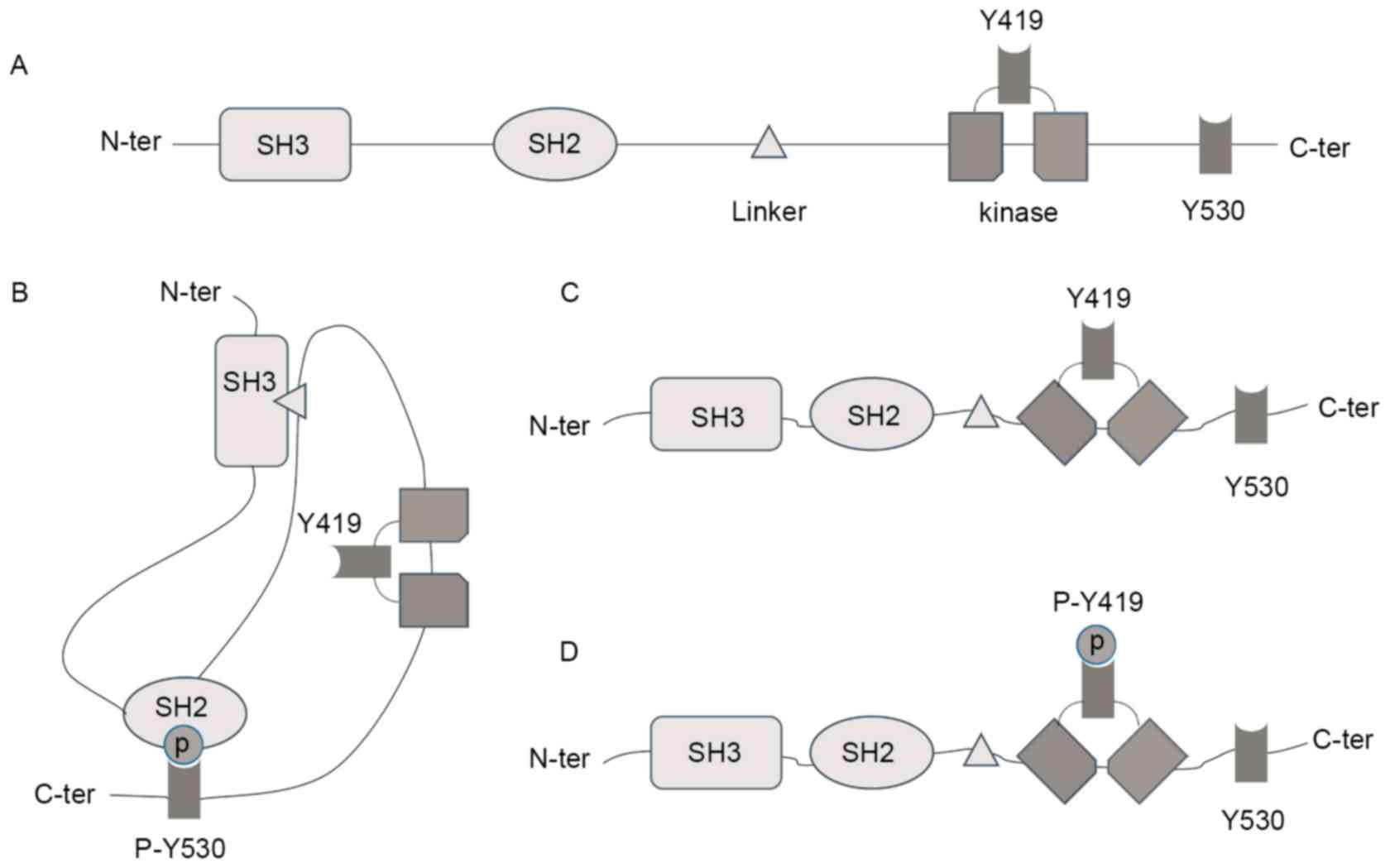
There are 11 SFKs, including Lck, Lyn, c-Src, Srm,
Blk, Brk, Fgr, Frk, Fyn, Hck and Yes (39). Among the different members, only
Lyn, c-Src, Fgr and Fyn are expressed in the mitochondria (40), and they share a similar domain
(Fig. 5A). The activity of Src
kinases is primarily regulated by the Y419 residue and the
C-terminal Y530 residue (41). The
phosphorylated Y530 binds to the SH2 domain, resulting in a closed
and inactive conformation, which masks the active Y419 residue
(Fig. 5B). In rat brain
mitochondria, tyrosine phosphatases, such as SHP-2 and PTP1B,
induce the dephosphorylation of Y530 residues (42). The dissociation between the SH2
domain and the Y530 residue exposes the Y419 residue (Fig. 5C). Src kinases are then activated
by auto-phosphorylation of Y419 (Fig.
