Introduction
Aganglionosis or Hirschsprung's disease (HD) is a
congenial intestinal dynamic disorder characterized by intestinal
submucosal and myenteric plexus parasympathetic ganglion cell loss,
leading to persistent diseased colonic convulsions, and
contractions in addition to the obstruction of intestinal contents
(1–4). This disease is a colonic motor
disorder with an incidence of 1:5,000 live births worldwide
(5–7). In previous studies, the failure of
neural crest stem cells (NCSCs) todifferentiate and migrate was
demonstrated to be associated with the poor development of the
enteric nervous system, and identified as the main cause of
aganglionosis (8,9).
Bone morphogenetic proteins (BMPs) are among the
most important proteins involved in the development of the enteric
nervous system (8–12). BMP/Smad signalling serve a key role
in NCSC migration at an early embryonic stage, while NCSC
differentiation occurs during the middle embryonic stages (8,11).
Eventually, BMP/Smad signalling determines the function of the
intestinal nervous system (10–12).
The current studies were designed to investigate the effects of the
downregulation of BMP4 gene expression in NCSCs using a
transgenic mouse model in which low expression level of the
BMP4 gene was governed by RNAi-BMP genomic sequences
(13–16). A series of pregnant mice at
6.5–14.5 days post coitum (dpc) with post-implantation staged mouse
embryos were injected via the tail vein with the pSES-small
interfering RNA (si)BMP4 plasmid to silence the BMP4
gene, and these procedures were applied to establish an animal
model (14). One of the objectives
of the present study was to expand current knowledge on the effect
of BMP4 on NCSCs during the middle stage of embryo
development. The current study also aimed to assess the
neurodevelopmental abnormalities associated with the knockdown of
BMP4.
Materials and methods
Animals and experimental groups
Balb/c mice (male 12, female 36; 8–12 weeks old;
weight 14–18 g) were purchased from the Animal Center of Chongqing
Medical University (Chongqing, China). The mice were kept in a
specific pathogen-free facility room at the Chongqing Children's
Hospital Animal Center (Chongqing, China), with 50±5% humidity and
a temperature of 25±2°C. Food and water were provided ad
libitum. Male and female mice were kept in single cages at a
1:2-1:4 ratio. A female mouse in which a vaginal plug was
identified the next dawn was marked as 0.5 dpc and housed
separately. The present study was approved by The Ethics Committee
of Chongqing Medical University.
Tail vein injections
Pregnant mice were randomly divided into the
following groups: Ringer's group (n=12), pSES group (n=12) and
RNAi-BMP4 group (n=12); these were injected with 10 µl/g
Ringer's solution, 50 ng/µl pSES empty vector and 50 ng/µl
pSES-SiBMP4 vector, respectively. The pSES vectors bore a
copy of the entire DsRed coding region, allowing fluorescent
detection of the delivered plasmids. At 11.5 dpc, the solution (at
a volume of 10 µl/g) was injected into the tail vein. A 31G needle
was used and the injectionusually performed within 5±1 sec.
Plasmids for siRNA were purchased from the Oncogene Laboratory,
Biological Sciences Division, University of Chicago and contained
the following 4 sites of RNAi to silence the BMP4 gene,
lowercase is the gene match to the cutting site):
(5′aGGTCCAGGAAGAAGAATAAtttt3′, mouse BMP4
simBMP4-site 1, sense strand; 3′aTTATTCTTCTTCCTGGACCtttt5′,
mouse BMP4 simBMP4-site 1, antisense strand.
5′aGAGCCATGCTAGTTTGATAtttt3′, mouse BMP4 simBMP4-site
2, sense strand; 3′aTATCAAACTAGCATGGCTCtttt5′, mouse BMP4
simBMP4-site 2, antisense strand.
5′aGGGAAAAGCAACCCAATTAtttt3′, mouse BMP4 simBMP4-site
3, sense strand; 3′aTAATTGGGTTGCTTTTCCCtttt5′, mouse BMP4
simBMP4-site 3, antisense strand.
