Introduction
Tuberculosis (TB) is a worldwide public health
concern caused by Mycobacterium tuberculosis (Mtb), and ~ a
third of the world's population is latently infected with Mtb
(1). The majority of infected
people do not present symptoms immediately, but ~10% of those
people develop an overt disease later in their lives. Latently
infected individuals represent a reservoir of infection and
potential reactivation of TB can be a source of transmission
(2). Macrophages represent a
primary target of infection and the most frequently infected cell
type by Mtb in host individuals. The initial interaction between
macrophages and Mtb determines the outcome of infection, but the
mechanism underlying the interaction between macrophages and Mtb
remains to be elucidated (3).
It has been demonstrated that during the latency
period, Mtb remains in a dormant or non-replicating state, and the
dormancy survival regulon (DosR), composed of 48 co-regulated
genes, is necessary for survival of dormant Mtb (4). However, the role of
dormancy-associated antigens in mediating interactions between Mtb
and macrophages remains to be elucidated. The 16-kDa α-crystallin
protein, (Rv2031c), also known as hspX, acr and Hsp16.3, is a
predominant protein produced by Mtb, accounting for up to 25% of
all proteins expressed during dormancy of Mtb (5). Rv2031c can be identified by mass
spectrometry in culture filtrates, membrane protein fractions and
whole cell lysates of Mtb (6).
Rv2031c has been hypothesized to enhance long-term stability of
proteins and cell structures, which in turn aids in maintaining
long-term survival of Mtb (7).
Rv2626c is a hypoxic response protein encoded by Mtb open reading
frame Rv2626c. Rv2626c is also one of the highly expressed
proteins by Mtb in hypoxic conditions and can be identified in
culture filtrates and lysates of Mtb (8), but the role served by Rv2626c remains
to be elucidated.
In the present study, a fusion protein of Rv2031c
and Rv2626c was expressed in a non-pathogenic, fast growing
Mycobacterium semegmatis (Ms), to describe the physiological
function of the fusion protein in mycobacteria and to investigate
its immuno-modulatory functions in macrophages.
Materials and methods
Strains of bacteria, media and growth
conditions
The Ms strain MC2 155 was purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
Bacillus Calmette-Guérin (BCG) was obtained from Shaanxi Research
Institute for Tuberculosis Control and Prevention (Shaanxi, China).
Mycobacterial strains Ms mc2155 and BCG were cultured in
Middle brook 7H9 broth and 7H10 agar (Difco Laboratories, Detroit,
MI, USA) containing albumin dextrose complex [5 g bovine serum
albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) 2 g glucose
and 0.85 g NaCl/l], 0.5% volume/volume (v/v) glycerol and 0.05%
Tween-80. E. coli DH5α (Takara Biotechnology Co., Ltd.,
Dalian, China) was cultured in Luria Bertani media (Takara
Biotechnology Co., Ltd.). Both E. coli and mycobacteria were
cultured at 37°C in an incubator, with agitation. Hygromycin
(Sigma-Aldrich; Merck KGaA) was added to certain treatment groups:
50 mg/ml to the E. coli culture and 15 mg/ml to the
mycobacteria culture. All recombinant (r)Ms strains were cultured
in the presence of 15 mg/ml hygromycin.
Construction of rMs strain expressing
rv2031c-rv2626c fusion protein
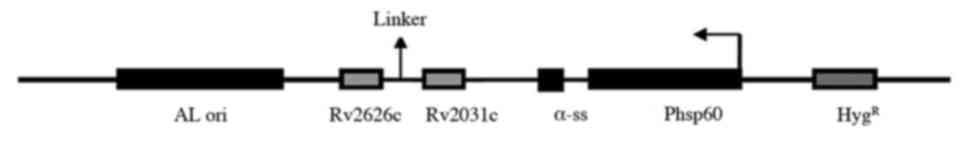
In order to construct a rMs strain expressing
Rv2031c-Rv2626 fusion protein, an expression vector was constructed
by cloning Rv2031c and Rv2626 genes into the E.
coli-Mycobacterium shuttle vector pDE22 (constructed in-house)
(Fig. 1) (9). Primers were designed based on
nucleotide sequences of Rv2031c and Rv2626c genes from the Mtb
H37Rv strain. Rv2031 gene was amplified using the following
primers: 5′-CGGGATCCATGGCCACCACCCTTC-3′
(BamHI site underlined; forward) and 5′-AGCGATATCGTTGGTGGACCGG-3′
(EcoRV site underlined; reverse). Rv2626c gene was amplified
using the following primers: 5′-AGCGATATCGGTGGCGGTAGCGGCGGTGGCTCCGGCGGTGGCAGCGGTGGCGGTAGCACCACCGCACGC-3′
(EcoRV site underlined; forward) and 5′-AGCAAGCTTCTAGCTGGCGAGGGC-3′
(HindIII site underlined; reverse). A 48-base pair sequence
encoding a hydrophobic linker (italics) was added in the linker
sequence between the 3′end of Rv2031c and the 5′end of Rv2626c to
ensure the correct folding of each protein. The following
thermocycling conditions were used for the polymerase chain
reaction (PCR): Following an initial denaturation at 95°C for 1
min, 30 cycles of 94°C for 45 sec, 65°C for 45 sec, 72°C for 50
sec; and a final extension at 72°C for 5 min. Sequences of all
resulting PCR products were validated by Sunny Biotechnology Co.
