Introduction
Isoflurane is commonly employed to maintain general
anesthesia during various types of surgery, owing to its properties
that allow a precise concentration to be delivered continuously
throughout in vivo experiments (1,2).
However, isoflurane may induce neurotoxicity, which in turn leads
to cognitive dysfunction or learning/memory impairment (3), and postoperative cognitive
dysfunction is one of the most common complications characterized
by cognitive decline following surgery using isoflurane (4). Notably, early isoflurane exposure may
induce long-term learning deficits and cognitive dysfunction in
children and rodents (5,6).
Hippocampal neuroplasticity has an important role in
cognitive functions such as learning and memory (7,8).
Previous studies have demonstrated that decreased hippocampal
neurogenesis contributes to spatial learning deficits in aging and
animal models of Alzheimer's disease (9,10).
In addition, decreased presynaptic and postsynaptic protein
expression, fewer synaptic contacts and less efficient synaptic
connections induced by adolescent ∆9-tetrahydrocannabinol treatment
are associated with cognitive impairment in adulthood (11). Furthermore, treadmill exercise
improves cognitive function by enhancing hippocampal
neuroplasticity, including increased expression of brain-derived
neurotrophic factor (BDNF) and enhanced cell proliferation in obese
mice (12).
Astrocytes, which actively interact with neurons at
synapses, are the most abundant cell type in the brain and have an
important role in supporting neuronal development (13,14).
In addition, astrocytes contribute to synaptic plasticity by
secreting factors that increase the number and function of
synapses, and also influence synaptic transmission across neuronal
circuits (15). Astrocyte-mediated
metaplasticity contributes to the hippocampal dysfunction that
underlies the impaired cognition involved in several neurological
diseases (16). Furthermore,
astrocytes are an important intermediary of septal cholinergic
modulation in the hippocampus, which has an important role in
hippocampus-dependent learning and memory (17). Notably, cognitive impairment
following isoflurane exposure is associated with impairment of
hippocampal activity and function in rodents (18). The majority of studies concerning
the cellular mechanisms of isoflurane toxicity have focused on the
survival, proliferation, differentiation and migration of
hippocampal neurons and neural stem cells (19,20).
However, the cellular effects of isoflurane on hippocampal
astrocytes and the associated molecular mechanism are not fully
understood.
TWIK-related K+ channel (TREK-1) is a
two-pore domain background K+ channel that is essential
for cell volume regulation and is therefore involved in the
regulation of cell proliferation, necrosis and apoptosis (21). TREK-1 is expressed throughout the
brain, particularly in neurons and astrocytes of the cortex,
cerebellum and hippocampus (22),
and a recent study reported high expression and partial function of
TREK-1 in astrocytes (23).
Notably, TREK-1 is activated by clinical concentrations of
isoflurane (24), and is involved
in the effects of isoflurane preconditioning (25,26).
A recent study reported that blocking TREK-1 using the
sortilin-derived peptide spadin induced an antidepressant-like
effect and also augmented protein kinase A-CREB-BDNF signaling in
the hippocampus (27).
Additionally, the essential role of astrocyte K+
channels in central nervous system homeostasis has been confirmed
in animal disease models, and emerging evidence indicates that
signaling mediated by astrocyte ion channels, such as TREK-1,
enables the interaction between astrocytes and neurons, which
subsequently regulates synaptic transmission and plasticity
(28). Thus, we hypothesize that
the activity of TREK-1 and associated factors, such as BDNF in
hippocampal astrocytes, may be involved in isoflurane-induced
cognitive dysfunction. The present study investigated the effects
of different isoflurane dosages on cell viability and the
expression of caspase-3, Bcl-2-associated X (Bax) and BDNF in
astrocytes following lentiviral-mediated TREK-1 manipulation.
Materials and methods
Astrocyte isolation and culture
Experiments were approved by the Institutional
Animal Care and Use Committee of the Fourth Military Medical
University. A total of 4 female and 2 male C57BL/6 J mice (20.0±1.2
g and 22.0±1.5 g; 7–9 weeks old) were purchased from the Laboratory
Animal Center of the Fourth Military Medical University (Xi'an,
China), and were housed on a 12-h light/dark cycle with ad
libitum access to food and water and bred within (2 female and
1 male in 1 cage) the Laboratory Animal Center of the Fourth
Military Medical University. Mouse pups were caged with the mother
and siblings under a 12-h light/dark cycle at room temperature
maintained at 22°C.
Astrocytes were harvested from the brains of 10
newborn (1-day-old) mice, as previously described (29–31).
Briefly, hippocampi were isolated in ice-cold dissection buffer
(Hanks' balanced salt solution; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) under a stereomicroscope. After the
meninges were removed, single cell suspensions were obtained by
mechanical dissociation. After filtering with a 200 molybdenum wire
mesh screen, cells were rinsed and resuspended in Dulbecco's
modified Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with 10% fetal bovine serum (FBS) (Gibco;
Thermo Fisher Scientific, Inc.) and plated in 75 cm2
flasks coated with poly-L-lysine (Corning Incorporated, Corning,
NY, USA). Cells were incubated at 37°C with 5% CO2 until
90% confluent. The medium was replenished 2 days after plating and
changed every 2 days. Astrocyte-enriched cultures were obtained by
shaking mixed glial cultures at a speed of 240 rpm to remove less
adhesive microglia and other cells for 24 h after 12 days of
incubation. Subsequently, astrocytes were digested with 0.25%
trypsin and 1 mM-EDTA, split into 6-well or 12-well plates and
incubated for ~3 days at 37°C prior to experiments.
Isoflurane administration
Cells were placed on 6-well plates at density of
3×105 per well and tand cultured in DMEM supplemented
with 10% FBS in an incubator with 5% CO2 at 37°C for ~3
days, which was followed by exposure to different concentrations of
isoflurane according to the clinical concentration of isoflurane
and previous studies (32,33). Briefly, identical airtight chambers
(Billups-Rothenberg, Inc., Del Mar, CA, USA) and content-certified
gas canisters containing 21% oxygen, and 79% nitrogen were
equilibrated to 37°C overnight in a heated room. Subsequently,
plates were randomly placed in airtight chambers flushed with
control gas (100% oxygen) at 4 l/min for ~5 min or flushed with gas
containing isoflurane (oxygen with isoflurane) at the same flow
rate until the isoflurane concentrations in the chamber reached the
set value and remained stable for 2 h at 0.5, 1.0 or 1.5 minimum
alveolar concentration (MAC) isoflurane (0.7%, 1.4% and 2.1%,
respectively). The chamber was then sealed and placed in an
incubator at 37°C for 2 h. Afterwards, the cells were removed and
returned to a normal culture (incubated at 37°C with 5%
CO2) at atmospheric conditions for 2 h for cell
viability analysis and other assays.
Cell viability determination
Cell viability was determined using a
2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo
phenyl)-2H-tetrazolium monosodium salt (WST-1) assay kit (Cell
proliferation Reagent WST-1; 11 644 807 001; Roche Diagnostics,
Indianapolis, IN, USA) according to the manufacturer's
instructions. In a preliminary experiment, three different WST-1
concentrations (WST-1/cell growth medium, 100, 200 and 300 µl/ml)
were employed with three different treatment durations (1, 2 and 3
h), and the results demonstrated that there was no significant
difference in the cell viability between cells treated with
different WST-1 concentrations and for different durations.
Therefore, for subsequent cell viability experiments, cells were
treated with 200 µl/ml WST-1 for 2 h, which was recommended in the
manufacturer's protocol. Briefly, 200 µl WST-1 (Cell proliferation
Reagent WST-1; 11 644 807 001, Roche Diagnostics) was added to each
well (with 3×105 cells in 1 ml growth medium) prior to
exposure to isoflurane, and cultures were treated with isoflurane
or O2 (control group) for 2 h at 37°C. The optical
density was measured at 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) immediately following
isoflurane administration. Data are presented as the mean of three
independent experiments that were performed at least five
times.
