Introduction
Chloroquine has been widely used as a potent
antimalarial and amebicidal drug since 1934, and has been proven to
be well tolerated in humans. Previous studies have suggested that
chloroquine may be regarded as an effective anticancer drug,
according to the ‘old drugs-new uses’ repositioning strategy, since
chloroquine has been reported to exert numerous biological effects,
including inhibiting cell growth and/or inducing cell death of
various types of cancer cells, overcoming chemoresistance,
increasing radiosensitivity and eliminating cancer stem cells
(1,2). The role of autophagy has previously
been demonstrated in cancer development and therapeutic resistance;
it serves a role as a protective cell mechanism by eliminating
excessive or unnecessary proteins and injured or aged organelles in
the microenviroment of tumor progression, as induced by nutrient
deprivation, hypoxia or therapeutic stress. Chloroquine has been
reported to act as an effective agent that inhibits autophagy by
interfering with the degradative functions of lysosomes, resulting
in the accumulation of damaged autolysosomes. The inhibition of
autophagy by chloroquine may lead to accelerated tumor cell death,
reduced chemoresistance and increased radiosensitivity (3).
Oral squamous cell carcinoma (OSCC) is the sixth
most common malignancy worldwide, and accounts for >90% of
cancers in the oral cavity (4).
Surgery, with or without adjuvant chemoradiotherapy, remains the
gold standard for treatment of OSCC, which exerts curative effects,
particularly during the early stages of OSCC. However, there are
serious side effects associated with current therapeutic regimens,
including no significant improvement of the 5-year survival rate,
and a significantly increased risk of developing subsequent primary
or recurrent tumors. Pignon et al conducted a meta-analysis
of 63 trials (10,741 patients) and reported that chemotherapy,
alongside locoregional treatment, provided an absolute survival
benefit of 4% at 2 and 5 years (5); however, the efficacy of neoadjuvant
chemotherapy, including cisplatin, 5-fluorouracil, paclitaxel and
carboplatin, remains controversial (6–8).
There remains an urgent demand for more effective
agents to better combat OSCC. The antimalarial drug chloroquine,
alone or in combination with other agents, has been reported to
exert antitumor efficacy on several types of cancer, including
breast, colon and liver cancer (9–14).
Therefore, the present study aimed to explore the effects of
chloroquine on OSCC, and the potential molecular targets and
pathways associated with chloroquine treatment of OSCC.
Materials and methods
Materials
Chloroquine, which was purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany), was solubilized in
phosphate-buffered saline (PBS; 0.1 M stock solution stored at
−20°C) and was used within 2 weeks.
Culture conditions
Two human oral squamous cancer cell lines (SCC25,
CAL27) were used in the present study. CAL27 (ATCC number:
CRL-2095) and SCC25 (ATCC number: CRL-1628) cell lines were
purchased from the American Type Culture Collection (Manassas, VA,
USA). SCC25 cells were routinely grown in Dulbecco's modified
Eagle's medium (DMEM)/F-12 (1:1; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and 2 mM L-glutamine. CAL27
cells were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.). Cells were maintained at 37°C in a humidified atmosphere
containing 95% air and 5% CO2. SCC25 and CAL27 cells
were seeded in cell plates and cultured for 24 h, after which the
medium was removed and replaced with DMEM/F-12 supplemented with
0.5% FBS for SCC25 cells, or with DMEM for CAL27 cells, with or
without chloroquine. Untreated cells were considered the control
group.
Cell proliferation assay
The effects of chloroquine on cell proliferation
were evaluated using the MTT assay (BioSource International, Inc.,
Camarillo, CA, USA). Cells (5×103 cells/well) were
plated into 96-well plates and treated with various doses of
chloroquine, or culture medium without chloroquine as a vehicle
control, for 24, 48 and 72 h at 37°C. Subsequently, an MTT assay
was conducted according to the manufacturer's protocol and
absorbance [optical density (OD)] was measured at 490 nm using a
microplate spectrophotometer (Thermo Fisher Scientific, Inc.). The
inhibitory effects of chloroquine on OSCC cell proliferation were
calculated using the following formula: Inhibitory
rate=(1-chloroquine-treated OD490/control
OD490)x100%.
Clonogenic assay
Clonogenic assays were performed as described
previously (15). Briefly, cells
were seeded in triplicate into 6-well plates at a density of 1,000
cells/well. The cells were then treated with 10 and 30 µM
chloroquine, or vehicle control, for 1 week at 37°C. Subsequently,
the cell colonies were stained with a solution containing 0.5%
crystal violet and 25% methanol, and washed three times with tap
water. Colonies consisting of >50 cells were counted under a
light microscope.
Cell cycle analysis
Flow cytometric analyses were performed as described
previously (15), in order to
determine the cell cycle distribution of chloroquine-treated and
untreated cells. A total of 2×105 OSCC cells were seeded
in 6-well plates and cultured for 24 h at 37°C, treated with or
without 10 and 30 µM chloroquine for 24 h, and harvested. Cells
were fixed and stained for total DNA content with a solution
containing 50 µg/ml propidium iodide (PI) and 100 µg/ml RNase I (BD
Biosciences, San Diego, CA, USA) in PBS for 30 min at 37°C. Cell
cycle distribution was immediately analyzed using a Cytomics FC500
MCL with CXP software 1.0 (Beckman Coulter, Inc., Brea, CA, USA).
The experiment was performed three times, and the ratio of cells in
G0/G1, intra-S and G2/M phases was
expressed as the mean ± standard deviation.