(Westmont, IL, USA) and the correct sequences were identical to
those reported by the GeneBank database. PCR products corresponding
to each gene were cloned into the multiple cloning site region of
the shuttle vector pDE22 using restriction endonucleases. The
resulting recombinant plasmids were transfected into competent Ms
cells by electroporation, and the transformed Ms were selected on
solid 7H10 agar containing hygromycin (50 µg/ml; Sigma-Aldrich,
Merck KGaA) for 3 days. Following selection, hygromycin-resistant
colonies were transferred to fresh middlebrook 7H9 media with 15
mg/ml hygromycin. The optical density was measured at a wavelength
of 600 nm (OD600nm) and when a colony reached
OD600nm = 1.0, cells were incubated at 42°C for 4 h.
A total of 2 ml of each cell culture was harvested
by centrifuging at 8,000 × g for 20 min at room temperature. The
supernatant was transferred into an Amicon ultrafiltration tube
with a membrane NMWL of 10 kDa, centrifuged at 3,000 × g at 4°C
until approximately 10 µl fluid remained in the chamber, before
adding 100 µl sterile-distilled water and centrifuged again at
3,000 × g at 4°C until ~10 µl fluid remained in the chamber. After
centrifugation, the upper chamber of the unltrfiltraion tube was
transferred to a fresh mirofuge tube and centrifuged at 3,000 × g
at 4°C for 2 min, and the volume in the tube was determined and
added to an equal volume of 2X SDS-PAGE sample buffer. The cell
pellet was resuspended in 1 ml sterile-distilled water and
re-centrifuged at 8,000 × g for 10 min at room temperature, then
resuspended in 100 µl sterile-distilled water and sonicated on ice
using 4 pulses for 15 sec on maximum output, then 100 µl 2X
SDS-PAGE sample buffer was added. A total of 10 µl samples were
loaded onto an 12% SDS-PAGE gel and proteins from gel were
electrotransferred to a polyvinylidene difluoride membrane (pore
size 0.2 µm; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 70 V
for 2 h at 4°C in Tris-Glycine transfer buffer composed of 25 mM
Tris, 192 mM glycine and 20% methanol at pH 8.3.
For immunoblotting, non-specific binding sites were
blocked with PBS containing 5% non-fat milk for 1 h at room
temperature. Blocked membranes were incubated overnight at 4°C in
PBS with mouse anti-Rv2031c monoclonal antibody (cat. no. ab64786,
dilution 1:500), mouse anti-Rv2626c monoclonal antibody (cat. no.
ab64786, dilution 1:500) (both from Abcam, Cambridge, UK). Washed
membranes were incubated 1 h at room temperature with IRDye 800CW
anti-mouse antibody (1:5,000; LI-COR Bioscience, Lincoln, NE, USA),
washed and immunodetection was performed using an ODYSSEY Infrared
Imaging system (LI-COR Bioscience). Following screening, positive
recombinant Ms strains were classified as rMs.
In vitro growth kinetics of rMs
To examine the growth pattern of rMs, rMs and Ms
strains were cultured until late exponential phase, diluted to
OD600 nm = 0.2 and cultured in Middlebrook 7H9. Growth
curves were generated by measuring alterations in OD600
nm over time for 57 h.
Macrophage infection
RAW264.7 murine macrophages (provided by Dr. Shi CH,
the Fourth Military Medical University, Xi'an, China) were cultured
at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% (v/v) fetal bovine serum (both from
Gibco; Thermo Fisher Scientific, Inc.), 1% L-glutamin and
antibiotics [60 mg/ml penicillin G sodium, 50 mg/ml streptomycin
sulphate and 30 mg/ml gentamycin sulphate, purchased from Leagene
Co., Beijing, China; www.leagene.bioon.com.cn). Cells were seeded in 6-well
plates at a density of 0.5×105 cells/well and used for
infection 24 h later. Exponentially growing bacteria cultured in
the presence of 15 mg/ml hygromycin were pelleted, washed and
resuspended in DMEM (without antibiotics) to OD600 nm =
1.0. Single cell suspensions of rMs and Ms strains were obtained by
passing cultures ~5–6 times through 26 gauge needles. Bacillary
viability was assessed at each step by colony-forming unit (CFU)
counts. Equal numbers of each strain were used to infect
macrophages at a multiplicity of infection (MOI) =10:1, selected
based on pilot infections (data not shown) with multiple MOIs that
we performed with cell lines used in the present study. Following
incubation with bacteria for 4 h, non-phagocytosed bacteria were
washed off using PBS. Cells were washed with PBS and post-infection
CFU counts were determined by lysing infected cells. Subsequently,
complete DMEM containing gentamycin (Gibco; Thermo Fisher
Scientific, Inc.) was added to eliminate extracellular bacteria.
