Introduction
Long non-coding RNAs (lncRNAs) are a class of small
non-protein coding transcripts longer than 200 nucleotides
(1). Molecular biology analyses
have suggested that lncRNAs participate in variety of processes of
cellular metabolism by regulation of different signal pathways in
many kinds cells (2). In recent
years, lncRNA have now become the new hotspots in an ocean of human
diseases including metabolic diseases, hereditary disease and
cancer (3–5). Evidences have indicated that lncRNAs
are associated with stem cell pluripotency, indicating lncRNA may
integrate into the pluripotency network, as well as prominent
questions in this emerging field (6,7).
Previous study has indicated that lncRNA can regulate cell
proliferation, apoptosis and differentiation of P19 cells by
regulating Mef2c gene (8).
Systematically profiling and annotating lncRNAs have showed that
lncRNAs provide valuable resources for both experimental biologists
and bioinformaticians in various human embryonic stem cell
(9).
Hair follicle stem cells (HFSc) belong to adult stem
cell and show remarkable proliferation ability in the static state.
Research has found that the HFSc have multidirectional
differentiation potential, which can differentiate into skin, hair
follicles, sebaceous glands and participates in skin wound healing
process (10). Previous study has
showed that generation of induced pluripotent stem cells can be
differentiated from hair follicle bulge neural crest stem cells
(11). Lien et al have
indicated that in vivo transcriptional governance of HFSc
can be regulated by Wnt regulators (12). Additionally, differentiation of
human HFSc into endothelial cells induced by vascular endothelial
and basic fibroblast growth factors has been clearly presented
(13). Notably, current state of
knowledge, the widening gap in translational research and future
challenges of human epithelial HFSc and their progeny need to be
further investigated in future.
In the present study, the potential signal pathways
of proliferation and differentiation of HFSc were analyzed in
vitro. Findings in this study demonstrated the importance of
transforming growth factor (TGF)-β1-mediated Wnt/β-catenin signal
pathways in the proliferation and differentiation of HFSc. We here
show that the proliferation and differentiation of HFSc is based on
the interaction of PlncRNA-1 with the TGF-β1 expression levels
thereby regulating the Wnt/β-catenin signal pathways in HFSc.
Materials and methods
Cells and reagents
HFSc and hair follicle cells (HFc) were purchased
from Beijing JingMeng High-Tech Stem Cell Technology Co., LTD
(Beijing, China). HFSc and hair follicle cells were cultured in MEM
medium (Gibco, CA, USA) supplemented with 10% fetal bovine serum
(FBS) (Invitrogen, CA, USA). All cells were cultured in a 37°C
humidified atmosphere of 5% CO2.
Transfection of PlncRNA-1 assay
PlncRNA-1 and negative control lncRNA-vector
(control) were obtained from GenePharma (Shanghai, USA). PlncRNA-1
was cloned into the pBabe vector to generate the PlncRNA-1 and
further used to transfect HFSc to establish the PlncRNA-1
overexpression cell line. Transfection of PlncRNA-1 or negative
control lncRNA-vector was performed using X-treme GENE RNA
transfection reagent (Roche, Switzerland). Transfection
concentrations were 100 nM for PlncRNA-1 and lncRNA-vector.
Cells proliferation and
differentiation
HFSc or PlncRNA-1 overexpression HFSc were cultured
and treated by TGF-β1 inhibitor LY2109761 (0.5 mg/ml) for 24 h at a
37°C humidified atmosphere of 5% CO2. Cells
proliferation was determined by 3H-Thymidine
incorporation and differentiation was analyzed by flow cytometry
referencing previous report (14–16).
Briefly, HFSc (250,000 cells/well) were seeded on TGF-β1 inhibitor
LY2109761 (0.5 mg/ml) coated plates and cultured until they reached
70–80% confluence. For HFSc differentiation analysis, HFSc were
incubated with FITC-labeled anti-CD34 antibody (1: 500; ab81289;
Abcam, Cambridge, UK) for 2 h at a 4°C. Cells were then washed PBS
and analyzed the percentage of FITC-positive HFSc using flow
cytometry.
Analysis cells cycle
To analyze the effects of PlncRNA-1 and/or
LY2109761on the cell cycle stage of HFSc, flow cytometry was
performed. Exponentially, culturing HFSc or PlncRNA-1
overexpression hair were treated with LY2109761 (0.5 mg/ml) for 24
h. Cells were washed and trypsinized and rinsed with
phosphate-buffered saline (PBS). All cells were fixed in 75%
ice-cold ethanol for 5 min and then washed with PBS three times.
The fixed cells were washed with RNase A (20 µg ml/l, Fermentas)
and stained propidium iodide (20 µg ml/l, Sigma-Aldrich, St. Louis,
MO, USA) for 10 min at 37°C. The percentages of cells in G1 phase
were analyzed using BD FACS Calibur (Becton Dickinson, NJ,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HFSc by using RNAeasy
Mini kit (QIAGEN, Gaithersburg, MD). 1 µg total RNA was used to
transcribe into cDNA by using the reverse transcription kit
(QIAGEN, Gaithersburg, MD) and confirmed quality by
electrophoresis. The cDNA (10 ng) was subjected to quantitative PCR
(Bio-Rad, Laboratories, Inc., Hercules, CA, USA) with SYBR-Green
Master Mix system (50 ng of genomic DNA, 200 µM dNTP, 2.5 units of
Taq DNA polymerase, and 200 µM primers) according to manufacturers'
protocols, followed by preliminary denaturation at 94°C for 2 min,
35 cycles at 94°C for 30 sec, annealing temperature reduced to 64°C
for 30 sec and 72°C for 10 min. All the forward and reverse primers
were synthesized by Invitrogen (PlncRNA-1, NR_038892.1; CD200,
KJ897199.1; CD133, HQ628627.1; OCT4, HQ907734.1; SOX2, AH011668.2;
TGF-β1, NM_001311325.1; Wnt3, DQ658158.1; β-catenin, M77013.1;
Axin2, AF205888.1; CyclinD1, NM_001086776.1 and Myc: NM_002467.4).