5′aGGGAAAAGCAACCCAATTAtttt3′, mouse BMP4 simBMP4-site
4, sense strand; 3′aTAATTGGGTTGCTTTTCCCtttt5′, mouse BMP4
simBMP4-site 4, antisense strand). At 1week [Ringer's group
F1 mice (n=16), pSES group (n=18) and RNAi-BMP4 group
(n=10)], 2 weeks [Ringer's group (n=15), pSES group (n=15) and
RNAi-BMP4 group (n=12)]and 4 weeks [Ringer's group (n=21),
pSES group (n=15) and RNAi-BMP4 group (n=7)] following
birth, the F1 mice were sacrificed by cervical dislocation. Then,
the target tissues were removed and rinsed in PBS at 4°C. Parts of
the tissues were stored at −80°C and used for western blotting. The
remaining samples were stored at 4–20°C in 4% paraformaldehyde.
Reverse
transcriptase-semi-quantitative polymerase chain reaction
(RT-sqPCR) detection of BMP4 and Smad4 genes
Total RNA was extracted from the colon using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. To generate cDNA, RT
was performed with Prime Script RT Enzyme mix reverse transcriptase
at 4°C (Invitrogen; Thermo Fisher Scientific, Inc.). Then, the cDNA
samples were amplified by PCR using the following cycling
conditions: 94°C for 5 min; followed by 39 cycles at 94°C for 30
sec; 58°C for 30 sec; 72°C for 30 sec; and a final step at 72°C for
5 min. Oligonucleotide primers were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.) as follows: BMP4 forward
(F), GACTTCGAGGCGACACTTCT and reverse (R), CCTGGGATGTTCTCCAGATG;
Smad4 F, CATTCCAGCCTCCCATTTCCAATC and R,
CACATAGCCATCCACAGTCACAAC; β-actin F, AAGATGACCCAGATCATGTTTGAGACC
and R, GCCAGGTCCAGACGCAGGAT. The amplified products were resolved
in ethidium bromide-stained 2% agarose gels. Densitometry was
performed using Quantity One software version 4.6.2. (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Western blotting analysis of BMP4
Protein extracts were prepared from the colon.
Tissue samples were homogenized in RIPA lysis buffer and
phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology, Shanghai, China), and proteins were directly
extracted according to the manufacturer's protocols. Protein
concentrations were determined using a Micro Bicinchoninic Acid
Protein Assay reagent (Beyotime Institute of Biotechnology).
Protein samples were then diluted to obtain equal (50 µg) protein
amounts and heated at 100°C in an equal volume of SDS loading
buffer (Beyotime Institute of Biotechnology) for 10 min. Proteins
were then separated by SDS-PAGE (5% spacer gel, 40 V, 50 min; 8%
separating gel, 80 V, 70 min). Proteins were then
electrophoretically transferred (Bio-Rad Laboratories, Inc.) onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA) for 1.5 h at 250 mA. To block non-specific binding, the
membranes were incubated with 5% bovine serum albumin (Kang Yuan
Biology, Tianjing, China) in Tris-buffered saline with Tween 20
(250 µl Tween20 in 500 ml PBS) at 37°C for 1.5 h. The membranes
were then incubated overnight at 4°C with rabbit anti-BMP4 primary
polyclonal antibody (GR49989-1; 1:400; Abcam, Cambridge, MA, USA)
and β-actin (4AH240911; 1:150; 4A Biotech Co., Ltd., Beijing,
China.). Next, the membranes were incubated at 4°C for 1 h with
peroxidase-conjugated secondary anti-rabbit IgG (TA130015; 1:4,000;
OriGene Technologies, Inc., Beijing, China), according to the
manufacturer's protocol. The protein of interest was visualized
using an enhanced chemiluminescence western blotting substrate
(Boster Biological Technology, Pleasanton, CA, USA) and its
relative expression was quantified using a Chemidoc XRS gel imaging
system Quantity One software version 4.6.2. (Bio-Rad Laboratories,
Inc.).
Immunohistochemistry
Colontissue was immediately fixed in 4% buffered
formalin for 48 h, embedded in paraffin, and sectioned at 5 µm.
