Introduction
Inflammation has been previously defined to have
important role in various pathologies, including gliomas (1–3).
Currently, there is no clear cause of glioma development. The
probable causative factors may involve infectious agents, dietary
factors or estrogens (3). Within
the various inflammatory cell populations of the nervous system, it
has been recognized that macrophages are one of the important
inflammatory components. However, there are conflicting results
regarding the contributory mechanisms of macrophages in the
inflammatory process.
Previous studies demonstrated that chronic
inflammation may be associated with high-grade gliomas and then
result in a high risk of glioma development (4,5).
Increased numbers of macrophages have been detected in chronic
inflammatory infiltrates of gliomas (1,6).
They have also been associated with tissue injuries. Compared with
normal tissues and cells, numerous inflammatory cytokines are
overexpressed in gliomas (1,7,8).
However, how immune infiltrates are involved in the progression and
prognosis of gliomas remains unclear. A high level of
tumor-associated macrophages (TAMs) is an independent predictor for
disease-free survival for ovarian cancer (9). Additionally, a polarized M2 phenotype
is produced by tumor-derived and T cell-derived cytokines in
macrophages which may be associated with cancer progression
(9). Decreased number of
macrophages reduced angiogenesis and tumor growth, and macrophages
regulate hormonal resistance in gliomas (10).
Bone morphogenic protein (BMP) and activin
membrane-bound inhibitor homolog (BAMBI) is a transmembrane
glycoprotein which shares certain homology with-type I receptors of
the transforming growth factor-β (TGF-β) family (11). The extracellular domain of BAMBI is
homologous to the protein sequence of T-box brain protein 1 (TBR1).
Thus, BAMBI is a pseudoreceptor of TBR1. BAMBI is widely-expressed,
including in nervous system and ovarian cancer (11,12).
BAMBI is also overexpressed in different types of cancer (11,13).
Upregulation of BAMBI is correlated with tumor growth and
metastasis via escape from growth arrest by TGFβ (14). Furthermore, BAMBI has been
demonstrated to be that co-expressed with BMP, TGF-β and activin,
which indicates a potential function of BAMBI in mediating
macrophage proliferation (15). In
addition, the expression of BAMBI is regulated by
lysosomal/autolysosomal degradation indicating that there is an
association between BAMBI and immune activities in renal
endothelial cells (16). In
addition, these effects of BAMBI may be crucial in cancer
progression via stimulating macrophage proliferation (16). However, the link between
immunomodulatory activity of BAMBI and inflammation-associated
glioma development requires further investigation.
It has been previously demonstrated that macrophages
have an important role in cancer (17). BAMBI has been demonstrated to be
highly expressed in macrophages (18). However, few studies have
investigated the direct effect of BAMBI on monocyte/macrophage
migration and differentiation, or the association between BAMBI
expression levels and macrophage density human glioma in specimens.
In the present study, the effect of BAMBI on monocytes and
macrophages was evaluated via cell migration assay, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting, and immunohistochemistry using gliomas tissues.
The results demonstrated that BAMBI increased monocyte migration
and activated macrophages in vitro, and that the BAMBI
expression levels were positively associated with macrophage
density in human glioma.
Materials and methods
Patients
Formalin-fixed, paraffin-embedded adjacent normal
tissue (n=27) and glioma (n=27; Gleason score, 6–7) specimens were
obtained from Hunan Provincial People's Hospital of Hunan Normal
University (Changsha, China). The characteristics of the patients
are presented in Table I. The
specimens were diagnosed and scored by specialized pathologists via
hematoxylin and eosin staining (Beyotime Institute of
Biotechnology, Haimen, China). Written informed consent was
obtained from each patient and the study was approved by the Ethics
Committees of Hunan Provincial People's Hospital of Hunan Normal
University.
 | Table I.Clinicopathological characteristics of
the patients within the study. |
Table I.
Clinicopathological characteristics of
the patients within the study.
| Characteristic | n |
|---|
| Sex |
|
| Male | 13 |
|
Female | 14 |
| Age (years) |
|
|
20–45 | 16 |
|
46–60 | 11 |
| Glioma histopathology
grade |
|
| I–II | 13 |
|
III–IV | 14 |
Cell lines
RAW 264.7 macrophages were purchased from American
Type Culture Collection (ATCC; Manassas, VA, USA) and the cells
were cultivated in Dulbecco's modified Eagle's medium (DMEM)
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo
Fisher Scientific, Inc.). THP-1 monocytes (ATCC) were cultured in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS.
Differentiation of monocytes into macrophages was induced using 50
nM phorbol myristate acetate (PMA; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) for 48 h (11). The U-87 glioma cell line (ATCC) was
cultivated in RPMI-1640 medium with 10% FBS. All the cells were
cultured at 37°C with 5% CO2.
Cell migration assay
The cell migration was assayed using 48-well Boyden
chambers (Neuro Probe, Inc., Gaithersburg, MD, USA). Monocytes
(2×106 cell/ml) were seeded on the top of the chamber
membrane. RPMI-1640 containing 10% FBS (Gibco-BRL, Grand Island,
NY, USA) and 1 or 10 nM BAMBI (Sino Biological, Inc., Beijing,
China) was added to the bottom of the well. RPMI-1640 without FBS
was added to the top chamber. Control wells received no treatment.
After incubation for 10 h, the migrated cells were stained using
Crystal Violet Staining Solution for 15 min at 37°C (Sangon Biotech
Co., Ltd., Shanghai, China). The migrated cells were imaged using a
Leica 400 light microscope (Leica Microsystems, Inc., Buffalo
Grove, IL, USA) and the number of cells in 10 fields was calculated
by Image-Pro Plus software, version 5.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
RT-qPCR
RNA was extracted from cells or tissues using RNeasy
Mini kit (Qiagen, Inc., Valencia, CA, USA). Total RNA (1 µg) was
reverse transcribed to cDNA using the Verso cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. The qPCR reaction was performed on an ABI 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using Premix Ex Taq™ PCR kit (Perfect Real-Time; Takara
Biotechnology Co., Ltd., Dalian, China) with the following
conditions: Initial denaturating for 30 sec at 95°C; then 40 cycles
of 30 sec at 95°C and 30 sec at 60°C. The primers used were as
follows: BAMBI forward, 5′-CTCAAATTCCCCACTCACCCA-3′ and reverse,
5′-GCTGATACCTGTTTCCTTGTCCTG−3′; CD68 forward,
5′-CACGCAGCACAGTGGACATTC-3′ and reverse, 5′-GCCTGGAGCCTCAGGGAGA-3′;
and GAPDH forward, 5′-AATCCCATCACCATCTTCCA-3′ and reverse,
5′-TGGACTCCACGACGTACTCA-3′. The relative expression levels were
calculated using the 2−ΔΔCq method (19). Each sample was assayed in
triplicate.