Relative mRNA expression changes were calculated by
2−ΔΔCt (17). The
results are expressed as the n-fold way compared to control.
Western blot analysis
HFSc were homogenized in lysate buffer containing
protease-inhibitor and were centrifuged at 8000 rpm/min at 4°C for
10 min. The supernatant of mixture were used for analysis of
purpose protein. The primary antibodies used in the immunoblotting
assays were: TGF-β1 (1:500; ab92486), Wnt (1:500; ab28472),
β-catenin (1:500; ab32572), and β-catenin (1:500; ab8227; all
Abcam). Horseradish peroxidase-conjugated secondary antibody
(Bio-Rad, Laboratories, Inc.) was used at a 1:5,000 dilution and
detected using a Western Blotting Luminol Reagent. The results were
visualized by using chemi-luminescence detection system (Amersham
Biosciences, Piscataway, NJ, USA).
Statistical analysis
All date were expressed as mean ± SD of triplicate
dependent experiments and analyzed by using student t-tests or
one-way ANOVA (Tukey HSD test). Significance was established with
the SPSS statistical (SPSS, USA) and GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA USA). *P<0.05 and
**P<0.01 was considered to indicate a statistically significant
difference.
Results
Effects of PlncRNA-1 expression on
cells cycle, proliferation and differentiation of HFSc
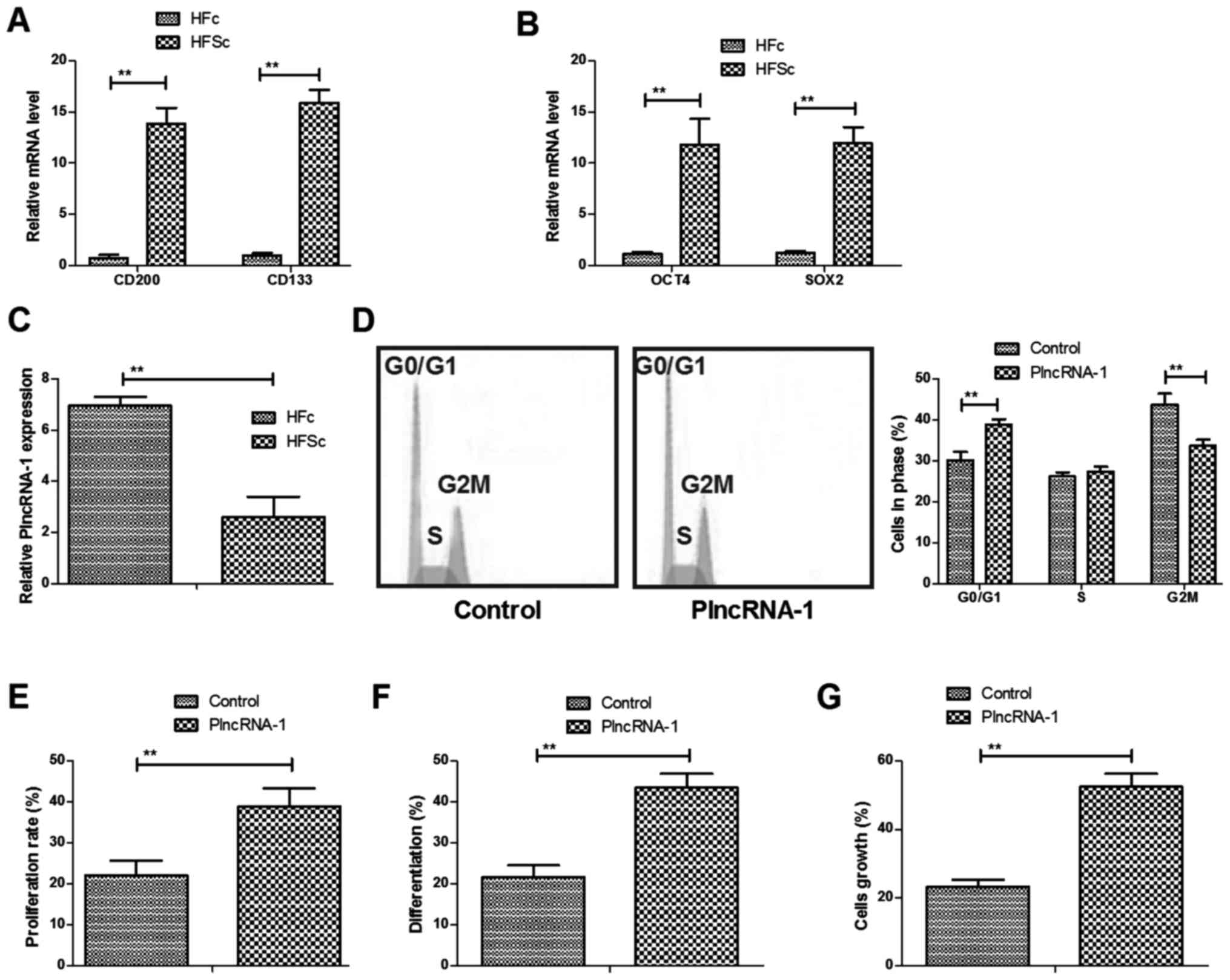
Previous studies have indicated that CD200 and CD133
are expressed in HFSc and OCT4 and SOX2 genes are representative
differentiation genes for HFSc (18–21).
