Introduction
Aloe, an important medicinal herb, has long been
used in traditional Chinese medicine to treat and cure a number of
diseases. Aloin (C21H22O9;
Fig. 1A), an anthrocyclic
glycoside compound, is one of the primary active ingredients (15 to
40%) in aloe plant juice (1).
Aloin has exhibited anti-angiogenesis, anti-inflammatory,
antimicrobial and antiviral pharmacological effects (2). In addition, Aloin has been
demonstrated to reduce cell proliferation and induce cell apoptosis
in different human tumor cells, including Jurkat T-cells, HeLaS3
human cervical cancer cells and A549 human lung cancer cells
(3,4). Therefore, Aloin may be effective as a
novel anticancer agent. However, the anticancer effect of Aloin
remains to be fully elucidated.
p53 serves a pivotal role in controlling cell cycle
progression and cell apoptosis, which is central to its function as
a tumor suppressor (5–8). In resting cells, p53 is a short-lived
protein that is highly regulated and maintained at low or
undetectable levels, due to degradation via the ubiquitin
proteasome signaling pathway (5).
A number of intracellular and extracellular stressors, including
irradiation, chemicals, oxygen-free radicals, hypoxia and very low
nucleotide levels, lead to rapid activation of the p53 protein
(9,10). Under these conditions, the p53
protein may be rescued from degradation, accumulate to high levels
and become phosphorylated on its N-terminal activation sites
(11). Among these phosphorylated
sites, phosphorylation of Serine (Ser)15 and 20 have been revealed
to be essential for the stabilization, induction and
transactivation functioning of p53. In addition, phosphorylation of
Ser392 is responsible for the nuclear import of p53.
Phosphorylation of Threonine 18 (Thr18) is crucial for regulating
interactions between p53 and its regulatory partner mouse double
minute 2 (MDM2) and specific genes, including AIP1, are induced
only when p53 is phosphorylated on Ser46 (12–16).
Phosphorylation of p53 may be mediated by numerous cellular
kinases, including ataxia telangiectasia mutated gene (ATM),
ataxia-telangiectasia and Rad3 related (ATR), DNA-dependent protein
kinase (DNA-PK) and the mitogen-activated protein kinases (MAPKs),
including p38, c-Jun N-terminal kinase and extracellular
signal-regulated kinases (ERK) (17–21).
In addition to phosphorylation, p53 protein may also
be regulated by the E3 ubiquitin ligase, MDM2, which binds to the
N-terminal transactivation domain (TAD) of p53 and inhibits its
transcriptional activities, targeting itself and p53 for
degradation by the proteasome (22). There is a large body of evidence
that suggests that MDM2 has a number of p53-independent effects.
One previous study revealed that MDM2 binds to and ubiquitinates
the retinoblastoma protein, resulting in its degradation and the
release of E2F1, which in turn promotes cell cycle progression
(11). Other studies have
demonstrated that MDM2 may physically interact with the internal
ribosome entry site of the X-linked inhibitor of apoptosis protein
(XIAP) 5′-untranslated region, resulting in translation of the
latter, which generates resistance to cancer therapy (11,23,24).
Therefore, targeting MDM2 itself or the MDM2-p53 interaction, may
improve outcomes of cancer therapy.
Following activation, p53 stimulates a large network
of signals that function through two major apoptotic pathways: The
extrinsic and intrinsic pathways. The former involves regulators of
cluster of differentiation (CD)95 (also known as Fas), which in
turn leads to a cascade of caspase activation, including caspase-3,
−8, −9 and −10. The extrinsic pathway involves regulators of CD95,
caspase-8 and −10, and proapoptotic effectors of B-cell lymphoma 2
(Bcl-2) homologous antagonist killer (BAK), Bcl-2 X-associated
protein (BAX), p53 upregulated modulator of apoptosis (PUMA) and
phorbol-12-myristate-13-acetate-induced protein 1 (NOXA), which
govern mitochondria-dependent cell apoptosis (25–27).
A previous study indicated that Aloin can induce
apoptosis in p53 proficient A549 cells and p53 deficient H1299
cells. In addition, the A549 cells were more susceptible to
Aloin-induced apoptosis than H1299 cells, suggesting the
involvement of p53 in Aloin-induced apoptosis (28). The aim of the present study was to
analyze the p53-dependent mechanisms involved in response to Aloin
treatment. Using the p53-proficient A549 cells, an Aloin-induced
apoptotic cell model was established. Subsequently, the potential
underlying molecular mechanisms were evaluated. The results
indicated that Aloin triggered the generation of reactive oxygen
species (ROS), which in turn activated c-Jun and p38-p53 molecular
pathways, and subsequently induced apoptosis via the intrinsic
apoptotic pathway.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), the TRIzol® RNA purification kit and
Fluo-4 probe were obtained from Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The ROS inhibitor (2R,
4R)-4-aminopyrrolidine-2,4-dicarboxylic acid (APDC) was supplied by
Enzo Life Sciences, Inc. (Farmingdale, NY, USA). The
dichlorodihydrofluorescein diacetate (H2DCF-DA) probe
was purchased from Thermo Fisher Scientific, Inc. MTT, Aloin and
rhodamine 123 were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). The commercial kits for protein isolation and
Bicinchoninic acid (BCA) protein quantification were products of
Bio-Rad Laboratories, Inc. (Hercules, CA, USA). An annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit was obtained from MultiSciences (Lianke) Biotech Co.,
Ltd (Hangzhou, Zhejiang, China). A SYBR® Green
polymerase chain reaction (PCR) Master Mix was purchased from
Applied Biosystems; Thermo Fisher Scientific, Inc. The
phosphorylated (p)-p38 and p-c-Jun inhibitors, SB203580 and
SP600125, were supplied by Beyotime Institute of Biotechnology
(Haimen, China). The PrimeScript Real Time Reagent kit was obtained
from Takara Biotechnology Co., Ltd. (Dalian, China).
Antibodies
Primary antibodies against caspase-3 (cat. no.
sc-56053), caspase-9 (cat. no. sc-81589), caspase-8 (cat. no.
sc-81661), caspase-10 (cat. no. sc-6186), CD95 (cat. no. sc-4276),
p53 (cat. no. sc-126), p53-Ser15 (cat. no. sc-101762), p53-Thr18
(cat. no. sc-135631), p53-Ser20 (cat. no. sc-18,079-R), p53-Ser46
(cat. no. sc-101764), p53-Ser392 (cat. no. sc-7997), p-DNA-PK (cat.
no. sc-101664), p-ATM (cat. no. sc-47739), p-ATR (cat. no.
sc-109912), p-p38 (cat. no. sc-7973), p-c-Jun (cat. no. sc-822) and
p-ERK (cat. no. sc-7976) were all purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The primary antibody against
β-actin, and the IRDye-conjugated anti-rabbit (cat. no. 926-32211),
anti-goat (cat. no. 926-32214) and anti-mouse (cat. no. 926-32280)
secondary antibodies were purchased from LI-COR Biosciences
(Lincoln, NE, USA).
Cell culture
A549 lung cancer cells were supplied by the Cell
Bank of Type Culture Collection of the Chinese Academy of Science
(Shanghai, China). The A549 cells were grown in DMEM medium
supplemented with 10% (v/v) FBS and incubated at 37°C in a
humidified (5% CO2) incubator.
Inhibitor pretreatments
For experiments that included pretreatment with
inhibitors of p-p38, p-c-Jun and ROS, A549 cells were pretreated
with SB203580 (30 µM), SP600125 (10 µM) or APDC (25 µM) for 30 min
at 37°C prior to Aloin (400 µM) treatment for 48 h at 37°C.
Inhibitors were diluted in PBS and added to the cell culture
supernatant. After 48 h Aloin treatment, the supernatant was
immediately removed and experiments were performed.
Cell proliferation assay
A549 cells were plated into 96-well plates at
5×103 cells per well. Following incubation for 24 h, the
cells were exposed to 0 to 400 µM concentrations of Aloin. At 24,
48 and 72 h following exposure, the supernatant was removed and 100
µl (500 µg/ml) MTT solution was added to each well. Following 4 h
of incubation at 37°C, the MTT solution from each well was replaced
with 150 µl dimethyl sulfoxide, which was pipetted up and down
several times to dissolve formazan crystals. The optical density
was measured at a wavelength of 570 nm for each well using a
microtiter plate reader (BioTek Instruments, Inc., Winooski, VT,
USA). The results were expressed as the percentage of the control
(0 µM Aloin).
Measurement of cell apoptosis
The Annexin V-FITC and PI double staining method was
used to analyze the percentage of cells undergoing apoptosis,
according to the manufacturer's protocol. Briefly, A549 cells were
seeded into 6-well plates at 2×105 cells per well.