Apoptosis analysis
OSCC cells (2×105) cultured in 6-well
plates were treated with or without 10 and 30 µM chloroquine for 24
h at 37°C. Harvested cells were stained with fluorescein
isothiocyanate (FITC)-conjugated Annexin V and PI for 5 min at room
temperature, according to the manufacturer's protocol (Annexin V
FITC Apoptosis Detection kit; BD Pharmingen; BD Biosciences). The
population of AnnexinV− PI− viable cells and
Annexin V+ apoptotic cells was evaluated by flow
cytometry; the latter were considered apoptotic cells. Data were
immediately collected using a Cytomics FC500 MCL with CXP software
1.0 (Beckman Coulter, Inc.). The experiment was performed three
times, and the ratio of apoptotic cells was expressed as the mean ±
standard deviation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To determine the mRNA expression levels of
microtubule-associated proteins 1A/1B light chain 3B (LC3B) and
cyclin D1 in the cells RT-qPCR was conducted. OSCC cells treated
with or without 10 and 30 µM chloroquine for 24 h at 37°C were
harvested, and total RNA from each sample was isolated with TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The concentration and quality of RNA was
measured using a spectrophotometer at 260 and 280 nm. Briefly, 2 µg
total RNA from each sample underwent RT (reaction volume, 20 ml),
using an RT system (Roche Diagnostics, Mannheim, Germany) according
to the manufacturer's protocol. SYBR Green Assay kit (Roche
Diagnostics) was used to conduct qPCR, according to the
manufacturer's protocol. The PCR primer sequences were designed by
Takara Biotechnology Co., Ltd. (Dalian, China), as follows: GAPDH,
forward AGGCTAGCTGGCCCGATTTC, reverse TGG CAA CAA TAT CCA CTT TAC
CAGA; LC3B, forward AAA CGC ATT TGC CAT CACA, reverse GGA CCT TCA
GCA GTT TAC AGT CAG; and cyclin D1, forward GAC TCT CAT TCG GGA TGA
TTG GA and reverse TTT GGT TCG GCA GCT TGCTA. qPCR was conducted
using the LightCycler480 instrument (Roche Diagnostics) and the
results were analyzed using LightCycler480 software 1.5 (Roche
Diagnostics). The thermal cycling conditions used during PCR
amplification were as follows: 5 min at 95°C, followed by 40–45
cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 20
sec and extension at 72°C for 20 sec, followed by 95°C for 5 sec
and 65°C for 1 min; the temperature was then increased from 65 to
95°C for the melt curve analysis and was then decreased to 40°C for
10 sec to cool the samples. Cyclin D1 and LC3 expression were
analyzed using the 2−∆∆Cq method, and expression levels
were normalized to GAPDH expression in each sample (16).
Immunofluorescence
OSCC cells seeded into 24-well plates were treated
with or without 10 and 30 µM chloroquine for 24 h at 37°C, after
which the cells were washed with PBS and fixed with methanol at
−20°C for 20 min. The cells were then permeabilized with 0.1%
Tween-20 for 15 min at room temperature and were blocked with 0.2%
bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) for 1
h at room temperature. The cells were incubated with anti-LC3B
antibody (2775S; 1:200 dilution; Cell Signaling Technology, Inc.,
Beverly, MA, USA) at 4°C overnight, were washed with PBS and were
then incubated with Alexa Fluor 647-conjugated goat anti-rabbit
antibody (ab150079; 1:1,000 dilution; Abcam, Cambridge, UK) for 1 h
at room temperature. DAPI (10236276001; 300 nM; Roche Diagnostics)
was used to counterstain nuclei for 1 min at room temperature.
Cells were examined under a fluorescence microscope (Leica
DMI4000B; Leica Microsystems GmbH, Wetzlar, Germany).
Western blot analysis
Cells were treated with chloroquine (0, 10 and 30
µM) in 6-well plates for 24 h at 37°C and were harvested by
scraping the culture dishes with a cell scraper. Subsequently,
cells were lysed with 150 µl radioimmunoprecipitation assay buffer
containing various protease inhibitors for 30 min on ice (Pierce;
Thermo Fisher Scientific, Inc.). Following centrifugation at 12,000
× g for 10 min at 4°C, the clear supernatant was collected and used
as the cell protein extract. Protein concentration was determined
using the bicinchoninic acid protein assay kit (Nanjing Keygen
Biotech Co., Ltd., Nanjing, China). Protein (40 µg) from control
cells and chloroquine-treated cells were separated by 12% (w/v)
SDS-PAGE and were electroblotted onto polyvinylidene fluoride
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). For
immunodetection, membranes were blocked with 5% non-fat milk in TBS
containing 0.1% Tween-20 for 1 h at room temperature prior to
incubation with rabbit monoclonal anti-LC3B (2775S) and anti-GAPDH
(2118) antibodies (1:1,000 dilution; Cell Signaling Technology,
Inc.) at 4°C overnight, followed by incubation with goat
anti-rabbit horseradish peroxidase (HRP)-conjugated secondary
antibody (sc-2004; 1:5,000 dilution; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) for 1 h at room temperature. Antibody
binding was determined using an enhanced chemiluminescence
substrate to HRP (Invitrogen; Thermo Fisher Scientific, Inc.). The
anti-LC3B (2775S) antibody produced two clear bands representing
LC3-I (16 kDa) and LC3-II (14 kDa). The expression levels of LC3-II
are expressed as the density measured by ImageJ 1.46 software
(National Institutes of Health, Bethesda, MD, USA), standardized to
the density of GAPDH.
Xenograft murine model analysis
The present study was approved by the ethics
committee of Guanghua School of Stomatology, Sun Yat-Sen University
(Guangzhou, China). A total of 10 female and 10 male BALB/c nude
mice (age, 4 weeks; weight, 14–16 g) were purchased from the
Guangdong Medical Laboratory Animal Center (Foshan, China), and
were bred in the Animal Care Unit of Sun Yet-sun University under
standard pathogen-free conditions. Mice were maintained under the
following conditions: Temperature, 20–23°C; relative humidity,
50–65%; 12-h light/dark cycle; free access to food and water. Each
mouse was subcutaneously inoculated with 5×106 CAL27
cells into the back next to the right front limb. After 10 days,
the xenografts were identifiable as a mass >6 mm in maximal
diameter in all recipients.
CAL27-bearing BABL/c nude mice were randomly
assigned into two groups (n=10/group). The treated mice were
intraperitoneally injected with 50 mg/kg chloroquine daily, whereas
the control mice were injected with normal saline. Tumor size was
determined by caliper measurement of the largest and perpendicular
diameters every 2 days, and tumor volumes were calculated according
to the following formula: Volume=axb2/2, where a refers
to the largest superficial diameter and b refers to the smallest
superficial diameter. The mice were sacrificed 24 days after
treatment, and the tumors were removed and weighed.