We compared these gene expression levels between HFSc and HFc. We
showed that gene expression levels of CD200 and CD133 were higher
in HFSc than HFc (Fig. 1A).
Differentiation genes of OCT4 and SOX2 were also overexpressed in
HFSc compared to HFc (Fig. 1B).
PlncRNA-1 expression was investigated in HFSc. We found that
PlncRNA-1 expression was downregulated in HFSc compared to HFc
determined by RT-qPCR (Fig. 1C).
Results showed that PlncRNA-1 transfection promoted cells cycle of
HFSc (Fig. 1D). We also found that
proliferation and differentiation of HFSc were stimulated by
PlncRNA-1 transfection (Fig. 1E and
F). PlncRNA-1 transfection also promoted growth of HFSc
(Fig. 1G). These results indicate
PlncRNA-1 regulates cells cycle, proliferation and differentiation
of HFSc.
Effects of PlncRNA-1 expression on
TGF-β1, Wnt and β-catenin expression levels in HFSc
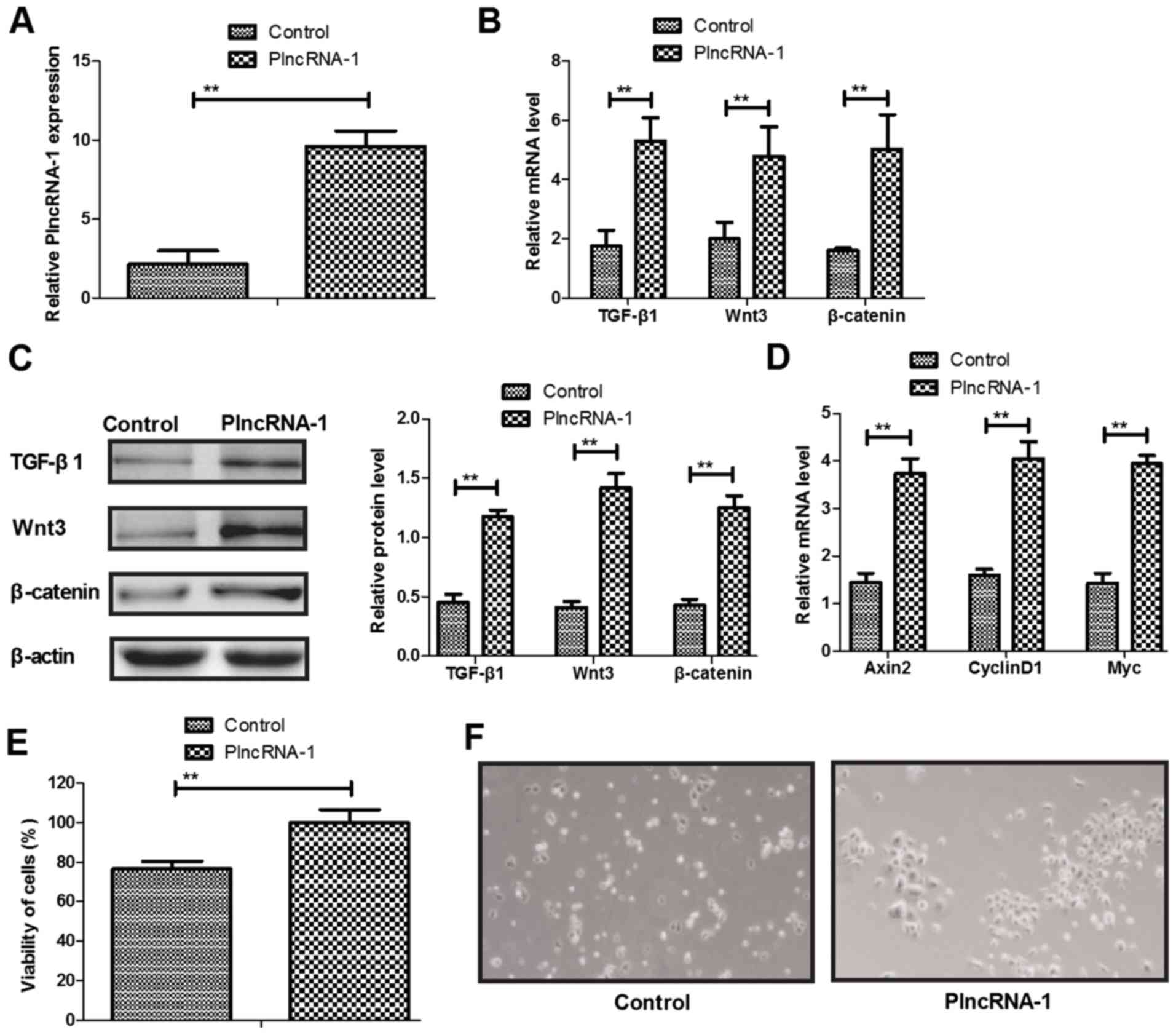
We analyzed TGF-β1, Wnt and β-catenin expression
levels in HFSc after PlncRNA-1 transfection. We confirmed PlncRNA-1
transfection increased PlncRNA-1 expression in HFSc (Fig. 2A). Results showed that TGF-β1, Wnt3
and β-catenin expression levels were upregulated by PlncRNA-1
transfection in HFSc (Fig. 2B and
C). We demonstrated that PlncRNA-1 transfection increased Wnt
signaling pathway downstream effectors Axin2, CyclinD1 and Myc gene
expression in HFSc compared to control (Fig. 2D). We found that PlncRNA-1
expression upregulation led to increasing of viability of HFSc
(Fig. 2E). The identity of the
stem cells demonstrated small colonies and the cells were round and
small, with uniform morphology (Fig.