Following incubation with or without the indicated concentrations
of Aloin (0 to 400 µM) for 48 h, the cells were trypsinized, washed
by PBS, extracted by centrifugation (1,500 × g for 10 min at
4°C), resuspended in binding buffer containing 1% (v:v) Annexin
V-FITC and 2% (v:v) PI and incubated in the dark at room
temperature for 20 min. Analysis was performed using a flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA). At least
1×105 cells were collected, recorded on a dot plot and
analyzed by ModFit v2.0.0 software (Verity Software House, Inc.,
Topsham, ME, USA).
Western blotting analysis
Expression levels of the target proteins were
analyzed by western blotting. Following incubation for 48 h,
1×107 A549 cells treated in the presence or absence of
Aloin (0 to 400 µM) were harvested and lysed with a
radioimmunoprecipitation assay lysis buffer supplied by Beyotime
Institute of Biotechnology (20 Mm Tris-HCl buffer, pH 7.5,
containing 1 mM protease inhibitor mix) for 30 min on ice.
Following this, the lysates were centrifuged at 1,500 × g
for 10 min at 4°C and the supernatant was collected as a whole cell
lysate for western blotting analysis. The whole cell lysate (~8 µg)
was mixed with a loading buffer [125 mM Tris-HCl, 4% sodium dodecyl
sulfate (SDS), 10% sucrose, 0.004% bromophenol blue (BPB) and 10%
mercaptoethanol], boiled at 95°C for 5 min and then gel
electrophoresis was performed with 8 to 12% SDS-PAGE gels. The
protein bands were transferred to polyvinylidene fluoride (PVDF)
membranes following manufacturer's protocol (EMD Millipore,
Billerica, MA, USA). The PVDF membranes were then blocked with TBS
containing 5% non-fat milk at 4°C for 1 h, rinsed with TBS with
Tween 20 and incubated with specific primary antibodies, which were
diluted in PBS (1:400), at 4°C overnight, followed by the
corresponding IRDye-conjugated secondary antibody, which were
diluted 1:2,000 in PBS, at room temperature for 1 h. Membranes were
detected using an Odyssey Infrared Imaging System and Odyssey v1.3
software (LI-COR Biosciences). The relative optical densities of
the target proteins were analyzed using Quantity One software v3.0
(Bio-Rad Laboratories, Inc.). Relative levels of the proteins were
normalized to the control b-actin bands in each series and for
protein loading. Each test was performed in at least
triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR)
The mRNA expression level of p53 was analyzed by
RT-qPCR following incubation of the A549 cells with or without the
indicated concentrations of Aloin (0 to 400 µM) for 48 h. Total RNA
from ~5×106 cells was isolated using a TRIzol reagent,
and cDNA was synthesized using the PrimeScript Real Time Reagent
kit according to the manufacturer's protocol. cDNA amplification
was performed at 95°C for 10 sec and 56°C for 30 sec (45 cycles) on
a 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.), using the QuantiTect SYBR® Green PCR
kit (Qiagen GmbH, Hilden, Germany) and 2 µl cDNA, according to the
manufacturer's protocol. Specific primers for p53 and β-actin were
custom-designed and synthesized by Takara Biotechnology Co., Ltd.
The results from three independent experiments are expressed as the
n-fold change of untreated (0 µM) cells and the relative mRNA level
of each sample was normalized to β-actin using the
2−∆∆Cq method (29).
Measurement of mitochondrial membrane
potential (MMP)
MMP was examined by staining A549 cells with
rhodamine 123. In healthy cells, rhodamine 123 accumulates and
aggregates in the mitochondria; the loss of MMP leads to the
release of rhodamine 123 from the mitochondria into the cytosol,
increasing intracellular fluorescence (30). Following incubation for 48 h,
5×105 cells A549 cells treated with or without the
indicated concentrations of Aloin (0 to 400 µM) were harvested and
resuspended in 200 µl PBS containing 10 µg/µl rhodamine 123 at 37°C
for 30 min in the dark. Following staining, the A549 cells were
rinsed three times with PBS and analyzed directly by flow cytometry
using ModFit software v2.0.0 (Verity Software House, Inc.). The
percentage of rhodamine 123 fluorescence positive cells was used to
evaluate the extent of mitochondrial depolarization.
Measurement of cytosolic
Ca2+ level
The level of Ca2+ released was measured
by staining A549 cells with Fluo-4, which is a fluorescent dye for
quantifying the levels of released mitochondrial Ca2+
(31). The A549 cells were
incubated with or without the indicated concentrations of Aloin (0
to 400 µM) for 48 h. A total of 1×106 cells were
harvested and resuspended in 200 µl PBS containing 2.5 µM Fluo-4,
prior to incubation at 37°C for 30 min in the dark. Following
staining, the A549 cells were rinsed three times with PBS and
analyzed directly by flow cytometry. The percentage of Fluo-4
fluorescence positive cells was used to evaluate the extent of
mitochondrial depolarization.
Measurement of intracellular ROS
generation
The generation of ROS was measured by staining A549
cells with a H2DCF-DA probe, which commonly reacts with
a number of ROS molecules including hydrogen peroxide, hydroxyl
radicals and peroxynitrite (32).
Following incubation for 48 h, 1×106 cells A549 cells
treated with or without the indicated concentrations of Aloin (0 to
400 µM) were harvested, resuspended in 200 µl PBS containing 10 µM
H2DCF-DA, and incubated at 37°C for 30 min in the dark.
Following staining, the A549 cells were rinsed three times with PBS
and analyzed directly by flow cytometry using ModFit software
v2.0.0 (Verity Software House, Inc.). The percentage of DCFH
fluorescence positive cells were used to evaluate the production of
ROS.
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons among groups were performed using one-way analysis of
variance. If the variation was significant, significant differences
between the means of control and of individual Aloin-treated cell
groups were analyzed by Fisher's least significant difference test.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using SPSS 19.0 (IBM
Corp., Armonk, NY, USA).
Results
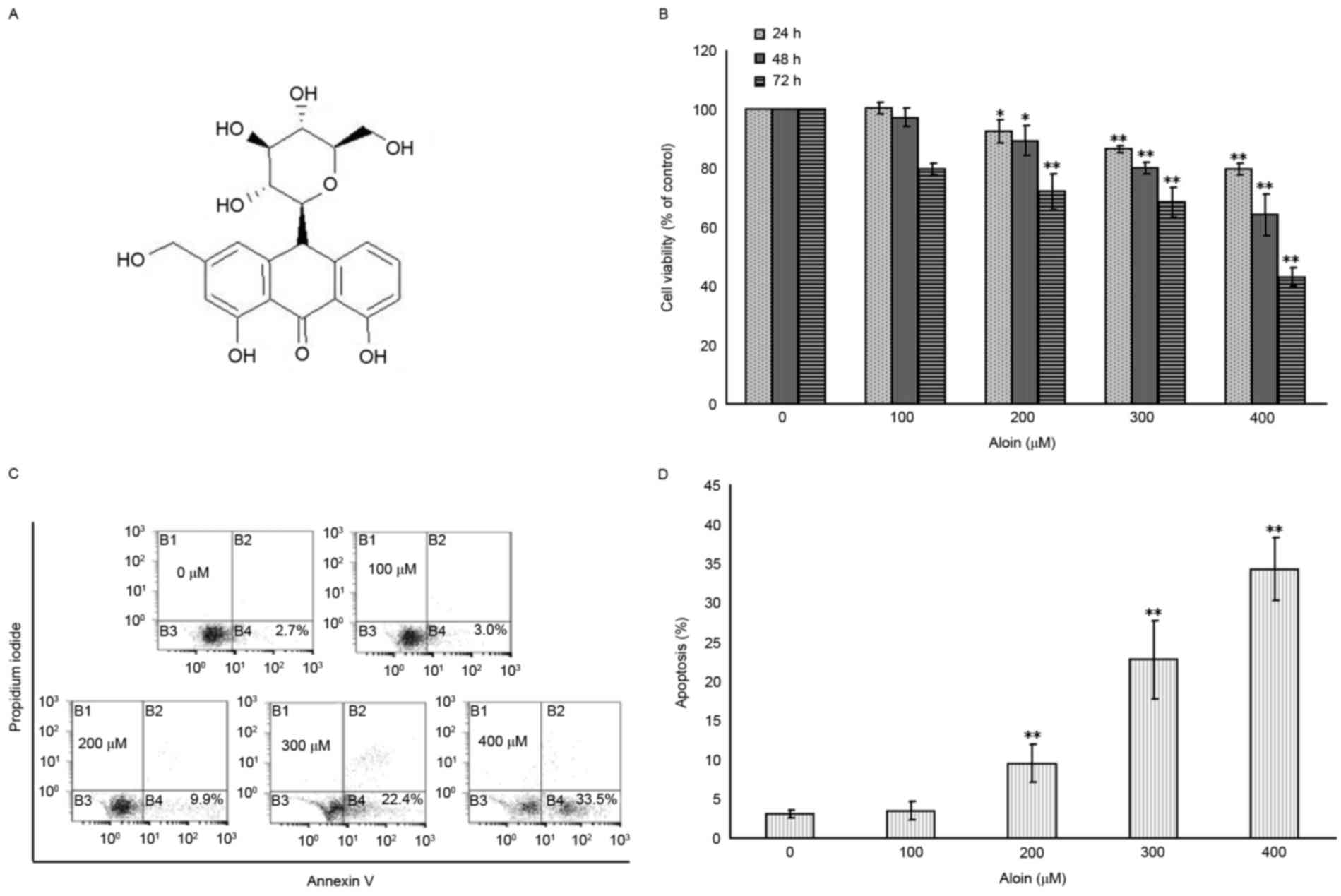
Aloin induces A549 cell viability
inhibition and apoptosis
To evaluate the inhibitory effects of Aloin on the
rate of A549 cell proliferation, cells were exposed to various
concentrations (0, 100, 200, 300 and 400 µM) of Aloin for 24, 48
and 72 h. As presented in Fig. 1B,
Aloin reduced A549 cell viability in a dose- and time-dependent
manner. When compared with the untreated (0 µM) group, the
viabilities of the 200, 300 and 400 µM Aloin treated groups were
all significantly reduced at the 24, 48 and 72 h time points;
however, no significant difference was observed in the cell
viabilities at 24, 48 and 72 h for the 100 µM Aloin-treated
group.