Western blotting
Protein expression was detected by western blot
analysis. Cells were lysed by radioimmunoprecipitation buffer
(Beyotime Institute of Biotechnology). The protein concentration in
each sample was determined using a Bradford kit (Beyotime Institute
of Biotechnology), 20 µg total protein was separated by 10%
SDS-PAGE (Beyotime Institute of Biotechnology). The proteins were
transferred onto a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA). The membrane was blocked with 4% skimmed milk
at room temperature for 1 h, and subsequently primary antibodies
were incubated with the membrane at room temperature for 2 h. The
primary antibodies used were as follows: Polyclonal primary rabbit
anti-iNOS antibody (cat. no. SAB4502012, 1:500 dilution;
Sigma-Aldrich; Merck Millipore); polyclonal primary rabbit
anti-interleukin (IL)-12 antibody (1:500 dilution, cat. no.
SAB1306460; Sigma-Aldrich; Merck Millipore), polyclonal primary
rabbit anti-IL-10 antibody (1:500 dilution, cat. no. SAB1410712;
Sigma-Aldrich; Merck Millipore), polyclonal primary rabbit
anti-arginase 1 antibody (1:500 dilution, cat. no. A6107;
Sigma-Aldrich; Merck Millipore); and polyclonal primary rabbit
anti-GAPDH antibody (1:2,000 dilution, cat. no. SAB2108266;
Sigma-Aldrich; Merck Millipore). Membranes were subsequently
incubated with secondary antibody (goat anti-rabbit IgG-peroxidase
antibody, cat. no. A0545, 1:2,000 dilution; Sigma-Aldrich; Merck
Millipore) at room temperature for 1 h. The blots were detected by
SignalBoost™ Immunoreaction Enhancer kit (EMD Millipore) and
visualized using a Kodak 440 digital imager (Kodak, Rochester, NY,
USA). The analysis was performed using Kodak Molecular Imaging
software, version 4.5 (Kodak). The relative protein levels were
calculated using Image-Pro Plus software, version 5.0 (Media
Cybernetics, Inc.) comparing the grayscale of the blots.
Immunohistochemistry
Tissue sections (6 µm) from representative paraffin
blocks were de-paraffinized and rehydrated using graded ethanol and
then blocked using 4% normal rabbit serum (Beyotime Institute of
Biotechnology) at room temperature for 15 min. Then, primary
monoclonal mouse anti-human CD68 antibody (1:200 dilution, cat. no.
ab201340; Abcam, Cambridge, MA, USA) and mouse monoclonal
anti-human BAMBI with 37°C for 1 h (1:200 dilution, cat. no.
ab57043; Abcam) were combined with the avidin-biotin blocking
solution and ABC staining kit (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), according to the manufacturer's instructions. The
sections were processed in a
3,3′-diaminobenzidine/H2O2 solution and
stained using hematoxylin (Beyotime Institute of Biotechnology).
The experiment with the reagents were used according to the
manufacturer's instructions. Staining was performed at room
temperature for 10 min. The stained sections were observed and
images captured using a Leica 400 light microscope (Leica
Microsystems, Inc.).
Green-fluorescent immunostaining was performed using
primary monoclonal mouse anti-human CD68 antibody (1:1,000
dilution, cat. no. ab201340; Abcam) and mouse monoclonal anti-human
BAMBI (1:1,000) at 37°C for 1 h. The secondary antibody was Goat
Anti-Mouse IgG H&L (FITC) (1:1,000, cat. no. ab6785; Abcam),
which was incubated at room temperature for 15 min. The stained
cells were observed and images captured using fluorescence
microscope (DMI 3000B; Leica Microsystems, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
from three independent experiments, each performed in triplicate or
quadruplicate. Statistical evaluation of data was performed using
Student's t-test, one-way analysis of variance (ANOVA) or repeated
measures ANOVA (SPSS 13.0 software; SPSS, Inc., Chicago, IL, USA).
