Introduction
Osteoarthritis has been one of the most popular
diseases that afflict many people in the aging population.
Currently, there is no effective therapy for the treatment of
osteoarthritis due to the inferior intrinsic repair capacity of the
damaged area of cartilage. Surgeons often use palliative measures
to relieve the symptoms. However, the pathophysiological change
during the disease progression cannot be restored by palliative
treatments. Thus, the final solution is the joint replacement
surgery. The development of tissue engineering provides new
insights into the treatment of osteoarthritis. 3D culture of
mesenchymal stem cells (MSCs) and chondrocytes in tissue
engineering can reduce cell dedifferentiation (1,2),
which have promoted the autologous implantation technology into the
clinical application (3,4). Since the interactions between bone
and cartilage sides are central to the progression of
osteoarthritis, many researchers consider osteoarthritis as a
disease of osteochondral tissue. Thus, several bi-phasic scaffolds
were investigated to anchor the cartilage phase of scaffold to
integrate better into the lesion area (5–7).
Since MSCs have great potential to proliferate and
differentiate into osteogenic and chondrogenic phenotypes in a
desired physiological environment, they are promising cell resource
for osteochondral tissue engineering (8–10).
In the bone and cartilage phases of scaffold, MSCs differentiate
into osteogenic lineage and chondrogenic lineage, respectively. The
different lineages of cells in the bi-phasic scaffold might have an
interaction through cytokine secretion. In the previous studies,
MSCs have showed the ability to secrete a wide range of bioactive
factors, e.g., matrix metalloproteinase, tissue inhibitor of
metalloproteinases, transforming growth factor (TGF)-β1 and
fibroblast growth factor-2 (11–14),
to influence cell behavior and tissue remodeling. However, there is
still controversy about the interactions between cells in bi-phasic
scaffold. Some researchers have studied the interactions between
osteoinduced MSCs and chondroinduced MSCs or interactions between
osteoinduced MSCs and chondrocytes under static conditions, which
cann't mimic the fluidic environment in vivo (15–17).
To enhance the therapeutic potential of MSCs and chondrocytes in
the treatment of osteoarthritis and other degenerative joint
diseases, it is meaningful to investigate the stimulation of cells
in the upper layer of bi-phasic scaffold to cells on the bottom
layer in a fluidic microenvironment which can better mimic the
mechanical characteristics in vivo.
The specific goals of this study were to investigate
the effects of osteoinduced MSCs, fluidic shear stress, or the
combination of them on the phenotype of chondroinduced MSCs and
chondrocytes, which can help illustrate the interaction of cells on
double layer in fluidic environment. Herein, we designed a novel
integrated lab-on-a-chip to introduce cells and perfusion systems
and thus mimic the fluidic flow in vivo. By using perfusion
systems and a semipermeable membrane, we studied the individual and
the combination effect of osteoinduced MSCs and shear stress on the
phenotype of chondroinduced MSCs and chondrocytes. Through the
computational fluid dynamics (CFD) method, we simulated the 3D flow
field and obtained a stable shear stress in the cell chambers. The
semipermeable membrane allows the transition of cytokines and thus
helps us to study the interactions between osteoinduced MSCs and
other related cells (chondrocytes, chondroinduced MSCs). The three
chambers on the bottom layer allow us to study the influence of
cells on the up layer to cells in three chambers separately at one
time. In a word, we designed a novel microfludic chip and decreased
the size of experimental device to micrometer level and reduced
cell numbers needed in one experiment, which may be helpful to
future clinical translation research.
Materials and methods
Isolation and culture of MSCs and
chondrocytes
All the experiments were approved by the Committee
on Animal Use and Care of the Dalian Medical University. The rats
were euthanized prior to the collection of tissue samples.
Articular cartilage chondrocytes were isolated from humeral heads,
femoral heads, and femoral condyles of male Sprague-Dawley rats
weighing 80–120 g, as reported (18). Briefly, chondrocytes were isolated
by digestion with 0.15% type II collagenase for 16 h and
resuspended in the Dulbecco's modified Eagle's medium/F-12
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) containing
10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences),
50 mg/ml ascorbic acid-2-phosphate (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), and 100 U/ml penicillin-streptomycin. The
primary chondrocytes obtained were used in the subsequent
experiments.
The primary rat MSCs were isolated from the
bilateral femurs and tibias at the same time. After the distal ends
of the bone were cut open, the marrow cavities were lavaged with
sterile phosphate-buffered saline (PBS). The cells were then
resuspended in the high-glucose Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% FBS (HyClone; GE Healthcare Life Sciences) and 100
U/ml penicillin-streptomycin. After incubation at 37°C in 5%
CO2 for 48 h, the medium was changed to remove the
nonadherent cells. After two passages, the attached MSCs were then
used in the following experiments.
The microdevice was coated with 100 mg/ml
fibronectin (Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature. Then, the chambers were washed with PBS. The
suspension of osteoinduced MSCs at a density of 1×105
cells/ml was injected gently from the inlet of top chamber to seed
cells on the polycarbonate membrane. After 12 h, a chondroinduced
MSCs suspension of 0.5×105 cells/ml, a chondrocyte
suspension of 1×105 cells/ml and a mixture of two cells
with the corresponding cell density were injected into the three
microchambers, respectively. The chip was incubated at 37°C for 12
h to allow the cell attachment. In groups 2 and 4, the inlets of
the bottom layer were connected to three peristaltic pumps (Longer
Pump BT100-2J; Longer Precision Pump Co., Ltd., Baoding, China).
The cells were exposed to 1 mmol/l cytochalasin D (CytoD;
Sigma-Aldrich; Merck KGaA) by pipetting medium containing CytoD
into the inlet of chip for 1 h to disrupt stress fibers before the
application of the flow stimulus.
In vitro induction of lineage
differentiation
To generate chondrogenic cells, MSCs were first
expanded for 5 days and then subjected to 14 days of
predifferentiation in a serum-free chondrogenic medium containing
DMEM-LG, 6.25 µg/ml insulin, 50 mg/l ascorbic acid,
1×10−7mol/l dexamethasone, 10 ng/ml TGF-β3 (PeproTech,
Inc., Rocky Hill, NJ, USA) and 1 µM ascorbate-2-phosphate (Wako
Chemicals USA, Inc., Richmond, VA, USA), 1% sodium pyruvate
(Invitrogen; Thermo Fisher Scientific, Inc.). To obtain Osteogenic
commitment MSCs, MSCs were cultured in a complete osteogenic medium
containing high glucose DMEM, 10% (v/v) FBS, 50 mg/l ascorbic acid,
10 mM β-glycerophosphate, 1×10−8 mol/l dexamethasone.
Osteoinduced MSCs were exposed to the osteogenic medium for 7
days.