Infected cells were transferred to fresh DMEM and incubated for 24
h at 37°C with 5% CO2. Following the incubation, 20 µl
MTT (Sigma-Aldrich; Merck KGaA) was added to each sample. Following
4 h of incubation with MTT, dimethyl sulfoxide was added and the
samples were incubated at 37°C for 10 min. Each sample was observed
under an optical microscope and the absorbance was measured at a
wavelength of 490 nm on a microplate reader (Omega Bio-Tek, Inc.,
Norcross, GA, USA). CFU counts were performed at 3, 6, 12 and 21 h
post infection by lysing 1×103 infected cells with 0.1%
Triton X-100 (Sigma-Aldrich; Merck KGaA) followed by dilution
plating on Middlebrook 7H10 agar, the results were expressed as
log10 CFU/103 cells.
Apoptosis and necrosis of
macrophages
RAW264.7 murine macrophages (105 cells)
were left uninfected as controls or infected with Ms or rMs at 10:1
MOI for 24 h. Macrophages were subsequently removed from plates
using accutase solution (Sigma-Aldrich; Merck KGaA), washed twice
in ice-cold PBS and stained with propidium iodide (PE)-conjugated
Annexin V and 7-aminoactinomycin D (7AAD), according to the
manufacturer's protocol (BD Biosciences). Cells were fixed in PBS
containing 5% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 20
min at room temperature and analyzed with a FACSCanto II cytometer
and FACSDiva software (version 6.1.2; BD Biosciences). Apoptosis
was expressed as the percentage of Annexin V-positive
7-AAD-negative cells, and necrosis was expressed as the percentage
of Annexin V and 7-AAD-double positive cells.
Cytokine and nitrite assays
Levels of interferon-γ (IFN-γ), tumor necrosis
factor-α (TNF-α) and interleukin (IL)-6 in macrophage culture
supernatants 24 h post infection were quantitated with a mouse
IFN-γ ELISA development kit (cat. no. 3321–1H-6), mouse TNF-α
development kit (cat. no. 3511-1H-6) and mouse IL-6 ELISA
development kit (cat. no. 3361-1H-6) according to the
manufacturer's instructions. All the kits were purchased from
Mabtech AB, Stockholm, Sweden. Estimation of nitric oxide (NO)
levels was performed using the Griess test. Equal volumes of cell
culture supernatants were transferred in duplicate into 96-well
culture plates and mixed with an equal volume of Griess reagent,
composed of 1% weight/volume (w/v) sulphanilamide, 0.1% (w/v)
napthyl-ethylenediamine hydrochloride and 2.5% (v/v)
H3PO4. Following incubation at room
temperature for 5 min, the absorbance was measured at a wavelength
of 540 nm using an Ultra Microplate Reader (Omega Bio-Tek, Inc.,
Norcross, GA, USA). The concentration of nitrate was calculated
using a NaNO2 standard curve.
Statistical analysis
All experiments were performed in triplicate.
Differences between groups were analyzed by one-way analysis of
variance using SPSS software (version 15.0; SPSS, Inc., Chicago,
IL, USA), followed by the Fisher-Tukey least significant difference
post hoc test. Data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of Rv2031c-Rv2626c fusion
protein in rMs
Total proteins from whole cell lysates of rMs and Ms
strains were obtained following induction at 42°C. Western blot
analysis revealed that a specific expression band ~34 kDa,
corresponding to the combined molecular weight of Rv2031c (16.3
kDa) and Rv2626c (16 kDa), was present in the cell lysate of rMs,
and absent in Ms cells (Fig. 2A).
The above results were further confirmed by western blot analysis
with anti-Rv2031c and anti-Rv2626 monoclonal antibodies, which
indicated that both Rv2031c and Rv2626c were correctly folded in
the Rv2031c-Rv2626c fusion protein (Fig. 2B).
