Introduction
Extracellular potassium homeostasis is maintained by
the regulation of renal potassium excretion, and is dependent on
the activity of potassium channels, which are expressed on the
apical membrane of epithelial tubular cells. Encoded by the renal
outer medullary potassium channel (ROMK) gene (also termed Kir 1.1
and KCNJ1) (1,2), ROMK channels are considered to be the
major route for the transport of potassium into the tubule lumen
(3,4). As ROMK channels usually exhibit a
high probability of being open, the physiologic augmentation of
channel activity is controlled by hormones and dietary potassium,
and is achieved predominantly by regulated alterations in the
number of active channels on the plasma membrane (5).
Hepatocyte growth factor (HGF) exerts multiple
biological activities, including mitogenic, motogenic and
morphogenic activities, on a variety of cells through its receptor,
Met. The multiple functions of HGF are important in organ
regeneration (6). In particular,
HGF is important for maintaining renal homeostasis (7,8). The
levels of HGF in the plasma increase following renal ischemia, and
the inhibition of HGF-Met signaling enhances tubular apoptosis in
the early stage of acute renal failure (ARF) (7) and inhibits the proliferation of
tubular cells in the late stage (8). However, whether HGF regulates the ion
channel activities remains to be elucidated, therefore, the present
study investigated the modulation of HGF on the expression,
phosphorylation and translocation of ROMK in renal tubular
cells.
Materials and methods
Materials
Human recombinant HGF was purified from the medium
of Chinese hamster ovary (CHO) cells transfected with human HGF
cDNA of the 5-amino acid deleted type (>98% of purity on
SDS-PAGE) as previously described (7,8). The
following antibodies were used: Anti-Tyr1234/1235-phosphorylated
Met (D26; cat. no. 3126; Cell Signaling Technology, Inc., Beverly,
MA, USA), anti-Met (SP260; cat. no. sc-162; Santa Cruz
Biotechnology, Inc., Danvers, MA, USA), anti-Met (B2; cat. no.
sc-8057; Santa Cruz Biotechnology, Inc.), anti-ROMK (cat. no.
APC001; Alomone Labs, Ltd., Jerusalem, Israel), anti-pS44/25-ROMK
(cat. no A1121; Assay Biotech Co., Inc., Sunnyvale, CA, USA),
anti-E-cadherin (cat. no. C20820, BD Biosciences, San Jose, CA,
USA), anti-GAPDH (cat. no. sc-32233, SCB), and anti-β-actin (cat.
no. A1978; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Membrane-bound ROMK and E-cadherin were biotinylated with
Sulfo-NHS-LC-biotin (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. The NRK-52E normal
rat kidney epithelial cell line was obtained from American Type
Culture Collection (Manassas, VA, USA) cultured in Dulbecco's
modified Eagle's medium (DMEM; Nacalai Tesque, Inc., Kyoto, Japan)
supplemented with 10% fetal calf serum. Cells were maintained and
grown at 37°C in 5% CO2 according to the supplier's
recommendations.
Immunoblotting
The cells were lysed on ice with lysis buffer,
containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 5
mM EDTA, protease inhibitor cocktail, 2 mM
Na3VO4 and 50 mM NaF. The protein
concentrations of cell lysates were measured by BCA protein assay
(Thermo Fisher Scientific, Inc.). For immunoblot assay of Met and
Tyr 1234/1235-phosphorylated Met, immunoprecipitation was performed
as previously reported (8,9). The cell lysates (300 µg/sample) were
incubated with anti-Met antibody (B2) at 4°C overnight and then
precipitated with protein G/A (GE Healthcare Life Sciences,
Chalfont, UK) for 2 h at 4°C. The immunoprecipitated lysates were
then subjected to SDS-PAGE and transferred onto PVDF membranes. The
primary antibodies (Met and Tyr 1234/1235-phosphorylated Met) were
applied to PVDF membranes overnight at 4°C at a concentration of
1:4,000, followed by a second reaction with horseradish
peroxidase-labeled antibodies (Dako, Glostrup, Denmark) at a
concentration of 1:4,000 for 1 h at room temperature. For the
immunoblot assay of ROMK, serine 44 phosphorylated ROMK and
E-cadherin, the cell lysates were subjected to SDS-PAGE, and then
detected as immunoblots in a similar method to total and tyrosine
1234/1235-phosphorylated Met. (1:4,000). The β-actin and GAPDH
antibodies were applied overnight at 4°C at a concentration of
1:8,000, and followed by a second reaction horseradish
peroxidase-conjugated antibodies (Dako) at a concentration of
1:8,000 for 1 h at room temperature. The signals were visualized on
PVDF membranes, using a kit (ECL system; GE Healthcare Life
Sciences). Densitometric quantification of the scanned band
intensities was performed by digitizing each band via densitometry
using ImageJ software version 1.47t (National Institutes of Health,
Bethesda, MD, USA) on a Macintosh computer.