Fabrication of microfluidic chip
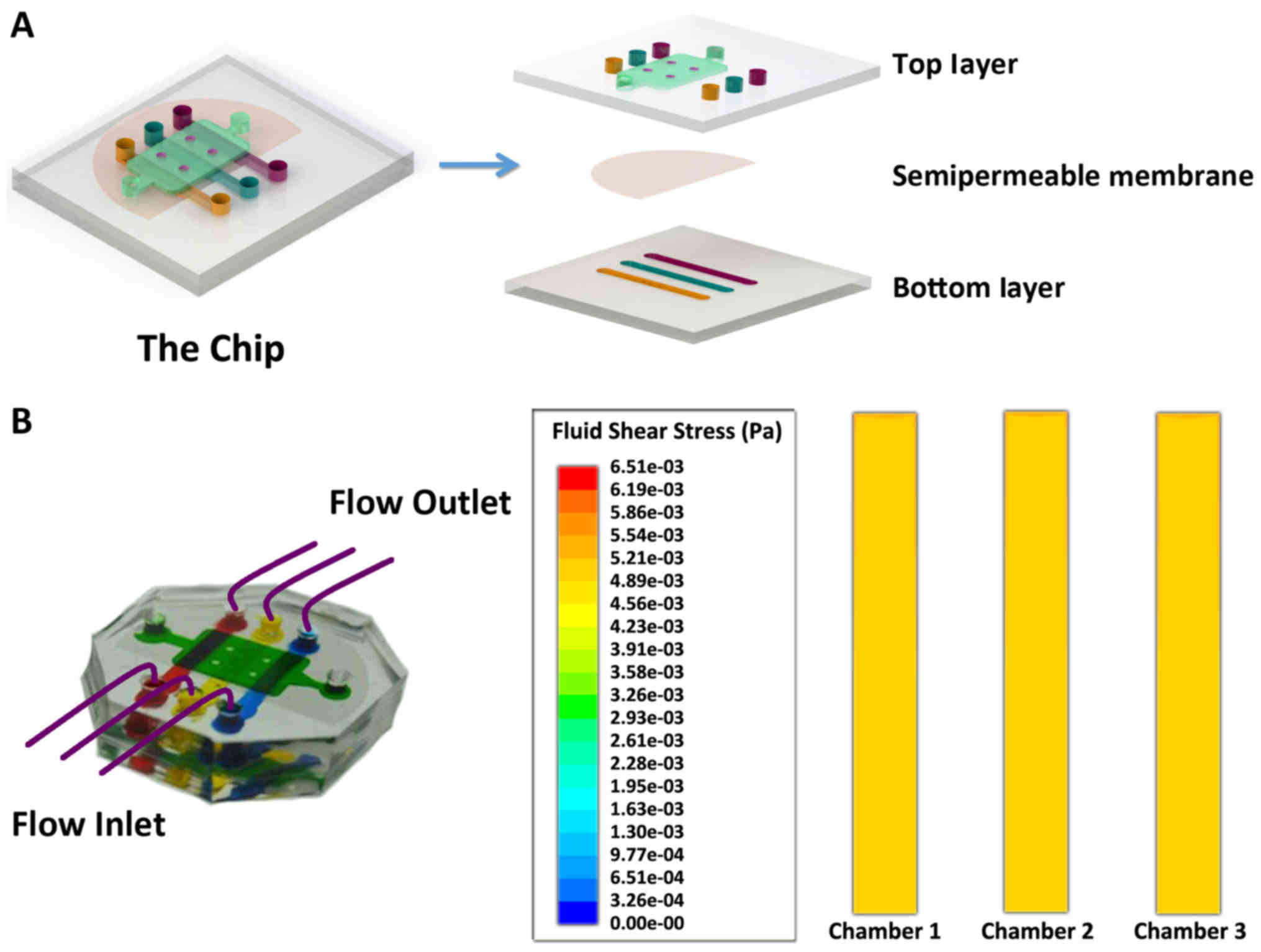
The schematic diagram of the device is as shown in
Fig. 1A. The device was composed
of two layers of polydimethylsiloxane (PDMS) with several
microchambers. The top layer of the chip has one cell culture
chamber which was 500 µm in height, 6 mm in width and 11 mm in
length and the bottom layer has three microchambers, each of which
was 500 µm in height, 2 mm in width and 16 mm in length. Between
two pieces of PDMS, there was a polycarbonate membrane (Whatman
Inc., Piscataway, NJ, USA), with the pore size of 0.4 µm, which
allowed the transition of cytokines and small molecular compounds
(19). Each chamber has one inlet
and one outlet, respectively. Perfusion system could be linked with
the inlet of each chamber to provide the fluid flow.
Microfluidic chip was fabricated using a
conventional microfabrication technique. The mask was designed by
AutoCAD 2007 (Autodesk, Inc., San Rafael, CA, USA) and printed on
transparencies with 4,000 dpi resolution. The master was fabricated
by photoresist SU-8 3035 through soft lithography technique. Then
the microfluidic chip was fabricated in PDMS by replicating molding
the master, followed by the curing process of PDMS at 80°C in an
oven for one hour. After that, PDMS layer was peeled off from the
master. A polycarbonate membrane and two pieces of PDMS were sealed
together after the treatment by oxygen plasma for 1 min.
Experimental design
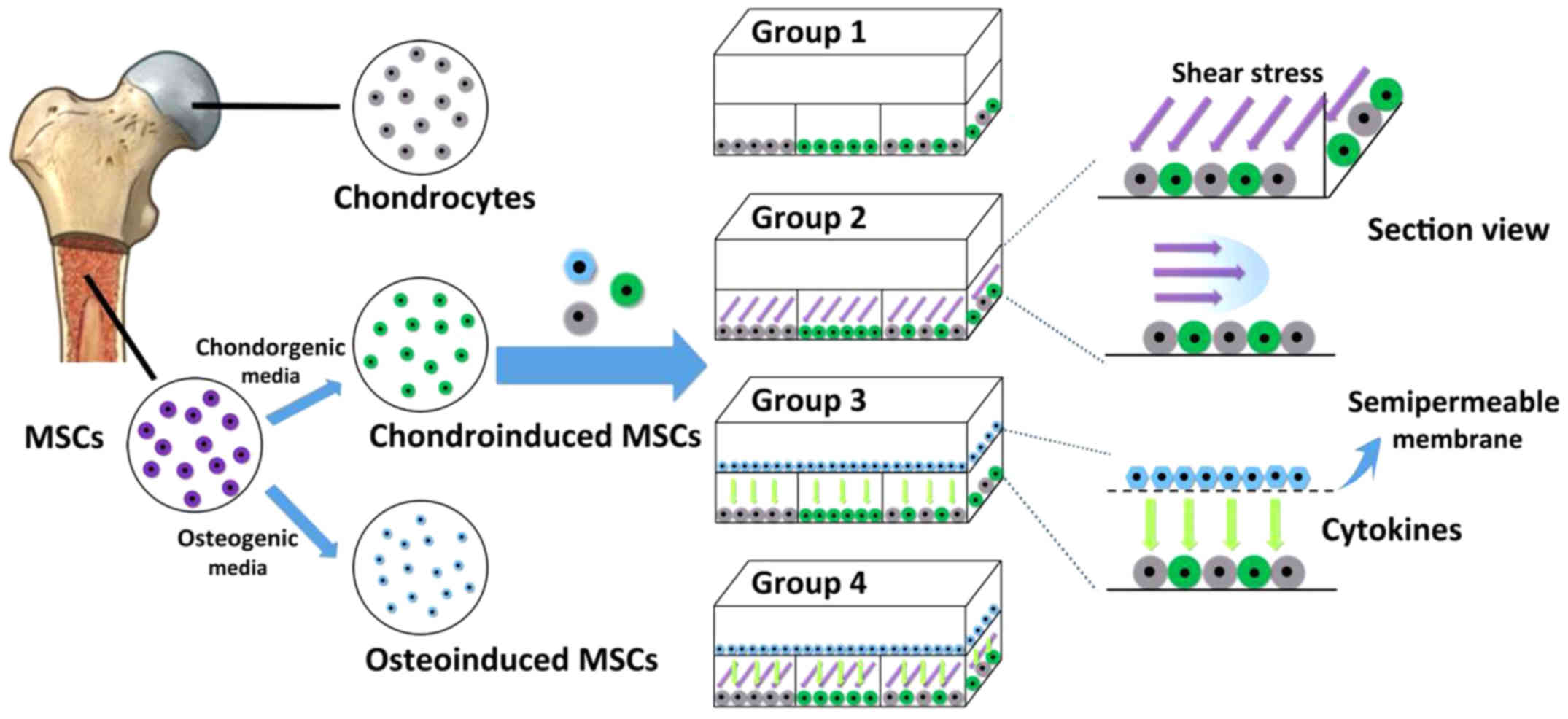
The overall experimental design is as shown in
Fig. 2. In this study, the top
layer of the chip was seeded with or without osteoinduced MSCs, and
the bottom layer of the chip was separately seeded with
chondroinduced MSCs, chondrocytes and a mixture of them in three
microchambers. MSCs were first expanded for 14 days in the general
medium and then incubated in the osteogenic medium for another 7
days to obtain osteogenic phenotype cells. Chondroinduced MSCs were
cultured for 14 days in the chondrogenic medium with TGF-β3 to
obtain chondroinduced MSCs. After 14 days' culture, the
chondroinduced MSCs were seeded on the bottom of the chip alone or
co-cultured with chondrocytes.
Numerical modeling of the shear
stress
To produce a certain shear stress (0.05
dyne/cm2) in the microchambers on the bottom layer of
the chip, three perfusion pumps were connected with the inlets of
the three microchambers. The cells cultured there were subjected to
the shear stress simultaneously. We assumed the flow as a model of
laminar and incompressible fluid. A finite volume method
(FVM)-based CFD could simulate the 3D flow field in the
microchambers with a known pressure at the inlet and outlet of the
microchamber. To avoid the computational rigor required to solve
Fourier series expansions, we used an approximate version in
algebraic form as follows: R=[12ηL/(1–0.63(h/w)] × (1/h3w).
In this formula, R is the hydraulic
resistance of the rectangular microchambers, η the dynamic
viscosity of the liquid, L the channel length, h and
w (always h<w) the channel height and
width, respectively. With a given perfusion rate, the Δp
could be obtained and used in CFD method. This result can be
summarized using the Hagen-Poiseuille equation, as follows:
Δp=QRH.