Immunocytochemistry
To verify the identity of astrocytes, primary
cultures were placed on poly-L-lysine-coated coverslips in 12-well
plates until 80% confluent. Astrocytes were fixed in 4%
paraformaldehyde for 30 min at 4°C and permeabilized in 0.3%
Triton-X-100 (T9284; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
for 10 min at room temperature (23°C) prior to immunocytochemistry.
Subsequently, astrocytes were blocked in 5% (w/v) bovine serum
albumin (Sigma-Aldrich; Merck KGaA) for 30 min at room temperature.
The primary antibody mouse anti-glial fibrillary acidic protein
(GFAP; 1:1,000; SAB5201113; Sigma-Aldrich; Merck KGaA) was diluted
in immune buffer (1% w/v bovine serum albumin and 0.3%
Triton-X-100) and incubated with astrocytes overnight at 4°C.
Subsequently, cells were washed with PBS and incubated for 2 h in
the dark at room temperature in the presence of fluorescent
secondary antibodies (Alexa Fluor 594 donkey anti-mouse IgG;
1:1,000; A-21203; Invitrogen; Thermo Fisher Scientific, Inc.).
Cells were then incubated with DAPI for 20 min at room temperature
to stain the cellular nuclei. Finally, the coverslips were mounted
onto slides in PBS/glycerol (vol/vol, 1:1). The preparations were
analyzed under a laser scanning confocal microscope (FV-1000;
Olympus Corporation, Tokyo, Japan), and the positive cells were
measured and quantitated using Image-Pro Plus software (version
6.0, Media Cybernetics, Inc., Rockville, MD, USA) in 6 fields of
view.
Virus infection and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Lentiviruses expressing a short hairpin RNA (shRNA)
targeting the sequence of the TREK-1 gene (Lv-shRNA-TREK-1), a
negative control lentivirus (Lv-shRNA-sham), lentivirus expressing
TREK-1 (Plenti-TREK-1-GFP) and a GFP control lentivirus
(Plenti-sham-GFP) were all purchased from Shanghai Genechem Co.,
Ltd. (Shanghai, China). Growing cells were seeded at
2×105 cells/well into 6-well plates or 1×105
cells/well into 12-well plates and incubated at 37°C for 3 days.
The transfection was performed using polybrene reagent (Shanghai
Genechem Co., Ltd.), according to the manufacturer's protocol.
Briefly, 20 µl of polybrene (5 mg/ml) and 50 µl of virus (storage
concentration of virus: 5.0×108 TU/ml for
Lv-shRNA-TREK-1, 4.5×108 TU/ml for Lv-shRNA-sham,
4.9×108 TU/ml for Plenti-TREK-1-GFP and
5.0×108 TU/ml for Plenti-sham-GFP) into 20 ml DMEM
medium (with 10% FBS) were mix gently, and the cells were cultured
with the mixture medium (1 ml/well for 6-well plates and 0.5
ml/well for 12-well plates) at 37°C for 24 h. At 24 h after
infection, the transfection mixture was replaced with fresh medium
(DMEM + 10% FBS) and cultured for a further 48 h at 37°C. Then,
Cells in 12-well plates were fixed in 4% paraformaldehyde and
stained with GFAP antibody to confirm the virus transfection
effects by immuno-fluorescent assay as before.
Total RNA was isolated from cells using RNAiso Plus
(Takara Bio, Inc., Otsu, Japan) and reverse transcribed (37°C for
15 min; 85°C for 5 sec and 4°C for 10 min) with a Prime-Script RT
reagent kit (Takara Bio, Inc.). Subsequently, the cDNA was
quantified by qPCR with SYBR Premix Ex Taq (Takara Bio, Inc., Otsu,
Japan). The following primer sequences were used: Mice GAPDH
forward, 5′-CCAATGTGTCCGTCGTGGATCT-3′ and reverse,
5′-GTTGAAGTCGCAGGAGACAACC-3′; BDNF forward,
5′-TCATACTTCGGTTGCATGAAGG-3′ and reverse,
5′-ACACCTGGGTAGGCCAAGTT-3′; caspase-3 forward,
5′-AACCAGATCACAAACTTCTGCAAA-3′ and reverse,
5′-TGGAGTCCAGTGAACTTTCTTCAG-3′; and TREK-1 forward,
5′-TCAAGCACATAGAAGGCTGG-3′ and reverse, 5′-ACGGATGTGGCAGCGTGG-3′.
The two-step qPCR program used was as follows: 1 cycle of 95°C for
30 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec,
and 1 cycle of 95°C for 15 sec, then maintained at 4°C.
Subsequently, the relative changes in gene expression of BDNF,
TREK-1, caspase-3 were analyzed by 2−ΔΔCq method
(34).
Western blot analysis
Cell samples were harvested from culture plates
following isoflurane exposure for the determination of TREK-1,
BDNF, caspase-3 and Bax protein levels. Cells were lysed in buffer
composed of 62.5 mM Tris-HCl, 2% w/v SDS, 10% glycerol, 50 mM
dithiothreitol and 0.1% w/v bromphenol blue. Insoluble materials
were separated by centrifugation at 4°C, 12,000 × g for 10 min and
protein levels in the supernatant were measured by the BCA method
(Invitrogen; Thermo Fisher Scientific, Inc.), and then the
supernatant was heated to 100°C for 10 min and then cooled on ice
for 30 min. Electrophoresis was performed by SDS-PAGE using a 10%
polyacrylamide gel (40 µg of total protein per lane). Separated
proteins were transferred onto nitrocellulose membranes, which were
subsequently blocked with 5% non-fat milk solution for 1 h at room
temperature under gentle agitation. After washing three times in
TBS with 0.5% Tween-20 (TBST; 10 min per wash), membranes were
incubated with primary antibodies against TREK-1 (1:500;
Sigma-Aldrich; Merck KGaA), BDNF (1:5,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), caspase-3 (1:500; Abcam,
Cambridge, UK), Bax (1:1,000; Abcam) and β-actin (1:2,000; Abcam)
overnight at 4°C. The membranes were washed three times in TBS and
incubated with peroxidase-conjugated antibodies in TBST for 1 h
(donkey anti-rabbit IgG; 1:10,000; Abcam). Subsequently, membranes
were washed three times for 10 min in TBST, and immunoreactive
bands were detected using SuperSignal West Pico Chemiluminescent
Substrate (34077; Thermo Fisher Scientific, Inc.), visualized on
X-ray films and densitometric analysis was performed with Bio-Rad
Quantity One1-D Analysis Software (1709600; Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean +
standard deviation. Comparisons were performed using a one-way or
two-way analysis of variance followed by Tukey post hoc tests for
multi-group comparisons, and isoflurane concentration was the
factor assessed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Isoflurane exposure decreases cell
viability in astrocytes in vitro
In the present study, primary mouse astrocytes were
isolated and cultured on poly-D-lysine-coated coverslips. After 7
days of culture, the cellular homogeneity of the primary cultures
was evaluated using immunocytochemistry for the glial marker GFAP,
and cells exhibited immunoreactivity for GFAP (Fig. 1A). Following treatment of the
cultured cells with different concentrations of isoflurane for 2 h,
cell viability was detected in a WST-1 assay. Compared with the
control group, isoflurane exposure at 0.5 MAC had no effect on the
viability of astrocytes. By contrast, 1.0 and 1.5 MAC isoflurane
treatments resulted in a significant reduction in cell viability
compared with the control group (P<0.01; Fig. 1B).
