Introduction
Uterine leiomyomas (ULs) are the most common tumors
of the female reproductive organs, occurring in ≤80% of all women
of reproductive age, with ≤30% of women complaining of severe
symptoms and seeking treatment (1,2). ULs
are associated with a range of symptoms, including
pressure-associated symptoms, uterine bleeding, dysmenorrhea and
infertility. It is well known that the growth of ULs is primarily
dependent on stimulation by sex steroid hormones, estrogen (E) and
progesterone (P4), secreted by the ovary. However, it is not clear
whether prostaglandins (PGs), including PGE2 and PGF2α, which are
produced in large amounts by the menstruating uterus, affect the
growth of ULs to the same extent as E or P4.
PGs are generated by almost every tissue in the body
and act as important messengers or effectors in a wide variety of
functions, particularly in the inflammatory response. Their
biosynthesis is significantly increased in inflamed tissues, such
as the menstruating uterus. Although a large amount of PGs are
produced in the uterus during the menstrual period, the precise
roles of PGs have not been extensively investigated (2). In a previous study, it was
demonstrated that the cyclooxygenase-2 (COX-2) inhibitor celecoxib
inhibited the proliferation of ULs by inhibiting the NF-κB pathway
in cultured leiomyoma cells (3).
The study indicated that PGs from the uterus may affect
proliferation through intracellular signaling pathways to some
extent. Therefore, PGs may not only serve a key role in the
generation of the inflammatory response, but they may also affect
the intracellular processes promoting tumor growth. However, that
study did not elucidate whether PGs induced cell proliferation or
inflammation to the same extent as ovarian sex steroids, namely E
and P4. Although PGE2 is one of the most abundant PGs in the body,
is widely described in animal species and exhibits useful
biological activities, it has not been clearly determined whether
it is able to stimulate UL growth.
MicroRNAs (miRNAs) are a novel class of regulators
that have been demonstrated to downregulate gene expression by
blocking mRNA translation and/or degrading the mRNA transcript,
depending on the level of complementarity between the miRNA and its
target (4). miRNAs are essential
for normal mammalian development and regulate genes involved in
cell division and differentiation, metabolism, stress responses and
apoptosis (5,6). This post-transcriptional regulation
appears to serve diverse and significant roles in multiple tissues
of the female reproductive system (7).
Little is known about the function of miRNAs in
human ULs. A number of studies have evaluated the levels of
subclasses of miRNAs through microarray expression analysis
(8,9), demonstrating that numerous miRNAs are
deregulated in leiomyomatous tissue compared with normal tissue.
The majority of the studies assessing the effect of sex hormones on
miRNA expression have been conducted via in vitro treatment
of human cell lines with estradiol (E2). The first report on E2
regulation of miRNAs was in 2005, with the correlation between
specific aberrant miRNA signatures, and E and P4 receptor status in
breast cancer (10). Although
there are numerous studies investigating in vitro the effect
of ovarian sex steroids on growth factors, various cytokines,
ECM-associated molecules, and cell proliferation and death in
primary and immortalized leiomyoma cell lines, there are inadequate
data on the regulation of miRNAs by E2 and P4 in the setting of
intact ULs (2). The aim of the
present study was to elucidate whether PGs affect UL growth
compared with ovarian sex steroids via in vitro culture of
leiomyoma and myometrial cells (LC and MC, respectively) obtained
from hysterectomized patients.
Materials and methods
Chemicals and reagents
E2, celecoxib (Cele), P4, PGE2, mifepristone (MF),
fulvestrant (ICI 182, 780) and PGF2α were purchased from
Sigma-Aldrich; Merck KGaA, (Darmstadt, Germany). Dulbecco's
modified Eagle's medium (DMEM), fetal bovine serum (FBS),
antibiotics-antimycotics and trypsin-EDTA were purchased from
Invitrogen; Thermo Fisher Scientific, Inc., (Waltham, MA, USA).
HBSS, collagenase and DNase were purchased from Invitrogen, and
HEPES was purchased from Sigma-Aldrich; Merck KGaA.