To determine whether the Aloin-induced inhibition of
A549 cell proliferation was the result of apoptosis induction, cell
apoptosis was assessed using flow cytometry. As presented in
Fig. 1C and D, compared with the
untreated group (0 µM), there was a significant increase in the
number of apoptotic A549 cells in the 200, 300 and 400 µM
Aloin-treated groups at 48 h. However, no significant difference
was detected in the level of cell apoptosis in the 100 µM
Aloin-treated group. Based on these findings, the 48-h time point
was selected for further analyses.
Aloin-induced apoptosis is associated
with the intrinsic pathway
To further elucidate the involvement of the
apoptosis pathway initiated by Aloin in A549 cells, the protein
expression levels of apoptosis-associated effectors including
cleaved caspase-8 and −10, CD95, BAK, BAX, PUMA and NOXA were
measured by western blotting. As presented in Fig. 2A, Aloin treatment failed to induce
activation of the extrinsic apoptosis pathway effectors,
cleaved-caspase-8 and −10, and CD95. However, compared with the
untreated (0 µM) group, treatment with 200, 300 and 400 µM Aloin
significantly increased the protein expression levels of the
intrinsic apoptosis pathway effectors, BAK, BAX, PUMA and NOXA
(Fig. 2B). In addition, as
presented in Fig. 2C, treatment
with 200, 300 and 400 µM Aloin also significantly increased the
protein expression levels of cleaved caspase-3 and −9, which are
downstream effectors of the intrinsic and extrinsic apoptosis
pathways.
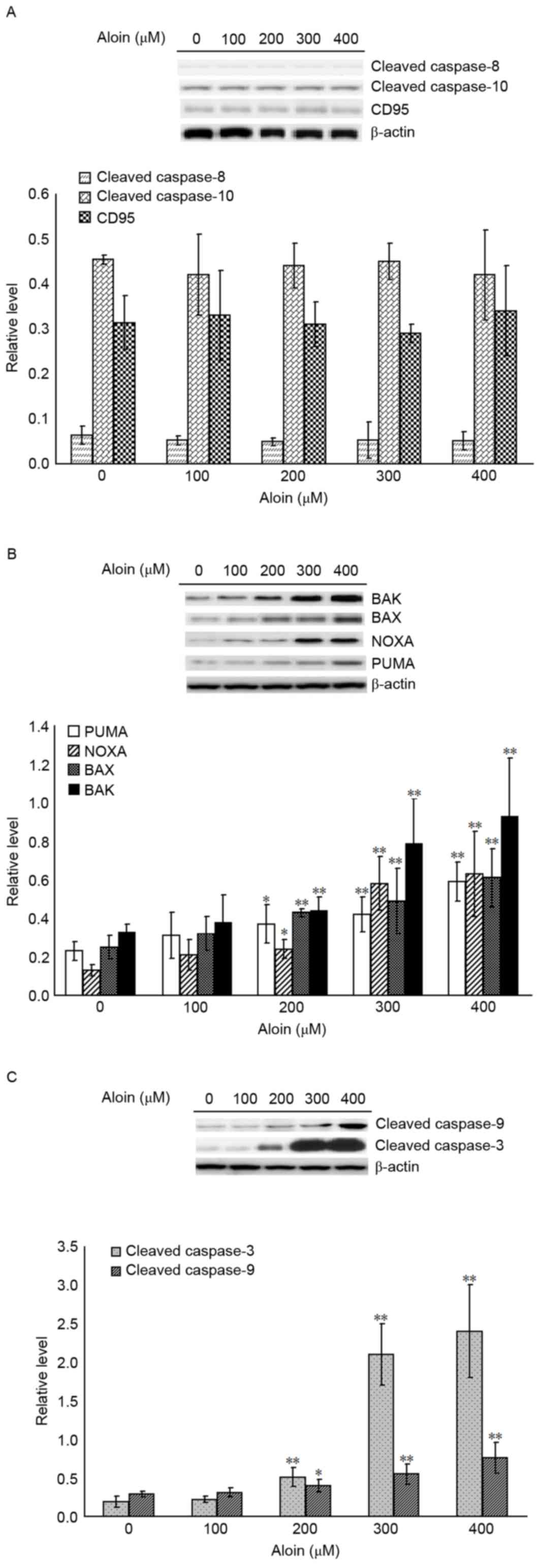 | Figure 2.Effects of Aloin on the extrinsic and
intrinsic apoptosis pathways. A549 cells were treated with or
without Aloin (0, 100, 200, 300 and 400 µM) for 48 h.
Representative western blot images and quantification of protein
expression levels of (A) cleaved caspase-8 and −10, and CD95, (B)
BAK, BAX, NOXA and PUMA, and (C) cleaved caspase-3 and −9. b-actin
served as an internal control. Data are presented as the mean ±
standard deviation. *P<0.05 and **P<0.01 vs. untreated (0 µM)
control cells. BAK, B-cell lymphoma 2 homologous antagonist killer;
BAX, B-cell lymphoma-2 X associated protein; NOXA,
phorbol-12-myristate-13-acetate-induced protein 1; PUMA, p53
upregulated modulator of apoptosis; CD95, cluster of
differentiation 95. |
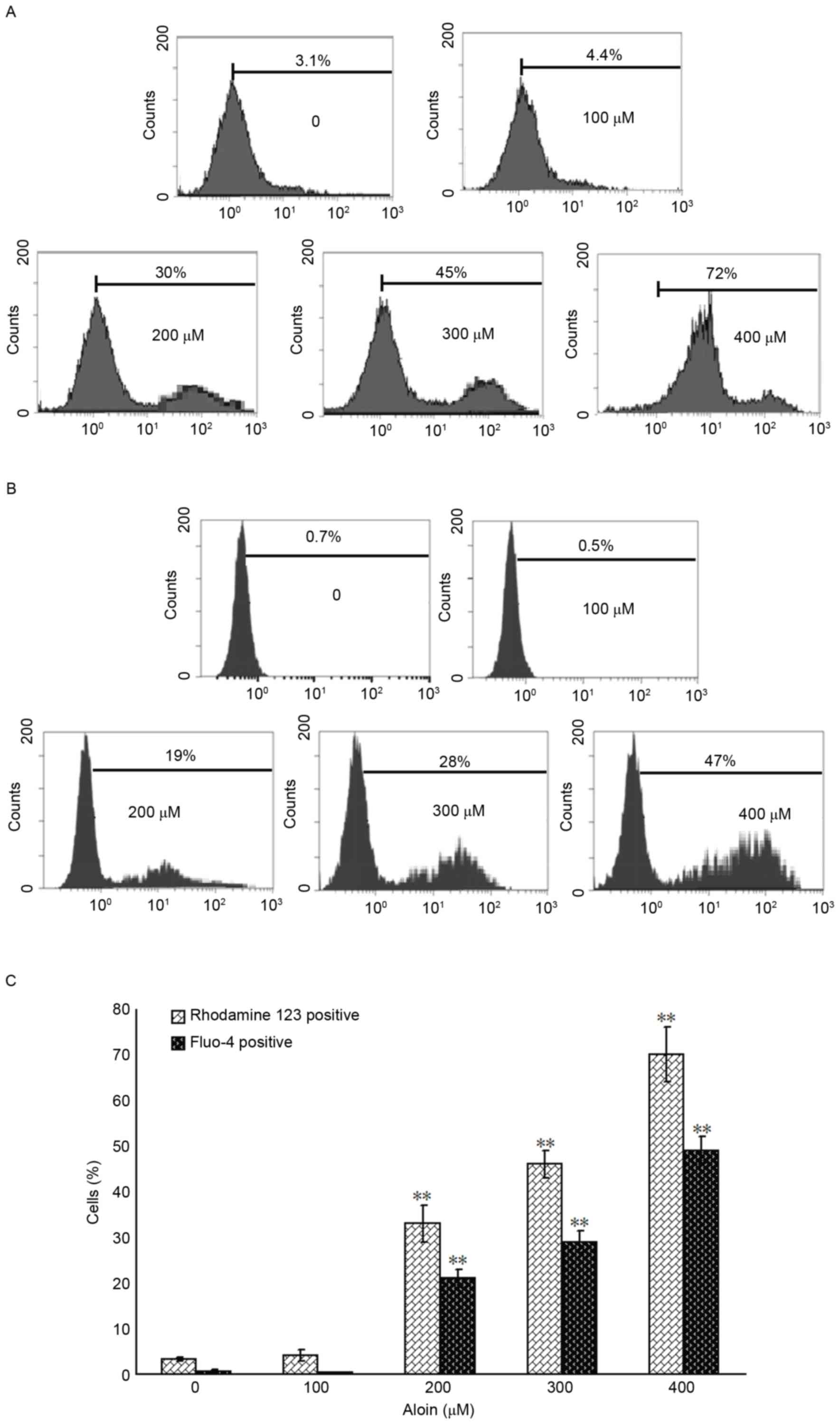
Aloin induces the loss of MMP and
release of mitochondrial Ca2+
To further define the role of the mitochondria in
Aloin-induced A549 cell apoptosis, the MMP and levels of released
mitochondrial Ca2+ were determined using flow cytometry.