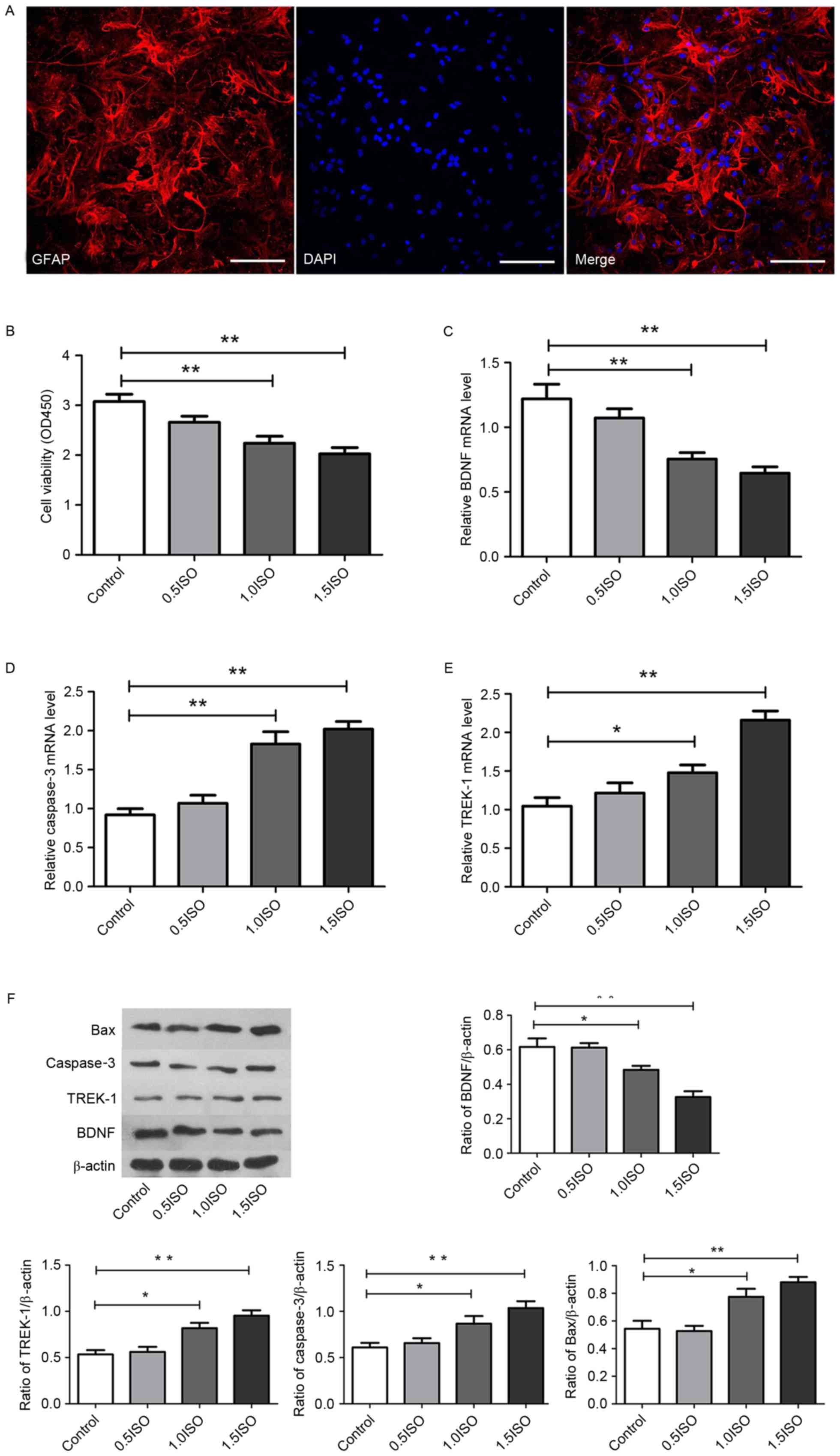 | Figure 1.Effect of isoflurane exposure on the
cell viability and gene expression of astrocytes. (A)
Representative microphotographs of GFAP staining (red), DAPI
staining (blue) and their merged images. Scale bar, 200 µm. (B)
Histograms demonstrating the effect of 0.5 (0.7%), 1.0 (1.4%) and
1.5 (2.1%) minimum alveolar concentration of isoflurane on the cell
viability. mRNA expression of (C) BDNF, (D) caspase-3 and (E)
TREK-1, as determined via reverse transcription-quantitative
polymerase chain reaction. (F) Representative protein bands and
densitometric analysis of BDNF, TREK-1, caspase-3 and Bax
expression relative to β-actin expression. n=6 per group;
*P<0.05 and **P<0.01, as indicated. GFAP, glial fibrillary
acidic protein; BDNF, brain-derived neurotrophic factor; TREK-1,
TWIK-related K+ channel; Bax, Bcl-2-associated X; OD,
optical density; ISO, isoflurane. |
Isoflurane exposure decreases BDNF
levels and increases Bax, caspase-3 and TREK-1 expression in
astrocytes
To investigate the potential molecular mechanism of
the effects of isoflurane in astrocytes, following treatment with
different concentrations of isoflurane for 2 h, RT-qPCR and western
blot analysis were performed to evaluate the expression of BDNF,
caspase-3, Bax and TREK-1. As demonstrated in Fig. 1C-F, in comparison with the control
group, the mRNA and protein expression of BDNF was significantly
decreased in the 1.0 MAC (P<0.05) and 1.5 MAC (P<0.01)
isoflurane-treated groups. The mRNA and protein expression of
caspase-3, and the protein expression of Bax, increased
significantly in the 1.0 MAC (P<0.05) and 1.5 MAC (P<0.01)
isoflurane-treated groups. Furthermore, the mRNA and protein
expression of TREK-1 increased significantly in the 1.0 MAC
(P<0.05) and 1.5 MAC (P<0.01) isoflurane-treated groups. No
significant differences were observed between the 0.5 MAC
isoflurane-treated group and the control group for any genes. These
results indicate that isoflurane administration may promote TREK-1
activity and apoptosis in astrocytes, and inhibit BDNF
expression.