The Pearson correlation coefficient (R) test was performed for
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
BAMBI induces the migration of
monocytes and macrophages in vitro
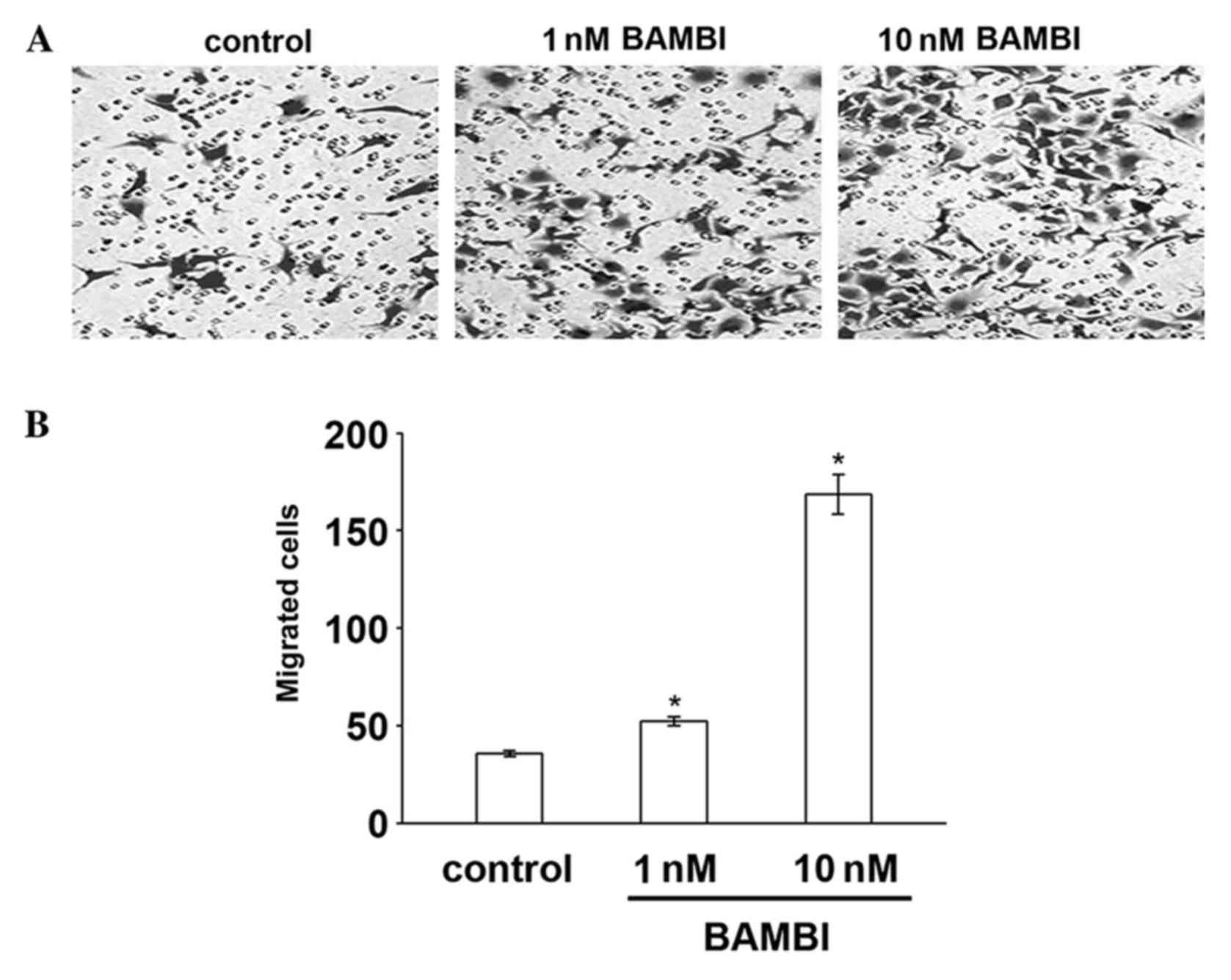
The present study assayed the effect of BAMBI on
monocyte cells migration using Boyden chambers. RAW 264.7 monocytes
were seeded in the top compartment of the chamber and enhancing
concentrations of recombinant human BAMBI was added to the bottom
compartment. The results demonstrated that BAMBI stimulated
monocyte migration in a dose-dependent manner compared with the
control cells (P=0.02; Fig. 1). To
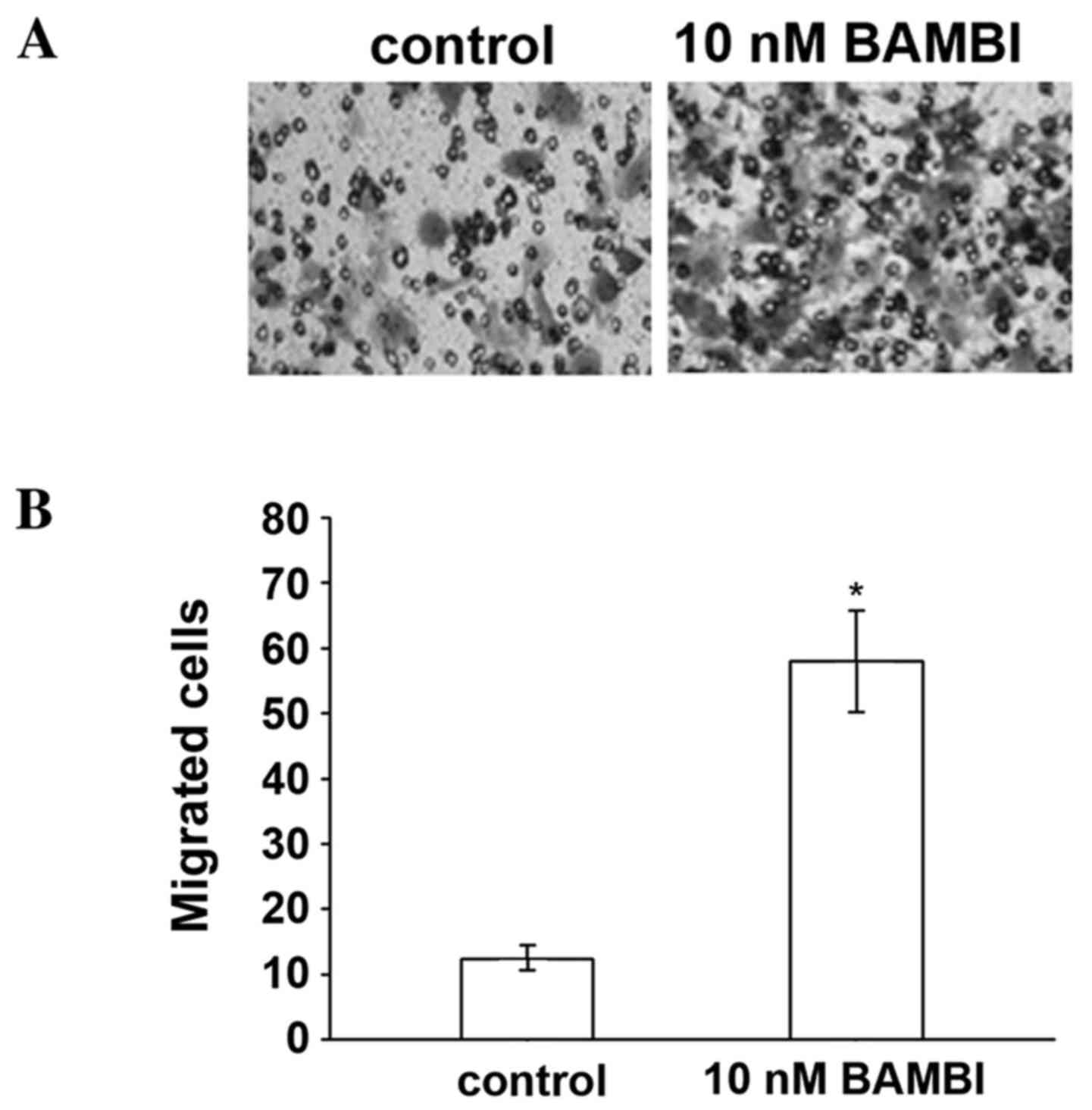
identify the on chemically-differentiated monocytes, PMA was used
to stimulate the monocytes to differentiate into macrophages. BAMBI
also significantly increased macrophage migration compared with the
control group (P=0.03; Fig. 2),
providing further evidence of the effects of BAMBI on inflammatory
cells.