Cell culture and treatment
UL tissues were obtained from patients undergoing
hysterectomy after obtaining their written informed consent in
accordance with the Ethics regulations of Dong-A University. The
present study had been approved by the institutional review board
of Dong-A University hospital in Busan, Republic of Korea. The
present study included premenopausal women aged 30–50 years, who
had not received any type of hormonal drug therapy to affect
uterine function within at least 3 months prior to surgery. The
tissues were minced and digested in collagenase solution (HEPES 25
mM, antibiotics 1X, collagenase 2 mg/ml and DNase 0.2 mg/ml) for 4
h at 37°C in a water bath. The digested tissues were passed through
gauze to filter fragmented tissues, and the cells were collected by
centrifugation and washed several times with PBS. The isolated
cells in suspension were seeded in a 100 cm2 dish in
culture medium (DMEM/F12) supplemented with 10% FBS and 1X
antibiotic-antimycotic solution at 37°C in a humidified atmosphere
containing 5% CO2 in air. The culture period was 72 h
until measuring the miRNA levels. Cells were used in experiments
between passages 4 and 8. Drugs were added as 100X stock in DMSO or
PBS. The culture duration and concentrations were determined
through repetitive MTT assay.
The cell stock concentrations of E2, P4, PGE2 and
PGF2α were 1 µM. The concentrations of their antagonists,
fulvestrant, MF and COX-2 inhibitor were 0.1 µM in the culture
media. Fulvestrant, MF and COX-2 inhibitor were administered
together with E2, P4, PGE2 and PGF2α.
RNA isolation and RT-qPCR
Total RNA was isolated using miRNeasy mini kit
(Qiagen GmbH, Hilden, Germany), according to the manufacturer's
protocol. cDNA was synthesized using a reverse transcription kit
from Clontech Laboratories, Inc., (Mountainview, CA, USA) according
to the manufacturer's protocol. qPCR was performed using SYBR green
reagent (Qiagen GmbH). Synthetic miRNA oligonucleotide primers were
designed for the mature miRNAs, and purchased from Genolution
Pharmaceuticals, Inc., (Song-pa, Seoul, South Korea). The details
of the sequences are presented in Table II. The expression of miRNAs was
normalized using U6 as an internal control. The Cq (quantification
cycle) values were calculated from the amplification curve. The
2−∆∆Cq method was used to determine the relative
quantification of miRNA expression. The miRNA experiment was
performed under the following conditions: 95°C for 5 min as initial
denaturation, followed by 40 cycles at 94°C for 15 sec, 55°C for 34
sec, and 70°C for 30 sec.
 | Table II.miR sequences known to be associated
with anti-apoptosis and anti-inflammation in cultured myometrial
and leiomyoma cells. |
Table II.
miR sequences known to be associated
with anti-apoptosis and anti-inflammation in cultured myometrial
and leiomyoma cells.
|
| Gene | Sequence
(forward) | Accession no. |
|---|
| Apoptosis-regulating
miRNAs | let-7a-1 |
TGAGGTAGTAGGTTGTATAGTT | MIMAT0000062 |
|
| miR-21 |
TAGCTTATCAGACTGATGTTGA | MIMAT0000076 |
|
| miR-26a |
TTCAAGTAATCCAGGATAGGCT | MIMAT0000082 |
|
| miR-200a |
TAACACTGTCTGGTAACGATGT | MIMAT0000682 |
|
Inflammation-regulating miRNAs | miR-93 |
CAAAGTGCTGTTCGTGCAGGTAG | MIMAT0000093 |
|
| miR-106b |
TAAAGTGCTGACAGTGCAGAT | MIMAT0000680 |
|
| miR-100 |
AACCGTAGATCCGAACTTGTG | MIMAT0000098 |
|
| miR-29 |
TAGCACCATTTGAAATCAGTGTT | MIMAT0000100 |
Statistical analysis
The data are expressed as the mean ± standard
deviation for numerical values. Differences in study participants'
characteristics were compared across subgroups with the analysis of
variance with Tukey's post-hoc test or Kruskal-Wallis test with
Dunn's post-hoc test, as appropriate. To determine if its
distribution was normal, the present study used Shapiro-Wilk's
test. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were carried out
using SPSS v.24.0 (IBM Corp., Armonk, NY, USA) statistical
software.
Results
PG effects on LC
The expression of four miRNAs (let-7a, miR-21,
miR-26a and miR-200a) known to be involved in regulating cellular
apoptosis was examined under treatment with sex steroids (E2 and
P4) and PGs. PGE2 induced higher expressions of the three miRNAs
(let 7a, miR26a and miR 200a), comparing with the NT or E2
significantly (Fig. 1). In
particular, there were no statistical differences of expression in
miR-21 group compared with other three miR groups (Fig. 1B). These findings indicated that
PGs exerted a more potent anti-apoptotic effect on LC than E2.