After some calculations, the pressure data obtained
were then used as the inlet and outlet pressure conditions to
simulate the 3D flow field in each culture chamber using the CFD
method. The computational domain was discretized using
approximately 52,000 hexahedral meshes and solved using FVM along
with the aforementioned inlet/outlet pressure conditions and
no-slip boundary conditions at the chamber walls. The density of
the perfusion medium was 993.2 kg/m3, and the viscosity
was 7.85×10−4 Pa·s at 37°C.
Colony formation
Cells were seeded at a density of 60
cells/cm2 and cultured for 14 days. Then the cells were
washed with PBS and fixed with acetone/methanol (1:1). 2% crystal
violet solution was used for 20 min staining. Excess stain was
removed with tap water.
Alkaline phosphatase staining and
Alcian Blue assay
After MSCs were exposed to the osteogenic medium for
7 days, the osteogenic differentiation was detected by alkaline
phosphatase (ALP) activity. After a wash with PBS, the cells were
fixed with 500 µl of 4% paraformaldehyde for 2 min. Fixed cells
were washed with 1 ml of washing buffer (0.05% Tween-20 in PBS w/o
calcium and magnesium) and incubated with BCIP®/NBT
substrate at RT for 10 min. After a wash cells staining for
alkaline phosphatase activity were observed under a microscope.
The chondrogenic differentiation was detected by
Alcian Blue Assay. After being fixed for 15 min with 4%
glutaraldehyde at room temperature, the cells were rinsed with 0.1
N HCl to decrease the pH to 1.0, stained 30 min with 1% Alcian blue
solution (Sigma-Aldrich; Merck KGaA), and rinsed twice with 0.1 N
HCl to remove nonspecific staining.
Live cell staining
The live cells on the bottom layer were stained by
plasma membrane dye DiI (Invitrogen; Thermo Fisher Scientific,
Inc.) and DiO (Invitrogen; Thermo Fisher Scientific, Inc.) to
illustrate the distribution of cells in the three chambers. The
cells were exposed to 10 µM DiI and DiO for 1 h and then cells were
rinsed with fresh medium before pipetting into the inlet of
chip.
Cell staining and image assay
The vimentin intermediate filament, collagen I,
collagen II and aggrecan were stained by immunofluorescence.
Chondroinduced MSCs and chondrocytes were fixed in a 4%
paraformaldehyde solution for 15 min, followed by permeabilization
with 0.1% Triton-X for 10 min. After being washed with PBS for 3
times, the samples were blocked with goat serum for 30 min at room
temperature and incubated with anti-intermediate filament
(BIOSYNTHESIS, China), anti-collagen II (Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China), anti-collagen I (Beijing
Biosynthesis Biotechnology Co., Ltd.) and anti-aggrecan (Beijing
Biosynthesis Biotechnology Co., Ltd.) overnight. Then they were
mixed with FITC-conjugated goat anti-rabbit IgG or TRITC-conjugated
goat anti-rabbit secondary antibodies (Zhongshan Golden Bridge,
Beijing, China) for 1 h at room temperature, respectively. Cell
nuclei were stained with 40, 6-diamidino-2-phenylindole (DAPI) for
the following 10 min. For the imaging of cell shape, the
intermediate filament was stained by using the same protocol. After
staining, the chip was washed with PBS for 2–3 times. The cells
were then observed on an Olympus fluorescence microscopy (Olympus
IX 71; Olympus Corporation, Tokyo, Japan Japan). The images were
analyzed using image Pro Plus 6.0 software. The angle of
orientation and the fluorescence area per cell were analyzed by the
intermediate filament staining with Image J to evaluate the effect
of shear stress.
Living and dead cells were distinguished by
different colors. Propidium iodide visualise the nucleus and more
than 100 nucleus in each group were picked randomly to check
cellular viability. For cell proliferation analysis, cells were
fixed and stained by DAPI to count the proliferation rate in each
group.
Quantitative PCR (qPCR)
After 7 days exposure to fluid flow stimulus, qPCR
was used to analyse the expression level of collagen I, collagen II
and aggrecan. After rinsing with cold PBS once, Total RNA was
extracted from the cultured cells with TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.), and first-strand complementary cDNA was
synthesized using the PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). qPCR was performed using
Power SYBR-Green PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) in combination with a 7500 Real-Time PCR
Detection System. β-actin was used as the internal control gene to
normalize the quantities of target gene expressions. Thermocycling
conditions were as follows: 95°C for 30 sec 40 cycles of
denaturation (95°C, 5 sec), annealing (60°C, 30 sec) and extension
(72°C, 30 sec). The primers used in this study were listed in
Table I. The relative mRNA level
was expressed as fold change relative to group 1 after
normalization to the expression of β-actin with 2−ΔΔCq
method (20).
 | Table I.Sequences of primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of primers used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | PCR primer
sequences (5′-3′) | GenBank
accession |
|---|
| Collagen I | Forward
CCCTGGTCTTGGAGGAAACT | NM_053304 |
|
| Reverse
GCACGGAAACTCCAGCTGAT |
|
| Collagen II | Forward
GGAAACTTTGCAGCCCAGAT | NM_012929 |
|
| Reverse
GCCTTGCATGACTCCCATC |
|
| Aggrecan | Forward
TGATGCATGGCATTGAGGAC | NM_022190 |
|
| Reverse
TCGGTCAAAGTCCAGTGTGT |
|
| β-actin | Forward
CAGCCATGTACGTAGCCATC | NM_031144 |
|
| Reverse
GTCTCCGGAGTCCATCACAA |
|
Statistical analysis
In this study, all the experiments were performed at
least in triplicate with different batches of microfluidic chips.
All the results were presented as mean ± standard deviation.
One-way ANOVA method followed by Tukey's post hoc test was used to
compare the statistically significant differences among different
groups. P<0.05 was considered to indicate statistical
significance.
Results
Computational simulation of fluid
shear stress
In this study, peristaltic pumps were connected the
inlets of the microchambers to drive the fluid flow through the
bottom layer of chip. The fluid flow was Poiseuille flow and we
assumed that the shear stress on the cells was equal to the shear
stress of the bottom wall of the chamber. When a perfusion flow
rate of 7 µl/h was applied to the microchip, a constant fluid flow
stimulus could be generated on the cells. From the simulation
results by FVM-based CFD (Fig.
1B), the central area of the chambers exhibited a uniform shear
stress distribution within the wall (the uniform yellow color means
a uniform 0.05 dyne/cm2 shear stress in three chambers,
1 dyne/cm2=0.1 pascals), and the average shear stress of
the bottom wall was approximately 0.05 dyne/cm2, which
is equal to the interstitial fluid flow in the cartilage space.
Phenotype of isolated MSCs and induced
MSCs
MSCs can rapidly proliferate under suitable
conditions with high proportion of FBS. When grown at low density,
MSCs are capable of forming colonies. Also, they can differentiate
into multiple lineages when induced by special induction medium.