Positive correlation between BAMBI
expression and macrophage levels
In order to validate the in vitro findings,
analysis was performed on a retrospective selection of gliomas
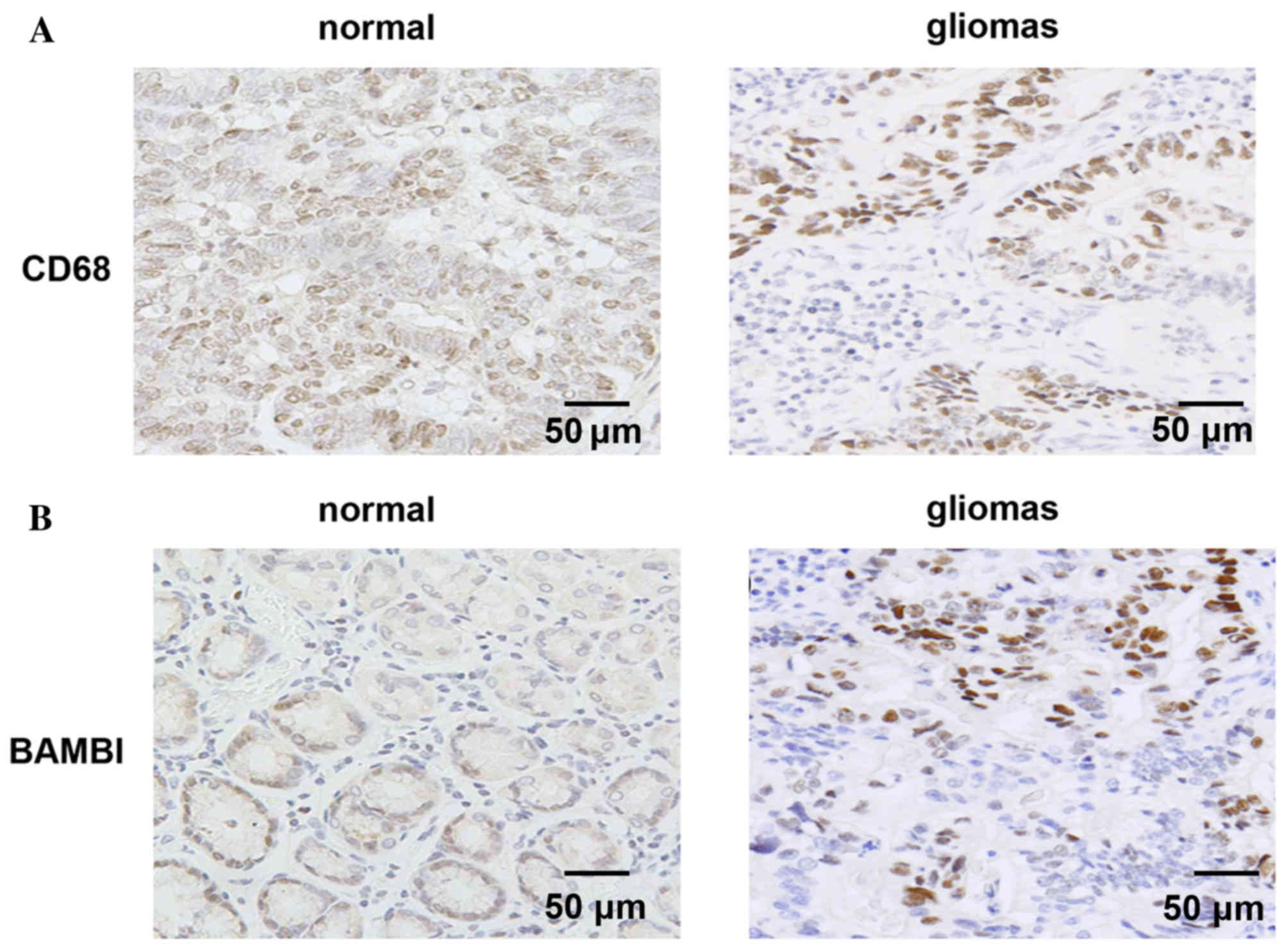
tissue specimens. CD68 is a macrophage-specific marker, and thus,
was used to detect macrophages. High levels of CD68 were observed
in glioma samples compared with the control specimens (Fig. 3A). Additionally, compared with
normal specimens, high levels of BAMBI were detected in the gliomas
tissues (Fig. 3B). The
localization of BAMBI and number of macrophages exhibited similar
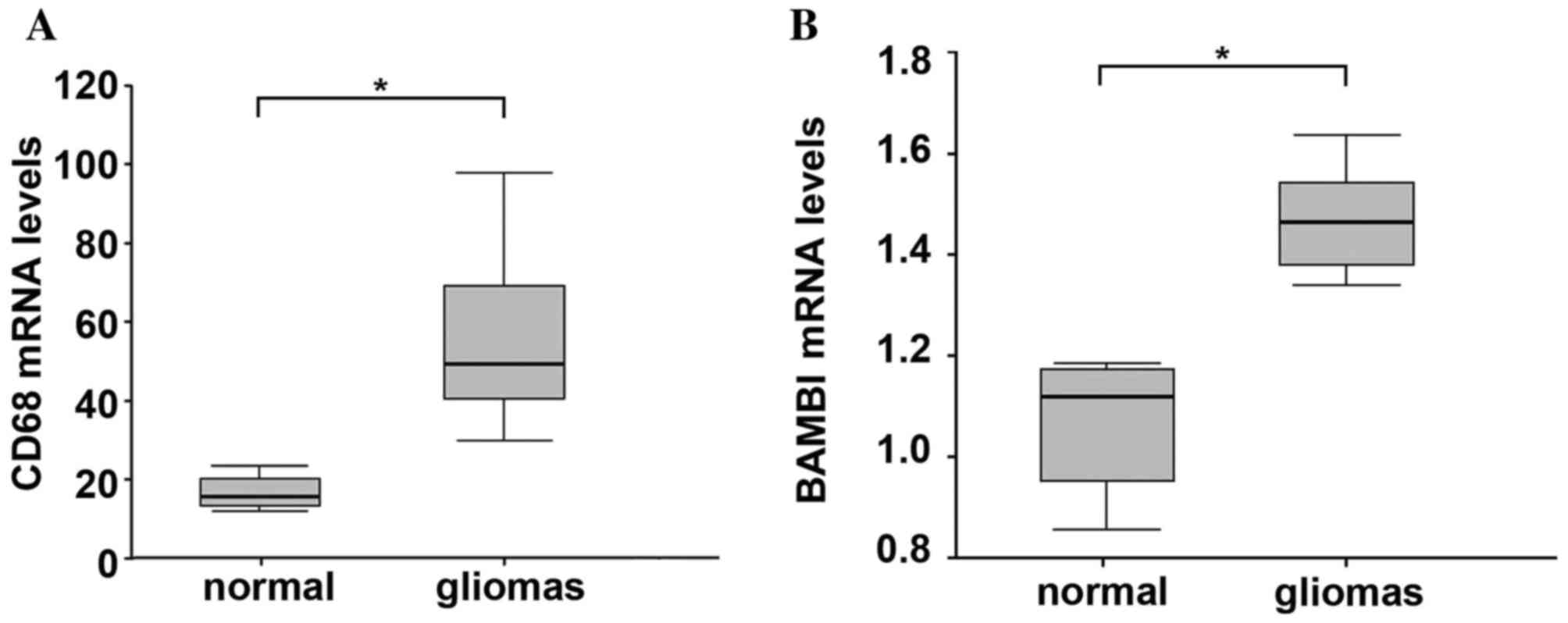
patterns in normal and glioma tissues. qPCR analysis demonstrated
that CD68 expression was significantly increased in glioma compared
with normal tissue, which is similar to the result of