However, the expression patterns of PGF2α differed from those of
E2, except for miR-26a that PGF2α induced as much as PGE2 (Fig. 1C). ICI did not reverse those
effects induced by E2 (Fig. 1). MF
and cele exhibited antagonistic action to P4 and PGE2 in only
let-7a (Fig. 1A). Another four
miRNAs (miR-29b, miR-93, miR-100 and miR-106b), known to regulate
cellular inflammation, were examined under the same conditions.
PGE2 did not induce the expressions of the four miRs as much as
those of non-treated control or E2 significantly (Fig. 2). According to the mean expression
fold ratio to E2, PGE2 exerted the most prominent anti-apoptotic
and anti-inflammatory effects compared with other agents (Table I).
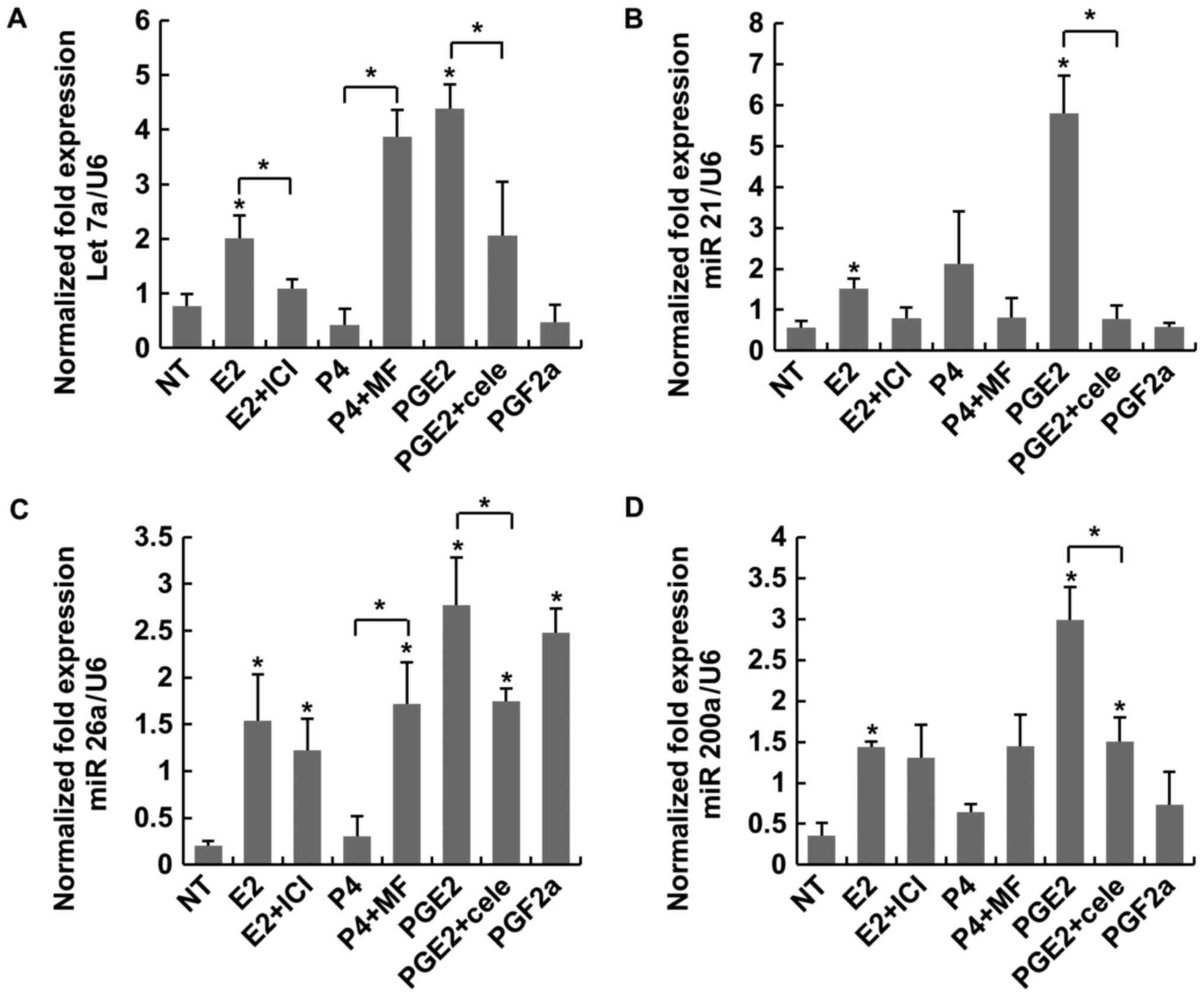 | Figure 1.Expression of the miRNAs let-7a,
miR-21, miR-26a and miR-200a was considered to regulate apoptosis
in LC. (A) PGE2 significantly increased the expression of let-7a.