2F), which showed that PlncRNA-1 transfection did not change
the stemness of HFSc. These results indicate that PlncRNA-1
promotes TGF-β1, Wnt and β-catenin expression levels in HFSc.
Effects of TGF-β1 inhibitor LY2109761
on proliferation and differentiation of HFSc
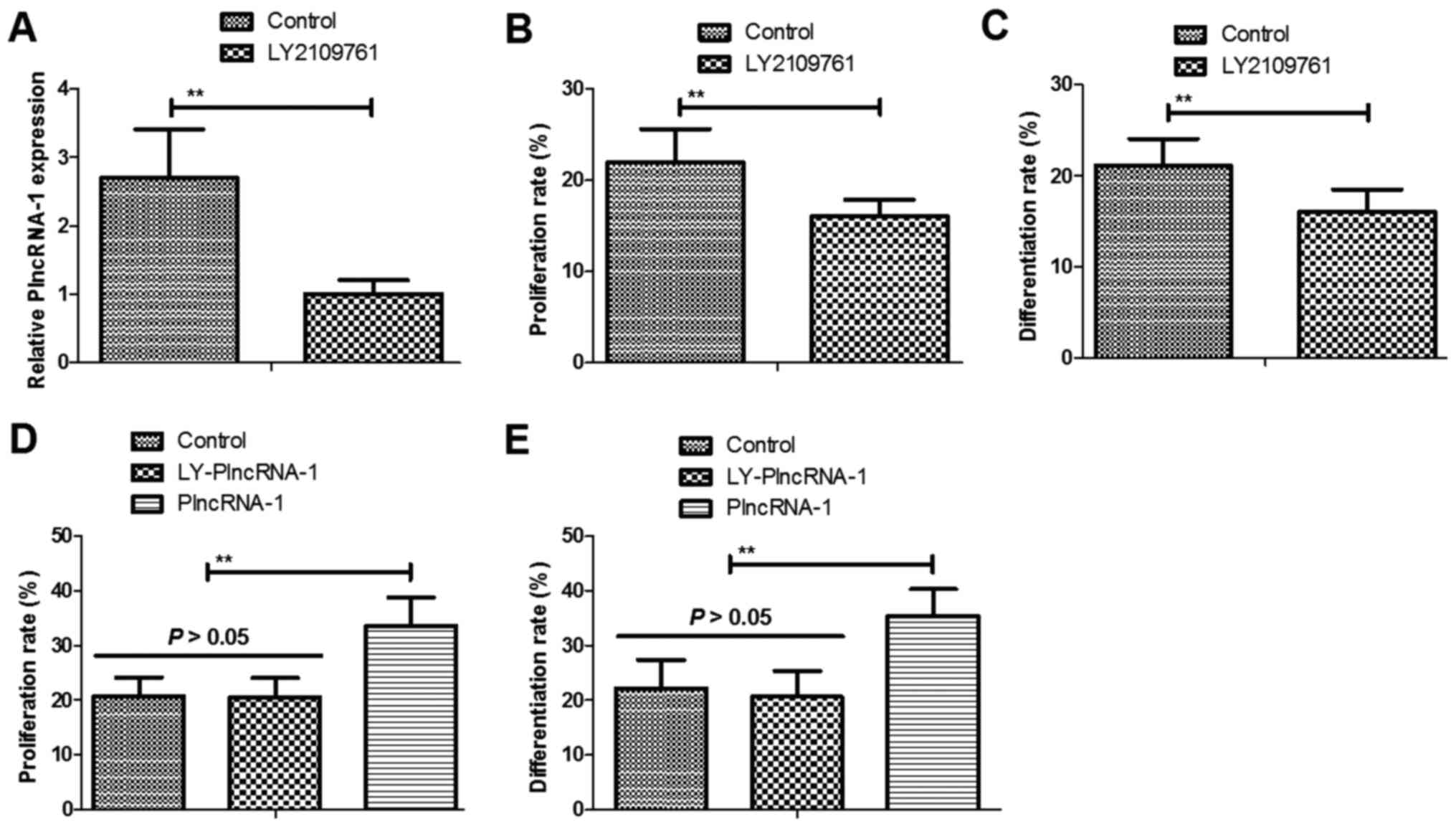
We next analyzed the effects of TGF-β1 inhibitor
LY2109761 on proliferation and differentiation of HFSc. We observed
that TGF-β1 inhibitor LY2109761 decreased PlncRNA-1 expression in