When compared with the untreated (0 µM) group, treatment with 200,
300 and 400 µM Aloin induced a significant disruption in the MMP
and release of mitochondrial Ca2+, as indicated by the
observed increase in the percentage of rhodamine 123 or Fluo-4
positive A549 cells, respectively (Fig. 3).
Aloin induces activation of p53
phosphorylation
It was hypothesized that Aloin-induced A549 cell
apoptosis may be p53-dependent. To investigate this, the levels of
p53 mRNA were measured using RT-qPCR. As presented in Fig. 4A, 100 to 400 µM Aloin did not exert
any directly inductive or inhibitory effects on p53 mRNA expression
following treatment for 48 h. Following this, the roles of Aloin in
the protein expression of p53 and its antagonist MDM2, and p53
protein phosphorylation status, were investigated. As presented in
Fig. 4B, compared with the
untreated (0 µM) group, there were no detectable differences in the
protein expression levels of p53, MDM2 or p53-Ser46. In contrast,
treatment with 200, 300 and 400 µM Aloin resulted in strong
activation of p53-Ser15, p53-Thr18, p53-Ser20 and p53-Ser392. These
observations indicated that the signaling system responsible for
p53 phosphorylation of Ser15, Thr18, Ser-20 and Ser-392 was
involved in Aloin-induced apoptosis in A549 cells.
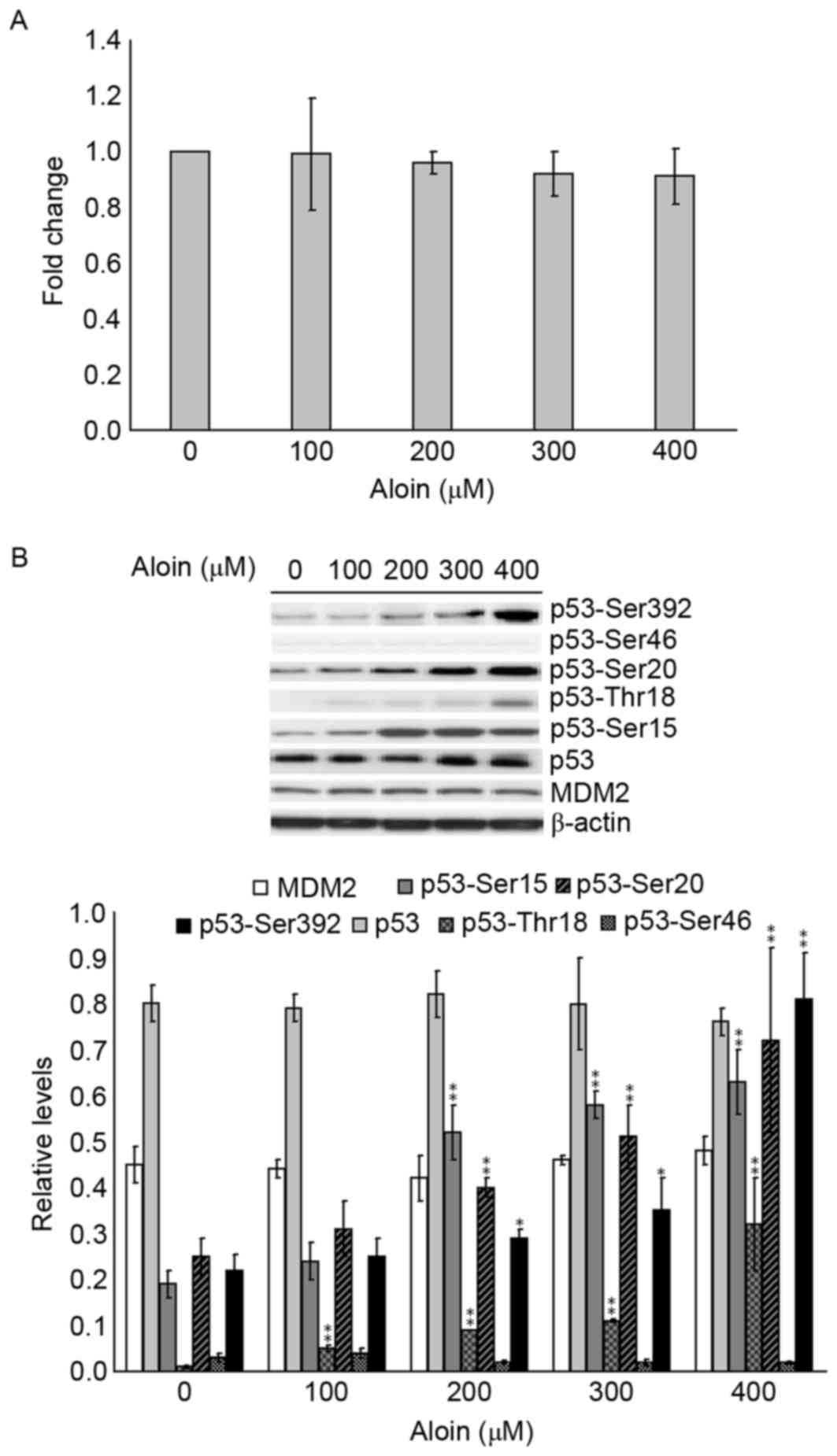 | Figure 4.Effects of Aloin on p53 mRNA and
protein expression levels, and p53 phosphorylation. A549 cells were
treated with or without Aloin (0, 100, 200, 300 or 400 µM) for 48
h. (A) p53 mRNA levels were determined by reverse
transcription-quantitative polymerase chain reaction. (B)
Representative western blot images and quantification of protein
expression levels of MDM2, p53-Ser15, p53-Ser20, p53-Ser392, p53,
p53-Thr18 and p53-Ser46 in A549 cells. b-actin served as an
internal control. Data are presented as the mean ± standard
deviation. *P<0.05 and **P<0.01 vs. untreated (0 µM) control
cells. MDM2, mouse double minute 2, Ser, serine; Thr,
threonine. |
p38 and c-Jun are responsible for
Aloin-induced p53 phosphorylation
As DNA-PK and MAPKs are traditionally phosphokinases
responsible for p53 phosphorylation, the extent of DNA-PK (p-ATM,
p-ATR and p-DNA-PK) and MAPK (p-p38, p-c-Jun and p-ERK) activation
in A549 cells following Aloin exposure were examined. As presented
in Fig. 5A, when compared with the
untreated (0 µM) group, treatment with 100 to 400 µM Aloin did not
alter the levels of p-ATM, p-ATR and p-DNA-PK. However, when
compared with untreated (0 µM) groups, treatment with 100 to 400 µM
and 300 to 400 µM Aloin for 48 h significantly increased p-c-Jun
and p-p38 levels, respectively. In addition, Aloin treatment did
not alter the protein expression levels of p-ERK. These results
suggest that c-Jun and/or p38-mediated cell signaling pathways may
contribute to Aloin activities in p53 phosphorylation.
