Introduction
Endothelial progenitor cells (EPCs) mobilized from
bone marrow into the peripheral blood have been observed to serve
an important role in endothelial repair and vascular regeneration
by incorporating into the site of vessel injury, differentiating
into endothelial cells, and releasing paracrine factors (1–3).
Thereby, EPC depletion may lead to endothelial dysfunction.
However, it has been demonstrated that the number of circulating
EPCs in patients with atherosclerosis (AS) was decreased (4) and studies have reported that the
number of EPCs may be associated with certain indicators of
atherosclerosis, including intima-media thickness, in healthy
adults (5,6). Angiotensin II (Ang II), the main
active effector of the renin-angiotensin system, serves an
important role in the pathobiology of AS (7). A previous study demonstrated that Ang
II is essential for EPC function, as Ang-II-induced oxidative
stress causes senescence of EPCs and endothelial dysfunction
(8). This, in turn, may accelerate
the development of AS. A recent study demonstrated that natural
extracts may restore the migration, adhesion and tube formation of
EPCs, which are diminished by Ang II (9). This provided novel insights into the
protection of EPCs from functional impairment.
Puerarin
(4–7-dihydroxy-8-beta-D-glucosylisoflavone), one of the principal
isoflavone glycosides extracted from the roots of Pueraria
lobata, has been clinically used for the treatment of diseases,
including hypertension, angina, myocardial infarction, arrhythmia,
cerebral infarction and diabetes (10,11).
A number of studies have revealed that puerarin may protect the
retina by inhibiting inflammation and neuronal damage induced by
pro-inflammatory factors (12),
may ameliorate endothelial dysfunction in isolated rat aortas
(13), and may inhibit the
endothelial inflammatory response (14). In addition, puerarin may function
as a defender against Ang II-mediated oxidative stress-induced cell
damage (15). The previous results
described above suggested that puerarin may possess therapeutic
potential for cardiovascular disorders, including AS. However, it
remains unclear whether puerarin may serve an antioxidant role in
Ang II-induced EPC damage. The present study, therefore, assessed
the potential protective effects of puerarin on Ang II-mediated EPC
injury, and investigated whether nuclear factor erythroid 2 like 2
(Nrf2) activation and extracellular signal-regulated kinase 1 and 2
(ERK1/2) phosphorylation may be involved.
Materials and methods
Identification of EPCs
EPCs were prepared as described previously (16). Peripheral blood mononuclear cells
were obtained from the peripheral blood of ten healthy volunteers.
Informed consent was provided by each participant and the above
procedure was approved by the Ethics Committee of Dongfang Hospital
(Beijing, China). Cells were isolated using Ficoll gradient
centrifugation and cultured in endothelial growth medium-2 (EGM-2;
Lonza Group, Ltd., Basel, Switzerland) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), in a humidified incubator with 5% CO2 at 37°C.
Following 7 days in culture, cells were incubated
with Dil-acetylated low-density lipoprotein (AcLDL; 10 µg/ml;
Molecular Probes; Thermo Fisher Scientific, Inc.) at 37°C for 1 h.
Cells were fixed with 4% paraformaldehyde at room temperature for
10 min and subsequently incubated with fluorescein
isothiocyanate-labeled lectin (UEA-1; 10 µg/ml; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 1 h. Following staining, the
slides were observed under an inverted fluorescent microscope
(Nikon Eclipse Ti-U; Nikon Corporation, Tokyo, Japan). Dual-stained
cells (positive for Dil-ac-LDL and UEA-1) were identified as
EPCs.
Treatment paradigm
For each experiment, cells were treated with human
1.0 µM Ang II (Sigma-Aldrich; Merck KGaA) for 24 h (17) with or without pre-treatment with
various concentrations (1, 10, and 100 µM) of puerarin (99% purity
as verified by high-performance liquid chromatography;
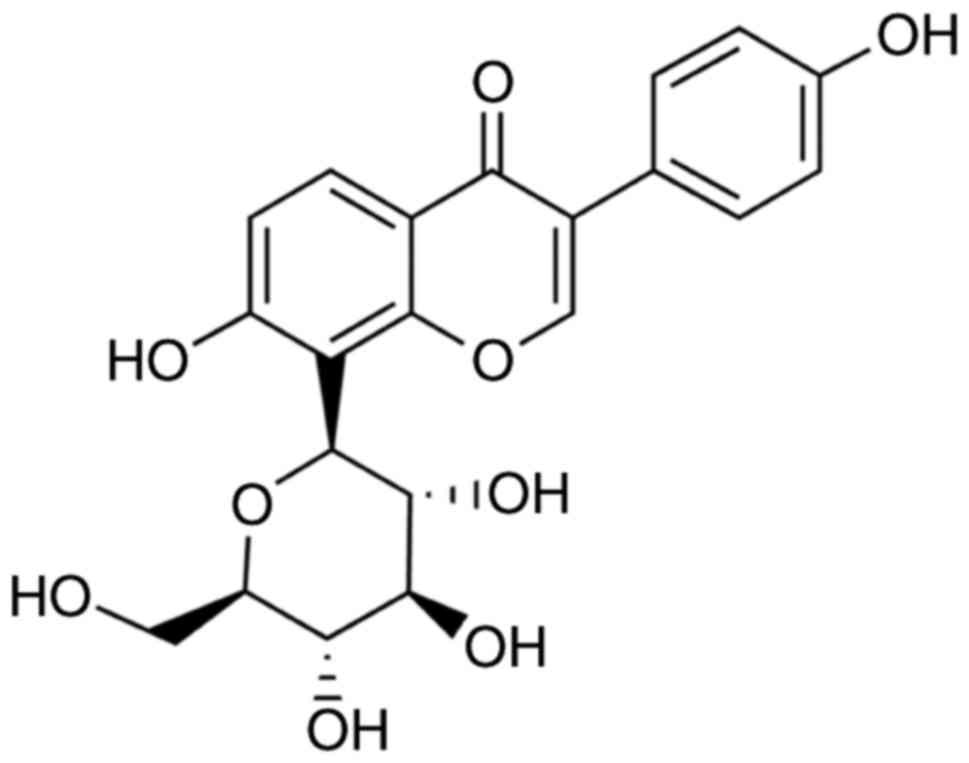
Sigma-Aldrich; Merck KGaA) for 24 h (18). The molecular structure of puerarin
is presented in Fig. 1. To
identify whether the ERK1/2 signaling pathway was involved in the
protective effect of puerarin, U0126 (10 µM; Cell Signaling
Technology, Inc., Danvers, MA, USA), a highly selective inhibitor
of ERK1/2, was added to cell cultures 30 min prior to treatment
with Ang II.