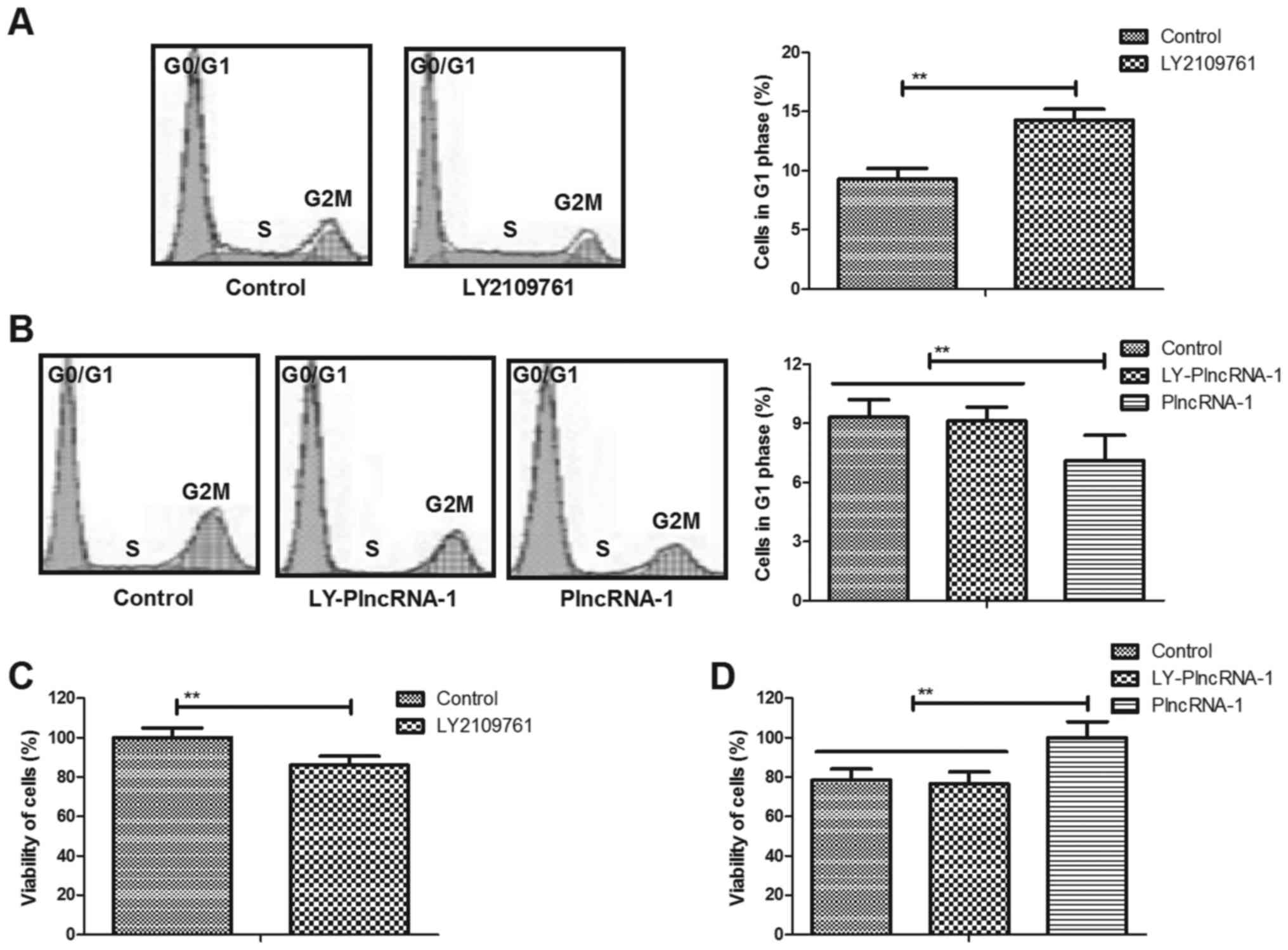
HFSc (Fig. 3A). As shown in
Fig. 3B and C, LY2109761 inhibited
proliferation and differentiation of HFSc. We also showed that
TGF-β1 inhibitor LY2109761 abolished PlncRNA-1 (LY-PlncRNA-1)
transfection-induced proliferation and differentiation of HFSc
(Fig. 3D and E). These results
indicate that TGF-β1 inhibitor LY2109761 abolishes
PlncRNA-1-regulated proliferation and differentiation of HFSc.