Overexpression of TREK-1 downregulates BDNF, and
upregulates Bax and caspase-3. To investigate whether the
aforementioned effects of isoflurane in astrocytes were due to the
upregulation of TREK-1, the present study overexpressed and knocked
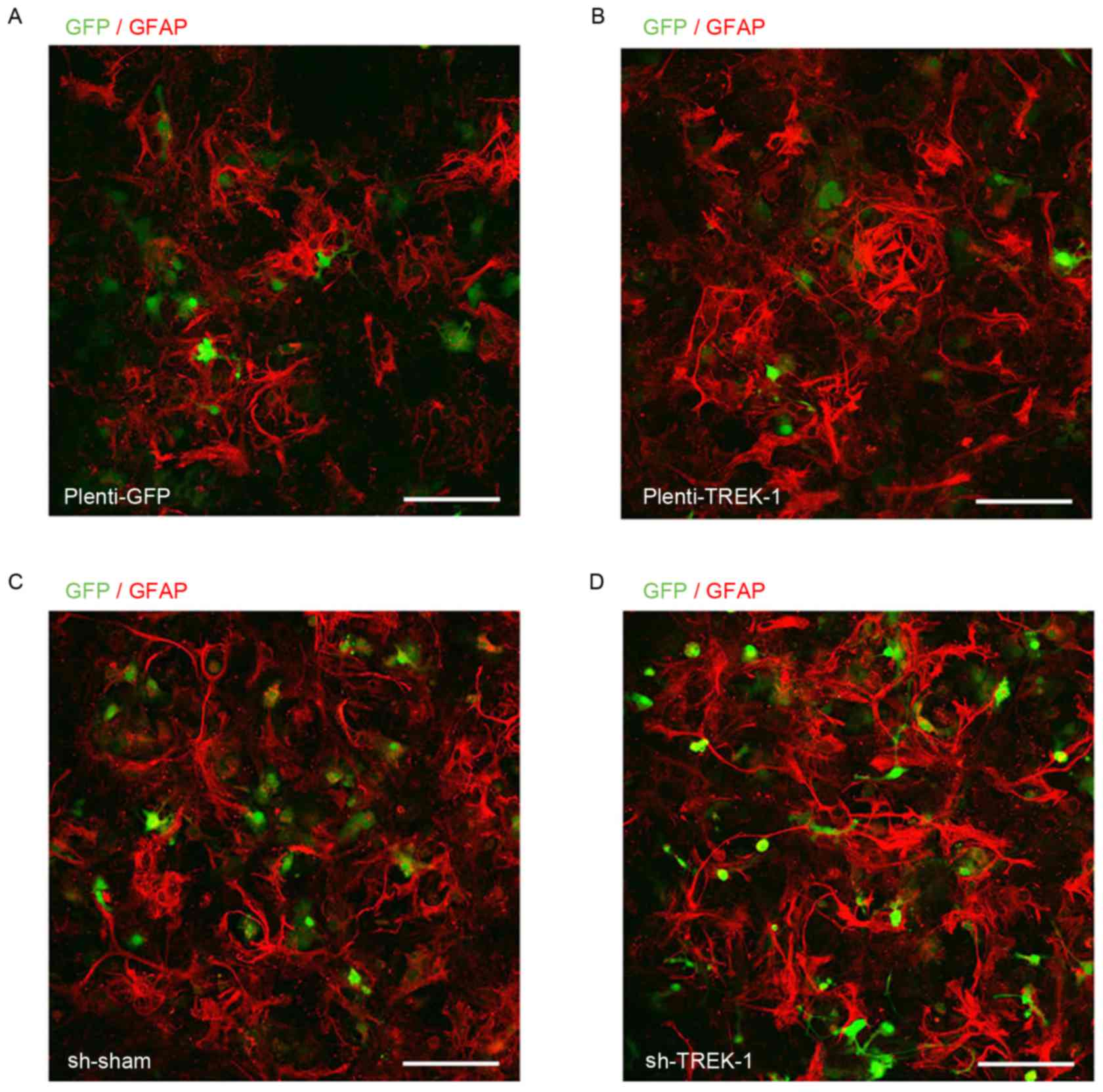
down TREK-1 in cultured astrocytes via lentivirus infection
(Fig. 2), and detected the mRNA
and/or protein expression of BDNF, Bax and caspase-3. As
demonstrated in Fig. 3,
Plenti-TREK-1-GFP infection significantly increased TREK-1
expression in astrocytes compared with Plenti-sham-GFP infection
cells (P<0.05), and TREK-1 overexpression exhibited similar
effects to the isoflurane-dependent changes in the expression of
BDNF, Bax and caspase-3, as TREK-1 overexpression reduced the
expression of BDNF, and increased the expression of Bax and
caspase-3, which was also observed for isoflurane treatment in
Fig. 1C-F. Compared with the
Plenti-sham-GFP group, the BDNF mRNA and protein expression
significantly decreased (P<0.05), while the Bax and caspase-3
expression was significantly increased (P<0.05) in the group
infected with Plenti-TREK-1-GFP. These results indicate that TREK-1
may have an important role in BDNF expression and apoptosis in
astrocytes.
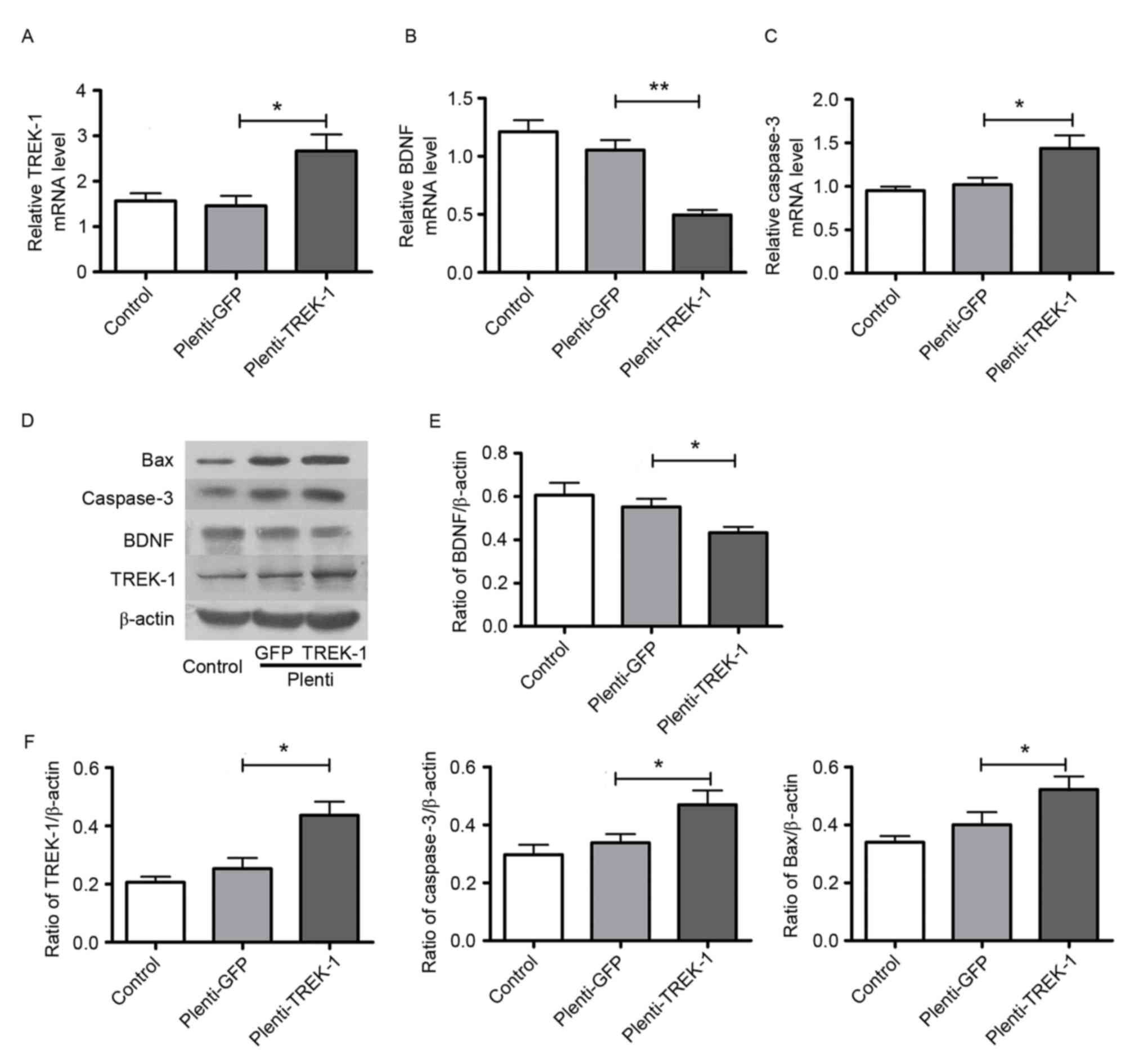 | Figure 3.Overexpression of TREK-1
downregulates BDNF, and upregulates Bax and caspase-3 expression.
Effect of Plenti-TREK-1-GFP infection on (A) TREK-1, (B) BDNF and
(C) caspase-3 mRNA levels in astrocytes. (D) Representative protein
bands for Bax, caspase-3, BDNF, TREK-1 and β-actin in sham and
TREK-1 overexpression lentivirus-transfected cells. (E)
Plenti-TREK-1-GFP infection decreased the protein expression of
BDNF in astrocytes. (F) Plenti-TREK-1-GFP infection increased the
protein expression of TREK-1, caspase-3 and Bax in astrocytes. n=6
per group; *P<0.05 and **P<0.01, as indicated. TREK-1,
TWIK-related K+ channel; BDNF, brain-derived
neurotrophic factor; Bax, Bcl-2-associated X; GFP, green
fluorescent protein. |
TREK-1 knockdown in astrocytes inhibits
isoflurane-dependent changes in BDNF, Bax, and caspase-3. To
determine whether suppressing TREK-1 expression may attenuate the
effects of isoflurane in astrocytes, cultured astrocytes were
infected with Lv-shRNA-TREK-1 and Lv-shRNA-sham prior to isoflurane
administration (Fig. 2). As
demonstrated in Fig. 4,
Lv-shRNA-TREK-1 infection significantly decreased TREK-1 mRNA and
protein expression (P<0.05) in astrocytes compared with
Lv-shRNA-sham cells, and inhibited the effect of 1.0 and 1.5 MAC
isoflurane on the expression of TREK-1, BDNF, Bax and caspase-3
(P<0.05).