Statistical analysis
The data are presented as the mean ± standard
deviation for at least three repeated individual experiments for
each group. Statistical analysis of the in vitro results was
determined by one-way analysis of variance followed by the least
significant difference test, whereas the in vivo experiments
were analyzed using a two-tailed Student's t test. Statistical
analyses were conducted using SPSS 16.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Chloroquine inhibits OSCC cell
proliferation and reduces colony formation in vitro
To determine the effects of chloroquine on human
OSCC cell growth in vitro, MTT and clonogenic assays were
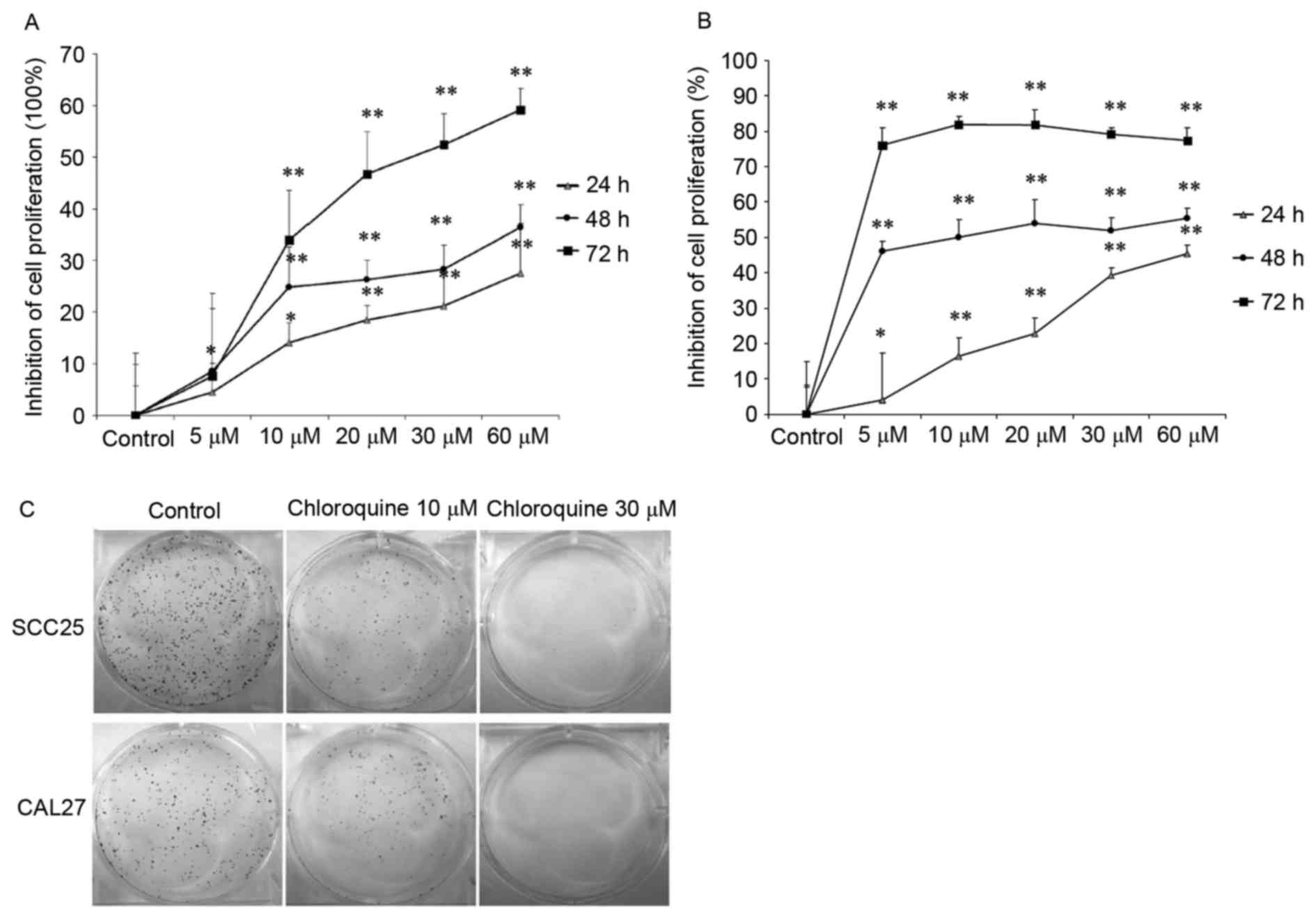
performed using two OSCC cell lines. The results demonstrated that
treatment with chloroquine (10, 20, 30 and 60 µM) significantly
inhibited cell proliferation in a dose- and time-dependent manner
in both OSCC cell lines (P<0.01; Fig. 1A and B). After treatment with 60 µM
chloroquine for 72 h, SCC25 and CAL27 cell growth was inhibited by
59.18 and 77.28%, respectively, compared with the untreated
controls. The half maximal inhibitory concentration
(IC50) values of chloroquine, following 48 h treatment,
were 29.95 and 17.27 µM in SCC25 and CAL27 cells, respectively.
Therefore, 10 and 30 µM chloroquine, which encompassed
concentrations above and below the IC50 values, were
used in further experiments. Subsequently, the present study
determined the colony-forming ability of SCC25 and CAL27 cells on
6-well cell culture plates in the presence or absence of
chloroquine for a period of 1 week. Results indicated a marked
decrease in OSCC cell colony formation following treatment with
chloroquine, in a dose-dependent manner (Fig. 1C). Chloroquine (10 µM) reduced the
colony formation of SCC25 and CAL27 cells by 34.9 and 38.2%,
respectively (P<0.05) (data not shown). In addition, chloroquine
(30 µM) significantly reduced the colony formation of SCC25 and
CAL27 cells by 98.1 and 99.4%, respectively (P<0.01) (data not
shown).