Effects of TGF-β1 inhibitor LY2109761
on Wnt and β-catenin expression levels in HFSc
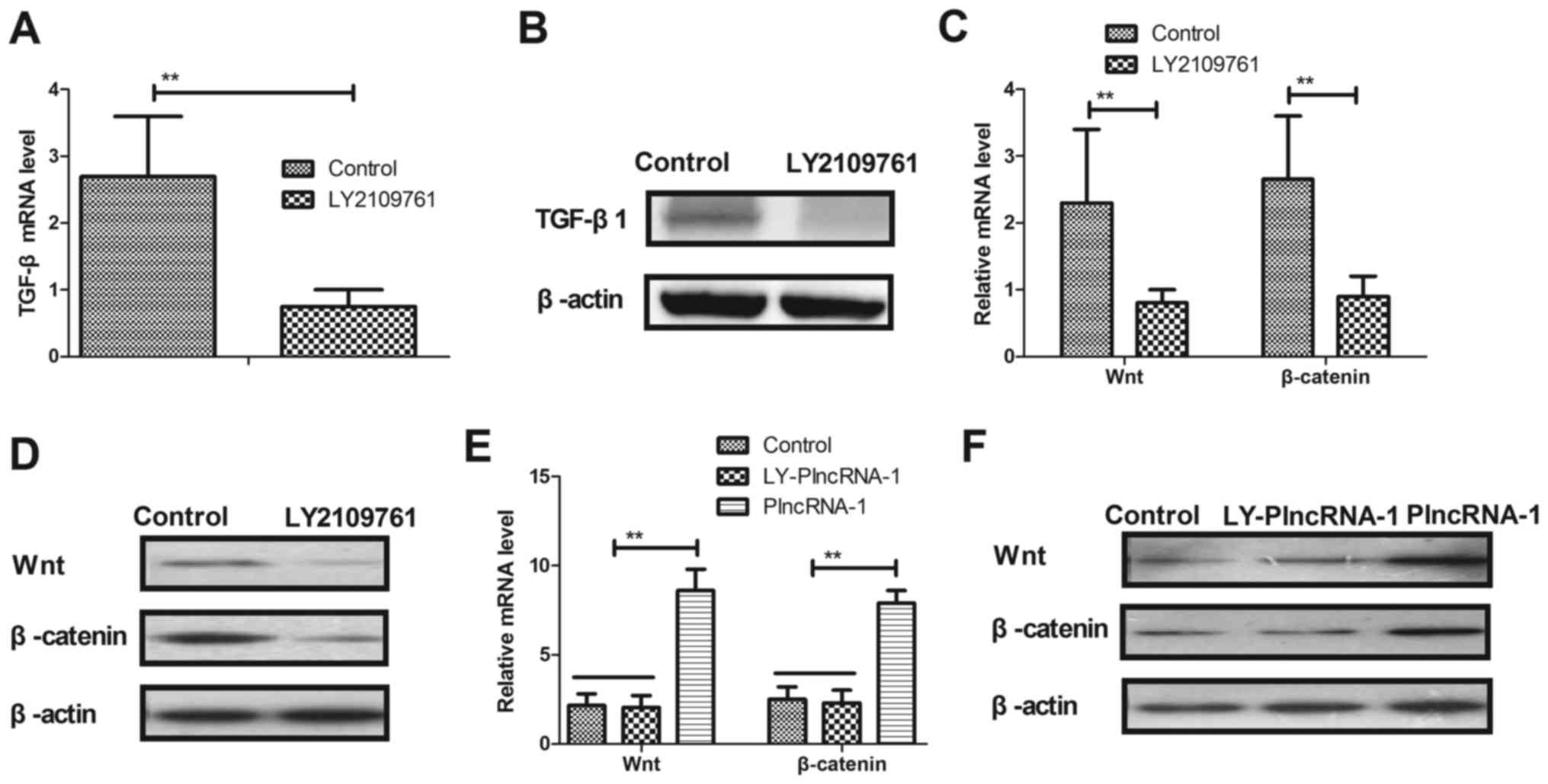
The effects of TGF-β1 inhibitor LY2109761 on Wnt and
β-catenin expression were investigated in HFSc. As shown in
Fig. 4A and B, TGF-β1 inhibitor
LY2109761 significantly decreased TGF-β1 expression in HFSc.
Results demonstrated that TGF-β1 inhibitor LY2109761 significantly
downregulated Wnt and β-catenin expression levels in HFSc (Fig. 4C and D). We observed that TGF-β1
inhibitor LY2109761 abolished PlncRNA-1 transfection-promoted Wnt
and β-catenin expression levels in HFSc (Fig. 4E and F). These results indicate
that TGF-β1 inhibitor LY2109761 downregulates Wnt and β-catenin
expression levels in HFSc.
Effects of TGF-β1 inhibitor LY2109761
on cells cycle of HFSc
We further analyzed effects of TGF-β1 inhibitor
LY2109761 on cells cycle of HFSc. As shown in Fig. 5A, TGF-β1 inhibitor LY2109761
inhibited cells cycle of HFSc compared to non-treated cells.
Results showed that TGF-β1 inhibitor LY2109761 abolished PlncRNA-1
transfection-promoted cells cycle of HFSc (Fig. 5B). We observed that TGF-β1
inhibitor LY2109761 also led to decreasing of viability of HFSc
(Fig. 5C). We also found that
TGF-β1 inhibitor LY2109761 abolished PlncRNA-1
transfection-increased viability of HFSc (Fig. 5D). These results suggest TGF-β1
inhibitor (LY2109761) canceled cells cycle of HFSc promoted by
PlncRNA-1 transfection.
Discussion
HFSc present more potential in the progression of
multilineage differentiation, which can be induced to differentiate
into neurons and glial cells, smooth muscle cells and melanocytes,
as well as melanocytes and keratinocytes (22,23).
Previous reports have investigated the role of PlncRNA-1 in
inducing apoptosis of human cancer cells (24–26).
However, no reports analyzed the regulatory effects of PlncRNA-1 in
the progression of HFSc. In this study, we investigated the role of
PlncRNA-1 on proliferation and differentiation of HFSc in
vitro. Results showed that PlncRNA-1 transfection significantly
promoted cells cycle, proliferation and differentiation of HFSc.
Previous reports have found that Wnt/β-catenin pathway involves in
the regulation of stem cells proliferation and differentiation
(27,28). Therefore, we assumed that PlncRNA-1
may regulate HFSc differentiation. Findings have found that
PlncRNA-1 can promote proliferation and differentiation of HFSc
through upregulation of TGF-β1-mediated Wnt/β-catenin signal
pathway.