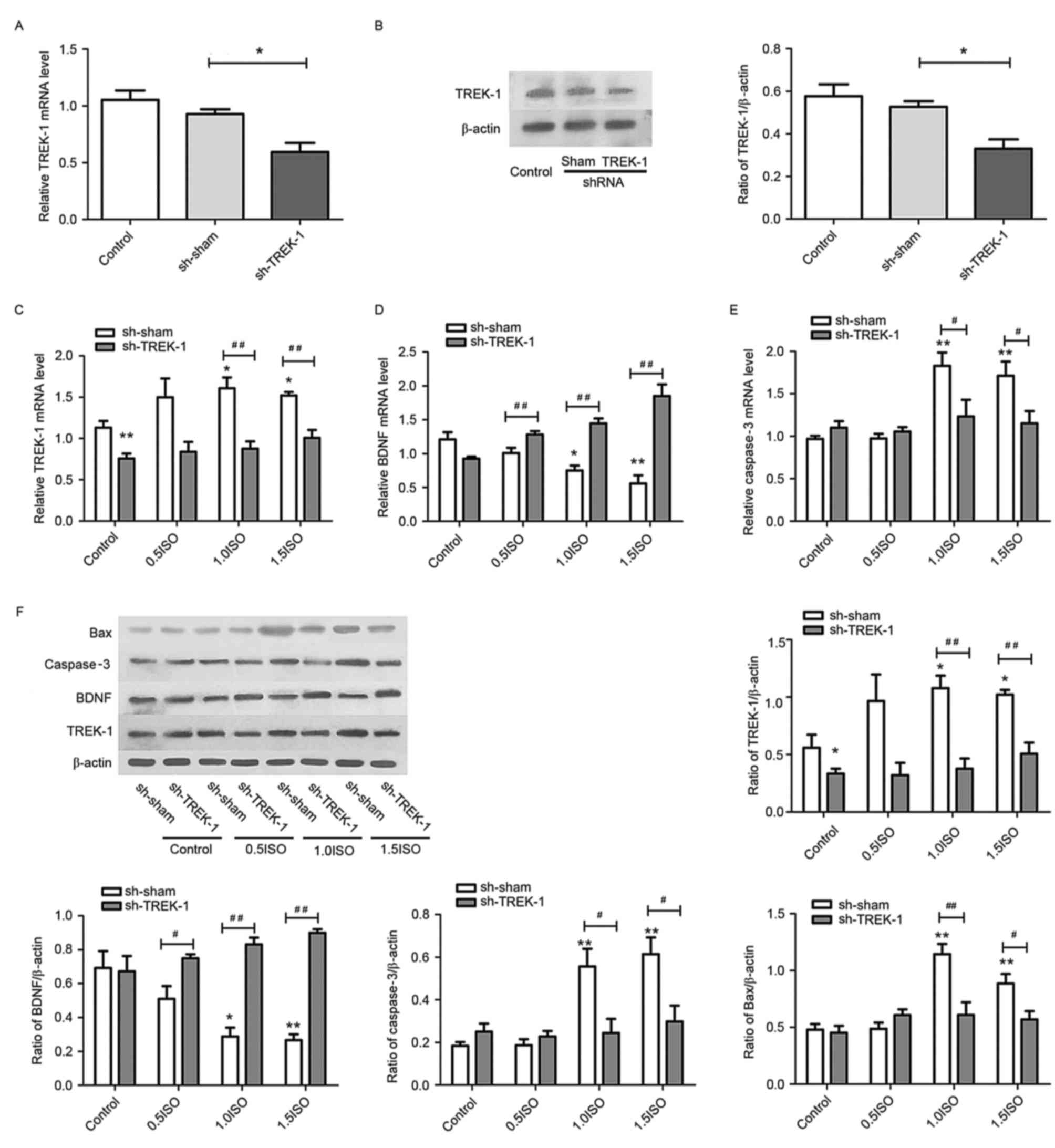 | Figure 4.TREK-1 knockdown in astrocytes
inhibited isoflurane-dependent changes in BDNF, Bax and caspase-3
expression. Evaluation of (A) mRNA and (B) protein levels of TREK-1
in astrocytes following Lv-shRNA-TREK-1 infection. mRNA levels of
(C) TREK-1, (D) BDNF, and (E) caspase-3 in Lv-shRNA-TREK-1 infected
astrocytes following exposure to 0.5 (0.7%), 1.0 (1.4%) and 1.5
(2.1%) MAC isoflurane. (F) Representative protein bands and
densitometric analysis of TREK-1, BDNF, caspase-3 and Bax
expression relative to β-actin in Lv-shRNA-TREK-1 infected
astrocytes following exposure to 0.5, 1.0 and 1.5 MAC isoflurane.
n=6 per group; *P<0.05 and **P<0.01 vs. control;
#P<0.05 and ##P<0.01 vs. sh-sham.
TREK-1, TWIK-related K+ channel; BDNF, brain-derived
neurotrophic factor; Bax, Bcl-2-associated X; Lv, lentivirus;
shRNA, short hairpin RNA; MAC, minimum alveolar concentration; sh-,
shRNA; ISO, isoflurane. |
Discussion
Anesthetic pharmacological agents are widely used
clinically for a controlled and reversible loss of consciousness
during surgery, which allows millions of individuals to safely
undergo surgery (35). However,
the side effects on brain development following exposure to general
anesthetics are attracting increased attention (36). Isoflurane is a commonly used
inhalation anesthetic, and treatment with 1.4–1.7% isoflurane for 2
h was reported to reduce acetylcholine levels in the brain and
result in learning/memory impairment in rats (32). In addition, exposure to high
concentrations of isoflurane (1.0 MAC) for 4 h induced caspase-3
and extracellular signal-regulated kinase 1/2 activation, and
inhibited N-methyl-D-aspartate (NMDA) receptor subunit NR2B protein
expression, leading to an impairment of spatial learning
performance in young adult C57BL/6 mice (33). Furthermore, neonatal isoflurane
exposure was demonstrated to disrupt hippocampal functions such as
learning and memory in humans (37). The mechanisms of isoflurane-induced
cognitive impairment are poorly understood. Potential candidate
mechanisms include an apoptotic cytotoxic effect (38–40)
or a sublethal alteration in circuit formation (41–43).
Previous studies have indicated that cognitive impairment following
isoflurane exposure is directly associated with its effects on
synaptic plasticity (19,20,44).
Currently, increasing evidence demonstrates that
astrocytes are responsible for controlling the formation,
maturation, function and elimination of synapses, processes that
are vital for the neural circuit formation and the processing of
information in the brain (45,46).
As a result, astrocyte dysfunction is reported to be involved in
several brain disorders that are associated with cognitive
impairment, therefore, astrocytes are considered to have a number
of roles in addition to their role in supporting cells (47,48)
Notably, previous studies have demonstrated that isoflurane
markedly disrupted the response of astrocytes to neuronal activity
by suppression of calcium transients in astrocytes (49), and astrocytes protected against
isoflurane-induced neurotoxicity by buffering pro-BDNF (50). Additionally, isoflurane alters the
ability of cultured astrocytes to support neuronal growth (51). In the present study, the results
demonstrated that brief clinical doses of isoflurane (1.0 MAC or
1.5 MAC for 2 h) significantly decreased astrocyte viability, and
this disruption may be critically involved in the cognitive
impairment previously reported following isoflurane exposure.