Cell proliferation assay
Cell proliferation was determined using the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) method. Cells were seeded into 96-well plates at
1×104 cells/well and incubated in serum-free EGM-2 for
24 h. Subsequently, media were removed, and cells were washed with
PBS and stimulated with 1.0 µM Ang II for 24 h. To evaluate the
effects of various concentrations of puerarin on the viability of
EPCs, CCK-8 (10 µl/well) was added to the wells at the end of the
experiment. Following incubation at 37°C for 2 h, the absorbance of
each well was determined using a microplate reader (Multiskan
Spectrum; Thermo Fisher Scientific, Inc.) at 450 nm. The degree of
cell proliferation was expressed as the percentage absorbance of
treated cells compared with control cells.
Cell migration assay
The effect of test substances on the migration of
EPCs was determined using uncoated Transwell chambers. A density of
~106 cells was seeded into the upper chambers and medium
containing 10% fetal bovine serum was used as a chemoattractant in
the lower chambers. Following 24 h of incubation, the medium was
removed, and the chambers were washed twice with PBS. The cells on
the upper surfaces of the inserts were removed and those which had
migrated to the lower surfaces were fixed with 4% paraformaldehyde
for 10 min and stained with 0.1% crystal violet for 15 min, both at
room temperature. Microphotographs were obtained using a digital
camera system (Olympus Corporation, Tokyo, Japan) at ×200
magnification.
Measurement of reactive oxygen species
(ROS) production and inflammation
For ROS detection, an Image-iT LIVE Green Reactive
Oxygen Species Detection kit (Invitrogen; Thermo Fisher Scientific,
Inc.) was used. EPCs (1×105/well) were incubated with
EGM-2 containing 10 µM 2,7-dichlorodihydrofluorescein diacetate for
30 min in 6-well plates and washed with PBS. The results were
obtained and analyzed using a FACSCalibur™ flow
cytometer (BD Biosciences, San Jose, CA, USA).
Tumor necrosis factor (TNF)-α and interleukin (IL)-6
concentrations in cell lysates were measured using TNF-α (cat. no.
ab181421; Abcam, Cambridge, UK) and IL-6 (cat. no. ab46042; Abcam)
enzyme-linked immunosorbent assay kits, according to the
manufacturer's instructions. Experiments were repeated three times
independently.
Western blot analysis
Treated cells were extracted using
radioimmunoprecipitation lysis buffer (Biyuntian; Beyotime
Institute of Biotechnology, Haimen, China) and protein
concentrations were determined using the bicinchoninic acid method
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Protein (30 µg) was separated by 10–12%
SDS-PAGE and transferred to polyvinylidene fluoride membranes.
Following blocking with 1% bovine serum albumin (MedChemExpress,
Monmouth Junction, NJ, USA) at room temperature for 1 h, the
membranes were incubated with the following primary antibodies
overnight at 4°C: Anti-β-galactosidase (β-gal; cat. no. ab168341;
1:500; Abcam), anti-intracellular adhesion molecule-1 (ICAM-1; cat.
no. ab20; 1:1,000; Abcam) anti-vascular cell adhesion molecule-1
(VCAM-1; cat. no. ab134047; 1:5,000; Abcam), anti-ERK1/2 (cat no.
9102; 1:10,000; Cell Signaling Technology, Inc.),
anti-phosphorylated (p-)ERK1/2 (cat no. 9101; 1:500; Cell Signaling
Technology, Inc.), anti-Nrf2 (1:1,000; cat no. 12721, Cell
Signaling Technology, Inc.) and anti-GAPDH (cat. no. ab8245;
1:1,000; Abcam). Subsequently, the membranes were incubated with
anti-rabbit IgG horseradish peroxidase-conjugated secondary
antibodies (cat. no. ab205718; 1:5,000; Abcam) for 2 h at room
temperature. The immune complexes were detected using an Enhanced
Chemiluminescence Plus kit (Biyuntian; Beyotime Institute of
Biotechnology). Bands were then analyzed using the Quantity One 4.0
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each
experiment was repeated three times independently.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 6.5 (GraphPad Software, Inc., La Jolla, CA, USA).