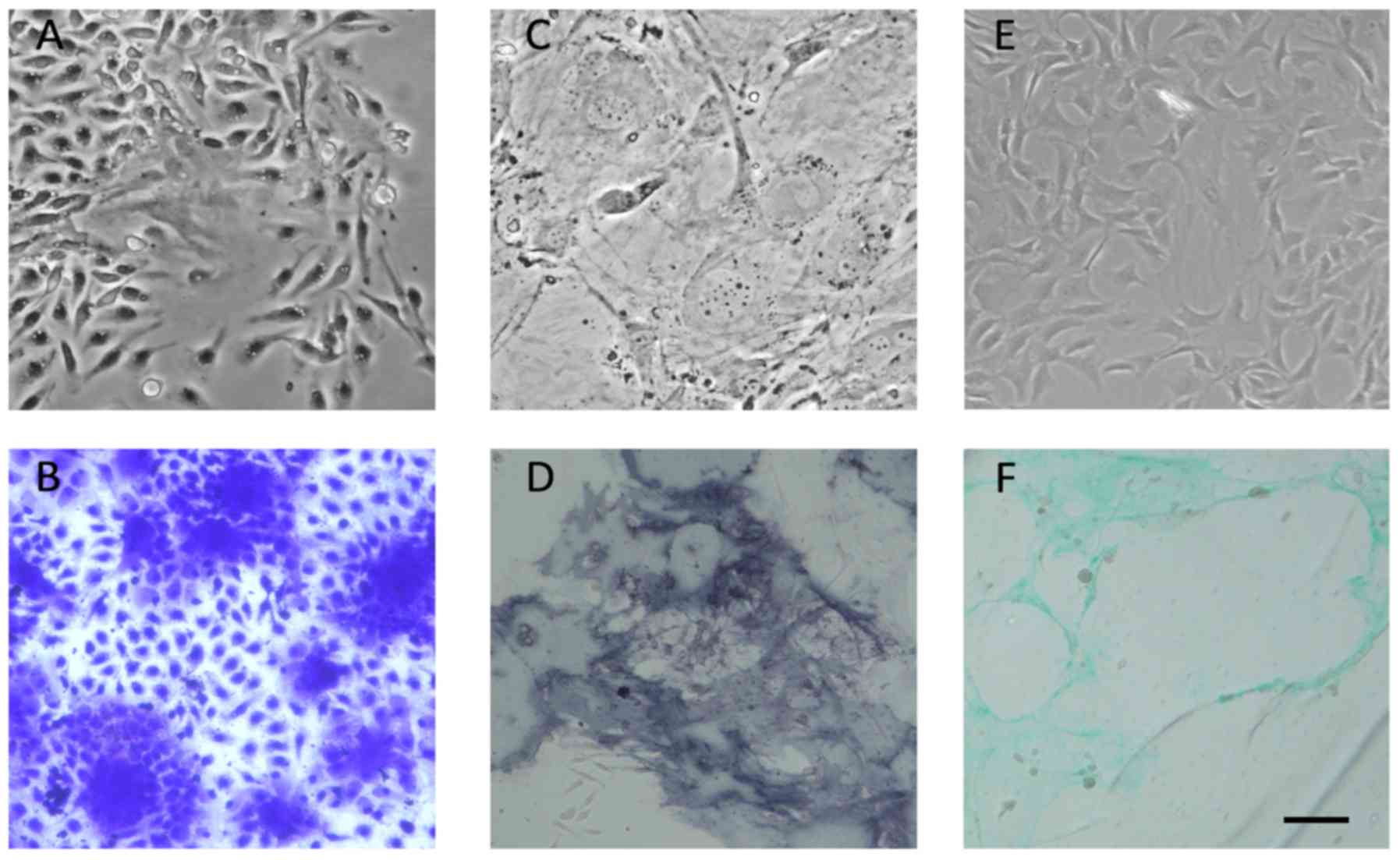
When isolated from bone marrow, primary MSCs showed colony-forming
capacity (Fig. 3A) and the
morphology of MSC colonies was also checked by crystal violet
staining (Fig. 3B). After
passaging, MSCs showed a fibroblast-like, spindle-shaped morphology
and a homogeneous phenotype (data not shown). Then we used
osteogenic and chondrogenic medium to induce MSCs. Induced
osteogenic commitment cells showed typical flattened morphology
after one week's induction (Fig.
3C). Also the osteogenic commitment differentiation was proved
by alkaline phosphatase staining (Fig.
3D). Besides, chondrogenic MSCs were induced after 14 days'
culture. The morphology was seen in the bright field picture
(Fig. 3E) and also proved by
Alcian Blue staining (Fig.
3F).
Effects of fluidic shear stress and
osteoinduced MSCs on the viability and proliferation of cells
The bottom layer of the chip had three cell culture
chambers, in which chondrocytes, chondroinduced MSCs and a mixture
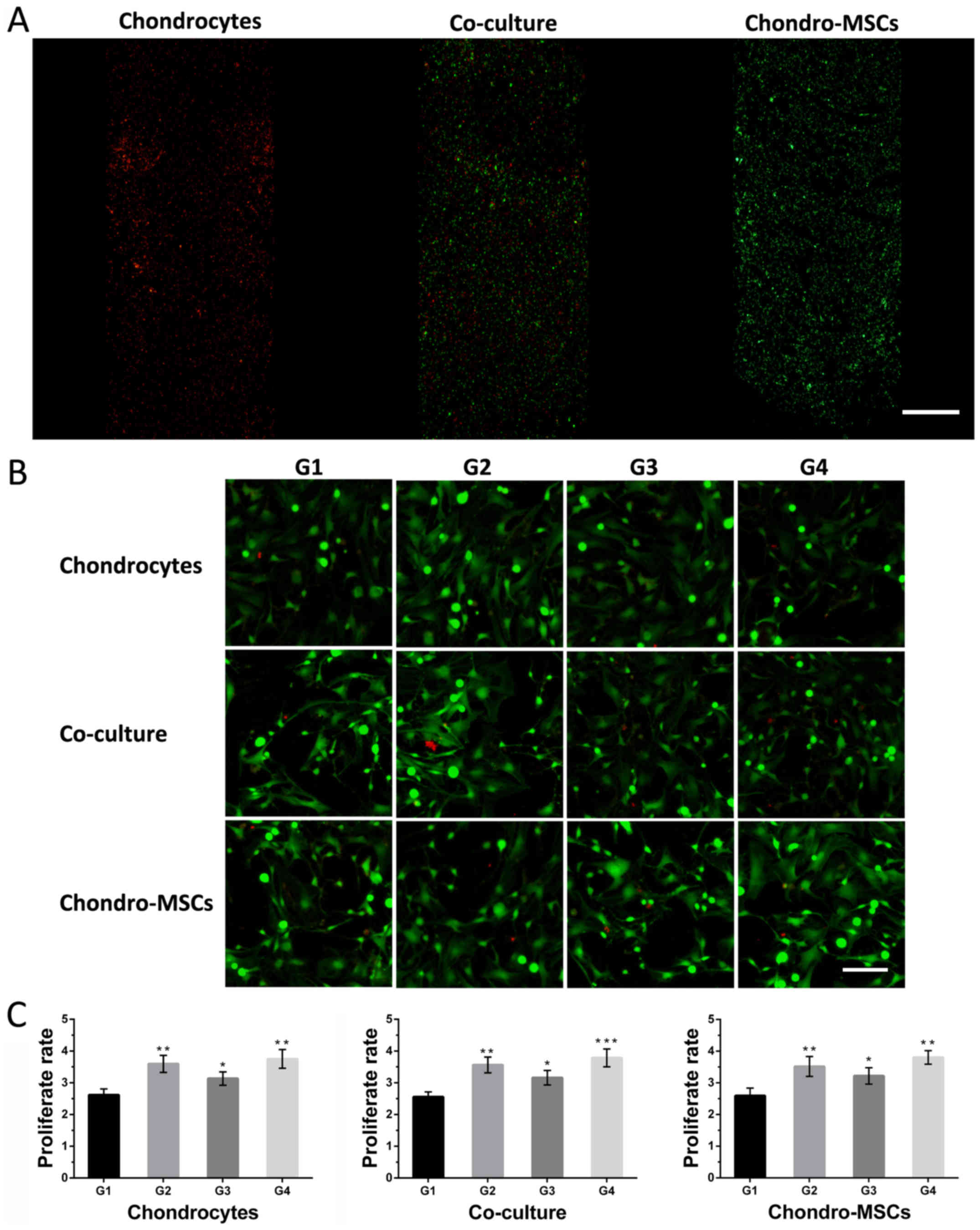
of them were seeded inside, respectively. Through the fluorescent
probe of DiI (red) and DiO (green), the distribution of cells on
the bottom layer could be clearly observed, which indicated the
uniform distribution of cells in 3 separated chambers (Fig. 4A). Besides, the viability of cells
in different groups was evaluated (Fig. 4B). Under different experiment
conditions, all these cells exhibited good state after 7 days'
culture. Very few dead cells (positive propidium iodide (PI)
staining) were observed, which indicated the feasibility of this
microfluidic chip for characterizing the stimulation of
osteoinduced MSCs and the shear stress to the cells on the bottom
layer. To analyze the effects of osteoinduced MSCs and fluid shear
stress on cell proliferation, the proliferation rates of cells in
different groups were analyzed by counting the cells (stained by
DAPI, data not shown) attached to the bottom of the chambers. As
shown in Fig. 4C, the
proliferation rates of cells in group 2/3/4 were higher than the
proliferation rate in group 1. These findings suggested that both
the given shear stress and the osteoinduced MSCs could affect the
proliferation rates of chondroinduced MSCs, chondrocytes and a
mixture of two cells.