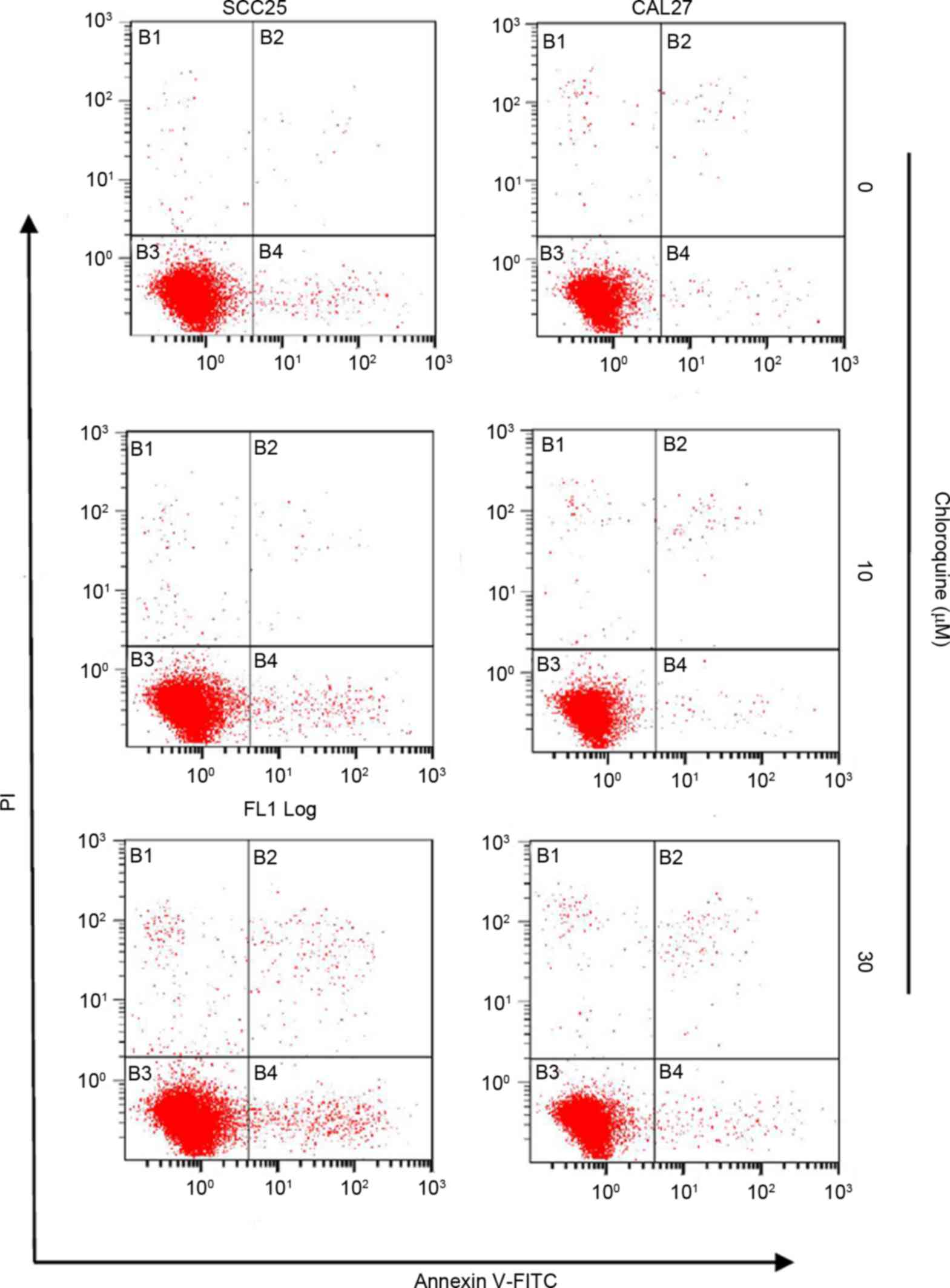
Chloroquine induces cell cycle arrest
but not apoptosis
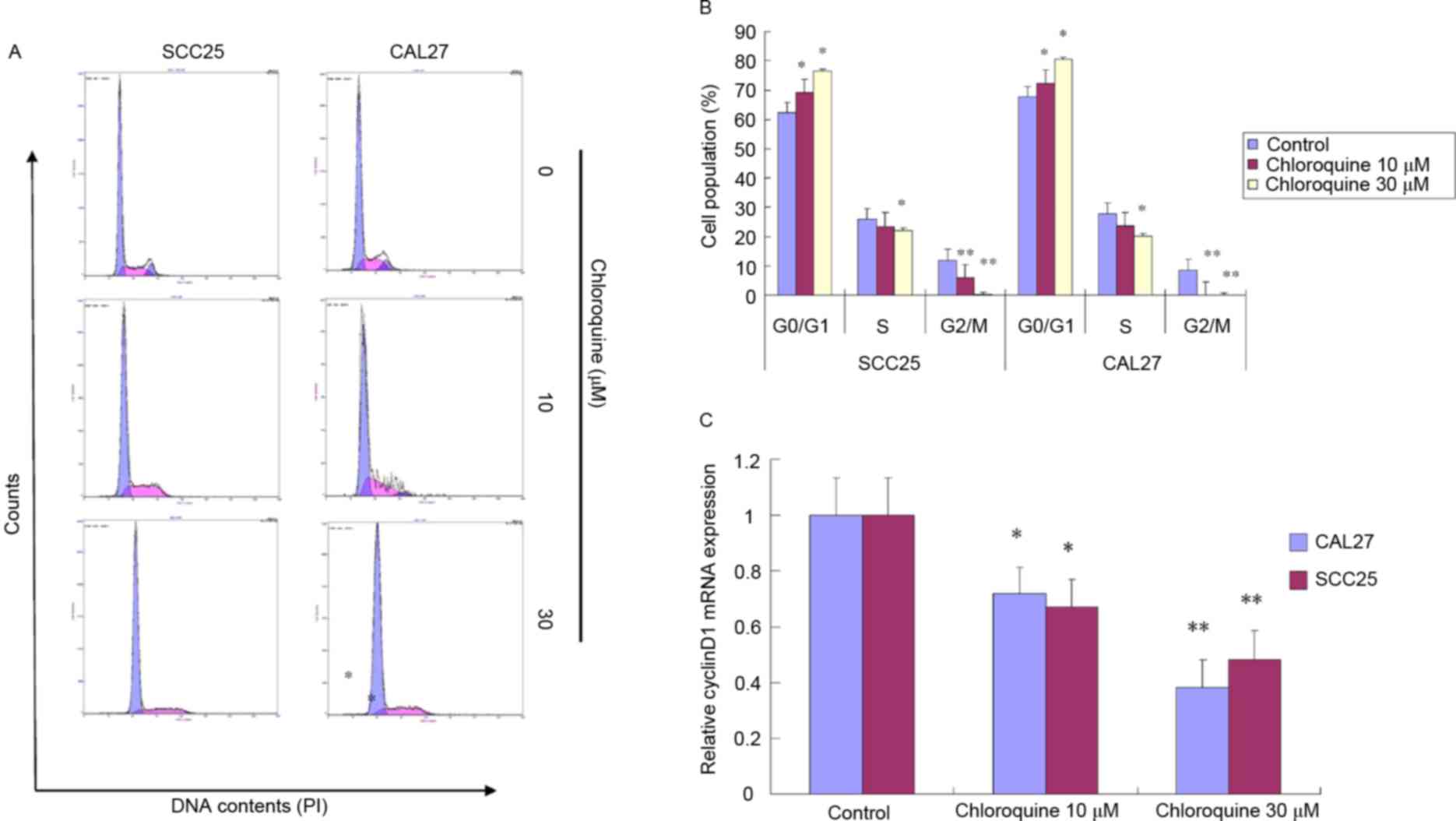
To further study the biological mechanisms
underlying chloroquine-induced growth inhibition, the present study
evaluated the alterations in cell cycle progression and apoptosis
by flow cytometric analysis (Figs.