immunohistochemistry analysis (P=0.01; Fig. 4A). Additionally, the BAMBI mRNA was
significantly increased in glioma samples compared with normal
tissues (P=0.02; Fig. 4B).
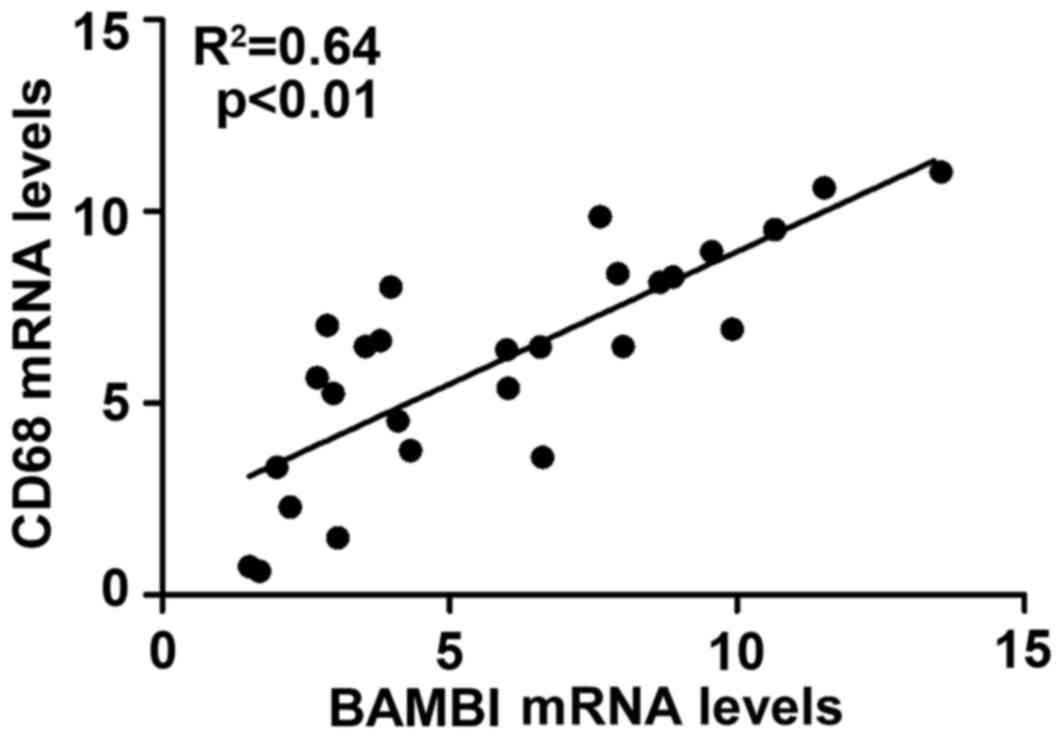
Furthermore, correlation analysis demonstrated that BAMBI is
positively correlated with CD68 in gliomas (P<0.01 and
R2=0.64; Fig. 5).
BAMBI promotes the differentiation of
macrophages
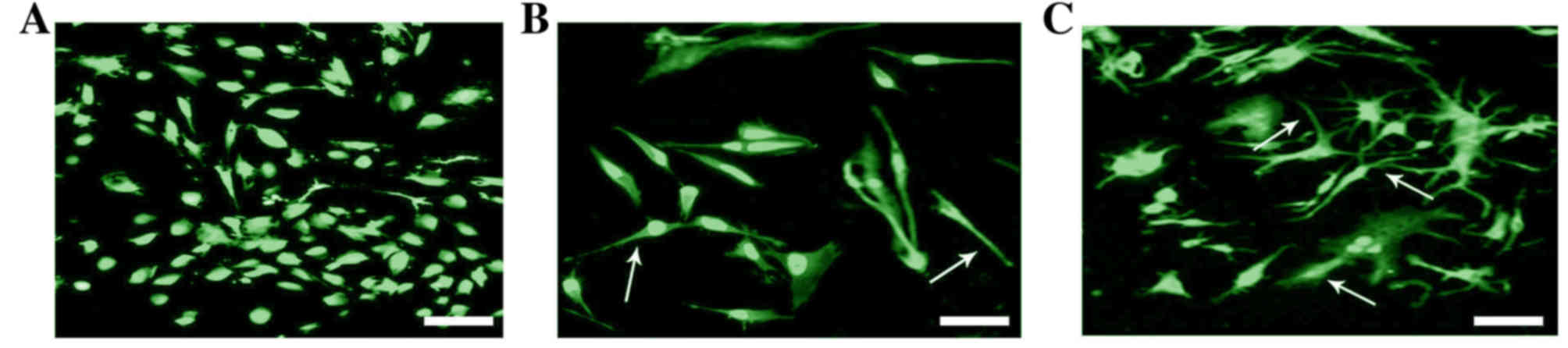
To determine whether BAMBI induces the proliferation
of macrophages during tumorigenesis, the co-treatment of BAMBI and
PMA was performed on RAW 264.7 macrophages (Fig. 6). The result demonstrated that
after 48 h treatment with BAMBI, certain cells were into
dendrite-like. With BAMBI and PMA treatment, the number of
dendrite-like cells was increased compared with BAMBI-only
treatment. This indicated that BAMBI and PMA promote the
differentiation of macrophages.