Effects of shear stress and
osteoinduced MSCs on the shape and morphology of cells
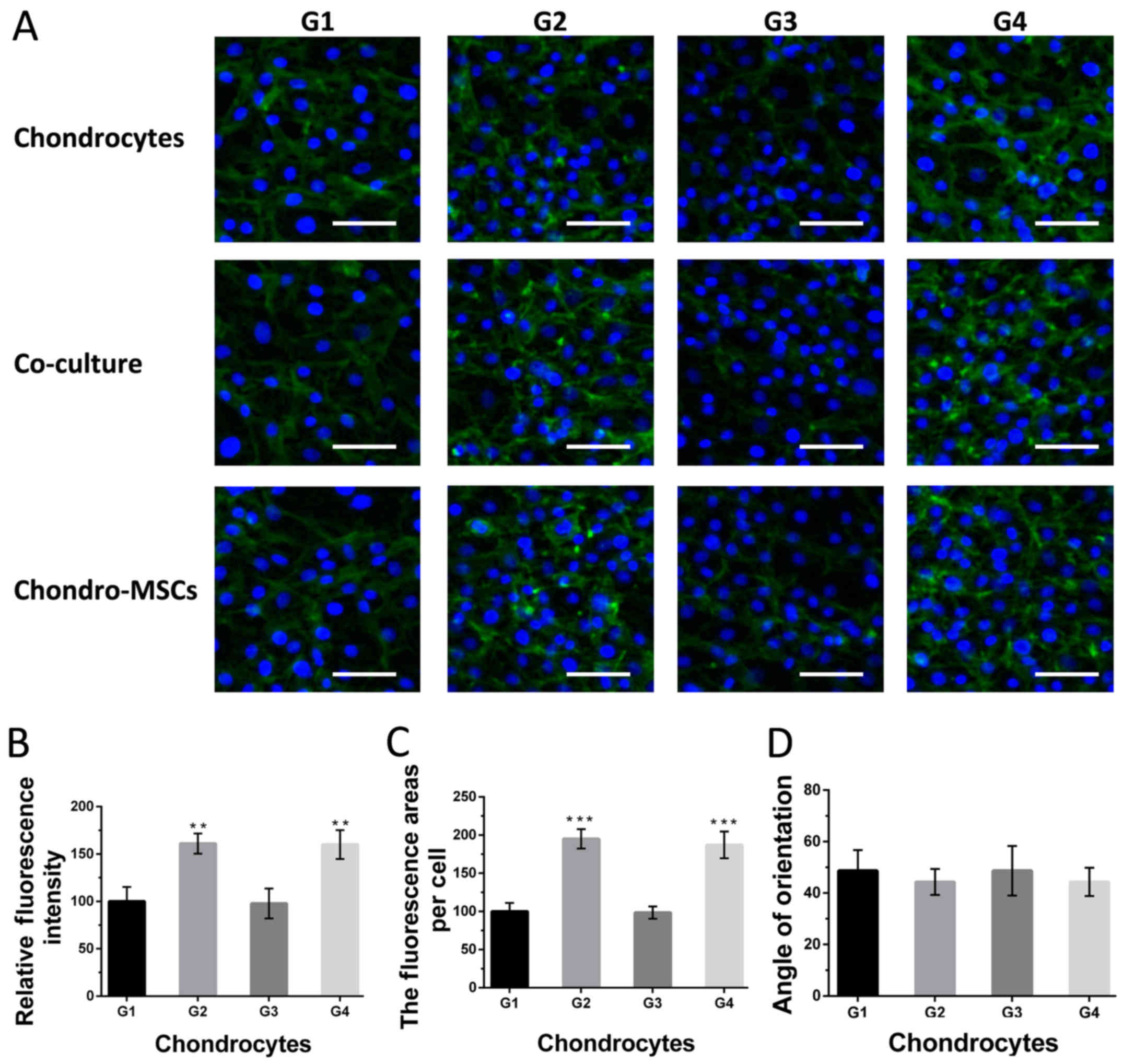
To characterize the shape and cytoskeleton change of
cells on the bottom layer in response to the shear stress, the
morphology change of chondroinduced MSCs, chondrocytes and a
mixture of them was evaluated by intermediate filament staining
(Fig. 5A). The results show that
chondrocytes showed significant differences on intermediate
filament expression under perfusion condition (group 2 and 4),
compared with that in group 1 (Fig.
5B). Besides, chondrocytes culture in group 2, 4 showed
remarkable morphological changes with spread adhering area per cell
on the substrate of the chips (Fig.
5C). Similar results were observed for chondroinduced MSCs and
the mixture of cells (data not shown). However, the angle of the
orientation induced by the change of cytoskeleton showed no
significant difference compared with those in group 1 (Fig. 5D), which indicated the fluid flow
may be not strong enough to change the angle of cellular
orientation.
Phenotypic variations of cells in
response to shear stress and osteoinduced MSCs
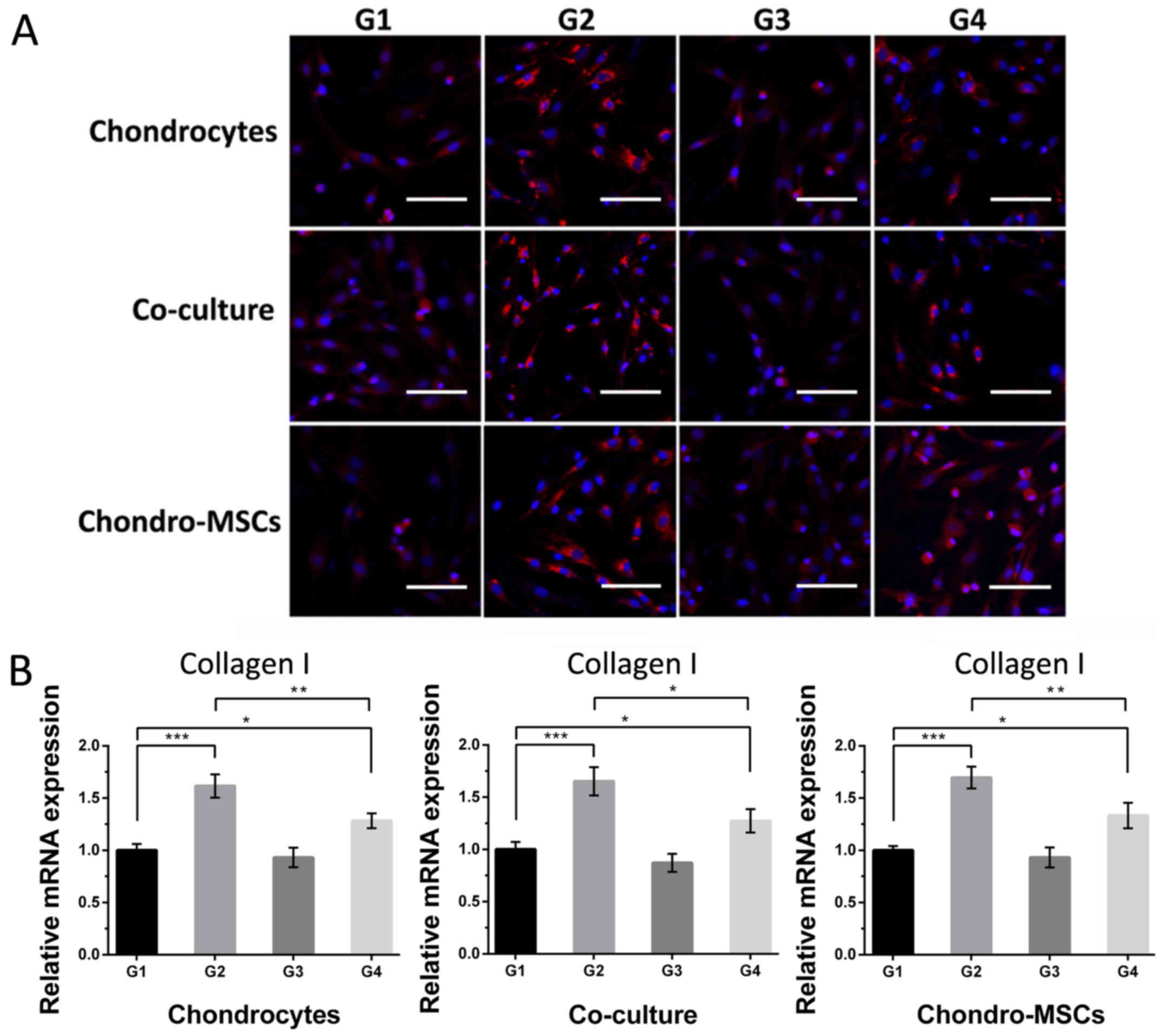
After 7 days' cultivation, collagen I, collagen II
and aggrecan, the markers for the dedifferentiated and
differentiated phenotype of chondrocytes and chondroinduced MSCs,
respectively, were characterized by immunofluorescence staining and
qPCR to evaluate the phenotypic changes of cells in different
groups. The detail data of the relative fluorescence intensities of
collagen I, collagen II and aggrecan can be seen in Figs. 6–8. In terms of collagen I, the cells in
group 1 were nearly negative (Fig.
6A). The mechanical loading provided by perfusion system in
group 2 enhanced the expression of collagen I, while osteoinduced
MSCs on the top layer weakened it. However, the cells in group 4,
in which the effects of osteoinduced MSCs and the shear stress were
combined, showed a weaker staining intensity compared with those in
group 2, but stronger than those in group 3, indicating the rescue
of the hyaline cartilage phenotype of cells by the stimulation of
osteoinduced MSCs. This tendency can also be seen in qPCR result
(Fig. 6B). Shear stress improved
the mRNA expression of collagen I and this improvement can be
reduced by stimulation of osteoinduced MSCs in Group 4 when
compared with Group 2.