It is established that astrocytes synthesize and
secrete various cytokines to regulate neural plasticity, which
include BDNF and glial cell line-derived neurotrophic factor (GDNF)
(52,53). BDNF belongs to the nerve growth
factor superfamily, which is essential for neuronal
differentiation, survival and hippocampal synaptic plasticity, and
is associated with cognitive functions such as learning and memory
(54). Previous studies have
indicated that the age-associated decline in BDNF signaling
contributes to age-associated memory deficits and cognitive
dysfunction (55,56), and decreased BDNF expression in the
brain was reported to be associated with the severity of cognitive
impairment (57). By contrast, a
previous study indicated that increased BDNF expression may have an
essential role in the recovery process of cognitive function
following injury to the adult central nervous system (58), and intracranial BDNF injection
effectively improved cognitive skill in rats with axonal injury
(59). Another study also reported
that exposure to an enriched environment restored cognitive
impairment induced by chronic cerebral hypoperfusion by
upregulating the protein levels of BDNF and NMDA receptor subunit 1
(60). Furthermore, the BDNF
signaling pathway is considered to be involved in the cognitive
deficits of aging mice (61), and
in isoflurane-induced apoptosis in the developing rat brain
(62). Xu et al (63) reported that BDNF was involved in
hippocampal cell apoptosis, and reduced BNDF levels promoted
hippocampal neuronal apoptosis in zinc deficient mice cell due to
upregulation of Bax and caspase-3 expression. Previous reports also
demonstrated that isoflurane exposure reduced the expression of
BDNF, and increased caspase-3 and Bax expression in the
hippocampus, which contributed to isoflurane-induced neuronal
apoptosis and cognitive impairments in rats (64,65).
GDNF belongs to the transforming growth factor-β
superfamily and has an important role in the development of the
dopaminergic nigrostriatal system, and astrocyte-derived GDNF is a
potent inhibitor of microglial activation (66). However, limited evidence concerning
the roles of GDNF in anesthetic-induced cognitive impairment is
available. The present study demonstrated that isoflurane decreased
BDNF, and increased Bax and caspase-3 expression in cultured
astrocytes, which is consistent with the results of previous
studies (62,67,68).
Furthermore, the results of the current study indicate that
isoflurane exposure-induced astrocyte apoptosis may be associated
with the downregulation of BDNF expression. However, the potential
role of GDNF in isoflurane-induced neurological damage in the
hippocampus is unclear and requires further investigation.
K+ channels containing a two-pore domain
(K2p) are a large family of hyperpolarizing channels that produce
background currents that prevent the depolarization of membranes
and cell excitability. These K+ channels have functions
in the cellular mechanisms of apoptosis, vasodilatation,
anesthesia, pain, neuroprotection and in the pathomechanism of
cognitive deficits (21). TREK-1
is a member of the TREK subfamily of K2p channels, which are
expressed throughout the central nervous system and activated by
polyunsaturated fatty acids and lysophospholipids, and inhibited by
neurotransmitters (69). TREK-1
may also be activated by volatile anesthetics and may be an
important target in the action of drugs such as isoflurane
(70). Several studies have
demonstrated that TREK-1 is involved in cognitive deficits
(71–73). The results of the current study
indicate that isoflurane administration in vitro
significantly upregulated TREK-1 expression in astrocytes.
Furthermore, overexpression of TREK-1 in astrocytes downregulated
BDNF, and upregulated Bax and caspase-3, while TREK-1 shRNA
effectively reversed the isoflurane-dependent changes in BDNF, Bax
and caspase-3 expression. These results indicate that isoflurane
exposure disrupts astrocyte viability and BDNF expression, and
induces apoptosis, and these effects may be mediated via the TREK-1
signaling pathway.
However, certain studies have reported that
isoflurane preconditioning or postconditioning may provide
neuroprotection against various damaging insults (74,75).
Thus, the role of isoflurane in brain development and function
remains controversial. Differences in the concentration and
duration time of isoflurane exposure, as well as differences
between in vitro and in vivo experimentation, may
have contributed to these discrepancies. However, the present study
did not investigate the roles of isoflurane and TREK-1 in
vivo, which is a potential limitation of the present study.
In conclusion, the results of the current study
demonstrated that brief exposure to isoflurane at 1.0 MAC or 1.5
MAC for 2 h increased the expression of TREK-1 and the
apoptosis-associated proteins caspase-3 and Bax, and decreased the
cell viability and expression of BDNF in vitro. However,
these changes were reversed by TREK-1 knockdown. Further
investigation is required to elucidate the detailed signaling
cascades that are involved in the regulation of TREK-1 in response
to isoflurane exposure for different cell types, and to confirm the
efficacy of TREK-1 knockdown in recovering cognitive dysfunction
in vivo. The present results provide evidence that
isoflurane-induced cognitive dysfunction may be associated with
TREK-1 dysfunction in hippocampal astrocytes and provides a
reference for the safe use of isoflurane anesthesia in infants and
children.
Acknowledgements
The present study was supported by the National
Natural Science Foundation (grant nos. 81571309 and 81401109) of
China and Project of Science and Technology of Social Development
in Shaanxi 21 Province (grant no. 2015SF005).
Glossary
Abbreviations
Abbreviations:
|
BDNF
|
brain-derived neurotrophic factor
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
GFAP
|
glial fibrillary acidic protein
|
|
TREK-1
|
TWIK-related K+ channel
|
References
|
1
|
Sall JW, Stratmann G, Leong J, McKleroy W,
Mason D, Shenoy S, Pleasure SJ and Bickler PE: Isoflurane inhibits
growth but does not cause cell death in hippocampal neural
precursor cells grown in culture. Anesthesiology. 110:826–833.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ballesteros KA, Sikorski A, Orfila JE and
Martinez JL Jr: Effects of inhaled anesthetic isoflurane on
long-term potentiation of CA3 pyramidal cell afferents in vivo. Int
J Gen Med. 5:935–942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang B, Tian M, Zhen Y, Yue Y, Sherman J,
Zheng H, Li S, Tanzi RE, Marcantonio ER and Xie Z: The effects of
isoflurane and desflurane on cognitive function in humans. Anesth
Analg. 114:410–415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang F, Zhu ZQ, Liu DX, Zhang C, Gong QH
and Zhu YH: Emulsified isoflurane anesthesia decreases
brain-derived neurotrophic factor expression and induces cognitive
dysfunction in adult rats. Exp Ther Med. 8:471–477. 2014.PubMed/NCBI
|
|
5
|
Zhu C, Gao J, Karlsson N, Li Q, Zhang Y,
Huang Z, Li H, Kuhn HG and Blomgren K: Isoflurane anesthesia
induced persistent, progressive memory impairment, caused a loss of
neural stem cells, and reduced neurogenesis in young, but not
adult, rodents. J Cereb Blood Flow Metab. 30:1017–1030. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mellon RD, Simone AF and Rappaport BA: Use
of anesthetic agents in neonates and young children. Anesth Analg.
104:509–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shetty AK: Hippocampal injury-induced
cognitive and mood dysfunction, altered neurogenesis and epilepsy:
Can early neural stem cell grafting intervention provide
protection. Epilepsy Behav. 38:117–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao N, Zhong C, Wang Y, Zhao Y, Gong N,
Zhou G, Xu T and Hong Z: Impaired hippocampal neurogenesis is
involved in cognitive dysfunction induced by thiamine deficiency at
early pre-pathological lesion stage. Neurobiol Dis. 29:176–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuhn HG, Dickinson-Anson H and Gage FH:
Neurogenesis in the dentate gyrus of the adult rat: Age-related
decrease of neuronal progenitor proliferation. J Neurosci.
16:2027–2033. 1996.PubMed/NCBI
|
|
10
|
Donovan MH, Yazdani U, Norris RD, Games D,
German DC and Eisch AJ: Decreased adult hippocampal neurogenesis in
the PDAPP mouse model of Alzheimer's disease. J Comp Neurol.