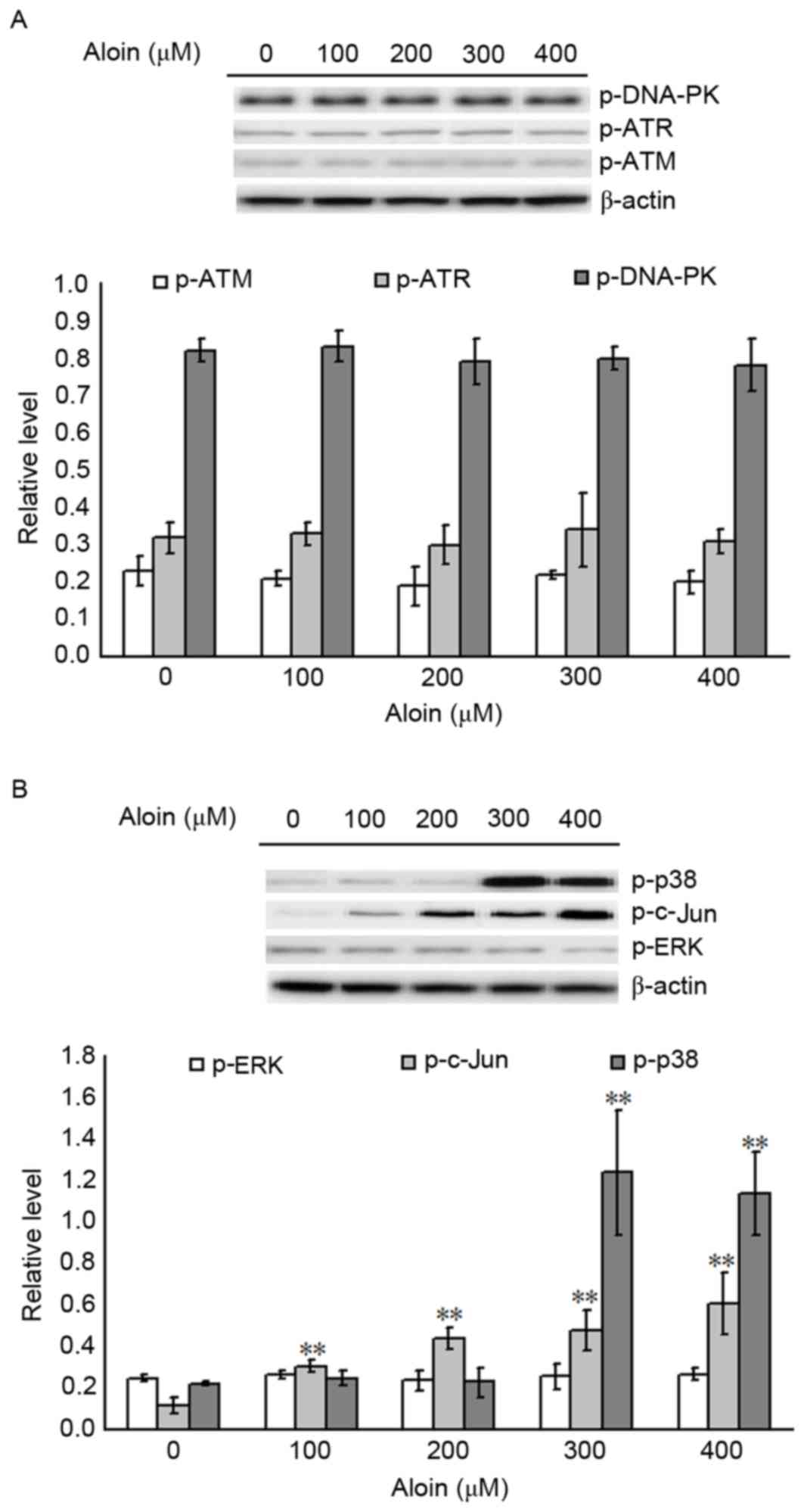 | Figure 5.Effects of Aloin on p53 upstream
effectors. A549 cells were treated with or without Aloin (0, 100,
200, 300 or 400 µM) for 48 h. Representative western blot images
and quantification of (A) p-DNA-PK, p-ATR and p-ATM and (B) p-p38,
p-c-Jun and p-ERK protein expression levels in A549 cells. β-actin
served as an internal control. Data are presented as the mean ±
standard deviation. **P<0.01 vs. untreated (0 µM) control cells.
p-, phosphorylated; DNA-PK, DNA-dependent protein kinase; ATR,
ataxia-telangiectasia and Rad3 related; ATM, ataxia telangiectasia
mutated gene; ERK, extracellular signal-regulated kinase. |
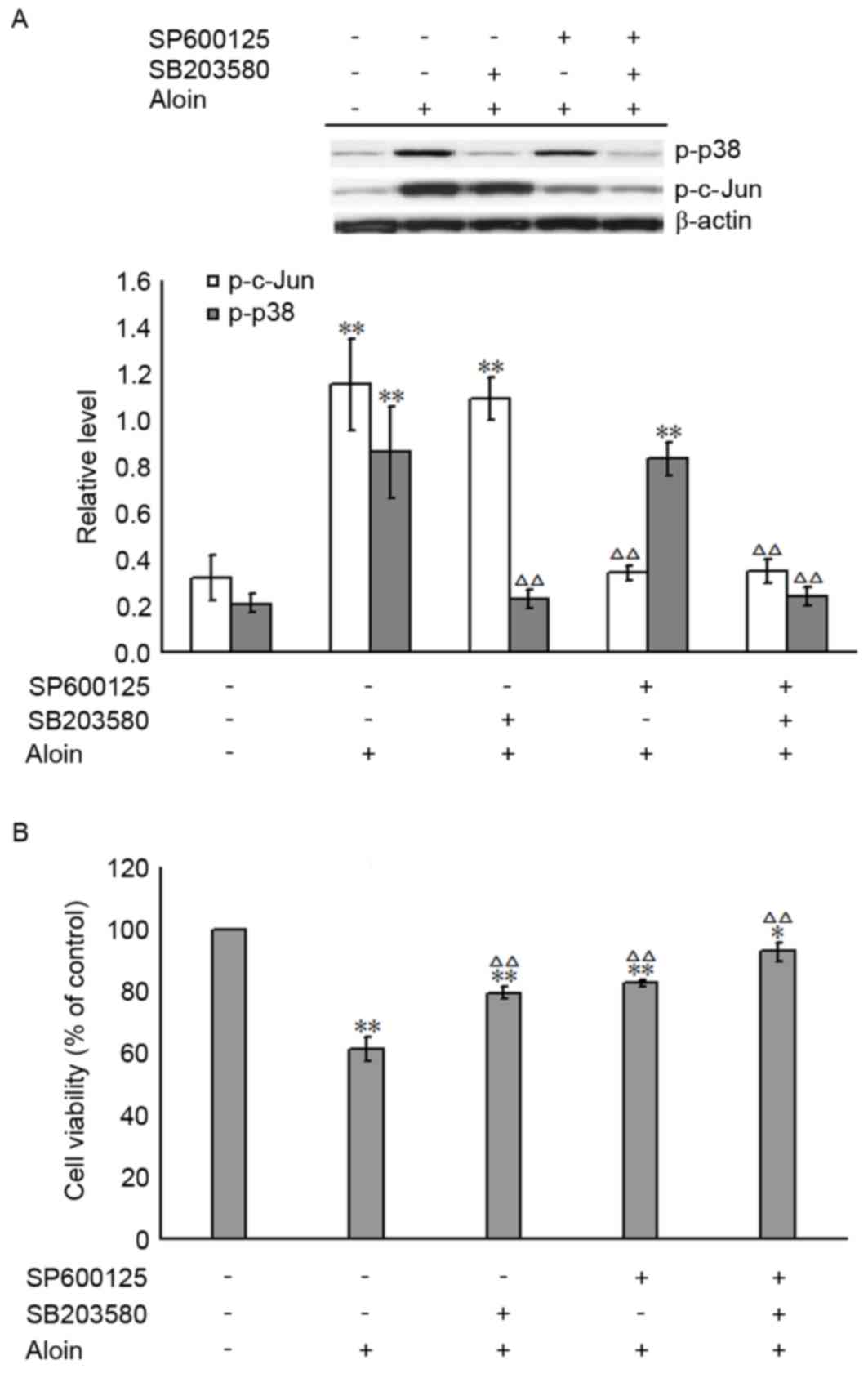
Inhibition of p-p38 and/or p-c-Jun
attenuates the effect of Aloin on A549 cell viability
To determine whether Aloin-induced A549 cell death
is attributable to the activation of p-p38 and/or p-c-Jun MAPKs,
the specific inhibitors of p-p38 and p-c-Jun, SB203580 (30 µM) and
SP600125 (10 µM), were used to pre-treat A549 cells for 30 min. The
inhibiting efficiencies of SB203580 and SP600125 are shown in
Fig. 6A. The levels of p-p38 and
p-c-Jun in the SB203580 and SP600125 pretreated groups were
significantly reduced when compared with the group treated with 400
µM Aloin only; the levels observed were nearly equal to those of
the untreated group (0 µM Aloin). The impact of SB203580 and
SP600125 on Aloin-induced A549 cell death was evaluated by MTT
assay. As presented in Fig. 6B,
SB203580 and SP600125 pretreatment, alone or in combination,
significantly attenuated the Aloin-induced decrease in A549 cell
viabilities. These results further support the hypothesis that
Aloin-induced A549 cell apoptosis is associated with the c-Jun- and
p38-mediated cell signaling pathways.