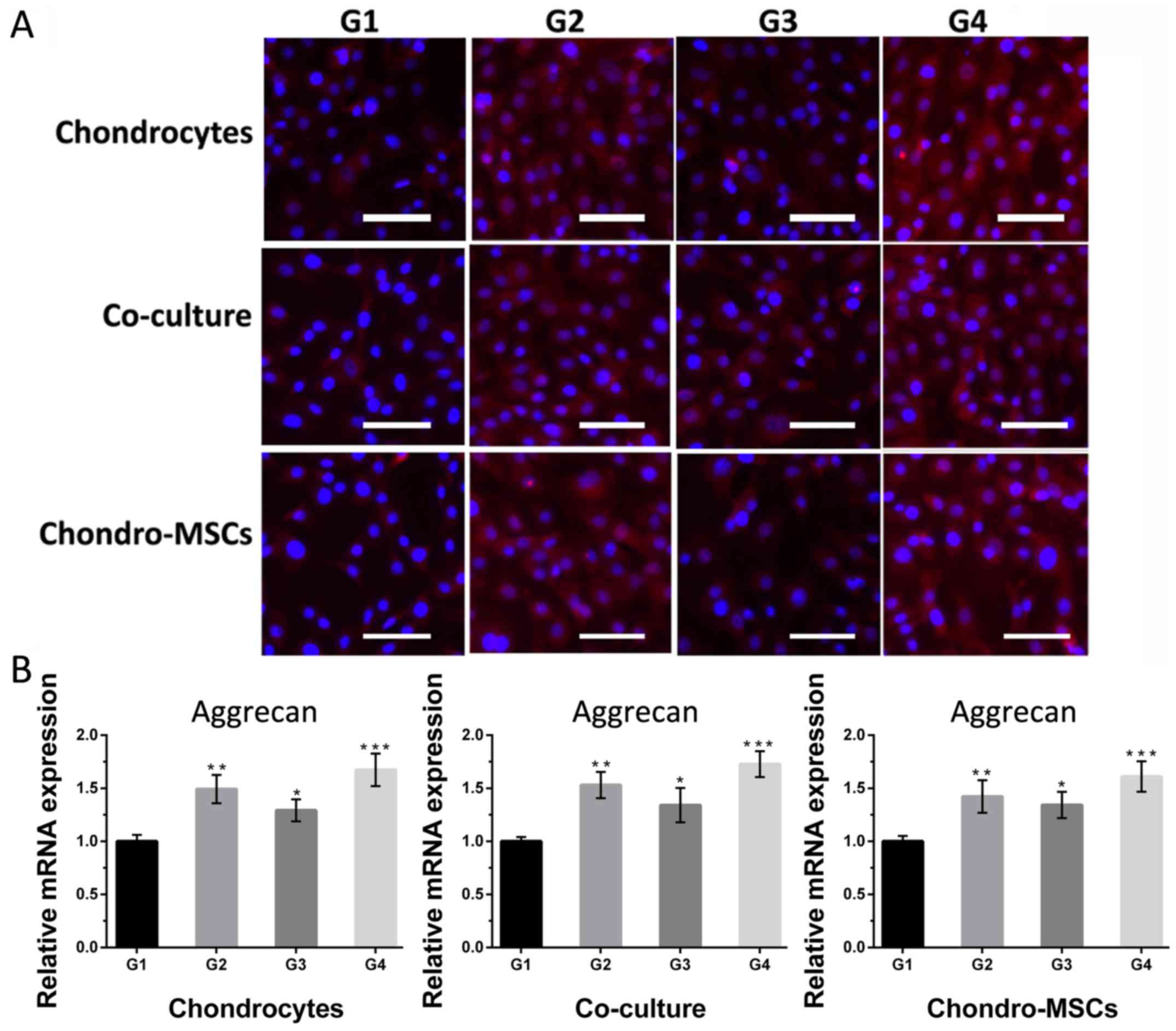
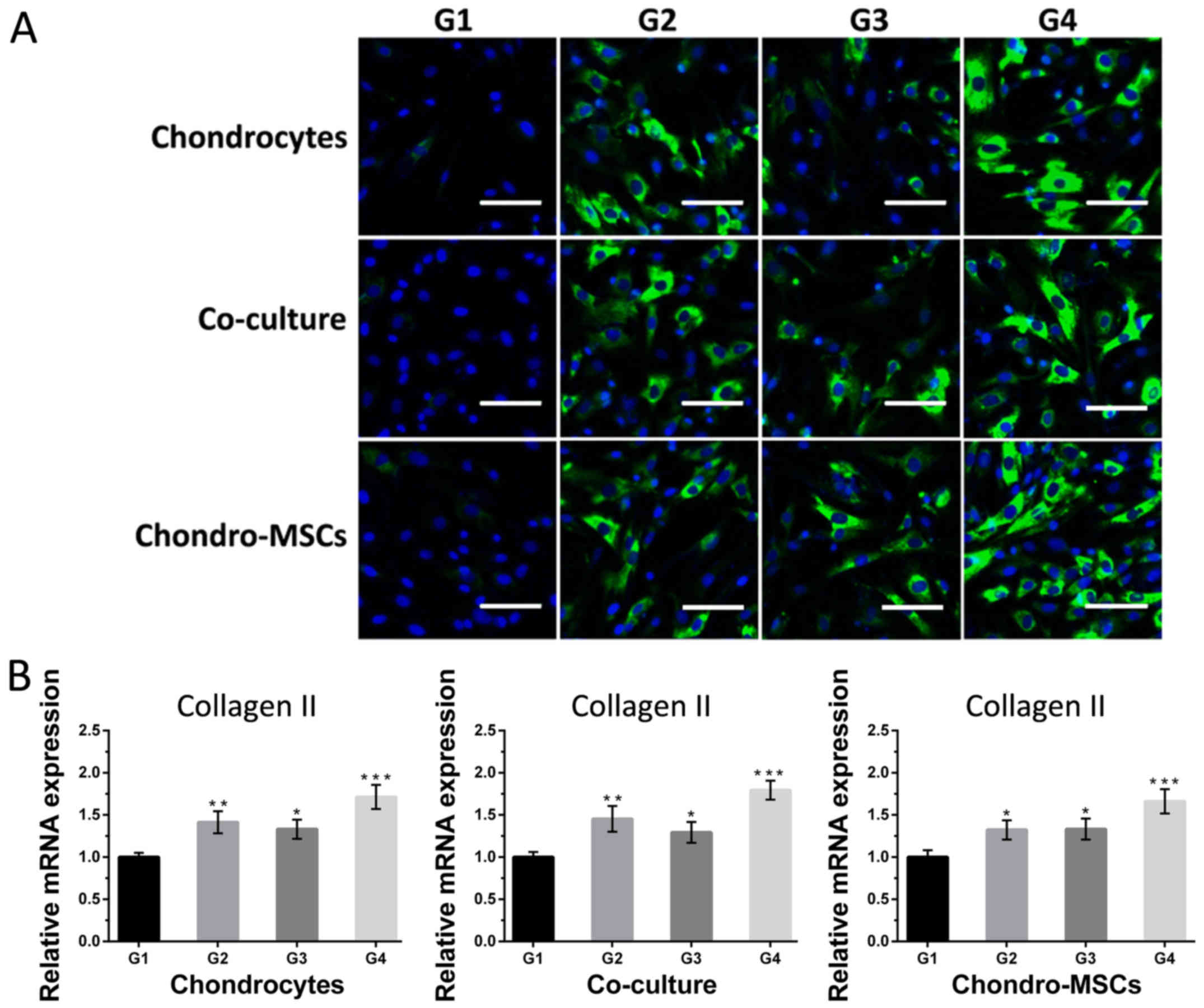
Then for collagen II and aggrecan, the cells in
group 1 were weakly positive (Figs.
7A and 8A). As for cells in
group 2 and 3, where there are perfusion systems on the bottom
layer and osteoinduced MSCs on the top layer, the expression of
collagen II and aggrecan was significantly higher than that in
group 1. Cells in group 4, which had perfusion systems and
osteoinduced MSCs simultaneously, the fluorescent intensity of
collagen II and aggrecan was the strongest compared with those in
other groups, indicating that osteoinduced MSCs on the top layer
and the shear stress provided by the perfusion system acted
synergistically to the cells on the bottom layer. Noncontact
co-culture with osteoinduced MSCs neutralized the negative effect
of the shear stress and even reversed the dedifferentiation
process. Figs. 7B and 8B showed the changes of mRNA levels of
collagen II and aggrecan in 4 groups. The collagen II and aggrecan
mRNA expression were significantly up-regulated by shear stress and
osteoinduced MSCs and the effects were synergistic.