Data are presented as the mean ± standard deviation. Comparisons
among groups were performed using one-way analysis of variance with
post hoc Bonferroni or post hoc Dunnett tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
Puerarin reverses the Ang II-induced
inhibition of EPC proliferation and migration
EPCs were cultured from human peripheral blood
mononuclear cells. Following 7 days in culture, isolated cells
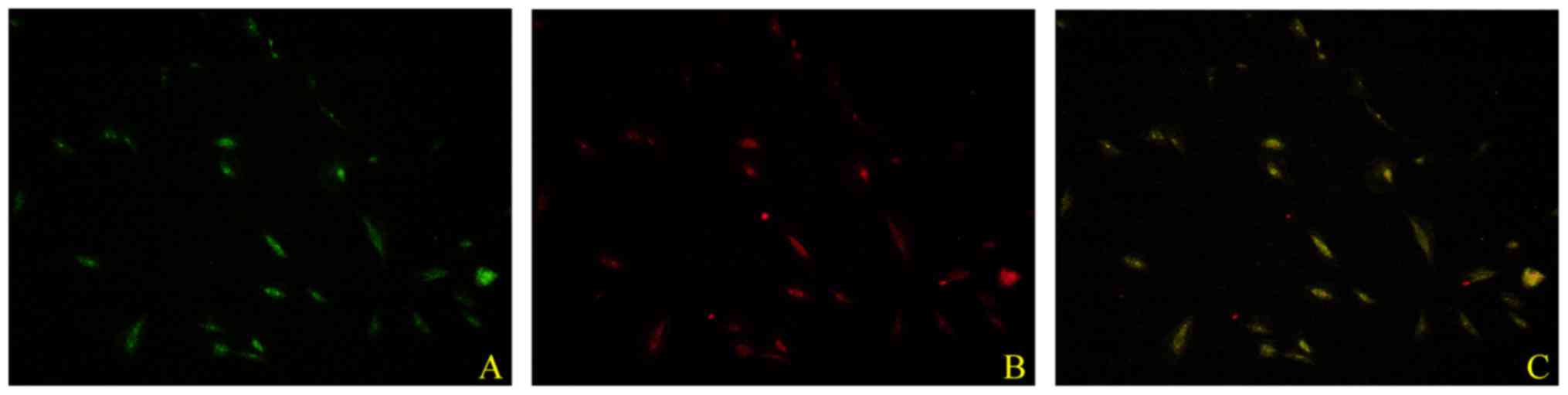
exhibited an endothelial cell-like morphology. As illustrated in
Fig. 2, EPCs were characterized by
double staining with Dil-AcLDL and UEA-1. Pre-incubation with 1.0
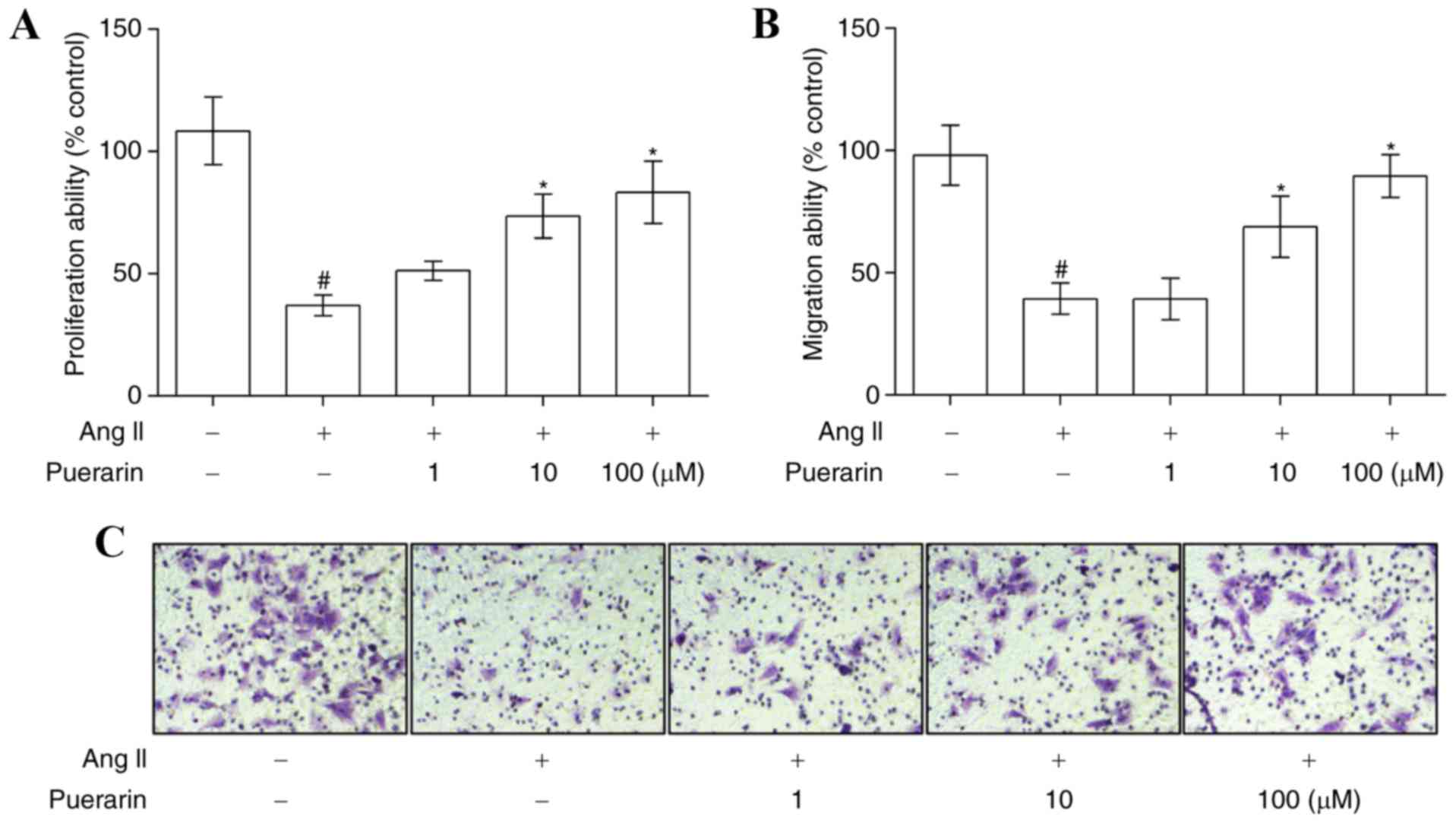
µM Ang II significantly suppressed EPC proliferation (Fig. 3A) and migration (Fig. 3B and C). However, puerarin reversed
the inhibitory effect caused by 1.0 µM Ang II, in a dose-dependent
manner, on cell proliferation and migration (Fig. 3; P<0.05).