2 and 3). Treatment with
chloroquine significantly decreased the percentage of cells in
G2/M phase and increased the percentage of cells in
G0/G1 phase in both OSCC cell lines compared
with control cells (P<0.05; Fig. 2A
and B). The chloroquine-associated G1 cell cycle
arrest was dose-dependent. However, the chloroquine-induced growth
inhibition was not associated with the induction of apoptosis.
Apoptosis was detected in <3% of OSCC cells treated with
chloroquine; however, there was an insignificant increase in the
number of apoptotic cells in the chloroquine groups compared with
in untreated cells (Fig. 3).
The present study also determined whether
chloroquine was able to induce alterations in molecular signaling
associated with the G1 checkpoint. The mRNA expression
levels of cyclin D1, as evaluated by RT-qPCR, were significantly
reduced in chloroquine-treated SCC25 and CAL27 cells, in a
dose-dependent manner, compared with the control cells (P<0.05;
Fig. 2C). Cyclin D1 promotes the
G1-S cell cycle transition; therefore, a reduction in
its expression may result in growth inhibition.
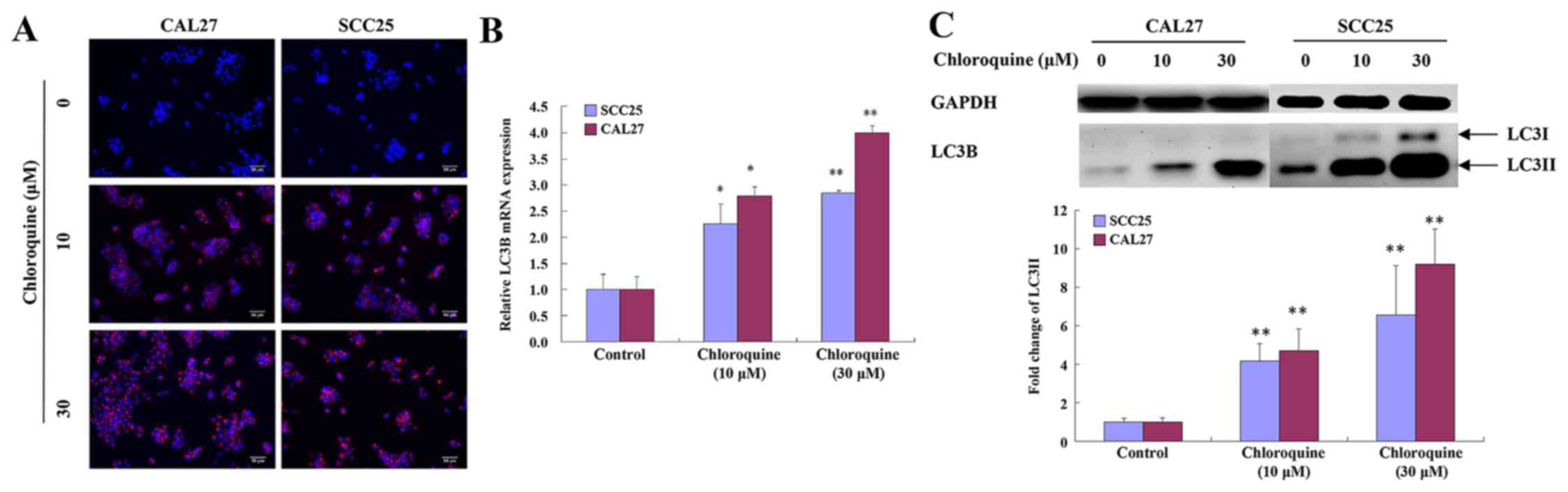
Chloroquine inhibits autophagy
Chloroquine has been reported to inhibit autophagy
by blocking fusion of the autophagosome and lysosome, thus
resulting in a marked accumulation of autophagic vacuoles in
numerous cell types, in which LC3 serves an important role. To
determine whether the effects of chloroquine on OSCC cells were
associated with autophagy, the levels of LC3 were detected.
Chloroquine-treated SCC25 and CAL27 cells exhibited clumped LC3
immunoreactivity, indicating a chloroquine-induced accumulation of
autophagic vacuoles, compared with relatively weak cytoplasmic LC3
expression in untreated cells. The subcellular distribution of LC3
immunoreactivity was monitored in SCC25 and CAL27 cells that were
exposed to 30 µM chloroquine for 24 h (Fig. 4A).
To confirm these immunocytochemical observations,
the mRNA expression levels of LC3B were evaluated by RT-qPCR. The
results demonstrated that the expression levels of LC3B were
increased in a dose-dependent manner following treatment of SCC25
and CAL27 cells with chloroquine (P<0.05; Fig. 4B). During autophagy, the cytosolic
version (LC3-I) of LC3 is converted to the lipidized form (LC3-II),
which is localized on the membrane of autophagosomes and
autolysosomes (17). The
expression levels of LC3-II were assessed by western blot analysis
of whole cell lysates from cells collected at 24 h post-treatment
with chloroquine (0, 10 and 30 µM). LC3-II levels were increased
after 10 µM chloroquine treatment and demonstrated a dose-dependent
increase. Representative data are presented for SCC25 and CAL27
cells (Fig. 4C).