Discussion
MSCs and chondrocytes are commonly used in cartilage
and bone tissue engineering (9,21–23).
MSCs can differentiate into osteogenic lineage in the bone scaffold
and chondrogenic lineage in the cartilage scaffold. Hence, the
great proliferative potential and the ability to adapt to different
phenotypes in vivo make MSCs a good candidate for
osteochondral tissue engineering. However, the results about the
interactions between MSCs and chondrocytes were conflicted
(24–26). It was reported that osteoinduced
MSCs could promote the extracellular matrix production of
chondrocytes, with the effect depending on the differentiation
states of MSCs (15). Another
study showed that chondroinduced MSCs within the chondral layer
exhibited enhanced chondrogenic phenotype when combined with
osteoinduced MSCs (16). However,
the studies on the interactions between osteoinduced MSCs and other
cells (chondrocytes and chondroinduced MSCs) were conducted under
static condition. When cells (e.g., myocardial cell, chondrocytes)
are subjected to the mechanical loading in vivo, the
cytoskeleton of cells were remodeled by the shear stress, resulting
in a better nutrient transportation (27,28).
In this study, we used a perfusion system to mimic the mechanical
microenvironment in vivo to study the combined effect of the
shear stress and non-contact co-culture of osteoinduced MSCs to
chondrocytes and chondroinduced MSCs metabolism.
The mechanical stimuli are important to regulate
cartilage metabolism and maintain chondrocytes function.
Microfabrication technology facilitates the regulation of the
biological stimuli at the cellular and subcellular levels. In this
work, we designed and fabricated a novel microchip by photo- and
soft-lithographic approaches. Compared with conventional cell
cultivation, this microfuidic chip not only reduced the cell
sources, but also generated a controllable flow stimulus through
tuning the input flow rate. Moreover, the transparent property of
PDMS made the cells on the bottom layer observable and the
semipermeable membrane between two layers allowed cytokines and
small molecular compounds to freely transfer from the top layer to
the bottom layer and prevented the cells from moving at the same
time.
The three microchambers on the bottom layer were
subjected to separated fluidic flow stress. The fluid shear stress
on the cell surface can be calculated by the Poiseuille flow model
in cylindrical microchamber. However, it is difficult to measure
the fluid shear stress in the rectangular microchamber. Here we
used hydromechanical CFD analysis to simulate the local fluid shear
stress in three microchambers, and the results showed that the
shear stress was uniform within the microchamber. Different
perfusion systems were applied to achieve a range of shear stress,
thus regulating the behaviors of MSCs and chondrocytes accordingly
(29–31). The interstitial level of fluid in
the intra-articular cartilage surface and different layers of
articular surface was in the range of 10−5 to
10−2 dyne/cm2 in the preceding researches
(32,33). In this work, the shear stress
applied was 0.05 dyne/cm2, which was consistent with the
level of interstitial fluid in the cartilage space.
Chondrocytes and chondroinduced MSCs are common
cells used in tissue engineering. Chondroinduced MSCs are
predifferentiated from MSCs and can facilitate the regeneration of
chronic osteochondral lesions in vitro. One study showed
that chondroinduced MSCs have superior regeneration ability
compared with chondrocytes (34).
Hence, in our study, we used both chondrocytes and chondroinduced
MSCs to study the stimulation of osteoinduced MSCs under a fluid
flow stimulus. The results showed that both chondrocytes and
chondroinduced MSCs were similarly affected by osteoinduced MSCs,
as well as their co-culture.
According to the results of our work, the
proliferation rates of cells on the bottom layer were increased by
the stimulus of shear stress on the bottom layer and the
osteoinduced MSCs on the top layer. The proliferation rate of cells
in group 4 showed the promotion effect was synergistic when the
shear stress and osteoinduced MSCs were combined. Cytoskeleton,
composed of intermediate filaments, actin and microtubules, is
considered as a key mechanotransduction factor involved in
morphology change in response to flow stimulus. In this study, we
evaluated the morphology change of cells by intermediate filament
staining. The cells exhibited obvious morphological changes with
flattened and increased adhering area per cell on the substrate.
Besides, the mean area and IF staining expression of cells under
perfusion condition were significant higher, indicating the
remodeling of the cells' cytoskeleton under appropriate flow
stimulus. However, the alignment of cells found in the previous
work (35) was not observed in our
work, suggesting that the level of the shear stress applied was not
effective enough to cause the morphological change.
According to abovementioned results, chondrocytes
showed phenotypic variation under the shear stress and the
stimulation of osteoinduced MSCs. The increasing expression of
collagen I in group 2 and 4 indicated the double-edged sword
function of fluid shear stress. The results were in accordance with
the previous studies (36,37). Interestingly, the dedifferentiation
effect brought by fluid shear stress could be neutralized by the
stimulation of chondrocytes, highlighting the importance of
osteoinduced MSCs in the maintaining of chondrocytes in tissue
engineering. Excessive flow stimulus could elicit the
proinflammatory cytokines release (38) and the matrix degradation of
chondrocytes (39). However, the
osteoinduced MSCs may secrete one or several soluble factors to
offset the inferior phenotypic variation induced by shear stress.
In terms of collagen II and aggrecan, the expression in group 2 and
3 showed significantly increasing compared with group 1. Besides,
the expression of collagen II and aggrecan in group 4 was higher
than that in group 2 and 3, demonstrating that not only both of the
shear stress and osteoinduced MSCs could promote the differentiated
phenotype of cells, but they also possessed synergistic effect on
the differentiated phenotype of chondrocytes when the shear stress
and the osteoinduced MSCs were combined.
Nevertheless, the cells on the bottom layer are not
cultured in a 3D matrix in our work, which means only the upper
surface of cells is exposed to the shear stress. Additionally, the
soluble factors (e.g., BMPs, IGFs, FGF, and Endothelin-1) that
mediate the ‘reverse’ process remain unknown. Further study will
explore the exact cytokines that influence the phenotypic variation
of cells. Furthermore, to better mimic the physiological properties
in vivo, the microfluidic chip will be modified to an
adoptable IIID cell culture on the bottom layer. Notwithstanding
these limitations, this new designed lab-on-a-chip device can
hurdle the obstacles of conventional method in the study of the
stimulation of osteoinduced MSCs on chondrocytes metaobolism in a
mechanical environment, which may help the development of clinical
transplantation.
This study presented a novel integrated microfluidic
chip to study the intercellular interactions between different
cells in a fluid flow-induced mechanical environment. Based on the
microfluidic chip, the morphological change, the proliferation rate
and the phenotypic responses of chondroinduced MSCs, chondrocytes
and the co-culture of them to osteoinduced MSCs in a mechanical
microenvironment were investigated. The results showed both
chondrocytes and chondroinduced MSCs were similarly affected by
osteoinduced MSCs. The shear stress (0.05 dyne/cm2) and
the osteoinduced MSCs were beneficial to maintain the phenotype of
chondrocytes, and the effects were synergetic. Besides, the
osteoinduced MSCs can reverse the dedifferentiate change induced by
the fluid flow. The microfluidic chip allows us to better mimic the
mechanical environment in vivo and minimizes the volume of
cell resources down to a micron scale, providing a convenient
evaluation tool in tissue engineering.
Acknowledgements
This study was supported by National Nature Science
Foundation of China (nos. 91543121, 81573394 and 81273483),
International Science and Technology Cooperation Program of China
(no. 2015DFA00740), Special Fund for Agro-scientific Research in
the Public Interest (no. 201303045), National scientific instrument
development project (Chinese Academy of Sciences), Key Laboratory
of Separation Science for Analytical Chemistry (Dalian Institute of
Chemical Physics, Chinese Academy of Sciences).
References
|
1
|
Caron M, Emans P, Coolsen M, Voss L,
Surtel D, Cremers A, van Rhijn LW and Welting TJ: Redifferentiation
of dedifferentiated human articular chondrocytes: Comparison of 2D
and 3D cultures. Osteoarthritis Cartilage. 20:1170–1178. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hubka KM, Dahlin RL, Meretoja VV, Kasper
FK and Mikos AG: Enhancing chondrogenic phenotype for cartilage
tissue engineering: Monoculture and coculture of articular
chondrocytes and mesenchymal stem cells. Tissue Eng Part B Rev.
20:641–654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang W, Zhuang A, Gu P, Zhou H and Fan X:
A review of the three-dimensional cell culture technique:
Approaches, advantages and applications. Curr Stem Cell Res Ther.