Previous study has revealed that the lncRNA GAS5
could regulate TGF-β-induced smooth muscle cell differentiation via
RNA-Smad binding element (29). In
this study, we demonstrated PlncRNA-1 transfection stimulated
TGF-β1 expression, which further stimulated proliferation and
differentiation of HFSc. However, TGF-β1 inhibitor LY2109761
blocked cells cycle of HFSc promoted by PlncRNA-1 transfection.
Evidences have showed that lncRNA can influence colorectal
carcinoma cell lines proliferation by regulating cyclin D1
expression (30). Liu et al
have indicated that miR-18b inhibits TGF-β1-induced HFSc
differentiation into smooth muscle cells by targeting SMAD2, which
provided novel insights into the regulatory mechanisms of
TGF-β-induced differentiation of HFSc (31). In addition, the lncRNA PARROT is
regarded as a regulator of c-Myc and upregulates proliferation and
translation of human mammary epithelial cells (32). We reported that PlncRNA-1
transfection increased Wnt signaling pathway downstream effectors
Axin2, CyclinD1 and Myc gene expression in HFSc compared to
control. Overall, our findings in this analysis showed that
PlncRNA-1 transfection led to upregulation of TGF-β1 that further
contributed to promotion of cells cycle for HFSc.
Proliferation and differentiation of HFSc are
essential for the trauma repair. Previous review has summarized the
Wnt signal transduction pathway in controlling the proliferation
and differentiation of HFSc (33).
Leiros et al also indicated that HFSc differentiation is
inhibited through cross-talk between Wnt/β-catenin and androgen
signaling in dermal papilla cells from patients with androgenetic
alopecia (34). Watabe et
al has showed that stimulation of embryonic stem cell-derived
endothelial cells with TGF-β resulted in phosphorylation of both
Smad2 and Smadl/5 (35). Our
results have indicated that inhibition of TGF-β1 expression
inhibited Wnt/β-catenin expression levels in HFSc, which further
resulted in inhibition of proliferation and differentiation of
HFSc. Notably, results demonstrated that TGF-β1 inhibitor LY2109761
blocked proliferation and differentiation of HFSc promoted by
PlncRNA-1 transfection, which provided potential insights to
understand molecular mechanism mediated by PlncRNA-1 in HFSc.
In conclusion, results indicate that TGF-β1
inhibitor LY2109761 could canceled PlncRNA-1 transfection-promoted
cells cycle, proliferation and differentiation of HFSc. Findings in
the current study indicate that PlncRNA-1 could regulate cells
cycle, proliferation and differentiation of HFSc via
TGF-β1-mediated Wnt/β-catenin signal pathway. Our findings will
help to understand the potential molecular mechanisms of
differentiative capacity of HFSc and show PlncRNA-1 may contribute
to the signal pathway about proliferation and differentiation of
HFSc.
References
|
1
|
Akhbari P, Whitehouse A and Boyne JR: Long
non-coding RNAs drive metastatic progression in melanoma (Review).
Int J Oncol. 45:2181–2186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang YK and Yu JC: Circulating microRNAs
and long non-coding RNAs in gastric cancer diagnosis: An update and
review. World J Gastroenterol. 21:9863–9886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Z: Long non-coding RNAs in
Alzheimer's disease. Curr Top Med Chem. 16:511–519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moyo B, Nicholson SA and Arbuthnot PB: The
role of long non-coding RNAs in hepatitis B virus-related
hepatocellular carcinoma. Virus Res. 212:103–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lazar DC, Morris KV and Saayman SM: The
emerging role of long non-coding RNAs in HIV infection. Virus Res.
212:114–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lammens T, D'Hont I, D'Herde K, Benoit Y
and Diez-Fraile A: Long non-coding RNAs in pluripotent stem cell
biology. Vet Q. 33:202–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ng SY and Stanton LW: Long non-coding RNAs
in stem cell pluripotency. Wiley Interdiscip Rev RNA. 4:121–128.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song G, Shen Y, Ruan Z, Li X, Chen Y, Yuan
W, Ding X, Zhu L and Qian L: LncRNA-uc.167 influences cell
proliferation, apoptosis and differentiation of P19 cells by
regulating Mef2c. Gene. 590:97–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang X, Hou M, Ding Y, Li Z, Ren L and Gao
G: Systematically profiling and annotating long intergenic
non-coding RNAs in human embryonic stem cell. BMC Genomics. 14
Suppl 5:S32013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohyama M: William J. Cunliffe Scientific
Awards. Advances in the study of stem-cell-enriched hair follicle
bulge cells: A review featuring characterization and isolation of
human bulge cells. Dermatology. 214:342–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma MS, Czepiel M, Krause T, Schäfer KH,
Boddeke E and Copray S: Generation of induced pluripotent stem
cells from hair follicle bulge neural crest stem cells. Cell
Reprogram. 16:307–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lien WH, Polak L, Lin M, Lay K, Zheng D
and Fuchs E: In vivo transcriptional governance of hair follicle
stem cells by canonical Wnt regulators. Nat Cell Biol. 16:179–190.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu ZC, Zhang Q and Li H: Differentiation
of human hair follicle stem cells into endothelial cells induced by
vascular endothelial and basic fibroblast growth factors. Mol Med
Rep. 9:204–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yabut O, Domogauer J and D'Arcangelo G:
Dyrk1A overexpression inhibits proliferation and induces premature
neuronal differentiation of neural progenitor cells. J Neurosci.