BAMBI induces differentiation of M1
macrophages
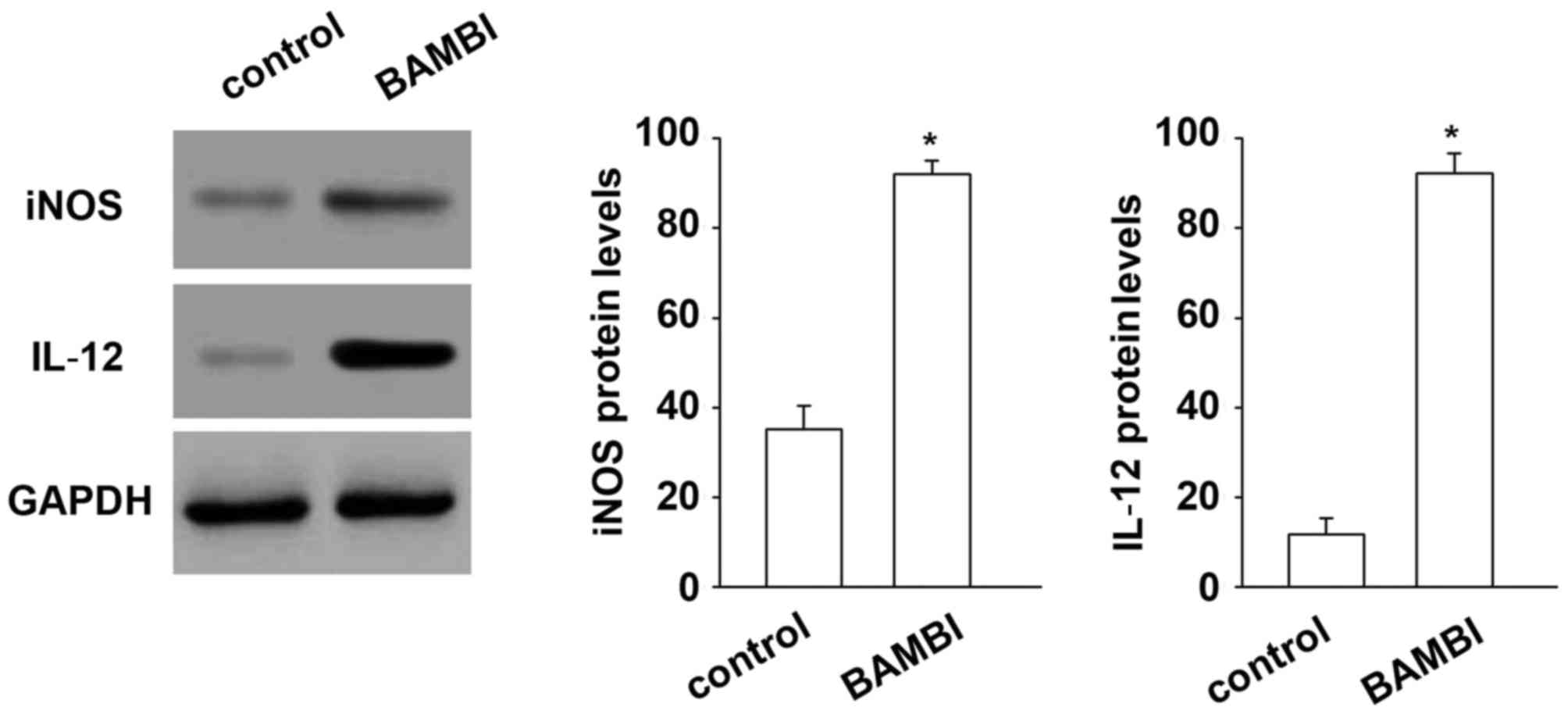
In order to improve the description of the
BAMBI-treated macrophage phenotype, the expression of specific M1
(iNOS and IL-12) and M2 (IL-10 and arginase 1) macrophages markers
was performed on BAMBI-treated RAW 264.7 cells. The results
demonstrated that the protein levels of specific markers of M1
macrophages (iNOS and IL-12) were increased significantly after by
with BAMBI compared with the control, indicating that BAMBI induces
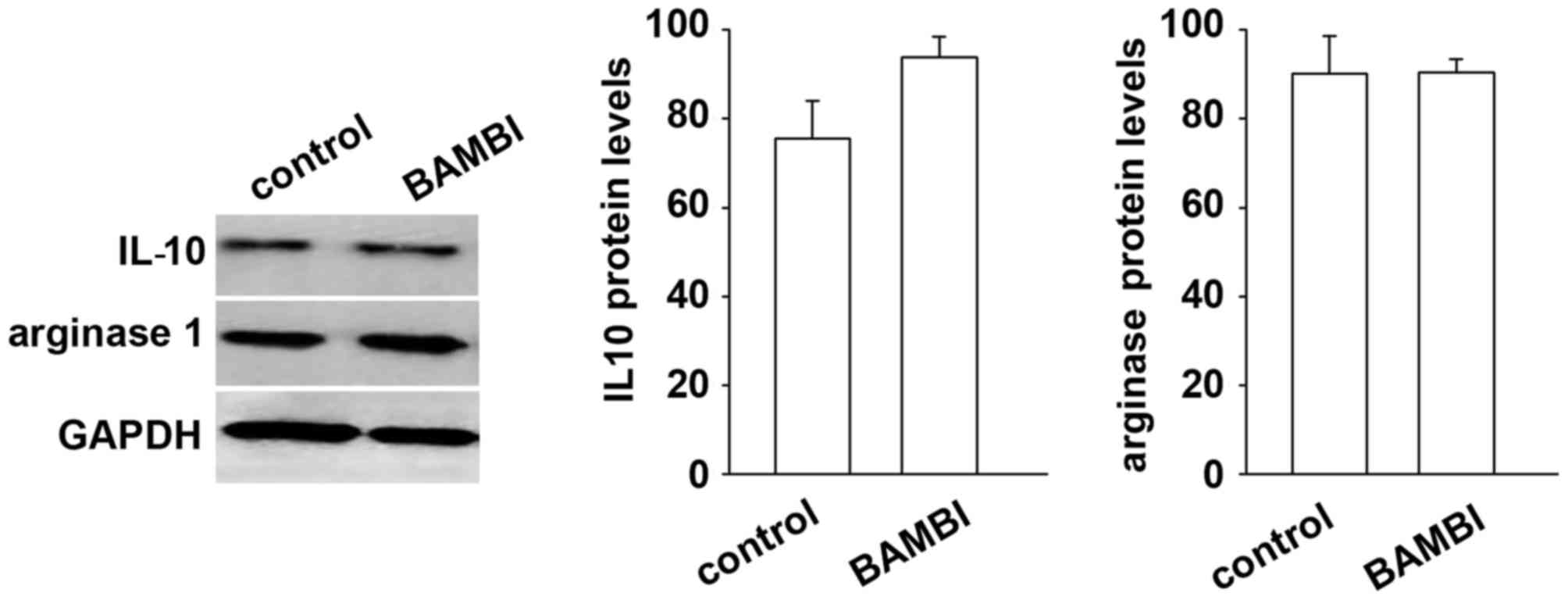
differentiation of monocytes to M1 type macrophages (Fig. 7). By contrast, the markers of M2
type macrophages, including IL-10 and arginase 1 were not affected
by BAMBI treatment (Fig. 8). These
results indicated that BAMBI induced differentiation of M1 type
macrophages.
Discussion
Gliomas consist of two key cellular compartments;
the epithelium and the surrounding stroma. Glioma cell invasion is
regulated by microregional extracellular matrix heterogeneity
(20,21). Among the inflammatory cells,
macrophages have been reported to be involved in various processes
associated with inflammation during tumorigenesis (22–24).
However, the specific mechanisms involved and the prognostic
importance of macrophages in tumorigenesis remains unclear;
investigating this is important to verify additional markers.
Higher expression of BAMBI in different types of cancer has been
reported previously (11,13) and has been suggested that BAMBI
modulates inflammation (16). To
the best of our knowledge, the present study is the first to
demonstrate that BAMBI stimulates the migration of monocytes and
macrophages in vitro. In addition, the expression levels of
BAMBI were correlated with macrophage density (indicated by CD68
expression) in human gliomas specimens. It was also illustrated
that BAMBI induced the expression of certain M1-specific markers,
which emphasized the inflammatory-modulating effect in glioma.
BAMBI expression has been previously reported to be
increased in colorectal (25) and
ovarian (26) cancer. In the
majority of these types of cancer, higher BAMBI expression
indicated poor prognosis. A previous study suggested that BAMBI
potentially regulated inflammation, particularly acting on
monocytes and macrophages. The role of macrophages in cancer
development is controversial. During cancer progression, polarized
M2 macrophages are induced by tumor-derived and T cell-derived
cytokines (9). The ratio of M2
macrophages is an indicator of poor prognosis (27). Additionally, tumor necrosis factor
was demonstrated to inhibit tumor growth in the brain by promoting
the recruitment of macrophages (28), which also indicated that