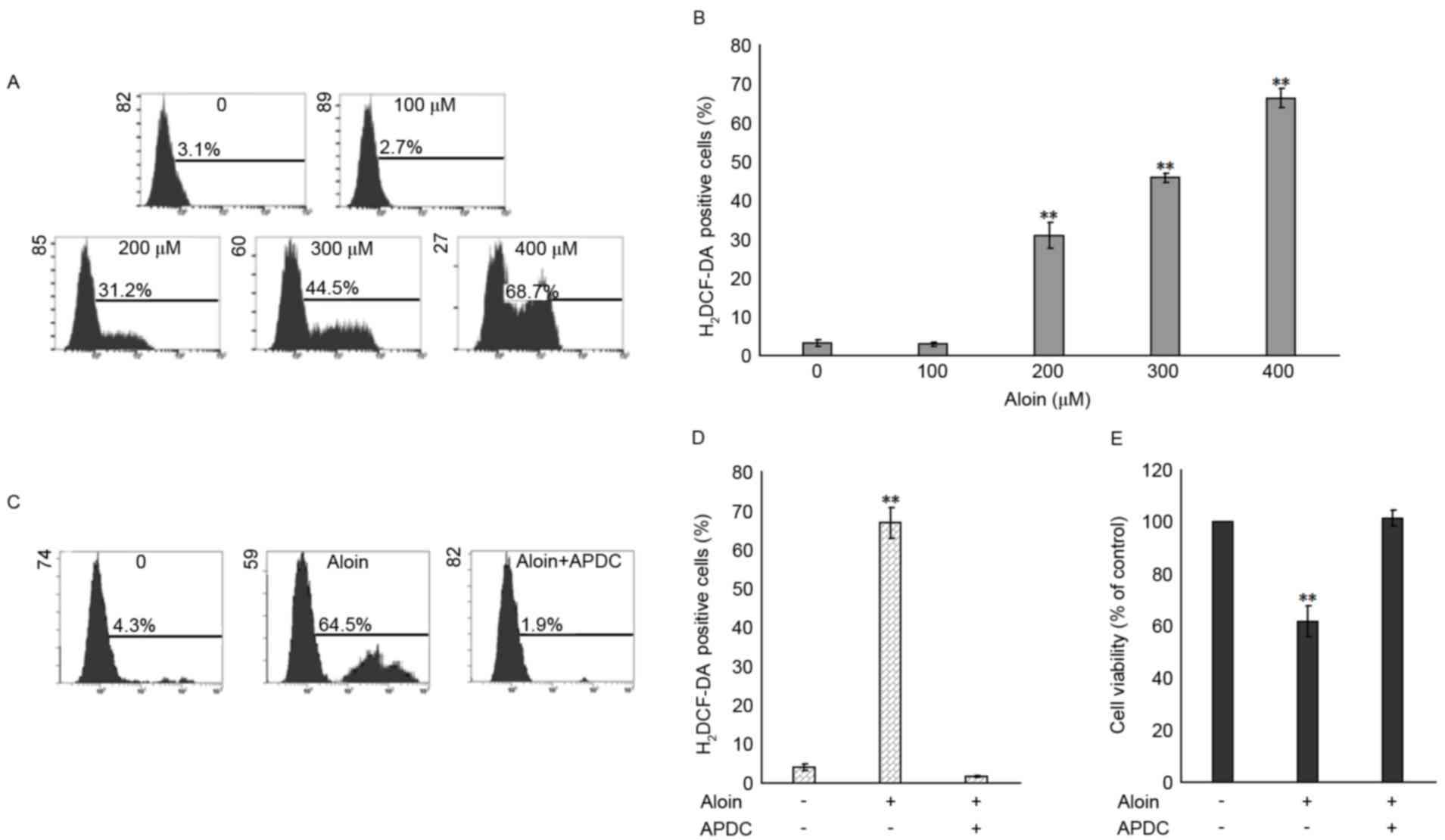
Aloin induces ROS production in A549
cells
It has been widely reported that the generation of
ROS induces the activation of MAPKs and subsequently, apoptosis
(33,34). To investigate whether ROS
generation is associated with Aloin-induced activation of c-Jun and
p38, and apoptosis, the levels of ROS in Aloin-treated A549 cells
were evaluated by flow cytometry following 48 h incubation and
H2DCF-DA probe labeling. As presented in Fig. 7A and B, when compared with the
untreated (0 µM) group, treatment with 200, 300 and 400 µM Aloin
induced a significant accumulation of ROS. To further define the
role of ROS in the Aloin-induced decrease in cell viability, the
ROS inhibitor, APDC (25 µM), was used for A549 cell pretreatment
for 30 min. As presented in Fig. 7C
and D, APDC pretreatment inhibited the 400 µM Aloin-induced
generation of ROS in A549 cells. When compared with the untreated
group, APDC pretreatment attenuated the 400 µM Aloin-induced
inhibition of A549 cell viability (Fig. 7E). These results indicated that ROS
generation may serve a crucial role in Aloin-induced apoptosis.
Discussion
Aloin, the main active component of aloe, has long
been used in traditional Chinese medicine. However, it has been
reported to induce apoptosis in a number of different cancer cell
types (2,3). In agreement with these previous
studies, the present study demonstrated that Aloin at
concentrations of 200 to 400 µM significantly inhibited the
proliferation rates of A549 cells 48 h post-treatment. These
results were further confirmed by flow cytometric analysis, which
demonstrated that 200 to 400 µM Aloin increased apoptosis in A549
cells following treatment for 48 h.
ROS have the ability to interact with cellular
proteins, lipids and DNA, resulting in oxidative stress, which in
turn stimulates extrinsic and/or intrinsic apoptosis (35,36).
ROS may also serve as cell signaling molecules and cause damage to
foreign bodies (37).
Overproduction of ROS may be induced by a number of intra- and
extracellular stressors (38–40).
In the present study, the production of ROS in Aloin-treated A549
cells was evaluated. Elevated ROS levels were concurrent with the
increased level of apoptosis. The results indicated that the
elevation in ROS may be an important initial cellular event that
occurs during Aloin-induced apoptosis. This was further confirmed
by experiments using the specific ROS inhibitor, APDC, which
attenuated the Aloin-induced inhibition of cell proliferation.
These results, coupled with the disruption of the MMP, increased
levels of cytosolic Ca2+ and activation of BAK, BAX,
PUMA and NOXA, suggested that the accumulation of ROS contributes
to the induction of mitochondria-dependent cell apoptosis under
these experimental conditions. These observations further confirmed
the hypothesis that chemotherapeutic agents may be selectively
toxic to tumor cells as they increase oxidative stress to cells and
induce apoptosis.
p53 may be activated by oncogene expression, DNA
damage and oxidative stress (41,42).
Under these conditions, phosphorylation of its N-terminus residues
may stabilize p53 and activate its anticancer activity via the
intrinsic and/or extrinsic apoptosis pathways (43,44).
In the present study, key observations were made concerning the
phosphorylation of p53 on the Ser15, Thr18, Ser20, Ser46 and Ser392
residues. With the concentrations that induced apoptosis, Aloin
exposure increased p53-Ser15, p53-Thr18, p53-Ser20 and p53-Ser392
protein expression levels, whereas phosphorylation of Ser46 was not
induced. In contrast, in Aloin-treated A549 cells, the mRNA and
protein expression levels of p53 were not increased.
Phosphorylation of Ser-15, −20 and −392, and Thr18, alone or in
combination, may be induced in response to a number of
chemotherapeutic agents, including camptothecin, cisplatin and
ionizing radiation (45–49). Therefore, the present study
demonstrated that Aloin also induces the phosphorylation of the p53
protein on Ser-15, −20 and −392, and Thr18. The specific roles of
each phosphorylated residue requires further determination.