Antigen retrieval was performed by boiling the sections in 0.01 M
sodium citrate in 1L PBS (pH 7.4) followed by a 20 min incubation
at room temperature, in 3% H2O2 for 20 min
and blocked in 5% BSA (Kangyuan Biology, Tianjing, China) for 20
min at room temperature. Following incubation in 5% normal serum
for 20 min at room temperature, sections were incubated with rabbit
anti-BMP4 primary polyclonal antibody overnight at 4°C (GR49989-1,
ab39973; 1:500; Abcam). Slides were then stained with goat
anti-rabbit secondary antibody (PV-6001, 1:1,500 dilution) from
OriGene Technologies, Inc. (Beijing, China) for 1 h at room
temperature. Detection was accomplished using a DAB kit (Beyotime
Institute of Biotechnology, Shanghai, China). Positive staining was
assessed by the degree of brown colour development. The integrated
optical density of positive staining was measured by NIS-Elements
Viewer version 4.0 (Nikon Corporation, Tokyo, Japan) using an
Eclipse 55i microscope (×40; Nikon Corporation).
Statistical analysis
The RT-PCR and western blotting greyscale values
were expressed as the mean ± standard deviation. The differences
among groups were analysed by a one-way analysis of variance and
t-tests implemented in SPSS software version 17.0 (SPSS, Inc.,
Chicago, IL, USA), followed by Student-Newman-Keuls-q test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
BMP4 and downstream gene
downregulation in F1 mouse colon is induced by administration of
the siBMP4 plasmid to pregnant mice
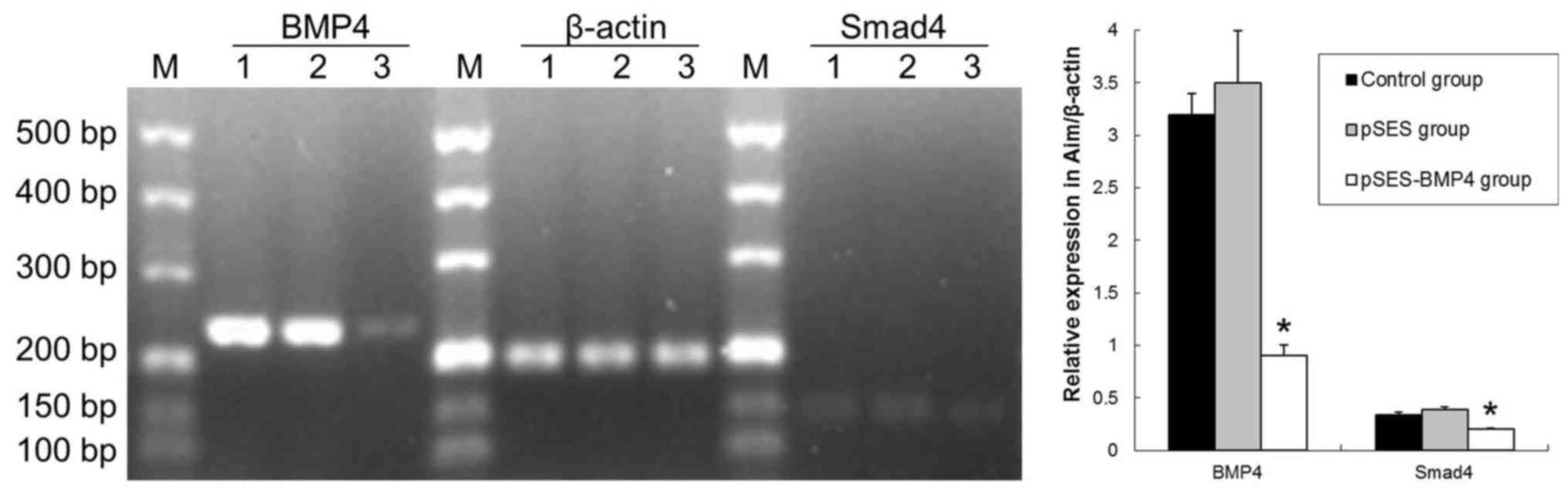
PCR revealed that BMP4 and Smad4 miRNA
in the colon was significantly lower in the RNAi-BMP4 group
compared with the control and pSES groups at 7 days after birth
(P<0.05; Fig. 1). A decrease in
BMP4-Smad4 in mice may lead to aganglionosis (9–11).