Puerarin attenuates Ang II-induced
EPCs senescence and adhesion
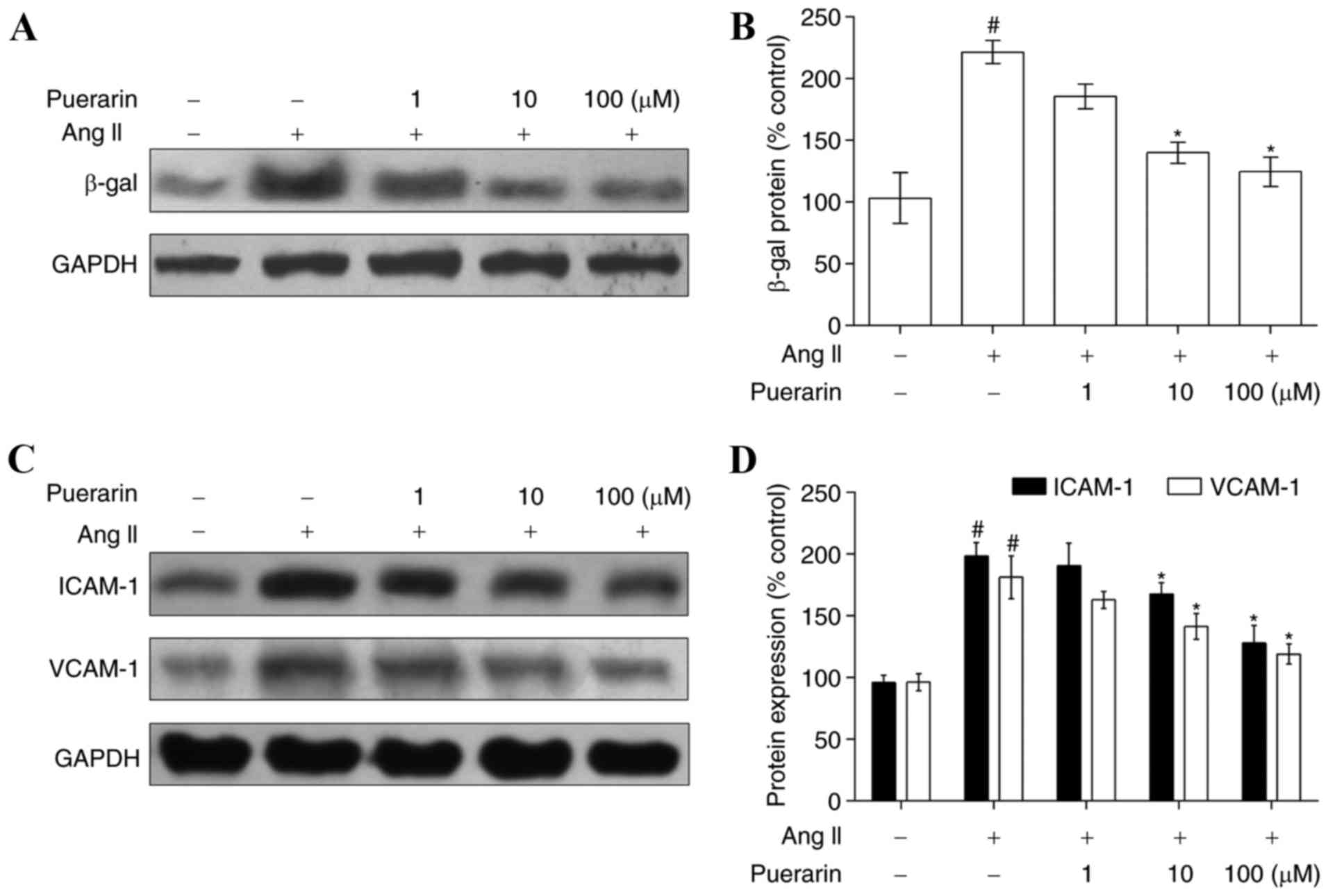
Compared with the control group, EPC senescence in
the Ang II group was accelerated, as characterized by the increased
level of β-gal (Fig. 4A and B). In
addition, compared with the control group, the expression of ICAM-1
and VCAM-1, two adhesion molecules, was increased in the Ang II
group (Fig. 4C and D). When EPCs
were incubated with increasing concentrations of puerarin for 24 h,
the expression of β-gal was significantly decreased, in addition to
the expression of ICAM-1 and VCAM-1 (Fig. 4; P<0.05).
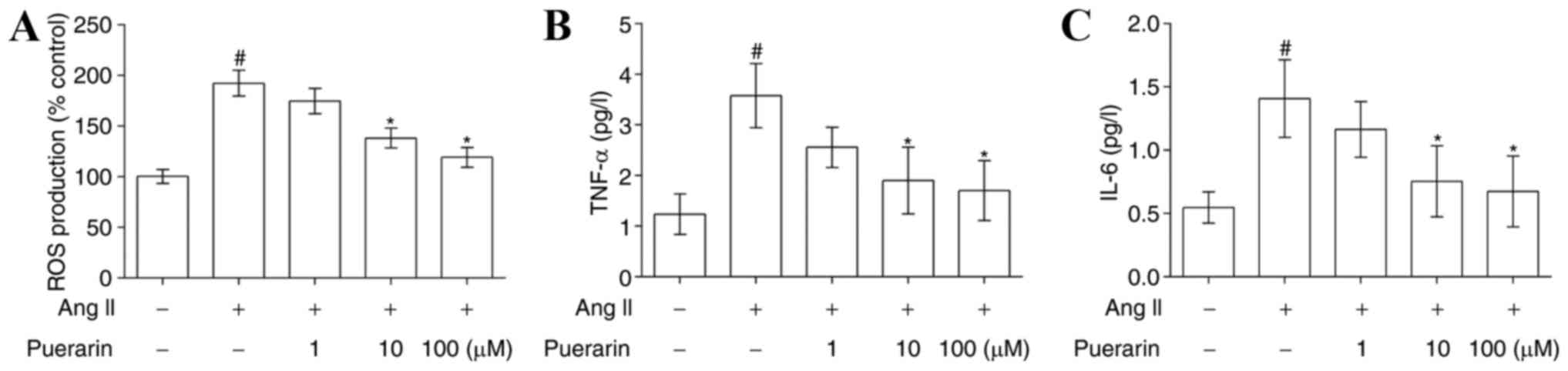
Puerarin suppresses Ang II-induced
oxidative stress and inflammation in EPCs
The results of the flow cytometry analysis presented
in Fig. 5A demonstrated that Ang
II significantly increased ROS production, compared with the
control group, and its effects were attenuated by treatment with
puerarin (P<0.05). In addition, the levels of TNF-α and IL-6
were significantly increased in the Ang II-treated EPCs compared
with control EPCs. Similarly, puerarin reduced the expression of
TNF-α and IL-6 in a dose-dependent manner (Fig. 5B and C; P<0.05).