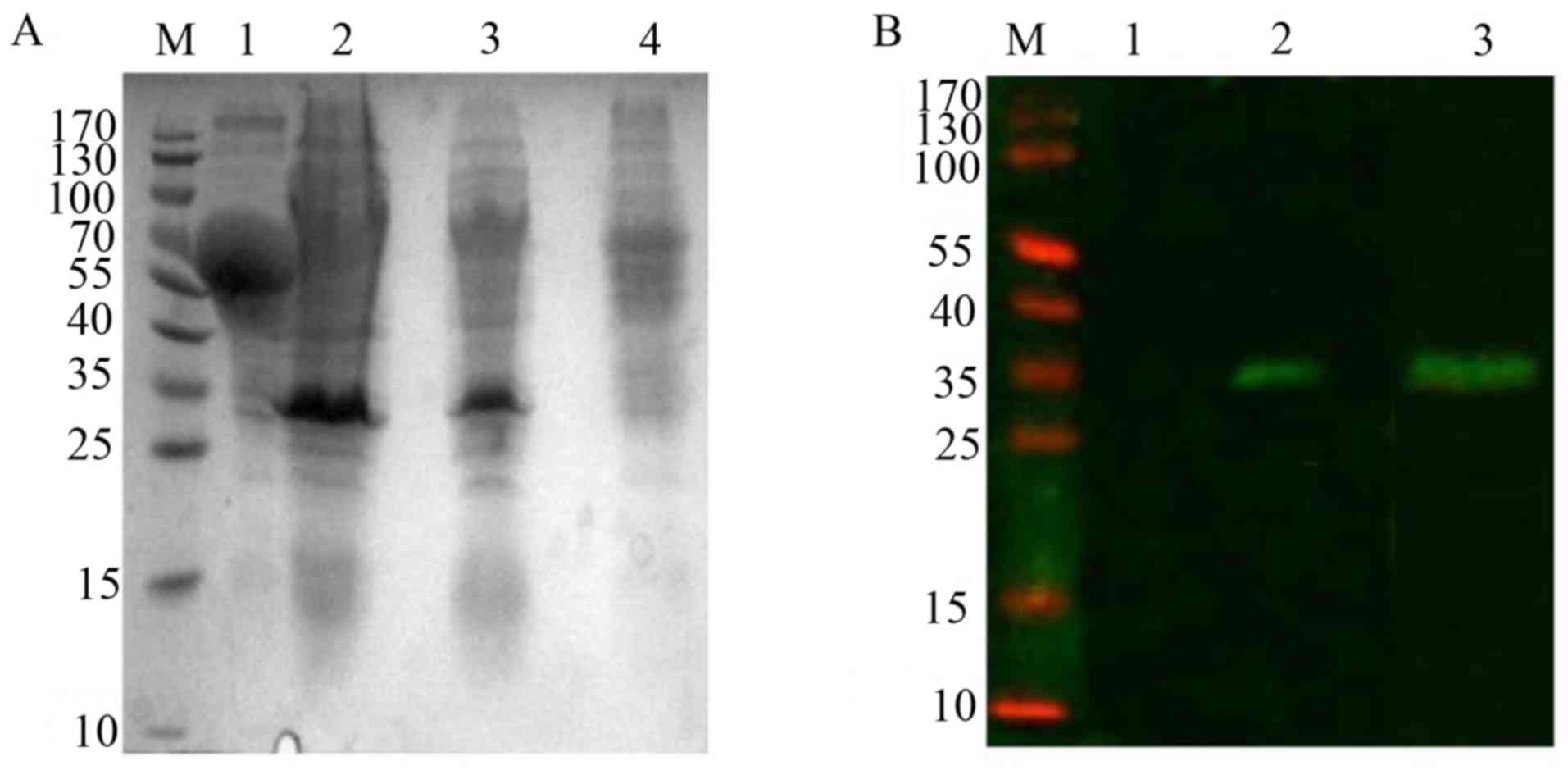 | Figure 2.Expression of Rv2031c-Rv2626c fusion
protein in rMs. (A) Western blot analysis of expression of Rv2031c
and Rv2626c fusion protein in rMs induced by heat shock at 42°C.
Lane M, molecular weight of standard protein markers; lane 1,
supernatant of rMs culture; lanes 2 and 3, cell lysates of rMs; and
lane 4, the cell lysate of Ms. (B) Western blot analysis of the
fusion protein by anti-Rv2031c mAb and anti-Rv2626c mAb. Lane M,
pre-stained protein markers; lane 1, the cell lysate of Ms; lane 2,
cell lysate of rMs stained with an anti-Rv2031 mAb; and lane 3,
cell lysate of rMs stained with anti-Rv2626c mAb. mAb, monoclonal
antibody; rMs, recombinant Mycobacterium smegmatis. |
Intracellular and in vitro growth
characteristics of rMs
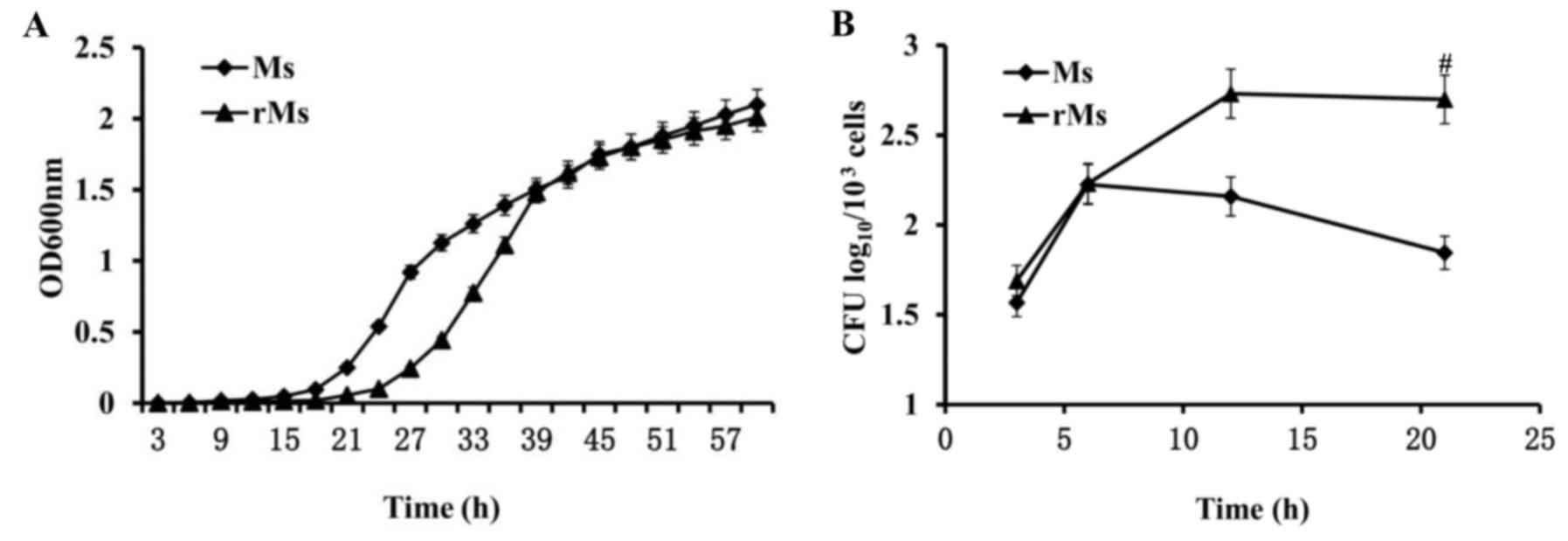
In order to determine whether the expression of
Rv2031c-Rv2626c fusion protein in rMs alters growth characteristics
of the strain, growth rates of rMs and Ms in vitro cultures
were identified by OD600 nm measurement. When log-phase
cultures of rMs and Ms were allowed to grow to saturation and were
equivalently diluted, the duration of the growth lag phase of rMs
was significantly prolonged in rMs compared with Ms (P<0.05).