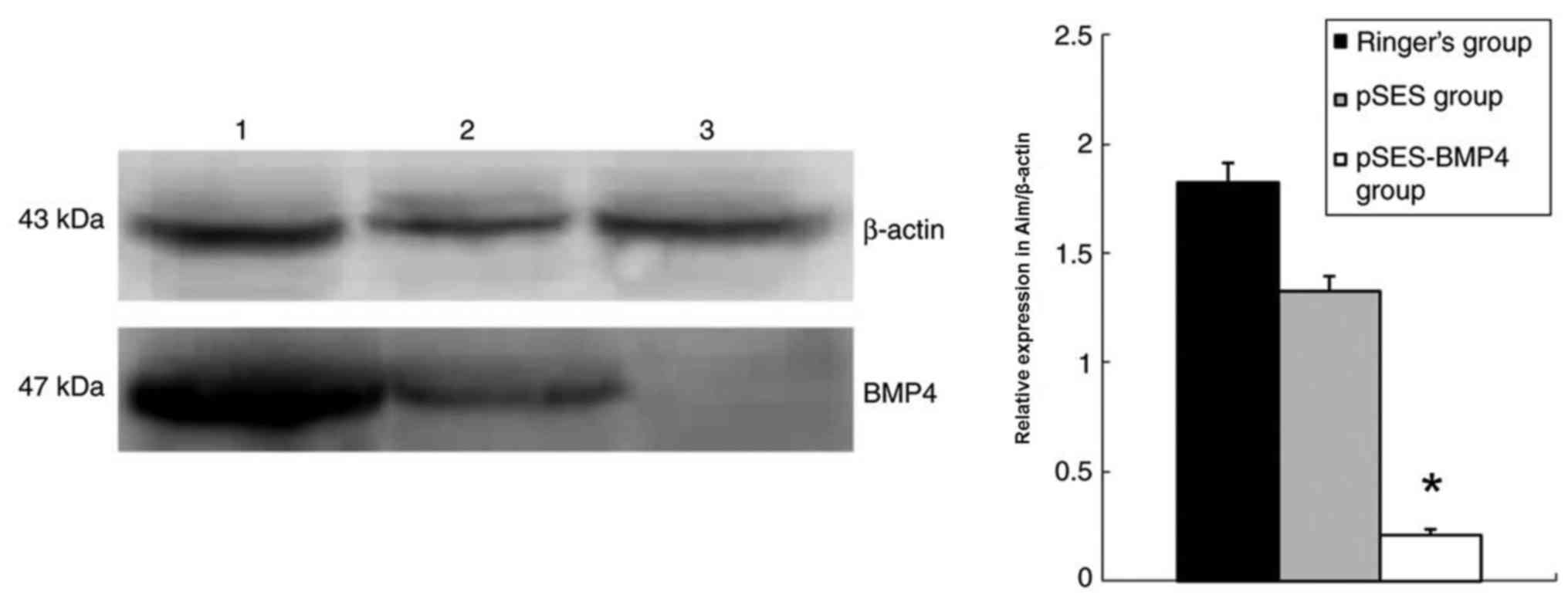
Western blotting results revealed that colon BMP4 in the
RNAi-BMP4 group was significantly lower compared with the
control and pSES groups (Fig.
2).