Immunofluorescence
The cells on cover glass were fixed with 4%
paraformaldehyde in PBS for 15 min, permeabilized with 0.2% Triton
X-100 in PBS for 5 min, and blocked with 5% goat serum in PBS for 1
h at room temperature. The cells were then incubated with primary
antibodies (ROMK and serine 44 phophorylated ROMK) overnight at 4°C
at a concentration of 1:200, followed by incubation with secondary
antibodies conjugated to Alexa Fluor 488 (Thermo Fisher Scientific,
Inc.) at room temperature for 20 min at a concentration of 1:600.
The nuclei were stained with propidium iodide (PI; Sigma-Aldrich;
Merck KGaA). Following washing with PBS, the cells were mounted
with crystal mount (Biomeda Corporation, Foster City, CA, USA), and
observed under an LSM5 Pascal confocal microscope (Zeiss AG,
Oberkochen, Germany).
Statistical analysis
Student's t-test was performed to evaluate the
significant differences of each group using Microsoft Excel for Mac
2011. Data are expressed as mean ± standard deviation. P<0.05
was considered to indicate statistically significant.
Results
HGF does not alter the expression of
ROMK, but phosphorylates serine-44 of ROMK in rat kidney epithelial
cells
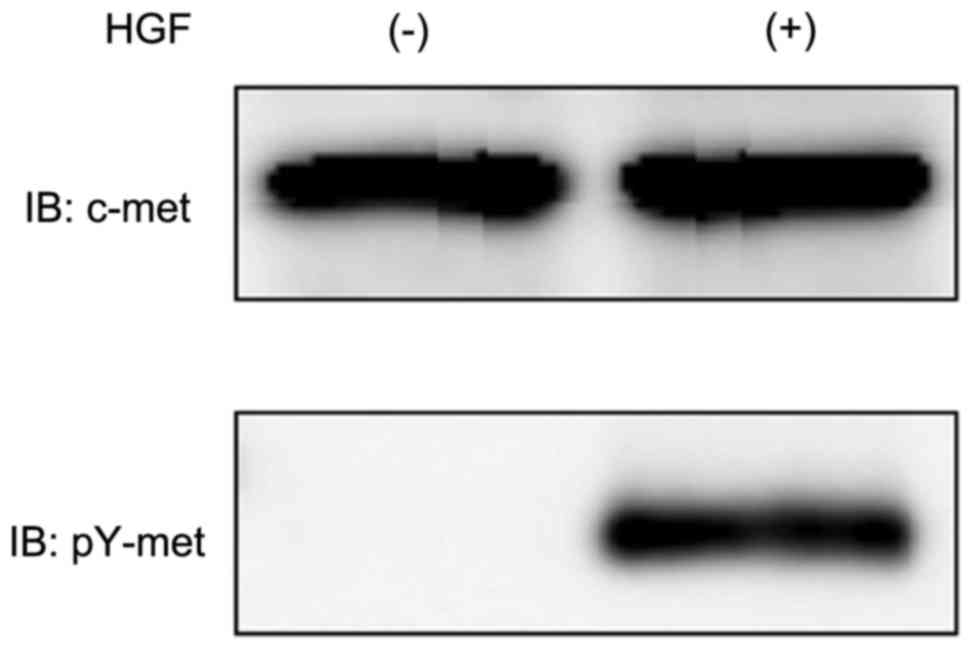
HGF led to an increase of Tyr
1234/1235-phosphorylated Met in the NRK-52E cells, determined using
western blot analysis (Fig. 1),
confirming it had an effect on the NRK-52E cells. Whether HGF
modulated the expression of ROMK was then investigated. Following
the addition of NRK-52E cells to 10 ng/ml of recombinant HGF, the
expression of ROMK was measured using western blot analysis.