macrophages have an anticancer effect via gene regulation.
The present study demonstrated that compared with
normal tissues, macrophage density was increased in glioma samples
(17,23). A previous study reported that the
total number of macrophages was positively correlated with
recurrence-free survival following radical treatment (29). The contradiction between these
finding may be due to the role of the two different macrophages
phenotypes: M1-type (classically activated) and M2-type
(alternatively activated) (30).
M1-type macrophages generally express iNOS, which is
tumor-cytotoxic. Tumor progression stimulates a phenotypic switch
to M2 macrophages that express arginase 1, and accelerate tumor
growth, survival and metastasis (31). Similarly, it has been previously
demonstrated that the density of iNOS-positive macrophages, which
infiltrate the stroma, is decreased in highly aggressive gliomas
compared with less aggressive cancer, which indicates that as
malignant potential of the cancer increased, the cytotoxic activity
of macrophages is reduced (32).
The present study demonstrated that treatment with BAMBI increased
the expression of M1-specific markers and had no effect on the
expression of M2 markers, which suggests that BAMBI promoted
differentiation of M1 macrophages. Thus, we hypothesize that higher
expression of BAMBI may inhibit tumorigenesis.
The outcomes of the present study are valuable as
they demonstrate the role of BAMBI in gliomas. BAMBI stimulated the
migration of monocytes and promoted their differentiation toward
the M1 inflammatory pathway. The results also indicate that
macrophages and BAMBI may exert important function in glioma.
References
|
1
|
Komohara Y, Ohnishi K, Kuratsu J and
Takeya M: Possible involvement of the M2 anti-inflammatory
macrophage phenotype in growth of human gliomas. J Pathol.
216:15–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abou-Ghazal M, Yang DS, Qiao W,
Reina-Ortiz C, Wei J, Kong LY, Fuller GN, Hiraoka N, Priebe W,
Sawaya R and Heimberger AB: The incidence, correlation with
tumor-infiltrating inflammation and prognosis of phosphorylated
STAT3 expression in human gliomas. Clin Cancer Res. 14:8228–8235.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Black KL, Chen K, Becker DP and Merrill
JE: Inflammatory leukocytes associated with increased
immunosuppression by glioblastoma. J Neurosurg. 77:120–126. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ali S, King GD, Curtin JF, Candolfi M,
Xiong W, Liu C, Puntel M, Cheng Q, Prieto J, Ribas A, et al:
Combined immunostimulation and conditional cytotoxic gene therapy
provide long-term survival in a large glioma model. Cancer Res.