The final log-phase rates were not significantly different
(Fig. 3A). To identify
intracellular growth characteristics of rMs and Ms, infectivity and
intracellular survival ability of rMs and Ms in RAW264.7 murine
macrophages were examined by CFU estimation of viable bacteria. The
results demonstrated that similar numbers of cells (1.99±0.09
log10 CFU for rMs and 1.86±0.10 log10 CFU for
Ms) were present 3 h following infection, suggesting that
infectivity of rMs was unaffected by transfection. However, 21 h
following infection, growth of rMs in macrophages was enhanced and
the number of viable bacteria in 103 macrophage cells
was equal to 2.70±0.14 lgCFU, which was significantly increased
compared with 1.85±0.07 lgCFU in macrophages infected with Ms
(P<0.05; Fig. 3B). The above
results revealed a significant difference in survival ability
inside macrophages between rMs and Ms, potentially associated with
expression of Rv2031c-Rv2626c fusion protein in rMs.
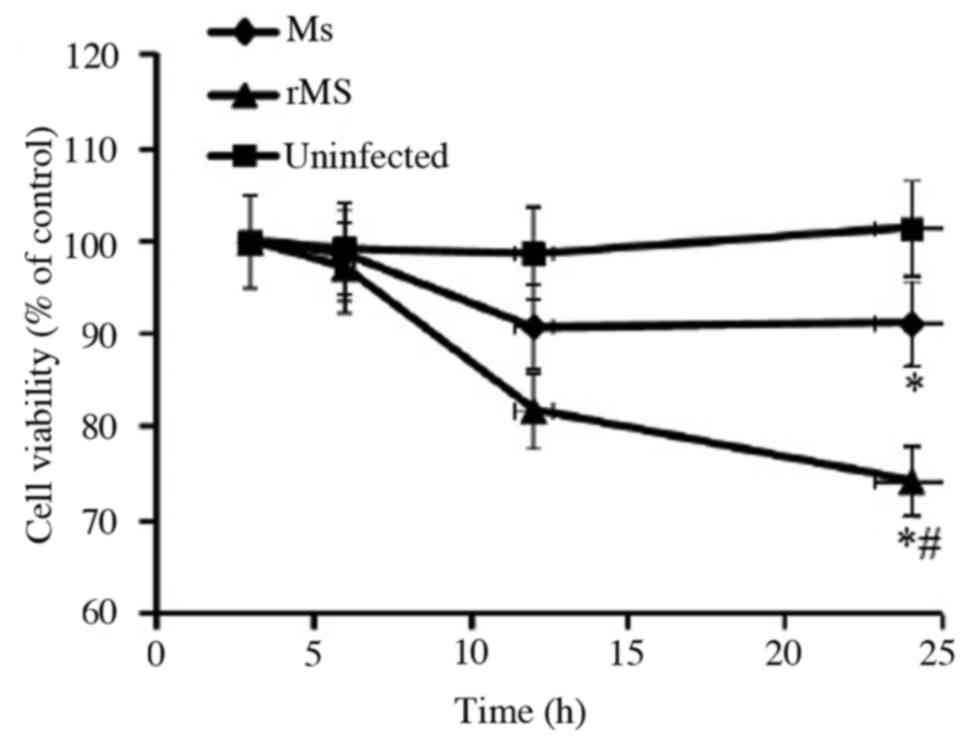
Effect of rMs on viability of
macrophages
To determine the effect of rMs on viability of
macrophages, MTT analysis was performed at different time points
following infection of RAW264.7 murine macrophages with rMs or Ms.
A total of 24 h following infection, the viability of macrophages
was equal to 78.8±3.9% in cells infected with rMs, 90.9±4.5% cells
infected with Ms and 101.4±5.1% in uninfected control cells. Both
rMs and Ms inhibited the viability of macrophages (P<0.05);
however, the viability of macrophages infected with rMs
demonstrated a significant decrease compared with Ms from 12 h
onwards (P<0.05; Fig. 4). The
above results indicate that rMs may be more virulent compared with
Ms.