(B) Statistical analysis of miR-21 across the groups. (C) PGE2
significantly increased the expression of miR 26a when compared
with NT. (D) PGE2 significantly increased the expression of
miR-200a when compared with NT. *P<0.05 vs. NT or indicated
bracket. LC, leiomyoma cells; PG, prostaglandin; E2, estradiol;
ICI, fulvestrant; P4, progesterone; NT, non-treated; MF,
mifepristone; Cele, celecoxib. |
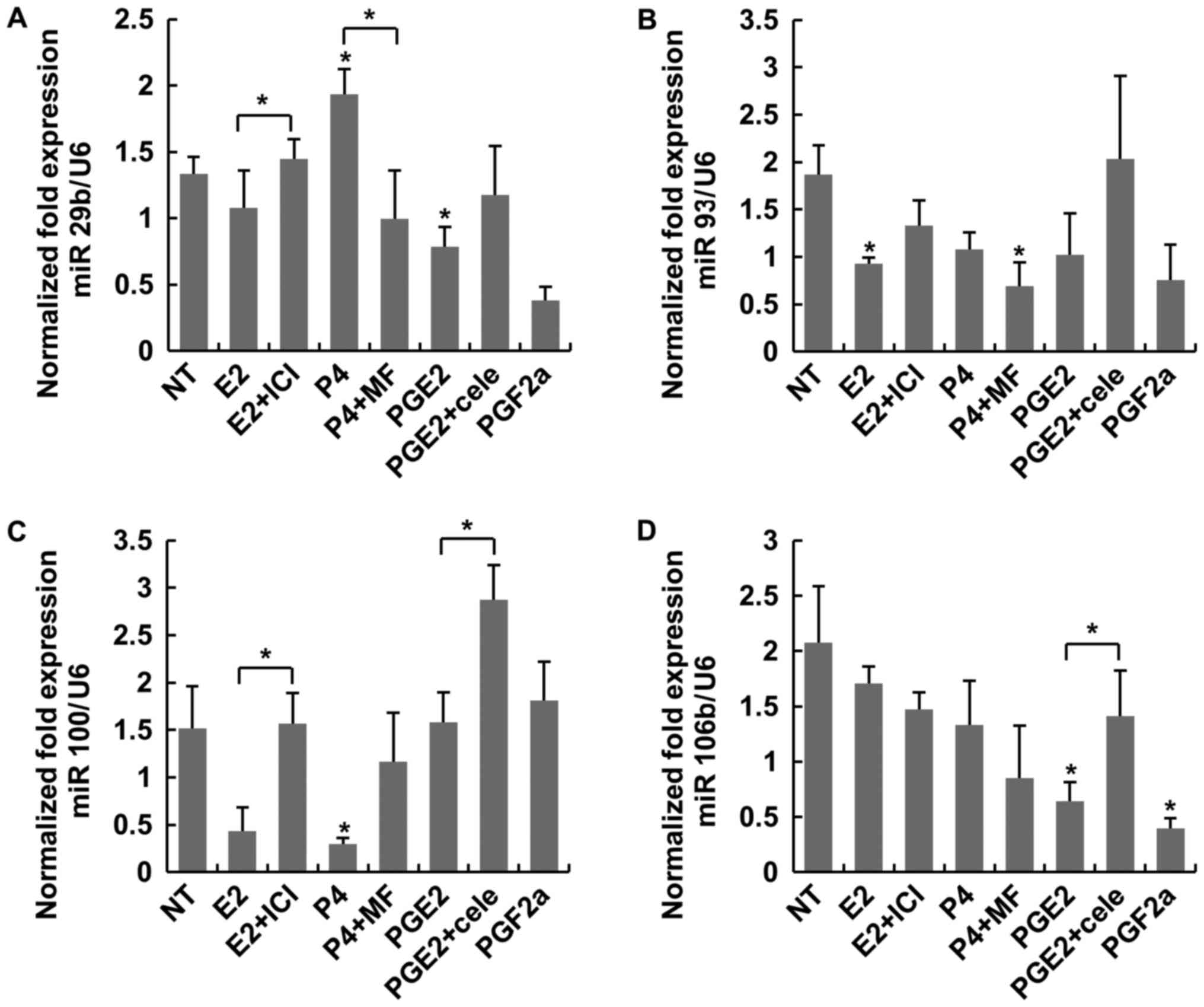 | Figure 2.Expression of the miRNAs miR-29b,
miR-93, miR-100 and miR-106b was considered to regulate
inflammation in LC. (A) P4 induced a higher expression of miR-29b
when compared with P4+MF. (B) No statistical differences were
observed between treatment groups. (C) PGE2+cele induced a higher
expression of miR-100 when compared with PGE2. (D) PGF2α
significantly decreased the expression of miR-106b when compared
with NT. *P<0.05 vs. NT or indicated bracket. LC, leiomyoma
cells; NT, non-treated; E2, estradiol; ICI, fulvestrant; P4,
progesterone; MF, mifepristone; PG, prostaglandin; Cele,
celecoxib. |
 | Table I.miR fold ratios of P4, PGE2 and PGF2α
relative to E2 treatment. |
Table I.
miR fold ratios of P4, PGE2 and PGF2α
relative to E2 treatment.
|
|
| miR fold ratio |
|---|
|
|
|
|
|---|
| Variables | miRNAs | NT/E2 | P4/E2 | PGE2/E2 | PGF2α/E2 |
|---|
| LC
(apoptosis-regulating miRNAs) | let-7a | 0.39 | 0.21 | 2.19 | 0.24 |
|
| miR-21 | 0.38 | 1.41 | 3.83 | 0.39 |
|