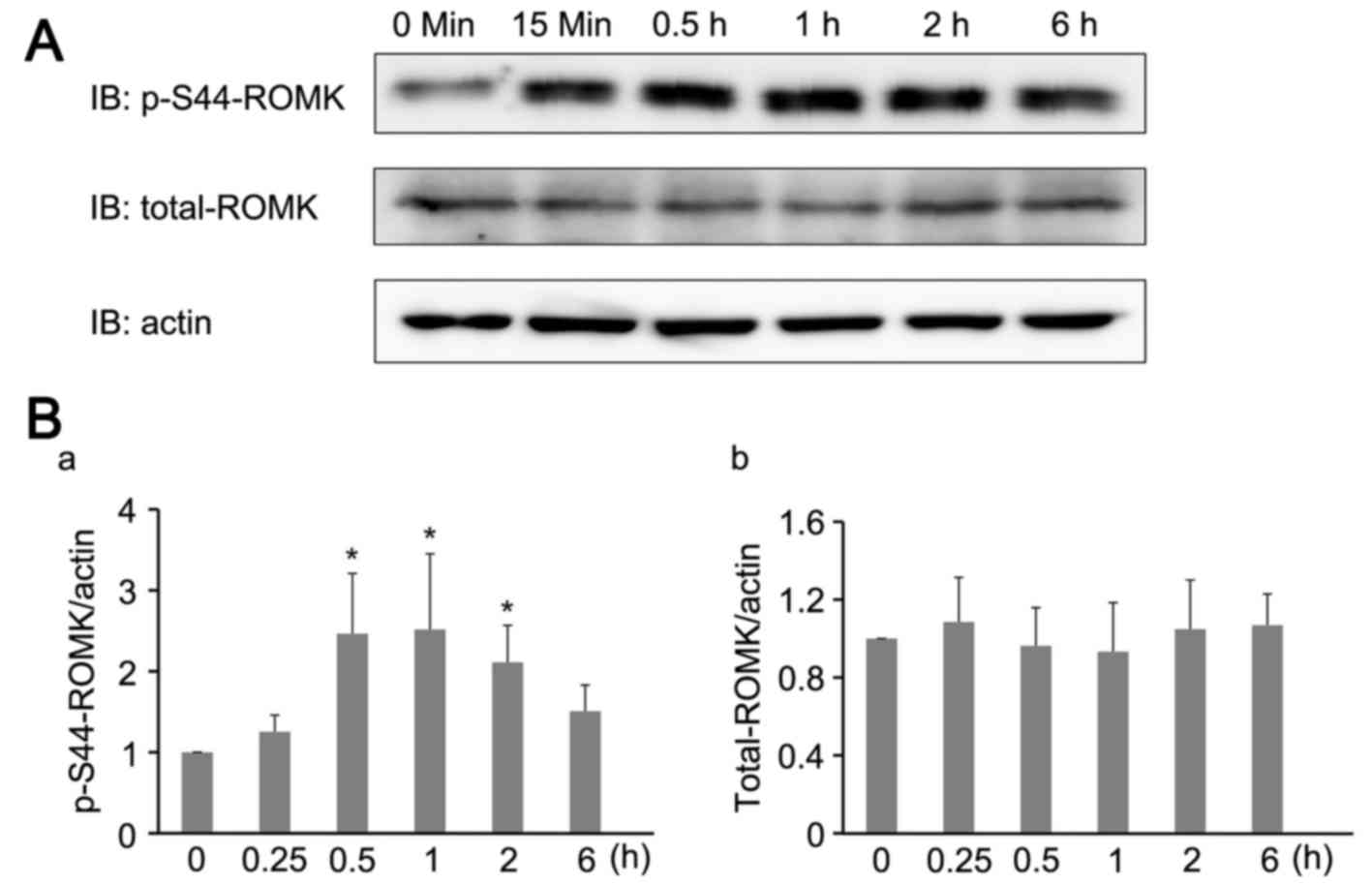
However, no alterations in the total expression of ROMK were
observed (Fig. 2A). ROMK activity
is dependent on direct phosphorylation by protein kinase A (PKA).