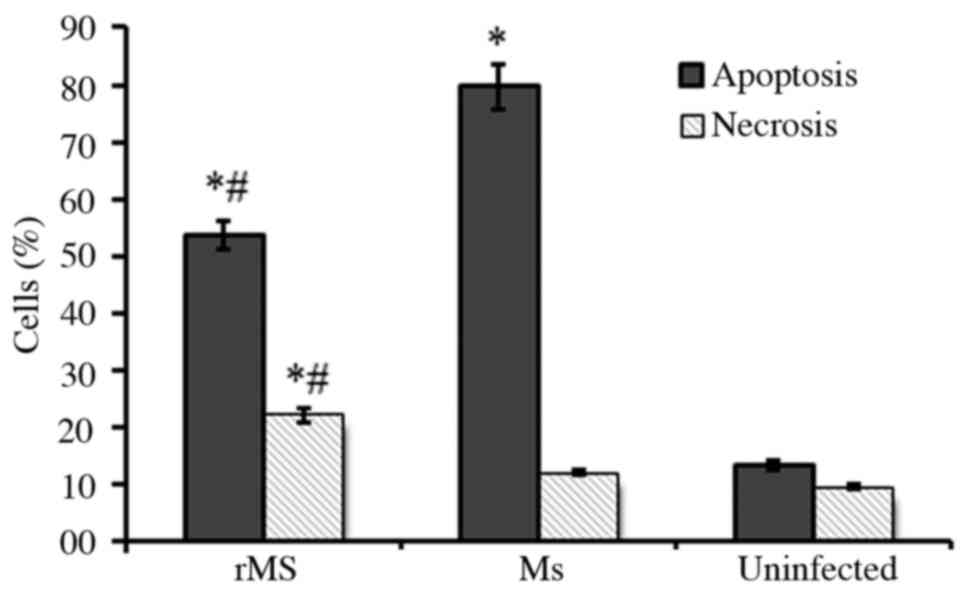
Apoptosis and necrosis of
macrophages
RAW264.7 murine macrophages infected with rMs or Ms
were stained with PE-conjugated Annexin V and 7AAD, and analyzed by
flow cytometry to identified apoptotic and necrotic cells. A total
of 24 h following infection, 50.6±3.2% macrophages infected with
rMs were apoptotic and 20.7±2.2% were necrotic, while 80.2±4.6% Ms
infected macrophages were apoptotic and 15.3±1.4% were necrotic.
Compared with Ms, rMs significantly inhibited apoptosis and induced
necrosis of infected macrophages (P<0.05; Fig. 5). The above results can be
associated with expression of Rv2031c-Rv2626c fusion protein by
rMS.
Modulatory effects of rMs on the
innate immunity of macrophages
To identify factors contributing to the increased
survival ability of macrophages infected with rMs, compared with
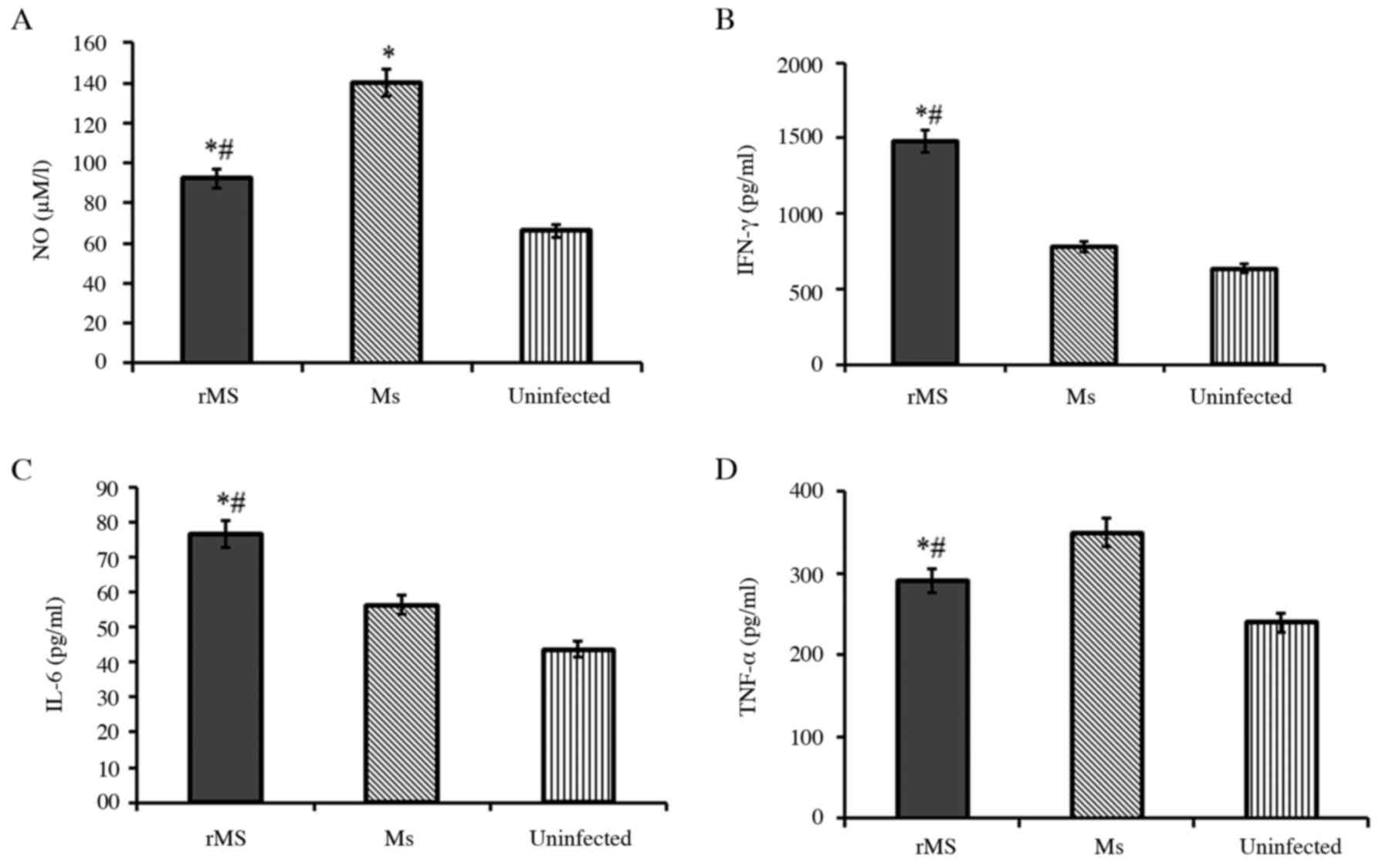
Ms, levels of nitric oxide were measured by Griess assay. NO is a
determinant of intracellular bacillary burden in host cells.
Following infection, NO was down-regulated in macrophages infected
with rMs, compared with macrophages infected with Ms (P<0.05;
Fig. 6A). Levels of IFN-γ, IL-6
and TNF-α in infected macrophages were determined by ELISA. The
results demonstrated that secretion of IFN-γ and IL-6 in
macrophages infected with rMs was significantly up-regulated
(P<0.05), but levels of TNF-α were down-regulated (P<0.05),
compared with macrophages infected with Ms. The above results
demonstrate that expression of Rv2031c-Rv2626c fusion protein in
rMs can modulate the innate immunity of macrophages infected with
rMs to favor intracellular survival of rMs.
Discussion
It has been demonstrated that DosR regulon, composed
of 48 co-regulated genes, is essential for the survival of Mtb in
macrophages (10).
Dormancy-associated antigens encoded by DosR genes in Mtb serve
physiological and immuno-modulatory functions of the host immune
system (11,12). In the present study, a fusion
protein of dormancy-associated antigens Rv2031c and Rv2626c was
expressed in a non-pathogenic strain of Ms. The results of the