Formation of aganglionosis in F1 mice
is induced by transplacental administration of RNAi-BMP4 to
pregnant mice at 11.5 days
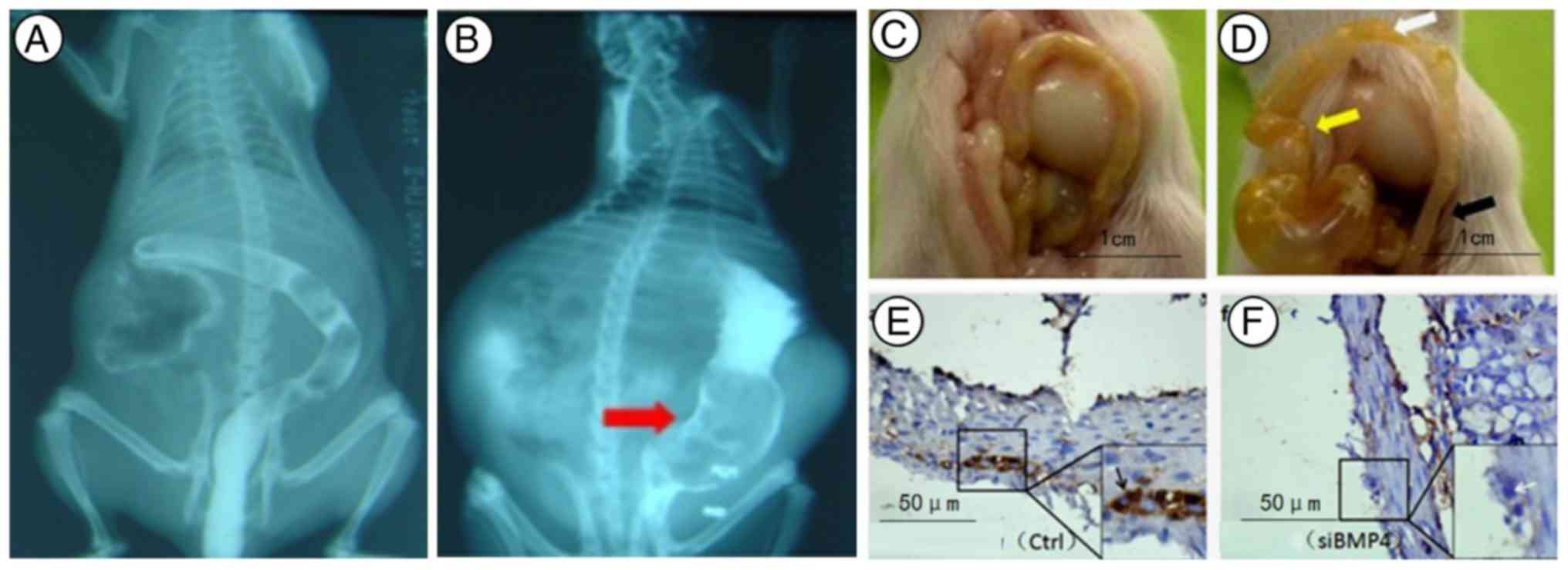
Barriers to stool discharge and abdominal distention
were observed in BMP4 knockdown old mice (n=35; 10 mice were
sacrificed at 1 week). With a barium enema, giant colons were
identified in 2-week-old mice from the RNAi-BMP4 group
(n=23; 12 mice were sacrificed at 1 week). Then, 4-week-old mice
died from cerebral ischaemia and were autopsied (n=7, some mice
succumbed naturally). Unfortunately, the mouse rectum was so small
that there was no suitable pressure probe. The spastic colon,
transitional colonand giant colon were exposed (Fig. 3). With immunocytochemistry, a small
number of glial-like cells were positive for BMP4 in the
colon of BMP4 knockdown mice. Additionally, absence or
dysplasia of neurons was observed in RNAi-BMP4 colons.
Discussion
There area number of ways to establish aganglionosis
in animal models (17–19). The principle methodis to affect the
migration, differentiation and proliferation of NCSCs or
artificially destroy the intestinal nervous system with drugs
(20). A high folic acid (FA) diet
during pregnancy leads to a gradual increase in serum FA in
pregnant mice and their offspring, causing aganglionosis in the
offspring (21). The level of FA
in the offspring reached the highest value with 160 mg/kg FA
feeding. It has also been demonstrated that a 0.1% benzalkonium
chloride enema may also be used to establish aganglionos is in
animal models. Ethylnitrosourea (ENU) is a type of artificial
synthesised compound that leads to random and single-base mutations
in a variety of organisms (22,23).
The offspring may end up with a severe mega colon phenotype due to
ENU-induced mutations in C57BL/6 male mice. Trisomy 16 mice, which
are likely to exhibit aganglionosis, are a type of genetic mouse
model with clinical manifestations similar to those identified in
the human trisomy 21 syndrome (24). This genetic mouse model, which does
not express the endothelin 3 gene, is also known as piebald and
spotted death mice and may develop defective intestinal aganglionic
syndrome or congenital megacolon (25,26).
However, at present, there is a lack of a genetic mouse models that
exhibits HD gene knockdown with unmistakeable implementation.