5D). As an inhibitor of phosphatases, vanadate attenuates the
dephosphorylation of Y530 and as a result, reduces the level of
activated SFKs.
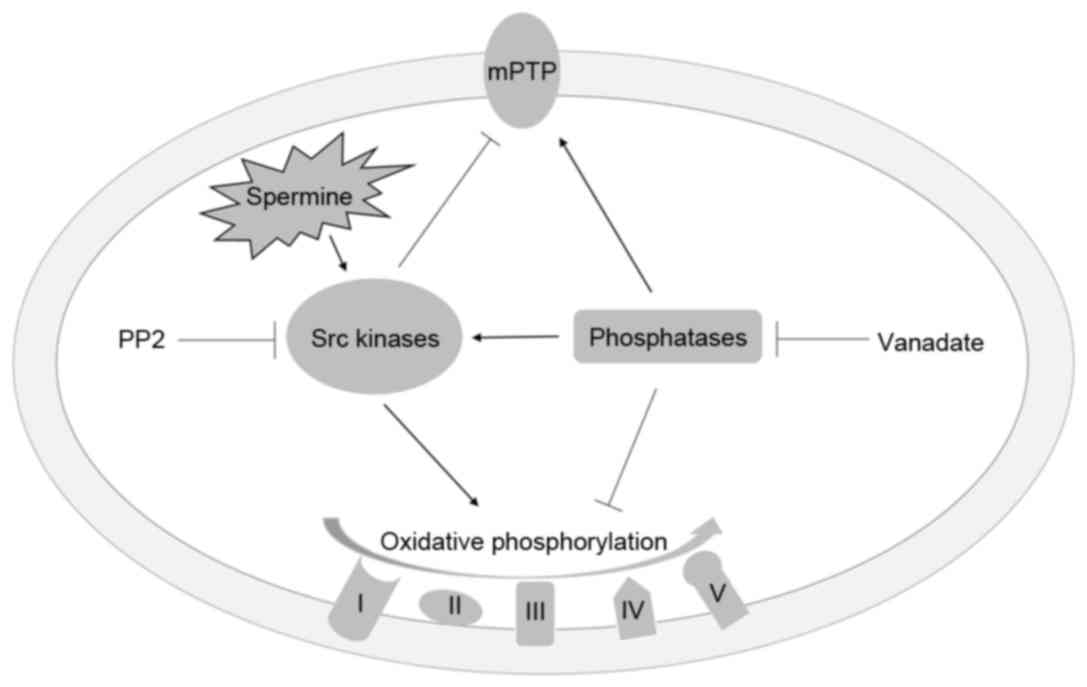
The mechanism of action of spermine within
mitochondria is illustrated in Fig.
6 according to the present experimental findings. Spermine
activates SFKs, and activated SFKs then induce the phosphorylation
of certain subunits of respiratory complexes to accelerate the
processes of electron transfer and ATP synthesis. Additionally, Src
kinases inhibit MPTP opening by regulating the function of VDAC and
AdNT. The phosphatases reverse the effect of SFKs by
dephosphorylation of tyrosine residues of the respiratory chain
complexes and MPTPs. However, SFKs are activated by phosphatases
through dephosphorylation of the Y530 residue. This may be due to
negative feedback within the mitochondria. Instead, in liver
mitochondria, which have no SFKs, the mitochondrial functions are
mainly regulated by the thiol groups and redox state (19). Therefore, the spermine presents
diverse mechanisms on distinct organs.
In summary, the results of the present study
demonstrate that spermine enhances respiratory processes and
reduces the degree of MPTP opening in isolated mitochondria of the
spinal cord. The mitochondria-protective properties of spermine
probably arise from the regulation on Src kinases, which are the
initiating signaling factors of the tyrosine kinase pathway.
Further work will evaluate whether secondary SCI is alleviated by
spermine in rat models and, if possible, identify the site of
action of spermine on SFKs.