present study demonstrated that expression of the fusion protein
Rv2031c-Rv2626c in rMs prolonged the duration of growth lag-phase
of rMs in vitro. The aforementioned data are consistent with
a previous report, in which overexpression of Rv2031c in Ms
resulted in a significant lag in growth of Ms (5). In order to determine whether
expression of Rv2031c-Rv2626c alters the intracellular survival
ability of Ms, macrophages were infected with rMs or Ms. Compared
with the Ms strain, infectivity of rMs was not affected by
expression of the fusion protein Rv2031c-Rv2626c. Virulence and
survival of rMs in macrophages were enhanced, the number of viable
cells in macrophages infected with rMs was markedly decreased and
the number of intracellular rMs bacteria increased. A previous
study reported that a Mtb mutant, in which Rv2031c gene was
replaced by a hygromycin resistance gene, was attenuated and
demonstrated inhibited growth in a macrophage model (7); however, Hu et al (13) reported that increased numbers of
CFU were observed in mice or macrophages that they were infected
with an unmarked Rv2031c deletion mutant of Mtb when compared with
the orginal Mtb strain.
It has been reported that virulent Mtb can inhibit
apoptosis and trigger necrosis of host macrophages to evade innate
immunity and delay the initiation of adaptive immunity (3). By contrast, attenuated Mtb and
non-pathogenic mycobacteria induce apoptosis of macrophages, an
innate defense mechanism that reduces bacterial viability (14,15).
Therefore, in the present study, apoptosis and necrosis of infected
macrophages were observed. The results of the present study
demonstrated that, compared with Ms, apoptosis of macrophages
infected with rMs was decreased, while necrosis was increased. NO
production is an antimicrobial mechanism employed by macrophages
(16). In the present study,
compared with macrophages infected with Ms, macrophages infected
with rMs demonstrated decreased NO levels in macrophages.
Therefore, it can be hypothesized that inhibition of NO enhanced
the survival of rMs in macrophages.