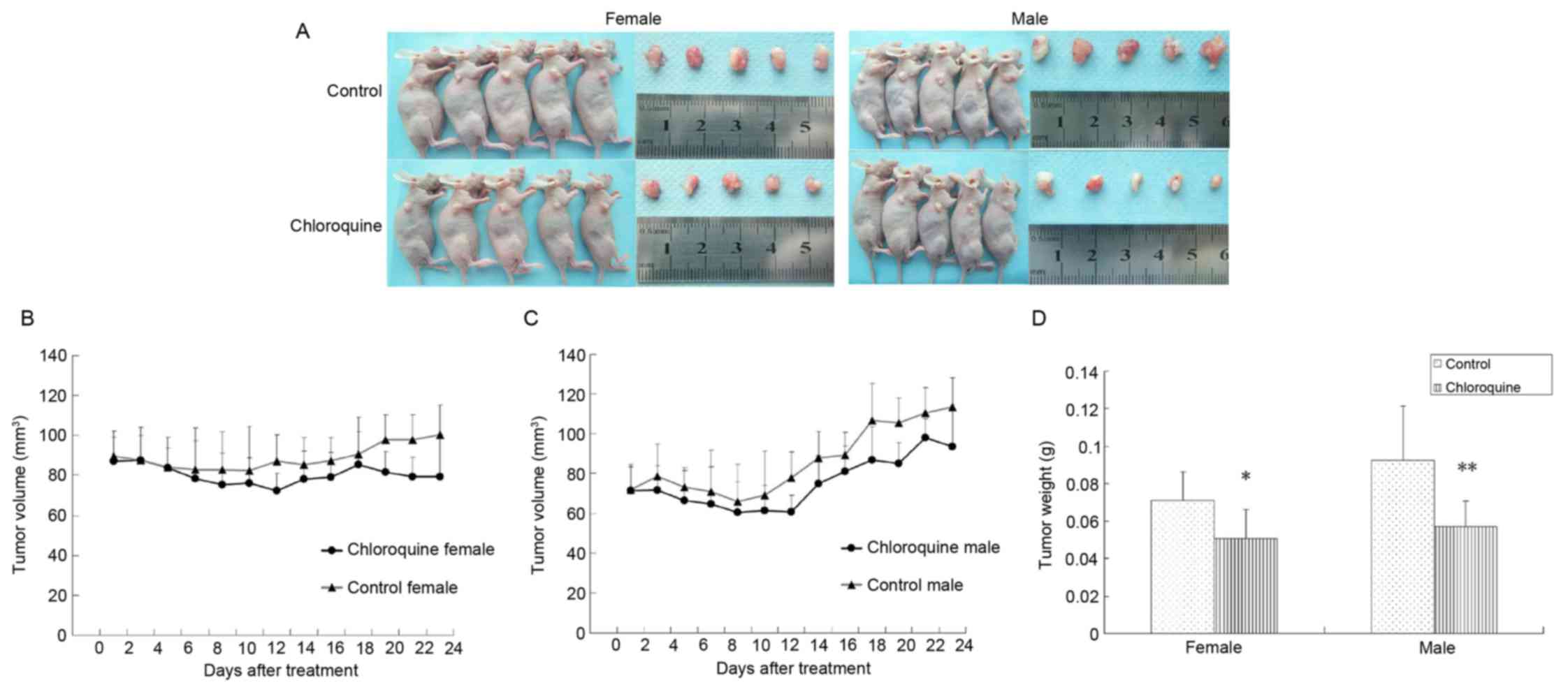
Chloroquine inhibits OSCC tumor growth
in vivo
To determine the antitumor activity of chloroquine
in vivo, CAL27-bearing BALB/c nude mice were used as an OSCC
xenograft nude murine model. The tumor volumes of female and male
mice were 86.44 and 64.28 mm3 on the 7th day,
respectively; therefore, the mice were randomly assigned into two
groups: The treatment group was treated with 50 mg/kg/day
chloroquine, and the control group was treated with normal saline
solution. During the 24 days of treatment, all mice appeared to be
healthy and there were no obvious signs or symptoms of drug
toxicity. Consistent with the in vitro results of the
present study, intraperitoneal administration of chloroquine
reduced tumor growth in female and male CAL27-bearing BALB/c nude
mice (Fig. 5A-C). In addition, the
mean weights of the excised tumors were ~25.68 and 38.28% lower in
chloroquine-treated female and male mice compared with in control
mice (P<0.05; Fig. 5D).
Discussion
Chloroquine is an important antimalarial and
antimycotic agent that is clinically safe and readily tolerated by
humans. Previous studies have suggested that chloroquine may exert
antitumor effects via cell cycle-, apoptosis-, proliferation- and
autophagy-associated mechanisms in several types of cancer cells
(9–14). In addition, it has been
demonstrated that chloroquine can overcome chemoresistance or
increase radiosensitivity and eliminate cancer stem cells (18–21).
However, the anticancer effects of chloroquine on OSCC remain to be
elucidated; OSCC is the sixth most common cancer worldwide, and
patients with OSCC have high levels of morbidity and mortality. Due
to poor prognosis, local recurrence and the difficulties associated
with functional reconstruction, OSCC is considered a great
therapeutic challenge. The present study aimed to investigate the
effects of chloroquine on human OSCC cells and to determine the
possible underlying mechanism. The results indicated that
chloroquine can effectively inhibit the proliferation and
colony-forming ability of OSCC cancer cells in vitro.
Furthermore, the anticancer effects of chloroquine were associated
with cell cycle arrest and autophagic inhibition in OSCC.
Subsequently, an in vivo study was conducted to confirm the
role of chloroquine as a potent therapeutic agent against OSCC.
Previous studies have reported that chloroquine
exerts antitumor effects on several types of cancer; however, the
underlying molecular target and mechanism remain to be elucidated
(9–14). The present study investigated the
effects of chloroquine on cell proliferation by MTT and clonogenic
assays. The results demonstrated that both OSCC cell lines treated
with chloroquine exhibited a reduction in proliferation in a dose-
and time-dependent manner. Furthermore, a dose-dependent decrease
in OSCC cell colony formation was induced following chloroquine
treatment. A meta-analysis previously demonstrated that
overexpression of cyclin D1 was significantly correlated with
increased tumor size, lymphoid node metastasis, tumor
differentiation, advancement of clinical stages, and adversely
influenced overall survival of patients with OSCC (22–24).