NCSCs originate from cells of the neural crest that
migrate in chains as they colonize the embryonic gut, eventually
forming the myenteric and submucosal plexus (27–29).
Failure of the neural crest cells to colonize the gut leads to
aganglionosis in the sigmoid colon, a pathological condition called
Hirschsprung's disease, also known as congenital megacolon, in
humans (28,29). At present, the mechanism associated
withthe signalling pathways that adjust NCSCs for migration to the
intestinal tract and differentiation in the enteric nervous system
remains unclear. The BMP signalling pathway mayinvolve the
migration process of NCSCs to the intestinal tract, in addition to
the proliferation and differentiation of intestinal ganglion cells
(30–32). Studies have suggested that
BMP signalling serves an important role in differentiating
NCSCs into enteric ganglia (8–12).
Therefore, an improved understanding of the signalling pathways
regulated by NCSCs and associated with the mechanisms of action is
important to investigate the pathogenesis of aganglionosis. BMPs
contribute to the largest subgroup of the TGF-β super family and
were originally identified by their ability to induce bone
development (27,28,31).
Additionally, BMPs are expressed in the nervous system through out
its differentiation. The mechanisms by which these BMPs regulate
the induction of the neuroectoderm, the CNS primordium, and finally
the neural crest, which gives rise to the NCSCs, have been reviewed
(11,33). Following neural tube closure, the
most dorsal aspect of the tube becomes a signalling centre for
BMPs, which directs the pattern of development of the dorsal spinal
cord. Additionally, certain data suggested that BMP4 was a
peripherally derived factor that may regulate the survival of motor
neurons (34).
The RNAi phenomenon was identified in fungi and
plants (15,35). The placenta is responsible for
transport between the mother and foetus and is a tissue barrier of
high permeability (14,16). The present study confirmed that
plasmid vector injected into the tail vein of pregnant mice was
able to be transferred to foetal mice through the blood-embryo
barrier. The plasmid vector transfection of tissues and organs
depends on the plasmid concentration, solvent volume, injection
velocity, and weight of pregnant mice. The plasmid vector achieved
good results when the injection concentrationswere 50 ng/µl and 10
µl/g, and when the injection time was 5 sec. The majority of human
aganglionosis cases have revealed an association with decreased
BMP-Smad4 (8,30,36).
F1 mice that received transplacental RNAi-BMP4 at 11.5 days
revealed disordered NCSCs. That is, the downregulation of
BMP4 in the middle embryo stage possibly resulted in
developmental problems in the peripheral nervous system. BMP4 was
also involved in the TGF-β/BMP/Smad-mediated signalling
cascade as a transcriptional repressor of Smad proteins.
In summary, knockdown by transplacental RNAi is a
powerful technique to study the effect of signalling pathways on
responding tissues at the middle embryonic stage (14,37).
However, different genes regulate embryonic development through
specific mechanisms, and different gene plasmids possess different
transfection efficiencies. Thus, deciding the dose and when to
intervene should be considered when exploring the function of a
novel gene (38). The results
presented here suggested that downregulation of the BMP4
transgene was an excellent prognostic factor of neurodevelopmental
inactivity in mice. This approach may be used to make an
aganglionosis mouse model. As the mouse colon is relatively short,
it is planned to use rabbits to research colon gene expression in
different regions in future studies.
Acknowledgements
This research was supported by National Natural
Science Foundation of China (grant nos. 81370474 and 81600398).
References
|
1
|
Nakamura H, Henderson D and Puri P: A
meta-analysis of clinical outcome of intestinal transplantation in
patients with total intestinal aganglionosis. Pediatric Surg Int.
33:837–841. 2017. View Article : Google Scholar
|
|
2
|
Li MH, Eberhard M, Mudd P, Javia L,
Zimmerman R, Khalek N and Zackai EH: Total colonic aganglionosis
and imperforate anus in a severely affected infant with
Pallister-Hall syndrome. Am J Med Genet A. 167:617–620. 2015.