Glossary
Abbreviations
Abbreviations:
|
RCR
|
respiratory control ratio
|
|
MPTP
|
mitochondrial permeability transition
pore
|
References
|
1
|
Hall ED and Springer JE: Neuroprotection
and acute spinal cord injury: A reappraisal. NeuroRx. 1:80–100.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lewen A, Matz P and Chan PH: Free radical
pathways in CNS injury. J Neurotrauma. 17:871–890. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chinopoulos C and Adam-Vizi V:
Mitochondrial Ca2+ sequestration and precipitation
revisited. FEBS J. 277:3637–3651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pivovarova NB and Andrews SB:
Calcium-dependent mitochondrial function and dysfunction in
neurons. FEBS J. 277:3622–3636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szabo I and Zoratti M: The mitochondrial
megachannel is the permeability transition pore. J Bioenerg
Biomembr. 24:111–117. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zoratti M and Szabó I: The mitochondrial
permeability transition. Biochim Biophys Acta. 1241:139–176. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sesso A, Marques MM, Monteiro MM,
Schumacher RI, Colquhoun A, Belizário J, Konno SN, Felix TB,
Botelho LA, Santos VZ, et al: Morphology of mitochondrial
permeability transition: morphometric volumetry in apoptotic cells.
Anat Rec A Discov Mol Cell Evol Biol. 281:1337–1351. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christofferson DE and Yuan J: Necroptosis
as an alternative form of programmed cell death. Curr Opin Cell
Biol. 22:263–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duprez L, Wirawan E, Vanden Berghe T and
Vandenabeele P: Major cell death pathways at a glance. Microbes
Infect. 11:1050–1062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McEwen ML, Sullivan PG, Rabchevsky AG and
Springer JE: Targeting mitochondrial function for the treatment of
acute spinal cord injury. Neurotherapeutics. 8:168–179. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dalla Via L, Di Noto V, Siliprandi D and
Toninello A: Spermine binding to liver mitochondria. Biochim
Biophys Acta. 1284:247–252. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siliprandi D, Toninello A and Dalla Via L:
Bidirectional transport of spermine in rat liver mitochondria.
Biochim Biophys Acta. 1102:62–66. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pezzato E, Battaglia V, Brunati AM,
Agostinelli E and Toninello A: Ca2+ -independent effects
of spermine on pyruvate dehydrogenase complex activity in energized
rat liver mitochondria incubated in the absence of exogenous
Ca2+ and Mg2+. Amino Acids. 36:449–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agostinelli E, Tempera G, Molinari A,
Battaglia V, Toninello A and Arancia G: The physiological role of
biogenic amines redox reactions in mitochondria. New perspectives
in cancer therapy. Amino Acids. 33:175–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sava IG, Battaglia V, Rossi CA, Salvi M
and Toninello A: Free radical scavenging action of the natural
polyamine spermine in rat liver mitochondria. Free Radic Biol Med.
41:1272–1281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cesaro L and Salvi M: Mitochondrial
tyrosine phosphoproteome: New insights from an up-to-date analysis.
Biofactors. 36:437–450. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lewandrowski U, Sickmann A, Cesaro L,
Brunati AM, Toninello A and Salvi M: Identification of new tyrosine
phosphorylated proteins in rat brain mitochondria. FEBS Lett.
582:1104–1110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pantic B, Trevisan E, Citta A, Rigobello
MP, Marin O, Bernardi P, Salvatori S and Rasola A: Myotonic
dystrophy protein kinase (DMPK) prevents ROS-induced cell death by
assembling a hexokinase II-Src complex on the mitochondrial
surface. Cell Death Dis. 4:e8582013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Battaglia V, Tibaldi E, Grancara S, Zonta
F, Brunati AM, Martinis P, Bragadin M, Grillo MA, Tempera G,
Agostinelli E and Toninello A: Effect of peroxides on spermine
transport in rat brain and liver mitochondria. Amino Acids.
42:741–749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Battaglia V, Grancara S, Satriano J,
Saccoccio S, Agostinelli E and Toninello A: Agmatine prevents the
Ca(2+)-dependent induction of permeability transition in rat brain
mitochondria. Amino Acids. 38:431–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sullivan PG, Dubé C, Dorenbos K, Steward O
and Baram TZ: Mitochondrial uncoupling protein-2 protects the
immature brain from excitotoxic neuronal death. Ann Neurol.