30:4004–4014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu D, Yi C, Zhang D, Zhang J and Yang M:
Inhibition of proliferation and differentiation of mesenchymal stem
cells by carboxylated carbon nanotubes. ACS Nano. 4:2185–2195.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Potapova IA, Gaudette GR, Brink PR,
Robinson RB, Rosen MR, Cohen IS and Doronin SV: Mesenchymal stem
cells support migration, extracellular matrix invasion,
proliferation and survival of endothelial cells in vitro. Stem
Cells. 25:1761–1768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sultani M, Azad Mokhtari T, Eshragian M,
Shadab A, Naseri M, Eilami O and Yavarian J: Multiplex SYBR Green
Real-Time PCR Assay for detection of respiratory viruses.
Jundishapur J Microbiol. 8:e190412015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cotsarelis G: Gene expression profiling
gets to the root of human hair follicle stem cells. J Clin Invest.
116:19–22. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kopp JL, Ormsbee BD, Desler M and Rizzino
A: Small increases in the level of Sox2 trigger the differentiation
of mouse embryonic stem cells. Stem Cells. 26:903–911. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan YX, Gu CH, Zhang YL, Zhong BS, Wang
LZ, Zhou ZR, Wang ZY, Jia RX and Wang F: Oct4 and Sox2
overexpression improves the proliferation and differentiation of
bone mesenchymal stem cells in Xiaomeishan porcine. Genet Mol Res.
12:6067–6079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hemmoranta H, Satomaa T, Blomqvist M,
Heiskanen A, Aitio O, Saarinen J, Natunen J, Partanen J, Laine J
and Jaatinen T: N-glycan structures and associated gene expression
reflect the characteristic N-glycosylation pattern of human
hematopoietic stem and progenitor cells. Exp Hematol. 35:1279–1292.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nowak JA, Polak L, Pasolli HA and Fuchs E:
Hair follicle stem cells are specified and function in early skin
morphogenesis. Cell stem cell. 3:33–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amoh Y, Li L, Katsuoka K and Hoffman RM:
Multipotent hair follicle stem cells promote repair of spinal cord
injury and recovery of walking function. Cell Cycle. 7:1865–1869.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Q, Cui ZL, Wang Q, Jin XB, Zhao Y,
Wang MW, Song W, Qu HW and Kang WT: PlncRNA-1 induces apoptosis
through the Her-2 pathway in prostate cancer cells. Asian J Androl.
19:453–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang CM, Wu QQ, Li SQ, Chen FJ, Tuo L, Xie
HW, Tong YS, Ji L, Zhou GZ, Cao G, et al: Upregulation of the long
non-coding RNA PlncRNA-1 promotes esophageal squamous carcinoma
cell proliferation and correlates with advanced clinical stage. Dig
Dis Sci. 59:591–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui Z, Ren S, Lu J, Wang F, Xu W, Sun Y,
Wei M, Chen J, Gao X, Xu C, et al: The prostate cancer-up-regulated
long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation
through reciprocal regulation of androgen receptor. Urol Oncol.
31:1117–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu K, Sun Y, Liu D and Ye S: Inhibition
of Wnt/β-catenin signaling by IWR1 induces expression of Foxd3 to
promote mouse epiblast stem cell self-renewal. Biochem Biophys Res
Commun. 490:616–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gong L, Song J, Lin X, Wei F, Zhang C,
Wang Z, Zhu J, Wu S, Chen Y, Liang J, et al: Serine-arginine
protein kinase 1 promotes a cancer stem cell-like phenotype through
activation of Wnt/β-catenin signalling in NSCLC. J Pathol.
240:184–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang R, Zhang G, Wang YC, Mei X and Chen
SY: The long non-coding RNA GAS5 regulates transforming growth
factor β (TGF-β)-induced smooth muscle cell differentiation via
RNA-Smad binding element. J Biol Chem. 292:14270–14278. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu ZQ, Zhang C, Lao XY, Wang H, Gao XH,
Cao GW, Zhou WP and Fu CG: Long non-coding RNA influences
radiosensitivity of colorectal carcinoma cell lines by regulating
cyclin D1 expression. Zhonghua Wei Chang Wai Ke Za Zhi. 15:288–291.
2012.(In Chinese). PubMed/NCBI
|
|
31
|
Liu X, Song L, Liu J, Wang S, Tan X, Bai
X, Bai T, Wang Y, Li M, Song Y and Li Y: miR-18b inhibits
TGF-β1-induced differentiation of hair follicle stem cells into
smooth muscle cells by targeting SMAD2. Biochem Biophys Res Commun.
438:551–556. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vučićević D, Gehre M, Dhamija S,
Friis-Hansen L, Meierhofer D, Sauer S and Ørom UA: The long
non-coding RNA PARROT is an upstream regulator of c-Myc and affects
proliferation and translation. Oncotarget. 7:33934–33947. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shao Y, Ni Z and Li Y: Wnt signal
transduction pathways and hair follicle stem cells. Sheng Wu Yi Xue
Gong Cheng Xue Za Zhi. 27:945–948. 2010.(In Chinese). PubMed/NCBI
|
|
34
|
Leirós GJ, Attorresi AI and Balañá ME:
Hair follicle stem cell differentiation is inhibited through
cross-talk between Wnt/β-catenin and androgen signalling in dermal
papilla cells from patients with androgenetic alopecia. Br J
Dermatol. 166:1035–1042. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Watabe T, Yamashita JK, Mishima K and
Miyazono K: TGF-beta signaling in embryonic stem cell-derived
endothelial cells. Methods Mol Biol. 330:341–351. 2006.PubMed/NCBI
|