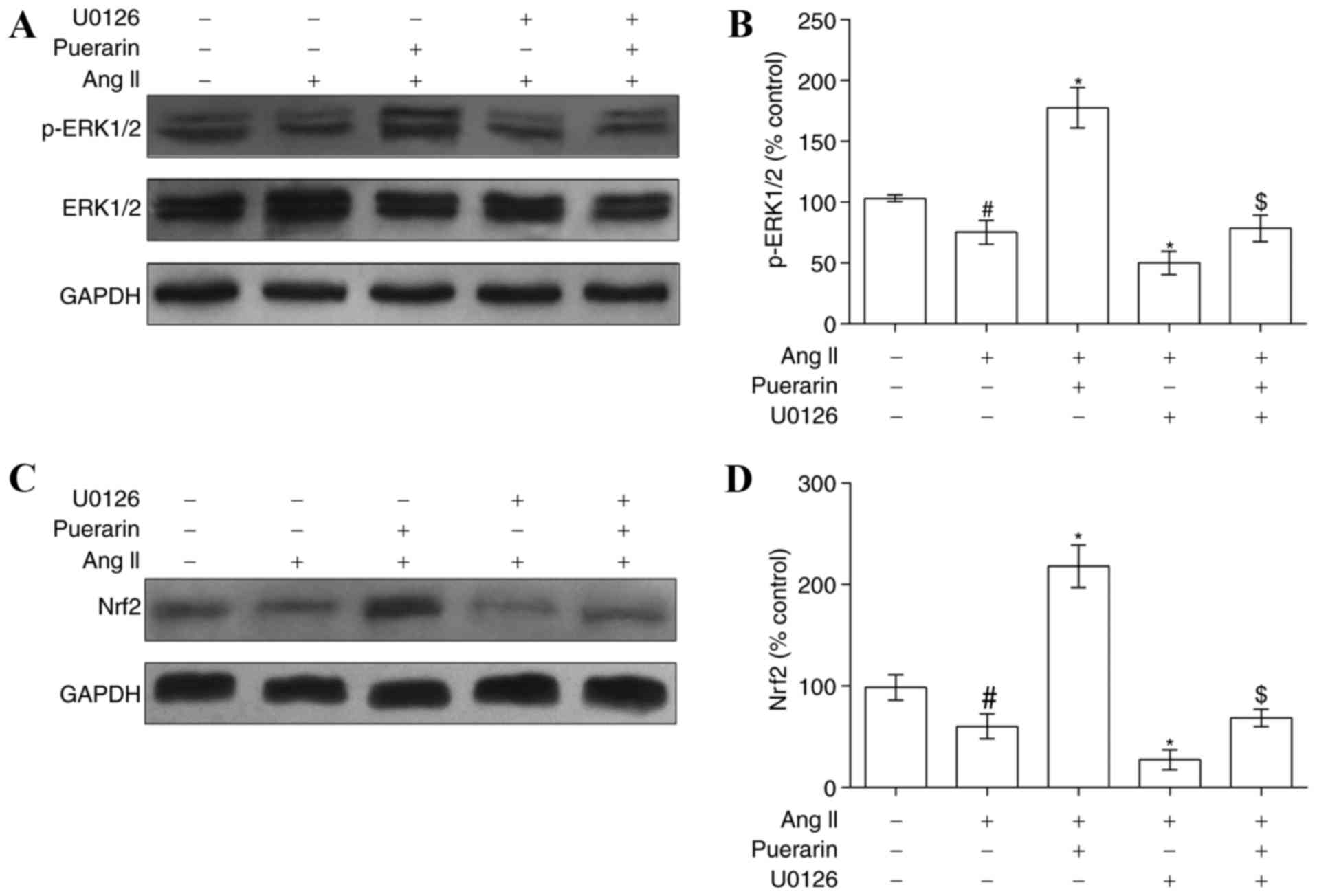
Puerarin alleviates Ang II-induced EPC
damage by activating ERK1/2-Nrf2
In order to elucidate the mechanism underlying the
above protective effects of puerarin on Ang II-induced EPC damage,
the present study investigated whether the ERK1/2-Nrf2 signaling
pathway was involved in this regulation. EPCs were exposed to Ang
II, puerarin or U0126, and western blotting was used to determine
the protein levels of ERK1/2, p-ERK1/2 and Nrf2. Ang II induced a
suppression of p-ERK1/2 and Nrf2 expression (Fig. 6). Notably, puerarin significantly
increased the p-ERK1/2 expression level and reversed the
suppression of Nrf2 protein expression mediated by Ang II. The
effects of puerarin may be blocked by the ERK1/2 inhibitor U0126
(Fig. 6).
In addition, cell proliferation and migration
following treatment with U0126 was decreased compared with
treatment with Ang II and puerarin. However, there were no apparent
alterations in the Ang II group compared with the Ang II + U0126
group or Ang II + puerarin + U0126 group (Fig. 7A). The effects of U0126 on cell
senescence and adhesion (Fig. 7B and
C) was consistent with the above. The levels of ROS production
and inflammatory cytokines (TNF-α and IL-6) were also decreased
when EPCs were exposed to U0126 (Fig.
7D-F). These results indicated that puerarin protected EPCs
from Ang II-induced damage by activating ERK1/2 and Nrf2.