| miR-26a | 0.13 | 0.20 | 1.80 | 1.61 |
|
| miR-200a | 0.25 | 0.45 | 2.08 | 0.51 |
|
| Mean | 0.29 | 0.57 | 2.48 | 0.69 |
| LC
(inflammation-regulating miRNAs) | miR-29b | 1.46 | 2.11 | 0.86 | 0.41 |
|
| miR-93 | 2.02 | 1.17 | 1.10 | 0.82 |
|
| miR-100 | 3.51 | 0.69 | 3.66 | 4.19 |
|
| miR-106b | 1.22 | 0.78 | 0.38 | 0.23 |
|
| Mean | 2.05 | 1.19 | 1.50 | 1.41 |
| MC
(apoptosis-regulating miRNAs) | let-7a | 1.34 | 1.97 | 0.13 | 0.33 |
|
| miR-21 | 2.22 | 22.34 | 10.29 | 6.27 |
|
| miR-26a | 1.80 | 0.67 | 1.02 | 2.09 |
|
| miR-200a | 0.47 | 0.99 | 2.03 | 0.50 |
|
| Mean | 1.46 | 6.49 | 3.37 | 2.30 |
| MC
(inflammation-regulating miRNAs) | miR-29b | 0.24 | 0.07 | 0.03 | 0.12 |
|
| miR-93 | 0.26 | 0.24 | 0.24 | 0.01 |
|
| miR-100 | 0.46 | 0.41 | 0.13 | 0.01 |
|
| miR-106b | 0.61 | 0.43 | 2.43 | 3.09 |
|
| Mean | 0.39 | 0.29 | 0.71 | 0.81 |
PG effects on MC
Overall, the miRNA expression patterns in MC
differed compared with those in LC. Among the four miRNAs
regulating MC apoptosis. PGE2 induced more expression of miR 200a
compared with NT significantly (Fig.
3D). In other three miRs, there were no antagonist actions to
sex-steroids or PGs. As regards miR-21, P4 induced higher
expression compared with NT and E2 (Fig. 3B). As presented in Fig. 4, antagonists like MF or cele
induced more expressions of miR 29b and miR 93 significantly. In
terms of the expression ratios in MC, there was no significance
among the miRs (Table I).