View Article : Google Scholar
|
|
3
|
Saida H, Hayet Z, Jamila C, Sana M, Samia
B, Amine K, Badii H, Imed K, Lassad S, Mongi M, et al: Familial
near-total intestinal aganglionosis. J Neonatal Surg. 6:62–63.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stenström P, Brautigam M, Borg H, Graneli
C, Lilja HE and Wester T: Patient-reported Swedish nationwide
outcomes of children and adolescents with total colonic
aganglionosis. J Pediatr Surg. 52:1302–1307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Narayanan SK, Soundappan SS, Kwan E, Cohen
RC, Charlton A and Cass DT: Aganglionosis with the absence of
hypertrophied nerve fibres predicts disease proximal to
rectosigmoid colon. Pediatr Surg Int. 32:221–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teerlink CC, Bernhisel R, Cannon-Albright
LA and Rollins MD: A population-based description of familial
clustering of Hirschsprung's disease. J Pediatr Surg S0022-3468.
1–30521. 2017.
|
|
7
|
Cheng S, Wang J, Pan W, Yan W, Shi J, Guan
W, Wang Y and Cai W: Pathologically assessed grade of
Hirschsprung-associated enterocolitis in resected colon in children
with Hirschsprung's disease predicts postoperative bowel function.
J Pediatr Surg. 52:1776–1781. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiou J, Su CY, Jan YH, Yang CJ, Huang MS,
Yu YL and Hsiao M: Decrease of FSTL1-BMP4-Smad signaling predicts
poor prognosis in lung adenocarcinoma but not in squamous cell
carcinoma. Sci Rep. 7:98302017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Modica S and Wolfrum C: The dual role of
BMP4 in adipogenesis and metabolism. Adipocyte. 6:141–146. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nemashkalo A, Ruzo A, Heemskerk I and
Warmflash A: Morphogen and community effects determine cell fates
in response to BMP4 signaling in human embryonic stem cells.
Development. 144:3042–3053. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sprott D and Chavakis T: A BMP4-angiomiR
connection in angiogenesis. Thromb Haemost. 117:650. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salie R, Niederkofler V and Arber S:
Patterning molecules; multitasking in the nervous system. Neuron.
45:189–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soares ML, Haraguchi S, Torres-Padilla ME,
Kalmar T, Carpenter L, Bell G, Morrison A, Ring CJ, Clarke NJ,
Glover DM and Zernicka-Goetz M: Functional studies of signaling
pathways in peri-implantation development of the mouse embryo by
RNAi. BMC Dev Biol. 5:282005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin X, Chen Z, Xiang L, Luo Q, Guo Z, Ding
X and Jin X: Colorectal polyp model established by transplacental
BMP4 RNAi. Mol Med Rep. 10:33–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'shea KS, De Boer LS, Slawny NA and
Gratsch TE: Transplacental RNAi: Deciphering gene function in the
postimplantation-staged embryo. J Biomed Biotechnol.
2006:186572006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gratsch TE, De Boer LS and O'Shea KS: RNA
inhibition of BMP-4 gene expression in postimplantation mouse
embryos. Genesis. 37:12–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heanue TA, Boesmans W, Bell DM, Kawakami
K, Vanden Berghe P and Pachnis V: A novel Zebrafish ret
heterozygous model of Hirschsprung disease identifies a functional
role for mapk10 as a modifier of enteric nervous system phenotype
severity. PLoS Genet. 12:e10064392016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gariepy CE, Williams SC, Richardson JA,
Hammer RE and Yanagisawa M: Transgenic expression of the
endothelin-B receptor prevents congenital intestinal aganglionosis
in a rat model of Hirschsprung disease. J Clin Invest.
102:1092–11101. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gasc JM, Clemessy M, Corvol P and Kempf H:
A chicken model of pharmacologically-induced Hirschsprung disease
reveals an unexpected role of glucocorticoids in enteric
aganglionosis. Biol Open. 4:666–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Villalba-Benito L, Torroglosa A, Fernández
RM, Ruíz-Ferrer M, Moya-Jiménez MJ, Antiñolo G and Borrego S:
Overexpression of DNMT3b target genes during enteric nervous system
development contribute to the onset of Hirschsprung disease. Sci
Rep. 7:62212017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu CQ, et al: Mice models of congenital
magacolon induced by high-dose folic acid diet at the duration of
pregnancy. Chinese Journal of Experimental Surgery. 28:1925–1926.