495:70–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rubino T, Realini N, Braida D, Guidi S,
Capurro V, Viganò D, Guidali C, Pinter M, Sala M, Bartesaghi R and
Parolaro D: Changes in hippocampal morphology and neuroplasticity
induced by adolescent THC treatment are associated with cognitive
impairment in adulthood. Hippocampus. 19:763–772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim TW, Choi HH and Chung YR: Treadmill
exercise alleviates impairment of cognitive function by enhancing
hippocampal neuroplasticity in the high-fat diet-induced obese
mice. J Exerc Rehabil. 12:156–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Secchiero P, Melloni E, di Iasio MG,
Tiribelli M, Rimondi E, Corallini F, Gattei V and Zauli G: Nutlin-3
up-regulates the expression of Notch1 in both myeloid and lymphoid
leukemic cells, as part of a negative feedback anti-apoptotic
mechanism. Blood. 113:4300–4308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schitine C, Nogaroli L, Costa MR and
Hedin-Pereira C: Astrocyte heterogeneity in the brain: From
development to disease. Front Cell Neurosci. 9:762015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barker AJ and Ullian EM: Astrocytes and
synaptic plasticity. Neuroscientist. 16:40–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jones OD: Astrocyte-mediated
metaplasticity in the hippocampus: Help or hindrance. Neuroscience.
309:113–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pabst M, Braganza O, Dannenberg H, Hu W,
Pothmann L, Rosen J, Mody I, van Loo K, Deisseroth K, Becker AJ, et
al: Astrocyte intermediaries of septal cholinergic modulation in
the hippocampus. Neuron. 90:853–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin D and Zuo Z: Isoflurane induces
hippocampal cell injury and cognitive impairments in adult rats.
Neuropharmacology. 61:1354–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nie H, Peng Z, Lao N, Dong H and Xiong L:
Effects of sevoflurane on self-renewal capacity and differentiation
of cultured neural stem cells. Neurochem Res. 38:1758–1767. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheung SW, Nagarajan SS, Bedenbaugh PH,
Schreiner CE, Wang X and Wong A: Auditory cortical neuron response
differences under isoflurane versus pentobarbital anesthesia. Hear
Res. 156:115–127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noel J, Sandoz G and Lesage F: Molecular
regulations governing TREK and TRAAK channel functions. Channels.
5:402–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ehling P, Cerina M, Budde T, Meuth SG and
Bittner S: The CNS under pathophysiologic attack-examining the role
of K2p channels. Pflugers Arch. 467:959–972. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu L, Wang W, Peng Y, Li J, Wang L and
Wang X: Electrophysiology and pharmacology of tandem domain
potassium channel TREK-1 related BDNF synthesis in rat astrocytes.
Naunyn Schmiedebergs Arch Pharmacol. 387:303–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel AJ, Honoré E, Lesage F, Fink M,
Romey G and Lazdunski M: Inhalational anesthetics activate
two-pore-domain background K+ channels. Nat Neurosci. 2:422–426.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang K and Kong X: Isoflurane
preconditioning induces neuroprotection by up-regulation of TREK1
in a rat model of spinal cord ischemic injury. Biomol Ther (Seoul).
24:495–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin X, Su B, Zhang H, Song W, Wu H, Chen
X, Zhang X, Dong H and Xiong L: TREK1 activation mediates spinal
cord ischemic tolerance induced by isoflurane preconditioning in
rats. Neurosci Lett. 515:115–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye D, Li Y, Zhang X, Guo F, Geng L, Zhang
Q and Zhang Z: TREK1 channel blockade induces an
antidepressant-like response synergizing with 5-HT1A receptor
signaling. Eur Neuropsychopharmacol. 25:2426–2436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Olsen ML, Khakh BS, Skatchkov SN, Zhou M,
Lee CJ and Rouach N: New insights on astrocyte ion channels:
Critical for homeostasis and neuron-glia signaling. J Neurosci.
35:13827–13835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Callaghan EL, Bassi JK, Porrello ER,
Delbridge LM, Thomas WG and Allen AM: Regulation of angiotensinogen
by angiotensin II in mouse primary astrocyte cultures. J Neurochem.
119:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Panickar KS, Jayakumar AR, Rao KV Rama and
Norenberg MD: Downregulation of the 18-kDa trans-locator protein:
Effects on the ammonia-induced mitochondrial permeability
transition and cell swelling in cultured astrocytes. Glia.
55:1720–1727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee J, Kim MS, Park C, Jung EB, Choi DH,
Kim TY, Moon SK and Park R: Morphine prevents glutamate-induced
death of primary rat neonatal astrocytes through modulation of
intracellular redox. Immunopharmacol Immunotoxicol. 26:17–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Xu Z, Feng C, Wang Y, Jia X, Wu A
and Yue Y: Changes of learning and memory in aged rats after
isoflurane inhalational anaesthesia correlated with hippocampal
acetylcholine level. Ann Fr Anesth Reanim. 31:e61–e66. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu J, Wang P, Zhang X, Zhang W and Gu G:
Effects of different concentration and duration time of isoflurane
on acute and long-term neurocognitive function of young adult
C57BL/6 mouse. Int J Clin Exp Pathol. 7:5828–5836. 2014.PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Uhrig L, Dehaene S and Jarraya B: Cerebral
mechanisms of general anesthesia. Ann Fr Anesth Reanim. 33:72–82.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vlisides P and Xie Z: Neurotoxicity of
general anesthetics: An update. Curr Pharm Des. 18:6232–6240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
DiMaggio C, Sun LS, Ing C and Li G:
Pediatric anesthesia and neurodevelopmental impairments: A Bayesian
meta-analysis. J Neurosurg Anesthesiol. 24:376–381. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jevtovic-Todorovic V, Hartman RE, Izumi Y,
Benshoff ND, Dikranian K, Zorumski CF, Olney JW and Wozniak DF:
Early exposure to common anesthetic agents causes widespread
neurodegeneration in the developing rat brain and persistent
learning deficits. J Neurosci. 23:876–882. 2003.PubMed/NCBI
|
|
39
|
Brambrink AM, Evers AS, Avidan MS, Farber
NB, Smith DJ, Zhang X, Dissen GA, Creeley CE and Olney JW:
Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque
brain. Anesthesiology. 112:834–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zou X, Liu F, Zhang X, Patterson TA,
Callicott R, Liu S, Hanig JP, Paule MG, Slikker W Jr and Wang C:
Inhalation anesthetic-induced neuronal damage in the developing
rhesus monkey. Neurotoxicol Teratol. 33:592–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang SQ, Fang F, Xue ZG, Cang J and Zhang
XG: Neonatal sevoflurane anesthesia induces long-term memory
impairment and decreases hippocampal PSD-95 expression without
neuronal loss. Eur Rev Med Pharmacol Sci. 17:941–950.
2013.PubMed/NCBI
|
|
42
|
Mintz CD, Barrett KM, Smith SC, Benson DL
and Harrison NL: Anesthetics interfere with axon guidance in
developing mouse neocortical neurons in vitro via a γ-aminobutyric
acid type A receptor mechanism. Anesthesiology. 118:825–833. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mintz CD, Smith SC, Barrett KM and Benson
DL: Anesthetics interfere with the polarization of developing
cortical neurons. J Neurosurg Anesthesiol. 24:368–375. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Culley DJ, Boyd JD, Palanisamy A, Xie Z,
Kojima K, Vacanti CA, Tanzi RE and Crosby G: Isoflurane decreases
self-renewal capacity of rat cultured neural stem cells.
Anesthesiology. 115:754–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Allen NJ, Bennett ML, Foo LC, Wang GX,
Chakraborty C, Smith SJ and Barres BA: Astrocyte glypicans 4 and 6
promote formation of excitatory synapses via GluA1 AMPA receptors.