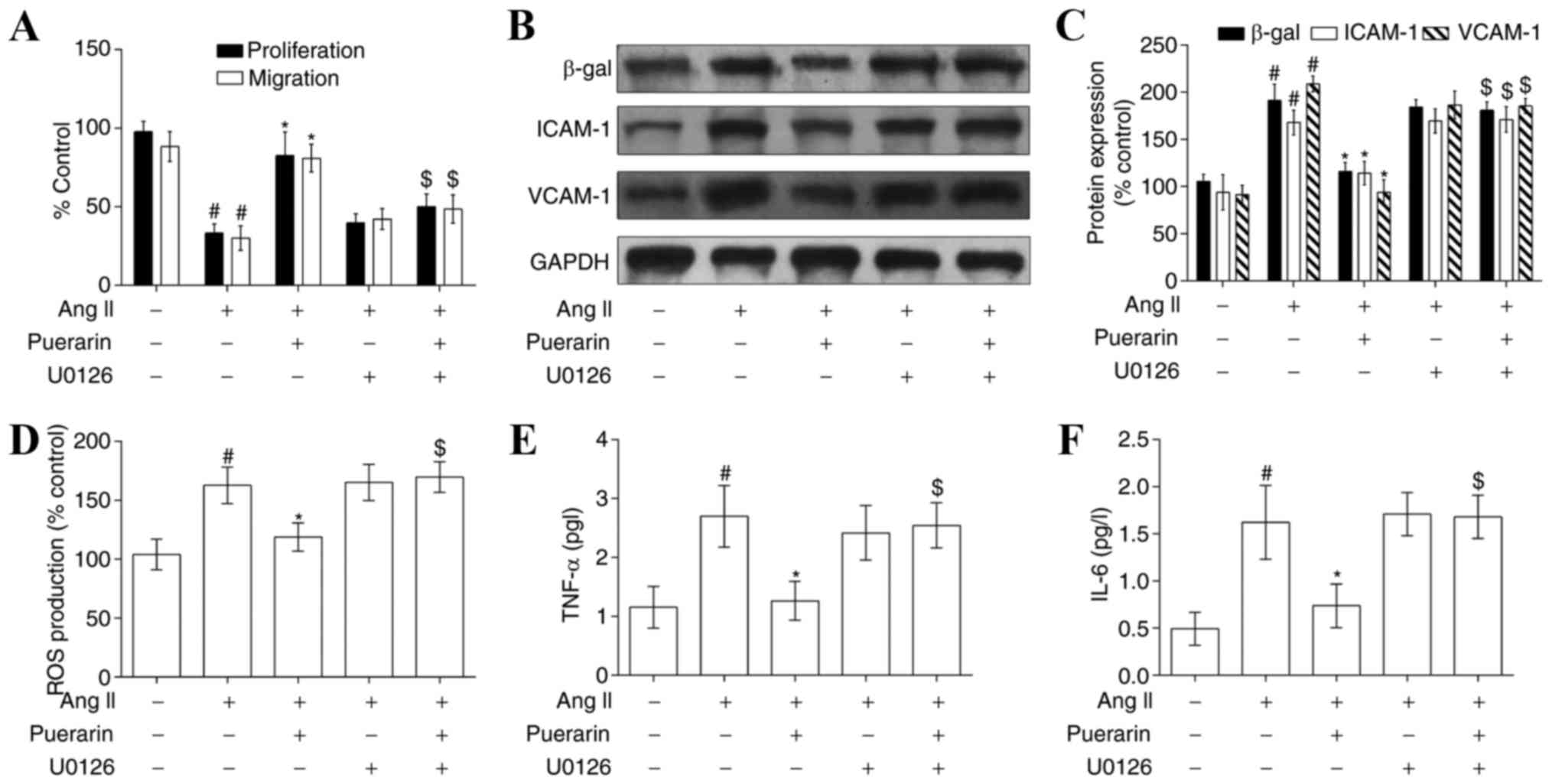 | Figure 7.Puerarin attenuates Ang II-induced
EPC dysfunction by activating the ERK1/2-nuclear factor erythroid 2
like 2 signaling pathway. (A) Cell proliferation and migration was
measured in the different groups. The protein expression of β-gal,
ICAM-1 and VCAM-1 was measured by (B) western blotting and (C)
densitometry, following treatment with puerarin or U0126 (an
inhibitor of ERK1/2) in Ang II-induced EPCs. (D) ROS levels were
detected by flow cytometry in the different groups. (E) TNF-α and
(F) IL-6 levels were detected by ELISA analysis in the different
groups. #P<0.05 vs. control; *P<0.01 vs. Ang II;
$P<0.05 vs. Ang II + puerarin. Ang II, angiotensin
II; EPC, endothelial progenitor cell; ERK1/2, extracellular
signal-regulated kinase; β-gal, β-galactosidase; ICAM-1,
intracellular adhesion molecule-1; VCAM-1, vascular cell adhesion
molecule-1; ROS, reactive oxygen species; TNF-α, tumor necrosis
factor-α; IL-6, interleukin-6. |
Discussion
The present study demonstrated that puerarin
protected EPCs from Ang II-induced cell damage by decreasing ROS
production and the expression of inflammatory cytokines. In
addition, the results of the present study suggested that the
protective effects of puerarin on EPC function may be dependent on
the ERK1/2-Nrf2 signaling pathway.
In patients with carotid AS, it has been observed
that the number of circulating EPCs is reduced and their function
is impaired (19). EPCs may be a
potential therapeutic target for vascular repair and regeneration,
due to their strong capacity for proliferation and differentiation
(20). However, studies have
reported that EPCs may be damaged by Ang II and there is evidence
that Ang II upregulates the levels of pro-inflammatory cytokines
(including IL-6, monocyte chemoattractant protein-1 and VCAM-1) via
the type 1 Ang II receptor, which may deteriorate the
atherosclerotic inflammatory response (21,22).