MDM2, as a target gene of p53, inhibits p53-induced
transactivation by inducing ubiquitination of the p53 N-terminus
and serving as a E3 ubiquitin ligase to mark p53 for rapid
degradation (5,50). In addition, MDM2 has
p53-independent anti-apoptotic functions (50,51),
and amplification of MDM2 genes or overexpression of the MDM2
protein are features of a number of tumor types (41). Thus, developing treatments to
reduce the levels of MDM2 may be viable strategies for cancer
therapy. Specific alkylating agents used in cancer chemotherapy
treatments have been reported to downregulate the expression of
MDM2 (52–54). For example, the DNA alkylating
agents mitomycin C and methylmethane sulfonate, induced a reduction
in MDM2 protein expression in RKO cells. These decreased levels of
MDM2 coincided with the downregulation of ubiquitinated p53 and
p53/MDM2 binding complexes (54).
In another study, triptolide, a natural product with anticancer
properties, induced apoptosis in lymphoblastic leukemia cells by
inhibiting the MDM2-XIAP cell signaling pathway (55). However, in the present study, the
expression of MDM2 in Aloin-treated A549 cells was not altered.
Together with the findings of previous studies, these results
indicated that inhibition of MDM2 may be cell-type-specific and/or
stress-type-dependent events.
The MAPK family includes c-Jun, p38 and ERKs
(56). Among the MAPK family,
activation of ERKs is usually involved in cell survival or
differentiation, whereas activation of c-Jun and p38 are commonly
associated with promoting cell apoptosis and death, particularly
under oxidative-stress conditions (57). In addition, phosphorylation of p53
may be mediated by c-Jun and p38 (17,58).
Consistent with previous studies, the present study demonstrated
that c-Jun and p38 were essential for the Aloin-induced inhibition
of cell proliferation. This was further confirmed by pretreatment
with c-Jun and the p38 MAPK specific inhibitors, SP600125 and
SB203580, alone or in combination, which significantly attenuated
the Aloin-induced inhibition of cell proliferation. However, the
interactions between c-Jun and p38, and p53 phosphorylation,
require further investigation in future studies.
In conclusion, the present study demonstrated that
Aloin treatment induces ROS accumulation in A549 cells, which in
turn triggers apoptosis via the intrinsic pathway. Aloin-induced
apoptosis was associated with p53 phosphorylation and was dependent
on c-Jun and p38 activation. These results provided mechanistic
insight into the therapeutic potential of Aloin in clinical
treatments for lung cancer; however, further study is required to
confirm these mechanisms in vivo.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation (grant no. 81000032).
Glossary
Abbreviations
Abbreviations:
|
MDM2
|
mouse double minute 2
|
|
ATM
|
ataxia telangiectasia mutated
|
|
ATR
|
ataxia-telangiectasia and Rad3
related
|
|
MAPKs
|
mitogen-activated protein kinases
|
|
ERK
|
extracellular signal-regulated
kinases
|
|
TAD
|
N-terminal trans-activation domain
|
|
XIAP
|
X-linked inhibitor of apoptosis
protein
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
PVDF
|
polyvinylidene fluoride
|
|
MMP
|
measurement of mitochondrial membrane
potential
|
|
ROS
|
reactive oxygen species
|
|
H2DCF-DA
|
dichlorodihydrofluorescein
diacetate
|
References
|
1
|
Gutterman Y and Chauser-Volfson E: The
content of secondary phenol metabolites in pruned leaves of Aloe
arborescens, a comparison between two methods: Leaf exudates and
leaf water extract. J Nat Med. 62:430–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tabolacci C, Rossi S, Lentini A,
Provenzano B, Turcano L, Facchiano F and Beninati S: Aloin enhances
cisplatin antineoplastic activity in B16-F10 melanoma cells by
transglutaminase-induced differentiation. Amino Acids. 44:293–300.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buenz EJ: Aloin induces apoptosis in
Jurkat cells. Toxicol In Vitro. 22:422–429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pronin AN, Xu H, Tang H, Zhang L, Li Q and
Li X: Specific alleles of bitter receptor genes influence human
sensitivity to the bitterness of aloin and saccharin. Curr Biol.
16:1403–1408. 2007. View Article : Google Scholar
|
|
5
|
Michael D and Oren M: The p53 and Mdm2
families in cancer. Curr Opin Genet Dev. 12:53–59. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang Y, Rao K, Yang G, Chen X, Wang Q,
Liu A, Zheng H and Yuan J: Benzo(a)pyrene induces p73 mRNA
expression and necrosis in human lung adenocarcinoma H1299 cells.
Environ Toxicol. 27:202–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Callén E, Jankovic M, Wong N, Zha S, Chen
HT, Difilippantonio S, Di Virgilio M, Heidkamp G, Alt FW,
Nussenzweig A and Nussenzweig M: Essential role for DNA-PKcs in DNA
double-strand break repair and apoptosis in ATM-deficient
lymphocytes. Mol Cell. 34:285–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saxena N, Ansari KM, Kumar R, Dhawan A,
Dwivedi PD and Das M: Patulin causes DNA damage leading to cell
cycle arrest and apoptosis through modulation of Bax, p(53) and
p(21/WAF1) proteins in skin of mice. Toxicol Appl Pharmacol.
234:192–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheikh MS and Fornace AJ Jr: Role of p53
family members in apoptosis. J Cell Physiol. 182:171–181. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Gao C, Chen Y, Yin J, Wang P and
Lv X: Expression pattern of the apoptosis-stimulating protein of
p53 family in p53+ human breast cancer cell lines. Cancer Cell
International. 13:1162013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deb SP, Singh S and Deb S: MDM2
overexpression, activation of signaling networks and cell
proliferation. Subcell Biochem. 85:215–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krzesniak M, Zajkowicz A, Matuszczyk I and
Rusin M: Rapamycin prevents strong phosphorylation of p53 on serine
46 and attenuates activation of the p53 pathway in A549 lung cancer
cells exposed to actinomycin D. Mech Ageing Dev. 139:11–21. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jabbur JR, Huang P and Zhang W: DNA
damage-induced phosphorylation of p53 at serine 20 correlates with
p21 and Mdm-2 induction in vivo. Oncogene. 19:6203–6208. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tichý A, Záskodová D, Zoelzer F, Vávrová
J, Sinkorová Z, Pejchal J, Osterreicher J and Rezácová M:
Gamma-radiation-induced phosphorylation of p53 on serine 15 is
dose-dependent in MOLT-4 leukaemia cells. Folia Biol (Praha).
55:41–44. 2009.PubMed/NCBI
|
|
15
|
Tampio M, Loikkanen J, Myllynen P,
Mertanen A and Vahakangas KH: Benzo(a)pyrene increases
phosphorylation of p53 at serine 392 in relation to p53 induction
and cell death in MCF-7 cells. Toxicol Lett. 178:152–159. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sluss HK, Armata H, Gallant J and Jones
SN: Phosphorylation of serine 18 regulates distinct p53 functions
in mice. Mol Cell Biol. 24:976–984. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee KB, Kim KR, Huh TL and Lee YM: Proton
induces apoptosis of hypoxic tumor cells by the p53-dependent and
p38/JNK MAPK signaling pathways. Int J Oncol. 33:1247–1256.
2008.PubMed/NCBI
|
|
18
|
Brown L and Benchimol S: The involvement
of MAPK signaling pathways in determining the cellular response to
p53 activation: Cell cycle arrest or apoptosis. J Biol Chem.
281:3832–3840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Wu L, Tashiro S, Onodera S and
Ikejima T: Reactive oxygen species mediate oridonin-induced HepG2
apoptosis through p53, MAPK and mitochondrial signaling pathways. J
Pharmacol Sci. 107:370–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salles-Passador I, Fotedar A and Fotedar
R: Cellular response to DNA damage. Link between p53 and DNA-PK. C
R Acad Sci III. 322:113–120. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gatz SA, Keimling M, Baumann C, Dörk T,
Debatin KM, Fulda S and Wiesmüller L: Resveratrol modulates DNA
double-strand break repair pathways in an ATM/ATR-p53- and
-Nbs1-dependent manner. Carcinogenesis. 29:519–527. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chène P: Inhibiting the p53-MDM2
interaction: An important target for cancer therapy. Nat Rev
Cancer. 3:102–109. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang X, Wu Z, Mei Y and Wu M: XIAP
inhibits autophagy via XIAP-Mdm2-p53 signalling. EMBO J.