PKA-acceptor sites in ROMK, embedded within the cytoplasmic
NH2-(Ser-44) and COOH-termini (Ser-219 and Ser-313)
require phosphorylation for full channel activity (10). The phosphorylation of the two
COOH-terminal sites is required to maintain a high probability of
the channel being in an open state (10). The phosphorylation of the
NH2-termination site has no effect on the probability of
channel opening, however, it appears to control the number of
active channels on the cell surface (10). In the present study, serine 44
phosphorylation of ROMK in the NRK-52E cells was examined.
Treatment with HGF enhanced the level of serine 44 phosphorylated
ROMK (Fig. 2B). The results of the
immunofluorescence assay also demonstrated the upregulated serine
44 phosphorylation of ROMK (Fig.
2C).
HGF translocates ROMK into the plasma
membrane in normal kidney cells
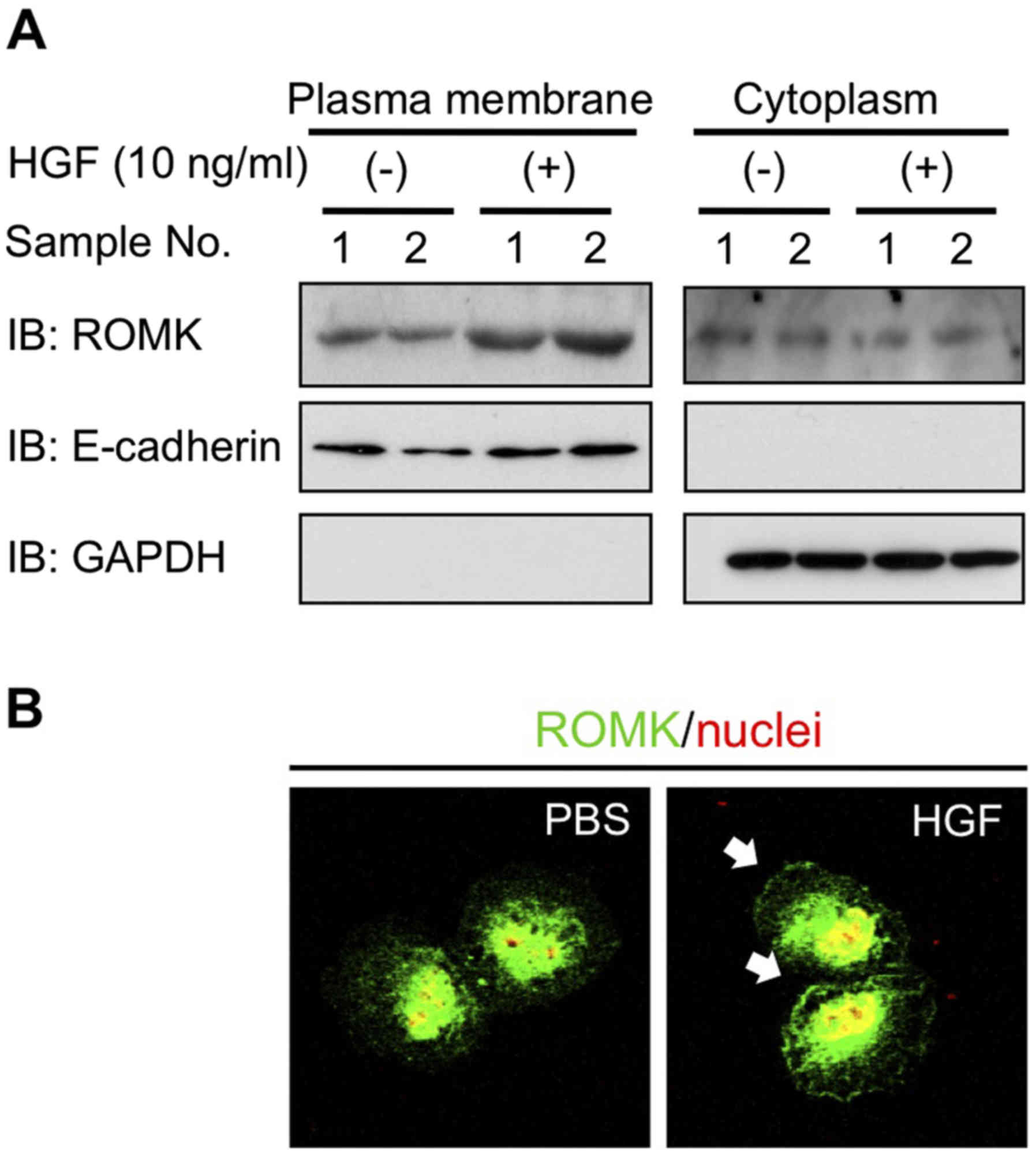
To investigate whether HGF-Met signaling induced the
movement of ROMK to the cell surface in NRK-52E cells, the present
study separated the protein constituents of cells into plasma
membrane and cytoplasm proteins. The present study confirmed that
the proteins derived from plasma membrane and cytoplasm were
E-cadherin and GAPDH using an immunoblot assay (Fig. 3A). The results of the western blot
analysis, revealed that the expression of ROMK on the plasma
membrane was increased in the HGF-treated NRK-52E cells (Fig. 3A). In addition, the results of the
immunofluorescence analysis indicated that HGF induced the
translocation of ROMK into the plasma membrane (Fig. 3B).