In addition, cyclin D1 polymorphism is associated with an increased
susceptibility to OSCC (25).
Therefore, cyclin D1 is considered a useful prognostic factor and
therapeutic target. In the present study, chloroquine induced
G0/G1 arrest in CAL27 and SCC25 cells 24 h
post-treatment, and downregulated the expression of cyclin D1.
Disturbed cell cycle progression may be a potential mechanism
through which chloroquine inhibits OSCC cell growth.
Apoptosis is an important mechanism of antitumor
drug-induced cell death, and the susceptibility of tumor cells to
apoptosis is an important determinant of chemotherapeutic efficacy.
Previous studies demonstrated that chloroquine is able to induce
the apoptosis of various cell types, including human lung cancer
cells, melanoma cells, colon cancer cells, breast cancer cells and
hepatocellular carcinoma cells, the majority of which are
adenocarcinomas (9–14). However, the present study detected
only a slight increase in the percentage of apoptotic cells
following treatment with 30 µM chloroquine. This contradiction may
be attributed to histological difference, which is one of the most
important factors associated with the different patient responses
to the same anticancer treatment. To the best of our knowledge, no
previous studies have reported the chloroquine-induced apoptosis of
squamous cell carcinoma cells; however, previous studies have
demonstrated that the addition of chloroquine can promote the
apoptosis of squamous cell carcinoma cells induced by cisplatin and
the flavonoid luteolin (26–28).
Therefore, future studies should aim to investigate the effects of
chloroquine on various squamous cell carcinoma cell lines, using
different monitoring methods.
It has previously been reported that targeting
autophagy may be considered a novel anticancer therapeutic
approach, and autophagy is a mediator of chemotherapy-induced cell
death in cancer (3,29). Both chloroquine and
hydroxychloroquine are derivatives of a 4-aminoquinoline nucleus;
hydroxychloroquine is considered the safer alternative, and both
drugs can inhibit autophagy by blocking lysosome acidification and
autophagosome degradation. Previous studies demonstrated that
chloroquine and hydroxychloroquine act as novel antitumor drugs in
various tumors by inhibiting autophagy; furthermore, chloroquine or
hydroxychloroquine has been used alongside chemoradiotherapy in
clinical trials for patients with refractory malignancies (1,3,29).
Autophagy is a frequent and early event during oral carcinogenesis,
which may affect the malignant process of OSCC. Beclin-1, or
autophagy-related 6, is an important mediator of autophagy, which
has been reported to be significantly downregulated in OSCC tumor
tissue, compared with normal tissue (30). Nomura et al demonstrated
that overexpression and altered subcellular localization of
autophagy-related 16-like 1 in oral premalignant lesions and
primary OSCC was correlated with lymphovascular invasion and
lymph-node metastasis (31). To
investigate the mechanism underlying the inhibitory effects of
chloroquine on proliferation, the present study examined whether
chloroquine could suppress autophagy, using the LC3 conjugation
system as an autophagosomal marker (17). The results indicated that
chloroquine induced LC3 accumulation and increased LC3 expression
at the mRNA and protein level, thus suggesting that chloroquine
treatment may induce accumulation of damaged autolysosomes and
suppress the progression of autophagy. This result is consistent
with the findings of Geng et al, which reported that
chloroquine may induce an accumulation of autophagic vacuoles in
five glioma cell lines, indicating that chloroquine-induced cell
death was associated with autophagy, but not with caspase-mediated
apoptosis (13).
The present study investigated the in vivo
antitumor effects of chloroquine against a CAL27-bearing BALB/c
nude mouse model. As referred to in a previous in vivo study
regarding the effects of chloroquine on colon cancer, 50 mg/kg
chloroquine was used in the present study (10). The results of the in vivo
study indicated that chloroquine effectively inhibited OSCC tumor
growth, without being associated with signs or symptoms of drug
toxicity. Notably, the present study is the first, to the best of
our knowledge, to indicate that chloroquine may inhibit OSCC tumor
growth in vitro and in vivo.
In conclusion, the results of the present study
indicated that chloroquine is a potent compound that may inhibit
OSCC cell proliferation and tumor growth, and warrants further
study as a therapeutic agent against human OSCC.
Acknowledgements
The present study was supported by the Project of
National Natural Sciences Foundation of China (grant no. 81272948)
and the Guangdong Medical Scientific Research Fund (grant no.
B2014446).
References
|
1
|
Kimura T, Takabatake Y, Takahashi A and
Isaka Y: Chloroquine in cancer therapy: A double-edged sword of
autophagy. Cancer Res. 73:3–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Bari MA: Chloroquine analogues in drug
discovery: New directions of uses, mechanisms of actions and toxic
manifestations from malaria to multifarious diseases. J Antimicrob
Chemother. 70:1608–1621. 2015.PubMed/NCBI
|
|
3
|
Yang ZJ, Takabatake Y, Takahashi A and
Isaka Y: The role of autophagy in cancer: Therapeutic implications.