Consistent with previous studies, the results of the present study
suggested that cell proliferation and migration were decreased by
Ang II, and it was demonstrated that 1.0 µM Ang II stimulated ROS
production to increase EPC senescence, and increased the expression
of ICAM-1 and VCAM-1, two adhesion molecules of EPCs. Similarly,
Han et al (23)
demonstrated that Ang II caused EPC damage by testing
proliferation, migration, adhesion, angiogenic capacity and tube
formation in EPCs. Notably, the present study identified that
puerarin was able to alleviate Ang II-mediated EPs injury.
There has been increasing interest in bioactive
molecules that modulate cellular homeostasis and biological
functioning. Puerarin, the principal isoflavone glycoside obtained
from the root of Pueraria lobata (kudzu), has been observed
to possess antioxidant (24),
anti-hypercholesterolemic (25)
and anti-hyperglycemic properties (26). In the present study, it was
observed that puerarin had the ability to attenuate Ang II-induced
EPC damage by decreasing ROS production. Similar to the results of
the present study, Lu et al (27) demonstrated that puerarin exerted
its protective action via the reduction of NADPH oxidase-derived
ROS overproduction and activation of the phosphatidylinositol
3-kinase (PI3K)/RAC-α serine/threonine protein kinase
(Akt)/endothelial nitric oxide synthase (eNOS) pathways, in amyloid
β40 peptide-induced vessel impairment. In addition, emerging
evidence has indicated the anti-inflammatory effects of puerarin in
Ang II-induced endothelial dysfunction. Li et al (18) demonstrated that puerarin inhibited
the expression of NADPH subunits and VCAM1, and increased the
phosphorylation of eNOS at Ser 1177 in Ang II-infused rats. A study
by Ji et al (28)
demonstrated that puerarin inhibited the inflammatory response in
atherosclerosis by suppressing the nuclear factor (NF)-κB signaling
pathway. This previous study additionally demonstrated that
puerarin induced the inhibition of adhesion molecules, including
VCAM-1 and ICAM-1, which serve a critical role in AS (29,30).
The present study demonstrated that puerarin downregulated the Ang
II-induced expression of ICAM-1 and VCAM-1 in EPCs. The mechanism
of action of puerarin against Ang II-induced EPC injury was further
investigated. As previously discussed, puerarin exerted antioxidant
and cytoprotective effects via the activation of a number of
signaling pathways, including the PI3K/Akt/eNOS (31), NF-κB (28) and peroxisome proliferator-activated
receptor pathways (23,32). It was observed that, accompanied by
the inhibition of inflammatory cytokines, puerarin activated the
ERK1/2-Nrf2 signaling pathway in Ang II-induced EPCs. Subsequent
chemical stressor analysis validated the hypothesis that puerarin
exerted its cellular protective function by activating the
ERK1/2-Nrf2 pathway. However, it remains to be completely
understood whether puerarin may exert its function via other
pathways and whether it affects EPC functioning in vivo.
In conclusion, the results of the present study
demonstrated that puerarin activated the ERK1/2-Nrf2 signaling
pathway, leading to cellular protection in EPCs exposed to Ang
II.
Acknowledgements
The present study was supported by the Projects of
Special Construction of Scientific Research of National Chinese
Medicine Clinical Research Base (grant no. JDZX2015287) and the
Basic Research Business Project of Beijing University of Chinese
Medicine (grant no. 2016-JYB-JSMS-056).
Glossary
Abbreviations
Abbreviations:
|
EPCs
|
endothelial progenitor cells
|
|
Ang II
|
angiotensin II
|
|
ROS
|
reactive oxygen species
|
|
β-gal
|
β-galactosidase
|
|
ICAM-1
|
intracellular adhesion molecule-1
|
|
VCAM-1
|
vascular cell adhesion molecule-1
|
|
TNF-α
|
tumor necrosis factor-α
|
|
ERK1/2
|
extracellular signal-regulated kinase
1/2
|
|
Nrf2
|
nuclear factor erythroid 2 like 2
|
References
|
1
|
Yin Y, Liu H, Wang F, Li L, Deng M, Huang
L and Zhao X: Transplantation of cryopreserved human umbilical cord
blood-derived endothelial progenitor cells induces recovery of
carotid artery injury in nude rats. Stem Cell Res Ther. 6:372015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu S, Malhotra A, Zhang L, Deng S, Zhang
T, Freedman NJ, Storms R, Peppel K, Goldschmidt-Clermont PJ and
Dong C: Human umbilical cord blood endothelial progenitor cells
decrease vein graft neointimal hyperplasia in SCID mice.
Atherosclerosis. 212:63–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ke X, Shu XR, Wu F, Hu QS, Deng BQ, Wang
JF and Nie RQ: Overexpression of the β2AR gene improves function
and re-endothelialization capacity of EPCs after arterial injury in
nude mice. Stem Cell Res Ther. 7:732016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Briasoulis A, Tousoulis D, Antoniades C,
Papageorgiou N and Stefanadis C: The role of endothelial progenitor
cells in vascular repair after arterial injury and atherosclerotic
plaque development. Cardiovasc Ther. 29:125–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fadini GP, Coracina A, Baesso I, Agostini
C, Tiengo A, Avogaro A and de Kreutzenberg SV: Peripheral blood
CD34+KDR+ endothelial progenitor cells are determinants of
subclinical atherosclerosis in a middle-aged general population.
Stroke. 37:2277–2282. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lau KK, Chan YH, Yiu KH, Li SW, Tam S, Lau
CP, Kwong YL and Tse HF: Burden of carotid atherosclerosis in
patients with stroke: Relationships with circulating endothelial
progenitor cells and hypertension. J Human Hypertens. 21:445–451.
2007. View Article : Google Scholar
|
|
7
|
Lin H, Pan S, Meng L, Zhou C, Jiang C, Ji
Z, Chi J and Guo H: MicroRNA-384-mediated Herpud1 upregulation
promotes angiotensin II-induced endothelial cell apoptosis. Biochem
Biophys Res Commun. 488:453–460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calò LA, Facco M, Davis PA, Pagnin E, Maso
LD, Puato M, Caielli P, Agostini C and Pessina AC: Endothelial
progenitor cells relationships with clinical and biochemical
factors in a human model of blunted angiotensin II signaling.