Discussion
By measuring the cell surface density and functional
activity of wild-type and phosphorylation-site mutant ROMK
channels, Yoo et al (11)
suggested that the phosphorylation of serine 44 markedly increases
functional channel density by recruiting on the plasma membrane,
rather than by activating silent channels already present on the
plasma membrane. In the present study, HGF enhanced ROMK
localization on the plasma membrane through the phosphorylation of
serine 44. Close inspection of the NH2-terminal PKA site
in ROMK has revealed that it also falls within a canonical serum-
and glucocorticoid-regulated kinase (SGK1) phosphorylation sequence
(11), which suggests that the
channels, and serine 44 in particular, may also be a target of
SGK1. SGK1 is a member of the protein kinase B/Akt family of
serine/threonine kinases in the renal collecting duct (12,13),
which has been shown to regulate the cell surface expression of
epithelial Na+ channels. Several pathways regulate SGK1,
including growth factor signaling, comprising HGF, and
stress-mediated signaling, and this is achieved through involvement
of the signaling pathways in the regulation of cell survival, and
cell-cell and cell-matrix interactions (14). Therefore, HGF activated ROMK
through the activation of SGK1, and may have also controlled the
number of functional ROMK at the plasma membrane.
Clinical crush syndrome occurs as a consequence of
traumatic events, for example during accidents. As a result of
muscle compression, myocytes are damaged, and this is followed by
the release of intracellular constituents into the systemic
circulation. Rhabdomyolysis, due to substantial cell necrosis in
injured muscles, causes the release of intracellular materials,
including potassium and myoglobin, into the systemic circulation,
which are important in early-and late-phase life-threatening
complications, including cardiac arrhythmia, myoglobinuric renal
failure and systemic inflammation (15). ARF due to crush-induced
rhabdomyolysis is the second most frequent cause of mortality
following traumatic impact in catastrophic earthquakes (16). The repeated administration of HGF
has been found to inhibit acute tubular necrosis in the renal
cortex, improve renal dysfunction and protect rats from death
caused by severe ARF (17). The
exogenous supply of HGF may be efficacious in combating ARF with
acute tubular necrosis, particularly rhabdomyolysis-induced ARF
(17). HGF has mitogenic,
motogenic and morphogenic functions, which accelerate the
regeneration of tubular epithelial cells and the reconstruction of
normal tissue architecture of the kidney. It also stimulates cation
transport through the activation of tubular
Na+−K+−ATPase (18). HGF may also be important in
potassium excretion and perform antihyperkalemic effects through
translocation of the ROMK potassium channel.
In the present study, it was found that HGF
treatment led to the increased serine 44 phosphorylation of ROMK
and the translocation of ROMK into the nucleus in normal kidney
cells. Future investigations aim to elucidate the effects of HGF on
potassium excretion.
Acknowledgements
The present study was supported by a grant from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan (grant no. 14454911 to Dr Takashi Kato).
References
|
1
|
Ho K, Nichols CG, Lederer WJ, Lytton J,
Vassilev PM, Kanazirska MV and Hebert SC: Cloning and expression of
an inwardly rectifying ATP-regulated potassium channel. Nature.