Mol Cancer Ther. 10:1533–1541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson NW, Jayasekara P and Amarasinghe
AA: Squamous cell carcinoma and precursor lesions of the oral
cavity: Epidemiology and aetiology. Periodontol 2000. 57:19–37.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pignon JP, Bourhis J, Domenge C and
Designé L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: Three meta-analyses of updated
individual data. MACH-NC collaborative group. Meta-analysis of
chemotherapy on head and neck cancer. Lancet. 355:949–955. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sturgis EM, Moore BA, Glisson BS, Kies MS,
Shin DM and Byers RM: Neoadjuvant chemotherapy for squamous cell
carcinoma of the oral tongue in young adults: A case series. Head
Neck. 27:748–756. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freier K, Engel M, Lindel K,
Flechtenmacher C, Mühling J, Hassfeld S and Hofele C: Neoadjuvant
concurrent radiochemotherapy followed by surgery in advanced oral
squamous cell carcinoma (OSCC): A retrospective analysis of 207
patients. Oral Oncol. 44:116–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wedemeyer I, Kreppel M, Scheer M, Zöller
JE, Büttner R and Drebber U: Histopathological assessment of tumour
regression, nodal stage and status of resection margins determines
prognosis in patients with oral squamous cell carcinoma treated
with neoadjuvant radiochemotherapy. Oral Dis. 20:e81–e89. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang PD, Zhao YL, Shi W, Deng XQ, Xie G,
Mao YQ, Li ZG, Zheng YZ, Yang SY and Wei YQ: Cell growth
inhibition, G2/M cell cycle arrest and apoptosis induced by
chloroquine in human breast cancer cell line Bcap-37. Cell Physiol
Biochem. 22:431–440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng Y, Zhao YL, Deng X, Yang S, Mao Y,
Li Z, Jiang P, Zhao X and Wei Y: Chloroquine inhibits colon cancer
cell growth in vitro and tumor growth in vivo via induction of
apoptosis. Cancer Invest. 27:286–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu T, Li P, Luo Z, Chen X, Zhang J, Wang
C, Chen P and Dong Z: Chloroquine inhibits hepatocellular carcinoma
cell growth in vitro and in vivo. Oncol Rep. 35:43–49. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan C, Wang W, Zhao B, Zhang S and Miao J:
Chloroquine inhibits cell growth and induces cell death in A549
lung cancer cells. Bioorg Med Chem. 14:3218–3222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geng Y, Kohli L, Klocke BJ and Roth KA:
Chloroquine-induced autophagic vacuole accumulation and cell death
in glioma cells is p53 independent. Neuro Oncol. 12:473–481.
2010.PubMed/NCBI
|
|
14
|
Lakhter AJ, Sahu RP, Sun Y, Kaufmann WK,
Androphy EJ, Travers JB and Naidu SR: Chloroquine promotes
apoptosis in melanoma cells by inhibiting BH3 domain-mediated PUMA
degradation. J Invest Dermatol. 133:2247–2254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Jia L, Kuang Z, Wu T, Hong Y, Chen
X, Leung WK, Xia J and Cheng B: The in vitro and in vivo antitumor
effects of clotrimazole on oral squamous cell carcinoma. PLoS One.
9:e988852014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pascolo S: Time to use a dose of
Chloroquine as an adjuvant to anti-cancer chemotherapies. Eur J
Pharmacol. 771:139–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lefort S, Joffre C, Kieffer Y, Givel AM,
Bourachot B, Zago G, Bieche I, Dubois T, Meseure D, Vincent-Salomon
A, et al: Inhibition of autophagy as a new means of improving
chemotherapy efficiency in high-LC3B triple-negative breast
cancers. Autophagy. 10:2122–2142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang DH, Choi DS, Ensor JE, Kaipparettu
BA, Bass BL and Chang JC: The autophagy inhibitor chloroquine
targets cancer stem cells in triple negative breast cancer by
inducing mitochondrial damage and impairing DNA break repair.
Cancer Lett. 376:249–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi DS, Blanco E, Kim YS, Rodriguez AA,
Zhao H, Huang TH, Chen CL, Jin G, Landis MD, Burey LA, et al:
Chloroquine eliminates cancer stem cells through deregulation of
Jak2 and DNMT1. Stem Cells. 32:2309–2323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Angadi PV and Krishnapillai R: Cyclin D1
expression in oral squamous cell carcinoma and verrucous carcinoma:
Correlation with histological differentiation. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 103:e30–e35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong LP, Zhu DW, William WN Jr, Liu Y, Ma
J, Yang CZ, Yang X, Wang LZ, Li J, Myers JN, et al: Elevated cyclin
D1 expression is predictive for a benefit from TPF induction
chemotherapy in oral squamous cell carcinoma patients with advanced
nodal disease. Mol Cancer Ther. 12:1112–1121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Yu D, Li H, Nie P, Zhu Y, Liu S,
Zhu M and Fang B: Cyclin D1 overexpression is associated with poor
clinicopathological outcome and survival in oral squamous cell
carcinoma in Asian populations: Insights from a meta-analysis. PLoS
One. 9:e932102014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Myo K, Uzawa N, Miyamoto R, Sonoda I, Yuki
Y and Amagasa T: Cyclin D1 gene numerical aberration is a
predictive marker for occult cervical lymph node metastasis in TNM
Stage I and II squamous cell carcinoma of the oral cavity. Cancer.
104:2709–2716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao XG, Sun RJ, Yang XY, Liu DY, Lei DP,
Jin T and Pan XL: Chloroquine-enhanced efficacy of cisplatin in the
treatment of hypopharyngeal carcinoma in xenograft mice. PLoS One.
10:e01261472015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verschooten L, Barrette K, Van Kelst S,
Romero N Rubio, Proby C, De Vos R, Agostinis P and Garmyn M:
Autophagy inhibitor chloroquine enhanced the cell death inducing
effect of the flavonoid luteolin in metastatic squamous cell
carcinoma cells. PLoS One. 7:e482642012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quan HY, Quan HY, Zhou LJ, Li AD and Zhang
ZB: Mechanism of chloroquine in promoting sensitivity of
chemotherapeutics in oral squamous cell carcinoma CAL-27 cell line
to cisplatin. Shanghai Kou Qiang Yi Xue. 24:30–36. 2015.(In
Chinese). PubMed/NCBI
|
|
29
|
Amaravadi RK, Lippincott-Schwartz J, Yin
XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT and White E:
Principles and current strategies for targeting autophagy for
cancer treatment. Clin Cancer Res. 17:654–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kapoor V, Paliwal D, Singh S Baskar,
Mohanti BK and Das SN: Deregulation of Beclin 1 in patients with
tobacco-related oral squamous cell carcinoma. Biochem Biophys Res
Commun. 422:764–769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nomura H, Uzawa K, Yamano Y, Fushimi K,
Ishigami T, Kouzu Y, Koike H, Siiba M, Bukawa H, Yokoe H, et al:
Overexpression and altered subcellular localization of
autophagy-related 16-like 1 in human oral squamous-cell carcinoma:
Correlation with lymphovascular invasion and lymph-node metastasis.
Hum Pathol. 40:83–91. 2009. View Article : Google Scholar : PubMed/NCBI
|