Hypertens Res. 34:1017–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parzonko A, Czerwińska ME, Kiss AK and
Naruszewicz M: Oleuropein and oleacein may restore biological
functions of endothelial progenitor cells impaired by angiotensin
II via activation of Nrf2/heme oxygenase-1 pathway. Phytomedicine.
20:1088–1094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong KH, Li GQ, Li KM, Razmovski-Naumovski
V and Chan K: Kudzu root: Traditional uses and potential medicinal
benefits in diabetes and cardiovascular diseases. J Ethnopharmacol.
134:584–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prasain JK, Peng N, Rajbhandari R and Wyss
JM: The Chinese Pueraria root extract (Pueraria lobata) ameliorates
impaired glucose and lipid metabolism in obese mice. Phytomedicine.
20:17–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teng Y, Cui H, Yang M, Song H, Zhang Q, Su
Y and Zheng J: Protective effect of puerarin on diabetic
retinopathy in rats. Mol Biol Rep. 36:1129–1133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng XH, Ni C, Zhu L, Shen YL, Wang LL and
Chen YY: Puerarin protects against high glucose-induced acute
vascular dysfunction: Role of heme oxygenase-1 in rat thoracic
aorta. Vascul Pharmacol. 50:110–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu W, Zhang Q, Yang X, Wang Y and Sun L:
Puerarin inhibits adhesion molecule expression in
tnf-alpha-stimulated human endothelial cells via modulation of the
nuclear factor kappaB pathway. Pharmacology. 85:27–35. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen G, Pan SQ, Shen C, Pan SF, Zhang XM
and He QY: Puerarin inhibits angiotensin II-induced cardiac
hypertrophy via the redox-sensitive ERK1/2, p38 and NF-kappaB
pathways. Acta Pharmacol Sin. 35:463–475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parzonko A, Oswit A, Bazylko A and
Naruszewicz M: Anthocyans-rich Aronia melanocarpa extract possesses
ability to protect endothelial progenitor cells against angiotensin
II induced dysfunction. Phytomedicine. 22:1238–1246. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Lin Y, Zhou H, Li Y, Wang A, Wang H
and Zhou MS: Puerarin protects against endothelial dysfunction and
end-organ damage in Ang II-induced hypertension. Clin Exp
Hypertens. 39:58–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong X, Shao L, Fu YM and Zou Y: Effects
of olmesartan on endothelial progenitor cell mobilization and
function in carotid atherosclerosis. Med Sci Monit. 21:1189–1193.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoder MC: Endothelial progenitor cell: A
blood cell by many other names may serve similar functions. J Mol
Med (Berl). 91:285–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bian F, Cui J, Zheng T and Jin S: Reactive
oxygen species mediate angiotensin II-induced transcytosis of
low-density lipoprotein across endothelial cells. Int J Mol Med.
2017. View Article : Google Scholar
|
|
22
|
Li W, Li Z, Chen Y, Li S, Lv Y, Zhou W,
Liao M, Zhu F, Zhou Z, Cheng X, et al: Autoantibodies targeting AT1
receptor from patients with acute coronary syndrome upregulate
proinflammatory cytokines expression in endothelial cells involving
NF-κB pathway. J Immunol Res. 2014:3426932014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han T, Liu M and Yang S: DJ-1 alleviates
angiotensin II-induced endothelial progenitor cell damage by
activating the PPARγ/HO-1 pathway. J Cell Biochem. 119:392–400.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong FL, Sun XH, Gan L, Yang XL and Xu
HB: Puerarin protects rat pancreatic islets from damage by hydrogen
peroxide. Eur J Pharmacol. 529:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan LP, Zhuang YL, Chan SW, Chen SL and
Shi GG: Analysis of the mechanisms underlying the
endothelium-dependent antivasoconstriction of puerarin in rat
aorta. Naunyn Schmiedebergs Arch Pharmacol. 379:587–597. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC and
Cheng JT: Antihyperglycemic effect of puerarin in
streptozotocin-induced diabetic rats. J Nat Prod. 66:788–792. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu XL, Liu JX, Wu Q, Long SM, Zheng MY,
Yao XL, Ren H, Wang YG, Su WW and Fai Cheung RT: Protective effects
of puerarin against Aß40-induced vascular dysfunction in zebrafish
and human endothelial cells. Eur J Pharmacol. 732:76–85. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji L, Du Q, Li Y and Hu W: Puerarin
inhibits the inflammatory response in atherosclerosis via
modulation of the NF-κB pathway in a rabbit model. Pharmacol Rep.
68:1054–1059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang CC, Chu CF, Wang CN, Wu HT, Bi KW,
Pang JH and Huang ST: The anti-atherosclerotic effect of tanshinone
IIA is associated with the inhibition of TNF- α-induced VCAM-1,
ICAM-1 and CX3CL1 expression. Phytomedicine. 21:207–216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spigoni V, Picconi A, Cito M, Ridolfi V,
Bonomini S, Casali C, Zavaroni I, Gnudi L, Metra M and Dei Cas A:
Pioglitazone improves in vitro viability and function of
endothelial progenitor cells from individuals with impaired glucose
tolerance. Plos One. 7:e482832012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hwang YP, Kim HG, Hien TT, Jeong MH, Jeong
TC and Jeong HG: Puerarin activates endothelial nitric oxide
synthase through estrogen receptor-dependent PI3-kinase and
calcium-dependent AMP-activated protein kinase. Toxicol Appl
Pharmacol. 257:48–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang OH, Kim SB, Mun SH, Seo YS, Hwang HC,
Lee YM, Lee HS, Kang DG and Kwon DY: Puerarin ameliorates hepatic
steatosis by activating the PPARalpha and AMPK signaling pathways
in hepatocytes. Int J Mol Med. 35:803–809. 2015. View Article : Google Scholar : PubMed/NCBI
|