65:7194–7204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dewey RA, Morrissey G, Cowsill CM, Stone
D, Bolognani F, Dodd NJ, Southgate TD, Klatzmann D, Lassmann H,
Castro MG and Löwenstein PR: Chronic brain inflammation and
persistent herpes simplex virus 1 thymidine kinase expression in
survivors of syngeneic glioma treated by adenovirus-mediated gene
therapy: Implications for clinical trials. Nat Med. 5:1256–1263.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishie A, Ono M, Shono T, Fukushi J,
Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, et al:
Macrophage infiltration and heme oxygenase-1 expression correlate
with angiogenesis in human gliomas. Clin Cancer Res. 5:1107–1113.
1999.PubMed/NCBI
|
|
7
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenman SJ, Shrikant P, Dubb L,
Benveniste EN and Ransohoff RM: Cytokine-induced expression of
vascular cell adhesion molecule-1 (VCAM-1) by astrocytes and
astrocytoma cell lines. J Immunol. 154:1888–1899. 1995.PubMed/NCBI
|
|
9
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gabrusiewicz K, Ellert-Miklaszewska A,
Lipko M, Sielska M, Frankowska M and Kaminska B: Characteristics of
the alternative phenotype of microglia/macrophages and its
modulation in experimental gliomas. PLoS One. 6:e239022011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sekiya T, Adachi S, Kohu K, Yamada T,
Higuchi O, Furukawa Y, Nakamura Y, Nakamura T, Tashiro K, Kuhara S,
et al: Identification of BMP and activin membrane-bound inhibitor
(BAMBI), an inhibitor of transforming growth factor-beta signaling,
as a target of the beta-catenin pathway in colorectal tumor cells.
J Biol Chem. 279:6840–6846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grotewold L, Plum M, Dildrop R, Peters T
and Rüther U: Bambi is coexpressed with Bmp-4 during mouse
embryogenesis. Mech Dev. 100:327–330. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blanco Calvo M, Bolós Fernández V, Medina
Villaamil V, Aparicio Gallego G, Díaz Prado S and Grande Pulido E:
Biology of BMP signalling and cancer. Clin Transl Oncol.
11:126–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sekiya T, Oda T, Matsuura K and Akiyama T:
Transcriptional regulation of the TGF-beta pseudoreceptor BAMBI by
TGF-beta signaling. Biochem Biophys Res Commun. 320:680–684. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Onichtchouk D, Chen YG, Dosch R, Gawantka
V, Delius H, Massagué J and Niehrs C: Silencing of TGF-beta
signalling by the pseudoreceptor BAMBI. Nature. 401:480–485. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xavier S, Gilbert V, Rastaldi MP, Krick S,
Kollins D, Reddy A, Bottinger E, Cohen CD and Schlondorff D: BAMBI
is expressed in endothelial cells and is regulated by
lysosomal/autolysosomal degradation. PLoS One. 5:e129952010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eccles SA: Macrophages and
cancerImmunological Aspects of Cancer. Springer; pp. 123–154. 1978,
View Article : Google Scholar
|
|
18
|
Marwitz S, Droemann D, Rupp J, Rohmann K,
Osbahr S, Ulmer AJ, Röschmann K, Abdullah M, Schultz H, Vollmer E,
et al: BAMBI-A TGF-β pseudoreceptor with possible functional
involvement in COPD and NTHI infection. Eur Respir J.
38:47522011.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bellail AC, Hunter SB, Brat DJ, Tan C and
Van Meir EG: Microregional extracellular matrix heterogeneity in
brain modulates glioma cell invasion. Int J Biochem Cell Biol.
36:1046–1069. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rutka JT, Apodaca G, Stern R and Rosenblum
M: The extracellular matrix of the central and peripheral nervous
systems: Structure and function. J Neurosurg. 69:155–170. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bach JP, Deuster O, Balzer-Geldsetzer M,
Meyer B, Dodel R and Bacher M: The role of macrophage inhibitory
factor in tumorigenesis and central nervous system tumors. Cancer.
115:2031–2040. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fritzmann J, Morkel M, Besser D, Budczies
J, Kosel F, Brembeck FH, Stein U, Fichtner I, Schlag PM and
Birchmeier W: A colorectal cancer expression profile that includes
transforming growth factor beta inhibitor BAMBI predicts metastatic
potential. Gastroenterology. 137:165–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pils D, Wittinger M, Petz M, Gugerell A,
Gregor W, Alfanz A, Horvat R, Braicu EI, Sehouli J, Zeillinger R,
et al: BAMBI is overexpressed in ovarian cancer and co-translocates
with Smads into the nucleus upon TGF-beta treatment. Gynecol Oncol.
117:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niino D, Komohara Y, Murayama T, Aoki R,
Kimura Y, Hashikawa K, Kiyasu J, Takeuchi M, Suefuji N, Sugita Y,
et al: Ratio of M2 macrophage expression is closely associated with
poor prognosis for angioimmunoblastic T-cell lymphoma (AITL).
Pathol Int. 60:278–283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Villeneuve J, Tremblay P and Vallières L:
Tumor necrosis factor reduces brain tumor growth by enhancing
macrophage recruitment and microcyst formation. Cancer Res.
65:3928–3936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimura S, Yang G, Ebara S, Wheeler TM,
Frolov A and Thompson TC: Reduced infiltration of tumor-associated
macrophages in human prostate cancer: Association with cancer
progression. Cancer Res. 60:5857–5861. 2000.PubMed/NCBI
|
|
30
|
Umemura N, Saio M, Suwa T, Kitoh Y, Bai J,
Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, et al:
Tumor-infiltrating myeloid-derived suppressor cells are
pleiotropic-inflamed monocytes/macrophages that bear M1- and
M2-type characteristics. J Leukoc Biol. 83:1136–1144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nelius T, Samathanam C, Martinez-Marin D,
Gaines N, Stevens J, Hickson J, de Riese W and Filleur S: Positive
correlation between PEDF expression levels and macrophage density
in the human prostate. Prostate. 73:549–561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsai CS, Chen FH, Wang CC, Huang HL, Jung
SM, Wu CJ, Lee CC, McBride WH, Chiang CS and Hong JH: Macrophages
from irradiated tumors express higher levels of iNOS, arginase-I
and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys.
68:499–507. 2007. View Article : Google Scholar : PubMed/NCBI
|