11:370–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin JW and Mooney DJ: Improving stem cell
therapeutics with mechanobiology. Cell Stem Cell. 18:16–19. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kon E, Robinson D, Verdonk P, Drobnic M,
Patrascu JM, Dulic O, Gavrilovic G and Filardo G: A novel
aragonite-based scaffold for osteochondral regeneration: Early
experience on human implants and technical developments. Injury. 47
suppl 1:S27–S32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimomura K, Moriguchi Y, Sugita N,
Koizumi K and Yasui Y: Current strategies in osteochondral repair
with biomaterial scaffoldMusculoskeletal research and basic
science. Springer; Cham: pp. 387–403. 2016, View Article : Google Scholar
|
|
7
|
Pina S, Ribeiro V, Oliveira JM and Reis
RL: Pre-clinical and clinical management of osteochondral
lesionsRegenerative strategies for the treatment of knee joint
disabilities. Springer; Cham: pp. 147–161. 2017, View Article : Google Scholar
|
|
8
|
Leach JK and Whitehead JR:
Materials-directed differentiation of mesenchymal stem cells for
tissue engineering and regeneration. ACS Biomater Sci Eng. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vo T, Shah SR, Lu S, Tatara AM, Lee EJ,
Roh TT, Tabata Y and Mikos AG: Injectable dual-gelling cell-laden
composite hydrogels for bone tissue engineering. Biomaterials.
83:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hung KC, Tseng CS, Dai LG and Hsu SH:
Water-based polyurethane 3D printed scaffolds with controlled
release function for customized cartilage tissue engineering.
Biomaterials. 83:156–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu CH and Hwang SM: Cytokine interactions
in mesenchymal stem cells from cord blood. Cytokine. 32:270–279.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cawston TE: Metalloproteinase inhibitors
and the prevention of connective tissue breakdown. Pharmacol Ther.
70:163–182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujimoto E, Ochi M, Kato Y, Mochizuki Y,
Sumen Y and Ikuta Y: Beneficial effect of basic fibroblast growth
factor on the repair of full-thickness defects in rabbit articular
cartilage. Arch Orthop Trauma Surg. 119:139–145. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schinköthe T, Bloch W and Schmidt A: In
vitro secreting profile of human mesenchymal stem cells. Stem Cells
Dev. 17:199–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lam J, Lu S, Meretoja VV, Tabata Y, Mikos
AG and Kasper FK: Generation of osteochondral tissue constructs
with chondrogenically and osteogenically predifferentiated
mesenchymal stem cells encapsulated in bilayered hydrogels. Acta
Biomater. 10:1112–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rothenberg AR, Ouyang L and Elisseeff JH:
Mesenchymal stem cell stimulation of tissue growth depends on
differentiation state. Stem Cells Dev. 20:405–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao G, Zhang XF, Hubbell K and Cui X:
NR2F2 regulates chondrogenesis of human mesenchymal stem cells in
bioprinted cartilage. Biotechnol Bioeng. 114:208–216. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gosset M, Berenbaum F, Thirion S and
Jacques C: Primary culture and phenotyping of murine chondrocytes.
Nat Protoc. 3:1253–1260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Green TR, Fisher J, Stone M, Wroblewski BM
and Ingham E: Polyethylene particles of a ‘critical size’ are
necessary for the induction of cytokines by macrophages in vitro.
Biomaterials. 19:2297–2302. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Markstedt K, Mantas A, Tournier I,
MartínezÁvila HC, Hägg D and Gatenholm P: 3D bioprinting human
chondrocytes with nanocellulose-alginate bioink for cartilage
tissue engineering applications. Biomacromolecules. 16:1489–1496.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwarz S, Elsaesser AF, Koerber L,
Goldberg-Bockhorn E, Seitz AM, Bermueller C, Dürselen L, Ignatius
A, Breiter R and Rotter N: Processed xenogenic cartilage as
innovative biomatrix for cartilage tissue engineering: effects on
chondrocyte differentiation and function. J Tissue Eng Regen Med.
9:E239–E251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mellor LF, Mohiti-Asli M, Williams J,
Kannan A, Dent MR, Guilak F and Loboa EG: Extracellular calcium
modulates chondrogenic and osteogenic differentiation of human
adipose-derived stem cells: A novel approach for osteochondral
tissue engineering using a single stem cell source. Tissue Eng Part
A. 21:2323–2333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen WH, Lai MT, Wu AT, Wu CC, Gelovani
JG, Lin CT, Hung SC, Chiu WT and Deng WP: In vitro stage-specific
chondrogenesis of mesenchymal stem cells committed to chondrocytes.
Arthritis Rheum. 60:450–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T,
Zhang W, Liu W, Cao Y and Zhou G: In vivo ectopic chondrogenesis of
BMSCs directed by mature chondrocytes. Biomaterials. 31:9406–9414.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu L, Prins HJ, Helder MN, van
Blitterswijk CA and Karperien M: Trophic effects of mesenchymal
stem cells in chondrocyte co-cultures are independent of culture
conditions and cell sources. Tissue Eng Part A. 18:1542–1551. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen CY, Chiang TS, Chiou LL, Lee HS and
Lin FH: 3D cell clusters combined with a bioreactor system to
enhance the drug metabolism activities of C3A hepatoma cell lines.
J Mater Chem B. 4:7000–7008. 2016. View Article : Google Scholar
|
|
28
|
Salinas M, Rath S, Villegas A,
Unnikrishnan V and Ramaswamy S: Relative effects of fluid
oscillations and nutrient transport in the in vitro growth of
valvular tissues. Cardiovasc Eng Technol. 7:170–181. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nie Z: Assessment of bioprocess shear
stress as a tool to enhance osteogenic induction of mesenchymal
cells (unpublished PhD thesis). University College London; 2017
|
|
30
|
Sonam S, Sathe SR, Yim EK, Sheetz MP and
Lim CT: Cell contractility arising from topography and shear flow
determines human mesenchymal stem cell fate. Sci Rep. 6:204152016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su YP, Chen CN, Chang HI, Huang KC, Cheng
CC, Chiu FY, Lee KC, Lo CM and Chang SF: Low shear stress
attenuates COX-2 expression induced by resistin in human
osteoarthritic chondrocytes. J Cell Physiol. 232:1448–1457. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park JY, Yoo SJ, Hwang CM and Lee SH:
Simultaneous generation of chemical concentration and mechanical
shear stress gradients using microfluidic osmotic flow comparable
to interstitial flow. Lab Chip. 9:2194–2202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen T, Buckley M, Cohen I, Bonassar L and
Awad HA: Insights into interstitial flow, shear stress and mass
transport effects on ECM heterogeneity in bioreactor-cultivated
engineered cartilage hydrogels. Biomech Model Mechanobiol.
11:689–702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marquass B, Schulz R, Hepp P, Zscharnack
M, Aigner T, Schmidt S, Stein F, Richter R, Osterhoff G, Aust G, et
al: Matrix-associated implantation of predifferentiated mesenchymal
stem cells versus articular chondrocytes: In vivo results of
cartilage repair after 1 year. Am J Sports Med. 39:1401–1412. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smith RL, Donlon BS, Gupta MK, Mohtai M,
Das P, Carter DR, Cooke J, Gibbons G, Hutchinson N and Schurman DJ:
Effects of fluid-induced shear on articular chondrocyte morphology
and metabolism in vitro. J Orthop Res. 13:824–831. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu F, Wang P, Lee NH, Goldring MB and
Konstantopoulos K: Prolonged application of high fluid shear to
chondrocytes recapitulates gene expression profiles associated with
osteoarthritis. PLoS One. 5:e151742010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Healy ZR, Lee NH, Gao X, Goldring MB,
Talalay P, Kensler TW and Konstantopoulos K: Divergent responses of
chondrocytes and endothelial cells to shear stress: Cross-talk
among COX-2, the phase 2 response and apoptosis. Proc Natl Acad Sci
USA. 102:pp. 14010–14015. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luong L, Duckles H, Schenkel T, Mahmoud M,
Tremoleda JL, Wylezinska-Arridge M, Ali M, Bowden NP, Villa-Uriol
MC, van der Heiden K, et al: Heart rate reduction with ivabradine
promotes shear stress-dependent anti-inflammatory mechanisms in
arteries. Throm Haemost. 116:181–190. 2016. View Article : Google Scholar
|
|
39
|
Trevino RL, Pacione CA, Malfait AM,
Chubinskaya S and Wimmer MA: Development of a cartilage
shear-damage model to investigate the impact of surface injury on
chondrocytes and extracellular matrix wear. Cartilage. 8:444–455.
2017. View Article : Google Scholar : PubMed/NCBI
|