Macrophages eliminate invading Mtb directly and
secrete cytokines to mediate host immune responses (17). The present study investigated
secretion of IFN-γ, TNF-α and IL-6 from infected macrophages. The
results of the present study demonstrated that levels of IFN-γ and
IL-6 markedly increased compared with macrophages infected with Ms,
while the levels of TNF-α decreased. TNF-α is an extrinsic mediator
of apoptosis, which has been demonstrated to have a negative impact
on the survival of mycobacteria within macrophages. More virulent
strains appear to inhibit expression of TNF-α (18). It can be hypothesized that reduced
apoptosis of macrophages infected with rMs may be associated with
inhibition of expression of TNF-α, but the underlying mechanism
remains to be elucidated.
In conclusion, the present study demonstrated that
expression of the fusion protein of dormancy-associated antigens
Rv2031c and Rv2626c in Ms can serve a physiological function of a
dormancy-associated antigen. The fusion protein also modulated the
innate immunity of host macrophages, favoring intracellular
bacillary survival. However, the mechanism underlying intracellular
survival mediated by dormancy-associated antigens in Mtb, remain to
be elucidated.
Acknowledgements
The present study was supported by the National
Science and Technology Major Project of China (grant no.
2012ZX10003008-007) and the National Natural Science Foundation of
China (grant no. 31501112).
References
|
1
|
World Health Organization (WHO): Global
Tuberculosis Report. WHO; Geneva: pp. 2042015
|
|
2
|
Gideon HP and Flynn JL: Latent
tuberculosis: What the host ‘sees’? Immunol Res. 50:202–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu PT and Modlin RL: Human macrophage
host defense against Mycobacterium tuberculosis. Curr Opin
Immunol. 20:371–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerasimova A, Kazakov AE, Arkin AP,
Dubchak I and Gelfand MS: Comparative genomics of the dormancy
regulons in mycobacteria. J Bacteriol. 193:3446–3452. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan Y, Crane DD and Barry CE III:
Stationary phase-associated protein expression in Mycobacterium
tuberculosis: function of the mycobacterial alpha-crystallin
homolog. J Bacteriol. 178:4484–4492. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Souza GA1, Arntzen MØ, Fortuin S,
Schürch AC, Målen H, McEvoy CR, van Soolingen D, Thiede B, Warren
RM and Wiker HG: Proteogenomic analysis of polymorphisms and gene
annotation divergences in prokaryotes using aclustered mass
spectrometry-friendly database. Mol Cell Proteomics.
10:M110.0025272011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan Y, Crane DD, Simpson RM, Zhu YQ,
Hickey MJ, Sherman DR and Barry CE III: The 16-kDa alpha-crystallin
(Acr) protein of Mycobacterium tuberculosis is required for
growth in macrophages. Proc Natl Acad Sci USA. 95:pp. 9578–9583.
1998; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenkrands I, Slayden RA, Crawford J,
Aagaard C, Barry CE III and Andersen P: Hypoxic response of
Mycobacterium tuberculosis studied by metabolic labeling and
proteome analysis of cellular and extracellular proteins. J
Bacteriol. 184:3485–3491. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao H, Bai Y, Xue Y, Wang L, Fan A, Ding X
and Xu Z: Expression, purification, and characterization of soluble
RpfD with high bioactivity as a recombinant protein in
Mycobacterium vaccae. Protein Expr Purif. 55:112–118. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boon C and Dick TP: How Mycobacterium
tuberculosis goes to sleep: The dormancy survival regulator DosR a
decade later. Future Microbiol. 7:513–518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh S, Saraav I and Sharma S:
Immunogenic potential of latency associated antigens against
Mycobacterium tuberculosis. Vaccine. 32:712–716. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Serra-Vidal MM, Latorre I, Franken KL,
Díaz J, de Souza-Galvão ML, Casas I, Maldonado J, Milà C, Solsona
J, Jimenez-Fuentes MÁ, et al: Immunogenicity of 60 novel
latency-related antigens of Mycobacterium tuberculosis.
Front Microbiol. 5:5172014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Y, Movahedzadeh F, Stoker NG and Coates
AR: Deletion of the Mycobacterium tuberculosis
alpha-crystallin-like hspX gene causes increased bacterial growth
in vivo. Infect Immun. 74:861–868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srinivasan L, Ahlbrand S and Briken V:
Interaction of Mycobacterium tuberculosis with host cell death
pathways. Cold Spring Harb Perspect Med. 4:pii: a022459. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fratazzi C, Arbeit RD, Carini C,
Balcewicz-Sablinska MK, Keane J, Kornfeld H and Remold HG:
Macrophage apoptosis in mycobacterial infections. J Leukoc Biol.
66:763–764. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrari CK, Souto PC, França EL and
Honorio-França AC: Oxidative and nitrosative stress on phagocytes'
function: From effective defense to immunity evasion mechanisms.
Arch Immunol Ther Exp (Warsz). 59:441–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Garra A, Redford PS, McNab FW, Bloom CI,
Wilkinson RJ and Berry MP: The immune response in tuberculosis.
Annu Rev Immunol. 31:475–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beham AW, Puellmann K, Laird R, Fuchs T,
Streich R, Breysach C, Raddatz D, Oniga S, Peccerella T, Findeisen
P, et al: A TNF-regulated recombinatorial macrophage immune
receptor implicated in granuloma formation in tuberculosis. PLoS
Pathog. 7:e10023752011. View Article : Google Scholar : PubMed/NCBI
|