2011.
|
|
22
|
Wagner JP, Sullins VF and Dunn JC: A novel
in vivo model of permanent intestinal aganglionosis. J Surg Res.
192:27–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujimura T, Shibata S, Shimojima N,
Morikawa Y, Okano H and Kuroda T: Fluorescence visualization of the
enteric nervous network in a chemically induced aganglionosis
model. PLoS One. 11:e01505792016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Busch LC and Kunel W: The
development of enteric nervous system in terisomy 16 mice with the
occurrence of congenital megacolon. National Med J China.
79:466–469. 1999.
|
|
25
|
Chen B, Ouyang HL, Wang WH, Yin YH, Yan
LN, Yang B and Xue ZF: Hirschsprung disease is associated with an
L286P mutation in the fifth transmembrane domain of the
endothelin-B receptor in the N-ethyl-N-nitrosourea-induced mutant
line. Exp Anim. 65:245–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frykman PK, Cheng Z, Wang X and Dhall D:
Enterocolitis causes profound lymphoid depletion in endothelin
receptor B- and endothelin 3-null mouse models of
Hirschsprung-associated enterocolitis. Eur J Immunol. 45:807–817.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rollo BN, Zhang D, Stamp LA, Menheniott
TR, Stathopoulos L, Denham M, Dottori M, King SK, Hutson JM and
Newgreen DF: Enteric neural cells from Hirschsprung disease
patients form Ganglia in autologous aneuronal colon. Cell Mol
Gastroenterol Hepatol. 2:92–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, He T, Liu J, Liu H, Zhou L, Hao W,
Sun Y and Wang X: Synergistic effects of overexpression of BMP2 and
TGFβ3 on osteogenic differentiation of bone marrow mesenchymal stem
cells. Mol Med Rep. 14:5514–5520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Yao D, Zhang J, Liu B, Zhang L, Feng
H and Li B: The effects of epidermal neural crest stem cells on
local inflammation microenvironment in the defected sciatic nerve
of rats. Front Mol Neurosci. 10:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldstein AM, Brewer KC, Doyle AM, Nagy N
and Roberts DJ: BMP signaling is necessary for neural crest cell
migration and ganglion formation in the enteric nervous system.
Mech Dev. 122:821–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuroyanagi G, Tokuda H, Yamamoto N,
Matsushima-Nishiwaki R, Mizutani J, Kozawa O and Otsuka T:
Resveratrol amplifies BMP-4-stimulated osteoprotegerin synthesis
via p38 MAP kinase in osteoblasts. Mol Med Rep. 12:3849–3854. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pavelock KA, Girard BM, Schutz KC, Braas
KM and May V: Bone morphogenetic protein down-regulation of
neuronal pituitary adenylate cyclase-activating polypeptide and
reciprocal effects on vasoactive intestinal peptide expression. J
Neurochem. 100:603–616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weber M, Apostolova G, Widera D,
Mittelbronn M, Dechant G, Kaltschmidt B and Rohrer H: Alternative
generation of CNS neural stem cells and PNS derivatives from neural
crest-derived peripheral stem cells. Stem Cells. 33:574–588. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chou HJ, Lai DM, Huang CW, McLennan IS,
Wang HD and Wang PY: BMP4 is a peripherally-derived factor for
motor neurons and attenuates glutamate-induced excitotoxicity in
vitro. PLoS One. 8:e584412013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Torres-Martinez S and Ruiz-Vazquez RM: The
RNAi Universe in Fungi: A varied landscape of small RNAs and
biological functions. Annu Rev Microbial. 71:371–391. 2017.
View Article : Google Scholar
|
|
36
|
Hegarty SV, O'Keeffe GW and Sullivan AM:
BMP-Smad 1/5/8 signalling in the development of the nervous system.
Prog Neurobiol. 109:28–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zou GM, Yu J, LeBron C and Fu Y: RNAi
knockdown of Ape1 gene in the differentiation of mouse embryonic
stem cells. Methods Mol Biol. 1622:131–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin X, et al: Down-regulation of the
target genes using trans-placental RNAi. J Med Mol Bio. 14:6–9.
2017.
|