362:31–38. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu M, Wang T, Yan Q, Yang X, Dong K,
Knepper MA, Wang W, Giebisch G, Shull GE and Hebert SC: Absence of
small conductance K+ channel (SK) activity in apical membranes of
thick ascending limb and cortical collecting duct in ROMK
(Bartter's) knockout mice. J Biol Chem. 277:37881–37887. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giebisch G: Renal potassium transport:
Mechanisms and regulation. Am J Physiol. 274:F817–F833.
1998.PubMed/NCBI
|
|
4
|
Palmer LG: Potassium secretion and the
regulation of distal nephron K channels. Am J Physiol.
277:F821–F825. 1999.PubMed/NCBI
|
|
5
|
Wang W, Sackin H and Giebisch G: Renal
potassium channels and their regulation. Annu Rev Physiol.
54:81–96. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura T and Mizuno S: The discovery of
hepatocyte growth factor (HGF) and its significance for cell
biology, life sciences and clinical medicine. Proc Jpn Acad Ser B
Phys Biol Sci. 86:588–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizuno S and Nakamura T: Prevention of
neutrophil extravasation by hepatocyte growth factor leads to
attenuations of tubular apoptosis and renal dysfunction in mouse
ischemic kidneys. Am J Pathol. 166:1895–1905. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohnishi H, Mizuno S and Nakamura T:
Inhibition of tubular cell proliferation by neutralizing endogenous
HGF leads to renal hypoxia and bone marrow-derived cell engraftment
in acute renal failure. Am J Physiol Renal Physiol. 294:F326–F335.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kato T, Funakoshi H, Kadoyama K, Noma S,
Kanai M, Ohya-Shimada W, Mizuno S, Doe N, Taniguchi T and Nakamura
T: Hepatocyte growth factor overexpression in the nervous system
enhances learning and memory performance in mice. J Neurosci Res.
90:1743–1755. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
MacGregor GG, Xu JZ, McNicholas CM,
Giebisch G and Hebert SC: Partially active channels produced by PKA
site mutation of the cloned renal K+ channel, ROMK2 (kir1.2). Am J
Physiol. 275:F415–F422. 1998.PubMed/NCBI
|
|
11
|
Yoo D, Kim BY, Campo C, Nance L, King A,
Maouyo D and Welling PA: Cell surface expression of the ROMK (Kir
1.1) channel is regulated by the aldosterone-induced kinase, SGK-1,
and protein kinase A. J Biol Chem. 278:23066–23075. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lang F and Cohen P: Regulation and
physiological roles of serum- and glucocorticoid-induced protein
kinase isoforms. Sci STKE. 2001:re172001.PubMed/NCBI
|
|
13
|
Náray-Fejes-Tóth A, Canessa C, Cleaveland
ES, Aldrich G and Fejes-Tóth G: SGK is an aldosterone-induced
kinase in the renal collecting duct. Effects on epithelial na+
channels. J Biol Chem. 274:16973–16978. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shelly C and Herrera R: Activation of SGK1
by HGF, Rac1 and integrin-mediated cell adhesion in MDCK cells:
PI-3K-dependent and -independent pathways. J Cell Sci.
115:1985–1993. 2002.PubMed/NCBI
|
|
15
|
Bosch X, Poch E and Grau JM:
Rhabdomyolysis and acute kidney injury. N Engl J Med. 361:62–72.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ukai T: The Great Hanshin-Awaji Earthquake
and the problems with emergency medical care. Ren Fail. 19:633–645.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagano T, Mori-Kudo I, Tsuchida A,
Kawamura T, Taiji M and Noguchi H: Ameliorative effect of
hepatocyte growth factor on glycerol-induced acute renal failure
with acute tubular necrosis. Nephron. 91:730–738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishibashi K, Sasaki S, Sakamoto H,
Nakamura Y, Hata T, Nakamura T and Marumo F: Hepatocyte growth
factor is a paracrine factor for renal epithelial cells:
Stimulation of DNA synthesis and NA,K-ATPase activity. Biochem
Biophys Res Commun. 182:960–965. 1992. View Article : Google Scholar : PubMed/NCBI
|