53:711–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kristal BS, Park BK and Yu BP:
4-Hydroxyhexenal is a potent inducer of the mitochondrial
permeability transition. J Biol Chem. 271:6033–6038. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salvi M, Battaglia V, Mancon M, Colombatto
S, Cravanzola C, Calheiros R, Marques MP, Grillo MA and Toninello
A: Agmatine is transported into liver mitochondria by a specific
electrophoretic mechanism. Biochem J. 396:337–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grancara S, Battaglia V, Martinis P,
Viceconte N, Agostinelli E, Toninello A and Deana R: Mitochondrial
oxidative stress induced by Ca2+ and monoamines:
Different behaviour of liver and brain mitochondria in undergoing
permeability transition. Amino Acids. 42:751–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bonaiuto E, Grancara S, Martinis P,
Stringaro A, Colone M, Agostinelli E, Macone A, Stevanato R,
Vianello F, Toninello A and Di Paolo ML: A novel enzyme with
spermine oxidase properties in bovine liver mitochondria:
Identification and kinetic characterization. Free Radic Biol Med.
81:88–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salvi M and Toninello A: Effects of
polyamines on mitochondrial Ca(2+) transport. Biochim Biophys Acta.
1661:113–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lapidus RG and Sokolove PM: Spermine
inhibition of the permeability transition of isolated rat liver
mitochondria: An investigation of mechanism. Arch Biochem Biophys.
306:246–253. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lapidus RG and Sokolove PM: The
mitochondrial permeability transition. Interactions of spermine,
ADP and inorganic phosphate. J Biol Chem. 269:18931–18936.
1994.PubMed/NCBI
|
|
29
|
Zhao YJ, Xu CQ, Zhang WH, Zhang L, Bian
SL, Huang Q, Sun HL, Li QF, Zhang YQ, Tian Y, et al: Role of
polyamines in myocardial ischemia/reperfusion injury and their
interactions with nitric oxide. Eur J Pharmacol. 562:236–246. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi YH and Park HY: Anti-inflammatory
effects of spermidine in lipopolysaccharide-stimulated BV2
microglial cells. J Biomed Sci. 19:312012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei C, Li HZ, Wang YH, Peng X, Shao HJ, Li
HX, Bai SZ, Lu XX, Wu LY, Wang R and Xu CQ: Exogenous spermine
inhibits the proliferation of human pulmonary artery smooth muscle
cells caused by chemically-induced hypoxia via the suppression of
the ERK1/2- and PI3K/AKT-associated pathways. Int J Mol Med.
37:39–46. 2016.PubMed/NCBI
|
|
32
|
Dalla Via L, Mammi S, Uriarte E, Santana
L, Lampronti I, Gambari R and Gia O: New furan side tetracyclic
allopsoralen derivatives: synthesis and photobiological evaluation.
J Med Chem. 49:4317–4326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grancara S, Zonta F, Ohkubo S, Brunati AM,
Agostinelli E and Toninello A: Pathophysiological implications of
mitochondrial oxidative stress mediated by mitochondriotropic
agents and polyamines: The role of tyrosine phosphorylation. Amino
Acids. 47:869–883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tahin QS, Blum M and Carafoli E: The fatty
acid composition of subcellular membranes of rat liver, heart, and
brain: Diet-induced modifications. Eur J Biochem. 121:5–13. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Augereau O, Claverol S, Boudes N, Basurko
MJ, Bonneu M, Rossignol R, Mazat JP, Gineste C, Hernandez A,
Ivarsson N, Cheng AJ, Naess K, Wibom R, Lesko N, Bruhn H, Wedell A,
Freyer C, et al: Cyclophilin D, a target for counteracting skeletal
muscle dysfunction in mitochondrial myopathy. Hum Mol Genet.
24:6580–6587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Crompton M: On the involvement of
mitochondrial intermembrane junctional complexes in apoptosis. Curr
Med Chem. 10:1473–1484. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Distler AM, Kerner J and Hoppel CL:
Post-translational modifications of rat liver mitochondrial outer
membrane proteins identified by mass spectrometry. Biochim Biophys
Acta. 1774:628–636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gineste C, Hernandez A, Ivarsson N, et al:
Cyclophilin D, a target for counteracting skeletal muscle
dysfunction in mitochondrial myopathy. Hum Mol Genet. 24:6580–6587.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roskoski R Jr: Src kinase regulation by
phosphorylation and dephosphorylation. Biochem Biophys Res Commun.
331:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salvi M, Brunati AM and Toninello A:
Tyrosine phosphorylation in mitochondria: a new frontier in
mitochondrial signaling. Free Radic Biol Med. 38:1267–1277. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hebert-Chatelain E: Src kinases are
important regulators of mitochondrial functions. Int J Biochem Cell
Biol. 45:90–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arachiche A, Augereau O, Decossas M, et
al: Localization of PTP-1B, SHP-2, and Src exclusively in rat brain
mitochondria and functional consequences. J Biol Chem.
283:24406–24411. 2008. View Article : Google Scholar : PubMed/NCBI
|