32:2204–2216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng M, Yang J, Xu X, Sebolt JT, Wang S
and Sun Y: Efficacy of MDM2 inhibitor MI-219 against lung cancer
cells alone or in combination with MDM2 knockdown, a XIAP inhibitor
or etoposide. Anticancer Res. 30:3321–3331. 2010.PubMed/NCBI
|
|
25
|
Lan YH, Chiang JH, Huang WW, Lu CC, Chung
JG, Wu TS, Jhan JH, Lin KL, Pai SJ, Chiu YJ, et al: Activations of
both extrinsic and intrinsic pathways in HCT 116 human colorectal
cancer cells contribute to apoptosis through p53-Mediated ATM/Fas
signaling by emilia sonchifolia extract, a folklore medicinal
plant. Evid Based Complement Alternat Med. 2012:1781782012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seitz SJ, Schleithoff ES, Koch A, Schuster
A, Teufel A, Staib F, Stremmel W, Melino G, Krammer PH, Schilling T
and Müller M: Chemotherapy-induced apoptosis in hepatocellular
carcinoma involves the p53 family and is mediated via the extrinsic
and the intrinsic pathway. Int J Cancer. 126:2049–2066.
2010.PubMed/NCBI
|
|
27
|
Liu T, Laurell C, Selivanova G, Lundeberg
J, Nilsson P and Wiman KG: Hypoxia induces p53-dependent
transactivation and Fas/CD95-dependent apoptosis. Cell Death
Differ. 14:411–421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee MC, Liao JD, Huang WL, Jiang FY, Jheng
YZ, Jin YY and Tseng YS: Aloin-induced cell growth arrest, cell
apoptosis, and autophagy in human non-small cell lung cancer cells.
Biomarkers and Genomic Medicine. 6:144–149. 2014. View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Camins A, Sureda FX, Gabriel C, Pallàs M,
Escubedo E and Camarasa J: Modulation of neuronal mitochondrial
membrane potential by the NMDA receptor: Role of arachidonic acid.
Brain Res. 777:69–74. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao XH, Zhao SS, Liu DY, Wang Z, Niu LL,
Hou LH and Wang CL: ROS-Ca(2+) is associated with mitochondria
permeability transition pore involved in surfactin-induced MCF-7
cells apoptosis. Chem Biol Interact. 190:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Chang F, Li F, Fu H, Wang J, Zhang
S, Zhao J and Yin D: Palmitate promotes autophagy and apoptosis
through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun.
463:262–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sato A, Okada M, Shibuya K, Watanabe E,
Seino S, Narita Y, Shibui S, Kayama T and Kitanaka C: Pivotal role
for ROS activation of p38 MAPK in the control of differentiation
and tumor-initiating capacity of glioma-initiating cells. Stem Cell
Res. 12:119–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu WH, Cheng YC and Chang LS:
ROS-mediated p38alpha MAPK activation and ERK inactivation
responsible for upregulation of Fas and FasL and autocrine
Fas-mediated cell death in Taiwan cobra phospholipase A(2)-treated
U937 cells. J Cell Physiol. 219:642–651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Colin DJ, Limagne E, Ragot K, Lizard G,
Ghiringhelli F, Solary É, Chauffert B, Latruffe N and Delmas D: The
role of reactive oxygen species and subsequent DNA-damage response
in the emergence of resistance towards resveratrol in colon cancer
models. Cell Death Dis. 5:e15332014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lapidus RG, Carter-Cooper BA, Sadowska M,
Choi EY, Wonodi O, Muvarak N, Natarajan K, Pidugu LS, Jaiswal A,
Toth EA, et al: Hydroxylated dimeric naphthoquinones increase the
generation of reactive oxygen species, induce apoptosis of acute
myeloid leukemia cells and are not substrates of the multidrug
resistance proteins abcb1 and abcg2. Pharmaceuticals (Basel).
9(pii): E42016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsieh CJ, Kuo PL, Hsu YC, Huang YF, Tsai
EM and Hsu YL: Arctigenin, a dietary phytoestrogen, induces
apoptosis of estrogen receptor-negative breast cancer cells through
the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic Biol
Med. 67:159–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sanada M, Kuroda K and Ueda M: ROS
production and apoptosis induction by formation of Gts1p-mediated
protein aggregates. Biosci Biotechnol Biochem. 75:1546–1553. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Derouet-Hümbert E, Drăgan CA, Hakki T and
Bureik M: ROS production by adrenodoxin does not cause apoptosis in
fission yeast. Apoptosis. 12:2135–2142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang H, Hou C, Zhang S, Xie H, Zhou W,
Jin Q, Cheng X, Qian R and Zhang X: Matrine upregulates the cell
cycle protein E2F-1 and triggers apoptosis via the mitochondrial
pathway in K562 cells. Eur J Pharmacol. 559:98–108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pei D, Zhang Y and Zheng J: Regulation of
p53: A collaboration between Mdm2 and Mdmx. Oncotarget. 3:228–235.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Walker G and Box N: Ribosomal stress, p53
activation and the tanning response. Expert Rev Dermatol.
3:649–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang J, Ahmed A and Ashcroft M: Activation
of a unique p53-dependent DNA damage response. Cell Cycle.
8:1630–1632. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pabla N, Huang S, Mi QS, Daniel R and Dong
Z: ATR-Chk2 signaling in p53 activation and DNA damage response
during cisplatin-induced apoptosis. J Biol Chem. 283:6572–6583.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheng F, Liu J, Teh C, Chong SW, Korzh V,
Jiang YJ and Deng LW: Camptothecin-induced downregulation of MLL5
contributes to the activation of tumor suppressor p53. Oncogene.
30:3599–3611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fraser M, Bai T and Tsang BK: Akt promotes
cisplatin resistance in human ovarian cancer cells through
inhibition of p53 phosphorylation and nuclear function. Int J
Cancer. 122:534–546. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Katayama A, Ogino T, Bandoh N, Takahara M,
Kishibe K, Nonaka S and Harabuchi Y: Overexpression of small
ubiquitin-related modifier-1 and sumoylated Mdm2 in oral squamous
cell carcinoma: Possible involvement in tumor proliferation and
prognosis. Int J Oncol. 31:517–524. 2007.PubMed/NCBI
|
|
48
|
Saito S, Goodarzi AA, Higashimoto Y, Noda
Y, Lees-Miller SP, Appella E and Anderson CW: ATM mediates
phosphorylation at multiple p53 sites, including Ser(46), in
response to ionizing radiation. J Biol Chem. 277:12491–12494. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yamauchi M, Suzuki K, Kodama S and
Watanabe M: Stabilization of alanine substituted p53 protein at
Ser15, Thr18, and Ser20 in response to ionizing radiation. Biochem
Biophys Res Commun. 323:906–911. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Momand J, Villegas A and Belyi VA: The
evolution of MDM2 family genes. Gene. 486:23–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ongkeko WM, Wang XQ, Siu WY, Lau AW,
Yamashita K, Harris AL, Cox LS and Poon RY: MDM2 and MDMX bind and
stabilize the p53-related protein p73. Curr Biol. 9:829–832. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yoou MS, Park CL, Kim MH, Kim HM and Jeong
HJ: Inhibition of MDM2 expression by rosmarinic acid in
TSLP-stimulated mast cell. Eur J Pharmacol. 771:191–198. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kao CL, Hsu HS, Chen HW and Cheng TH:
Rapamycin increases the p53/MDM2 protein ratio and p53-dependent
apoptosis by translational inhibition of mdm2 in cancer cells.
Cancer Lett. 286:250–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Inoue T, Geyer RK, Yu ZK and Maki CG:
Downregulation of MDM2 stabilizes p53 by inhibiting p53
ubiquitination in response to specific alkylating agents. FEBS
Lett. 490:196–201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang M, Zhang H, Liu T, Tian D, Gu L and
Zhou M: Triptolide inhibits MDM2 and induces apoptosis in acute
lymphoblastic leukemia cells through a p53-independent pathway. Mol
Cancer Ther. 12:184–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vitale I, Senovilla L, Galluzzi L, Criollo
A, Vivet S, Castedo M and Kroemer G: Chk1 inhibition activates p53
through p38 MAPK in tetraploid cancer cells. Cell Cycle.
7:1956–1961. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang JQ, Li YM, Liu T, He WT, Chen YT,
Chen XH, Li X, Zhou WC, Yi JF and Ren ZJ: Antitumor effect of
matrine in human hepatoma G2 cells by inducing apoptosis and
autophagy. World J Gastroenterol. 16:4281–4290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Taylor CA, Zheng Q, Liu Z and Thompson JE:
Role of p38 and JNK MAPK signaling pathways and tumor suppressor
p53 on induction of apoptosis in response to Ad-eIF5A1 in A549 lung
cancer cells. Mol Cancer. 12:352013. View Article : Google Scholar : PubMed/NCBI
|