Nature. 486:410–414. 2012.PubMed/NCBI
|
|
46
|
Kucukdereli H, Allen NJ, Lee AT, Feng A,
Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH,
et al: Control of excitatory CNS synaptogenesis by
astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci
USA. 108:E440–E449. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clarke LE and Barres BA: Emerging roles of
astrocytes in neural circuit development. Nat Rev Neurosci.
14:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hong S, Xin Y, HaiQin W, GuiLian Z, Ru Z,
ShuQin Z, HuQing W, Li Y and Yun D: The PPARγ agonist rosiglitazone
prevents cognitive impairment by inhibiting astrocyte activation
and oxidative stress following pilocarpine-induced status
epilepticus. Neurol Sci. 33:559–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thrane AS, Thrane V Rangroo, Zeppenfeld D,
Lou N, Xu Q, Nagelhus EA and Nedergaard M: General anesthesia
selectively disrupts astrocyte calcium signaling in the awake mouse
cortex. Proc Natl Acad Sci USA. 109:18974–18979. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Creed MS, Sun XY and Rona GG: Astrocytes
protect against isoflurane neurotoxicity by buffering
pro-brain-derived neurotrophic factor. Anesthesiology. 123:810–819.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ryu YK, Khan S, Smith SC and Mintz CD:
Isoflurane impairs the capacity of astrocytes to support neuronal
development in a mouse dissociated co-culture model. J Neurosurg
Anesthesiol. 26:363–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu W, Zhu H, Wang Y, Li G, Wang L and Li
H: Reactive Transformation and Increased BDNF Signaling by
Hippocampal Astrocytes in Response to MK-801. PLoS One.
10:01456512015. View Article : Google Scholar
|
|
53
|
Rocha SM, Cristovão AC, Campos FL, Fonseca
CP and Baltazar G: Astrocyte-derived GDNF is a potent inhibitor of
microglial activation. Neurobiol Dis. 47:407–415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hong Y, Zhao T, Li XJ and Li S: Mutant
Huntingtin impairs BDNF release from astrocytes by disrupting
conversion of Rab3a-GTP into Rab3a-GDP. J Neurosci. 36:8790–8801.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang XY, Chen DC, Xiu MH, Haile CN, Luo
X, Xu K, Zhang HP, Zuo L, Zhang Z, Zhang X, et al: Cognitive and
serum BDNF correlates of BDNF Val66Met gene polymorphism in
patients with schizophrenia and normal controls. Hum Genet.
131:1187–1195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kuczewski N, Porcher C and Gaiarsa JL:
Activity-dependent dendritic secretion of brain-derived
neurotrophic factor modulates synaptic plasticity. Eur J Neurosci.
32:1239–1244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Siuda J, Patalong-Ogiewa M, Żmuda W,
Targosz-Gajniak M, Niewiadomska E, Matuszek I,
Jędrzejowska-Szypułka H and Rudzińska-Bar M: Cognitive impairment
and BDNF serum levels. Neurol Neurochir Pol. 51:24–32.
2017.PubMed/NCBI
|
|
58
|
Dixon KJ and Sherrard RM: Brain-derived
neurotrophic factor induces post lesion trans-commissural growth of
olivary axons that develop normal climbing fibers on mature
Purkinje cells. Exp Neurol. 202:44–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Willson ML, McElnea C, Mariani J, Lohof AM
and Sherrard RM: BDNF increases homotypic olivocerebellar
reinnervation and associated fine motor and cognitive skill. Brain.
131:1099–1112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun H, Zhang J, Zhang L, Liu H, Zhu H and
Yang Y: Environmental enrichment influences BDNF and NR1 levels in
the hippocampus and restores cognitive impairment in chronic
cerebral hypo-perfused rats. Curr Neurovasc Res. 7:268–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu J, Zhang M, Li H, Sun X, Hao S, Ji M,
Yang J and Li K: BDNF pathway is involved in the protective effects
of SS-31 on isoflurane-induced cognitive deficits in aging mice.
Behav Brain Res. 305:115–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lu LX, Yon JH, Carter LB and
Jevtovic-Todorovic V: General anesthesia activates BDNF-dependent
neuro-apoptosis in the developing rat brain. Apoptosis.
11:1603–1615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu H, Gao HL, Zheng W, Xin N, Chi ZH, Bai
SL and Wang ZY: Lactational zinc deficiency-induced hippocampal
neuronal apoptosis by a BDNF-independent TrkB signaling pathway.
Hippocampus. 21:495–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ji M, Dong L, Jia M, Liu W, Zhang M, Ju L
and Yang J, Xie Z and Yang J: Epigenetic enhancement of
brain-derived neurotrophic factor signaling pathway improves
cognitive impairments induced by isoflurane exposure in aged rats.
Mol Neurobiol. 50:937–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang WY, Luo Y, Jia LJ, Hu SF, Lou XK,
Shen SL, Lu H, Zhang HH, Yang R, Wang H, et al: Inhibition of
aberrant cyclin-dependent kinase 5 activity attenuates isoflurane
neurotoxicity in the developing brain. Neuropharmacology. 77:90–99.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Campos FL, Cristovão AC, Rocha SM, Fonseca
CP and Baltazar G: GDNF contributes to oestrogen-mediated
protection of midbrain dopaminergic neurones. J Neuroendocrinol.
24:1386–1397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cho HJ, Sung YH, Lee SH, Chung JY, Kang JM
and Yi JW: Isoflurane induces transient anterograde amnesia through
suppression of brain-derived neurotrophic factor in hippocampus. J
Korean Neurosurg Soc. 53:139–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tyler WJ, Alonso M, Bramham CR and
Pozzo-Miller LD: From acquisition to consolidation: On the role of
brain-derived neurotrophic factor signaling in
hippocampal-dependent learning. Learn Mem. 9:224–237. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lesage F, Terrenoire C, Romey G and
Lazdunski M: Human TREK2, a 2P domain mechano-sensitive K+ channel
with multiple regulations by polyunsaturated fatty acids,
lysophospholipids and Gs, Gi, and Gq protein-coupled receptors. J
Biol Chem. 275:28398–28405. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Heurteaux C, Guy N, Laigle C, Blondeau N,
Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey
G and Lazdunski M: TREK-1, a K+ channel involved in neuroprotection
and general anesthesia. EMBO J. 23:2684–2695. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Moha Ou Maati H, Veyssiere J, Labbal F,
Coppola T, Gandin C, Widmann C, Mazella J, Heurteaux C and Borsotto
M: Spadin as a new antidepressant: Absence of TREK-1-related side
effects. Neuropharmacology. 62:278–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Borsotto M, Veyssiere J, Moha Ou Maati H,
Devader C, Mazella J and Heurteaux C: Targeting two-pore domain
K(+) channels TREK-1 and TASK-3 for the treatment of depression: A
new therapeutic concept. Br J Pharmacol. 172:771–784. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mazella J, Pétrault O, Lucas G, Deval E,
Béraud-Dufour S, Gandin C, El-Yacoubi M, Widmann C, Guyon A, Chevet
E, et al: Spadin, a sortilin-derived peptide, targeting rodent
TREK-1 channels: A new concept in the antidepressant drug design.
PLoS Biol. 8:10003552010. View Article : Google Scholar
|
|
74
|
Zhang HP, Sun YY, Chen XM, Yuan LB, Su BX,
Ma R, Zhao RN, Dong HL and Xiong L: The neuroprotective effects of
isoflurane preconditioning in a murine transient global cerebral
ischemia-reperfusion model: The role of the Notch signaling
pathway. Neuromolecular Med. 16:191–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yin J, Li H, Feng C and Zuo Z: Inhibition
of brain ischemia-caused notch activation in microglia may
contribute to isoflurane post-conditioning-induced neuroprotection
in male rats. CNS & neurological disorders drug targets.
13:718–732. 2014. View Article : Google Scholar : PubMed/NCBI
|