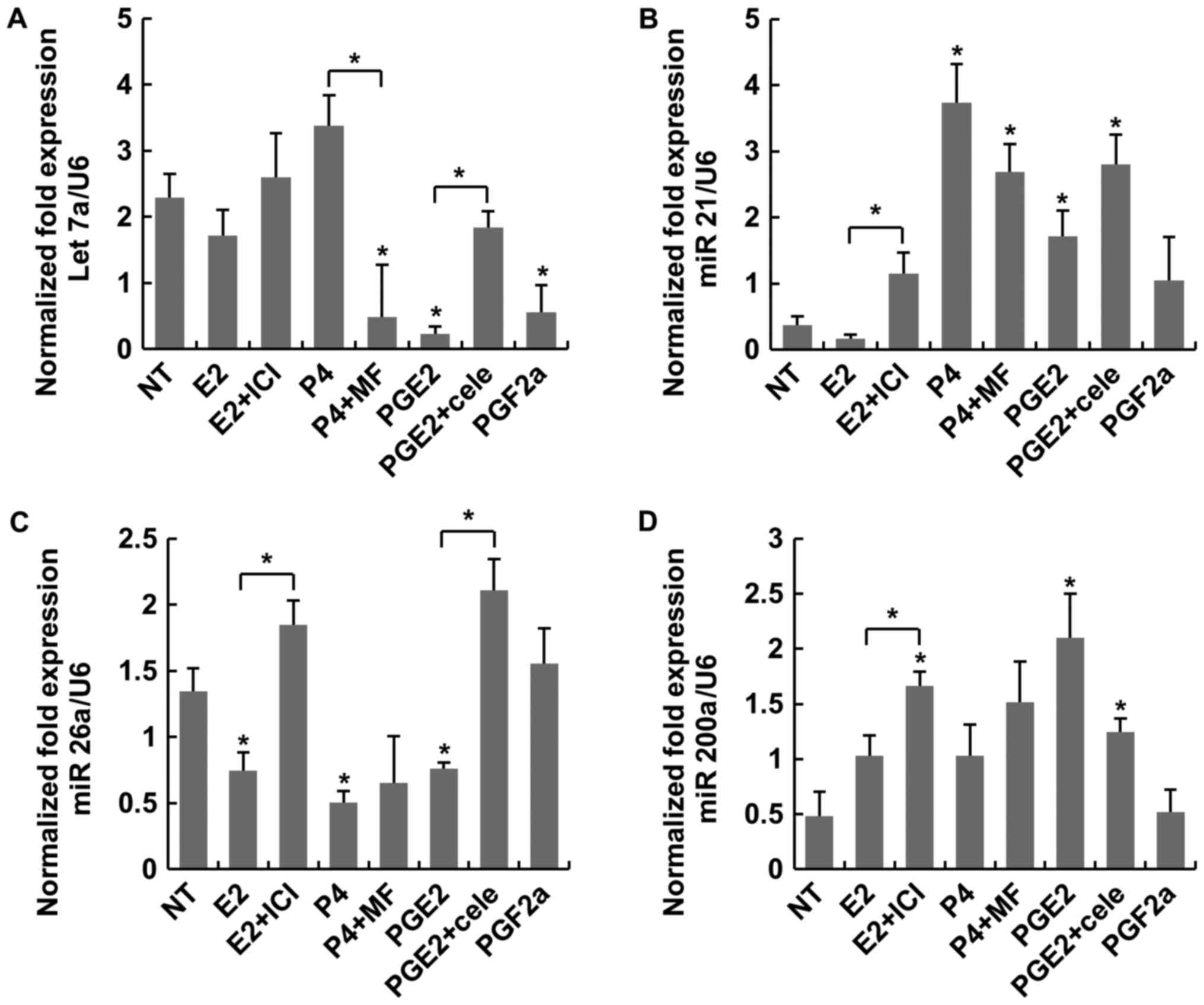 | Figure 3.Expression of the miRNAs let-7a,
miR-21, miR-26a and miR-200a was considered to regulate apoptosis
in MC. (A) Statistical differences were not observed between PGE2
and E2. (B) P4 induced a higher expression of miR-21 than NT. (C)
No statistical differences were observed between PGE2 and E2 for
miR-26a expression. (D) PGE2 induced a higher expression of
miR-200a when compared with NT. MC, myometrial cells; NT,
non-treated; E2, estradiol; ICI, fulvestrant; P4, progesterone; MF,
mifepristone; PG, prostaglandin; Cele, celecoxib. *P<0.05 vs. NT
or indicated bracket. |
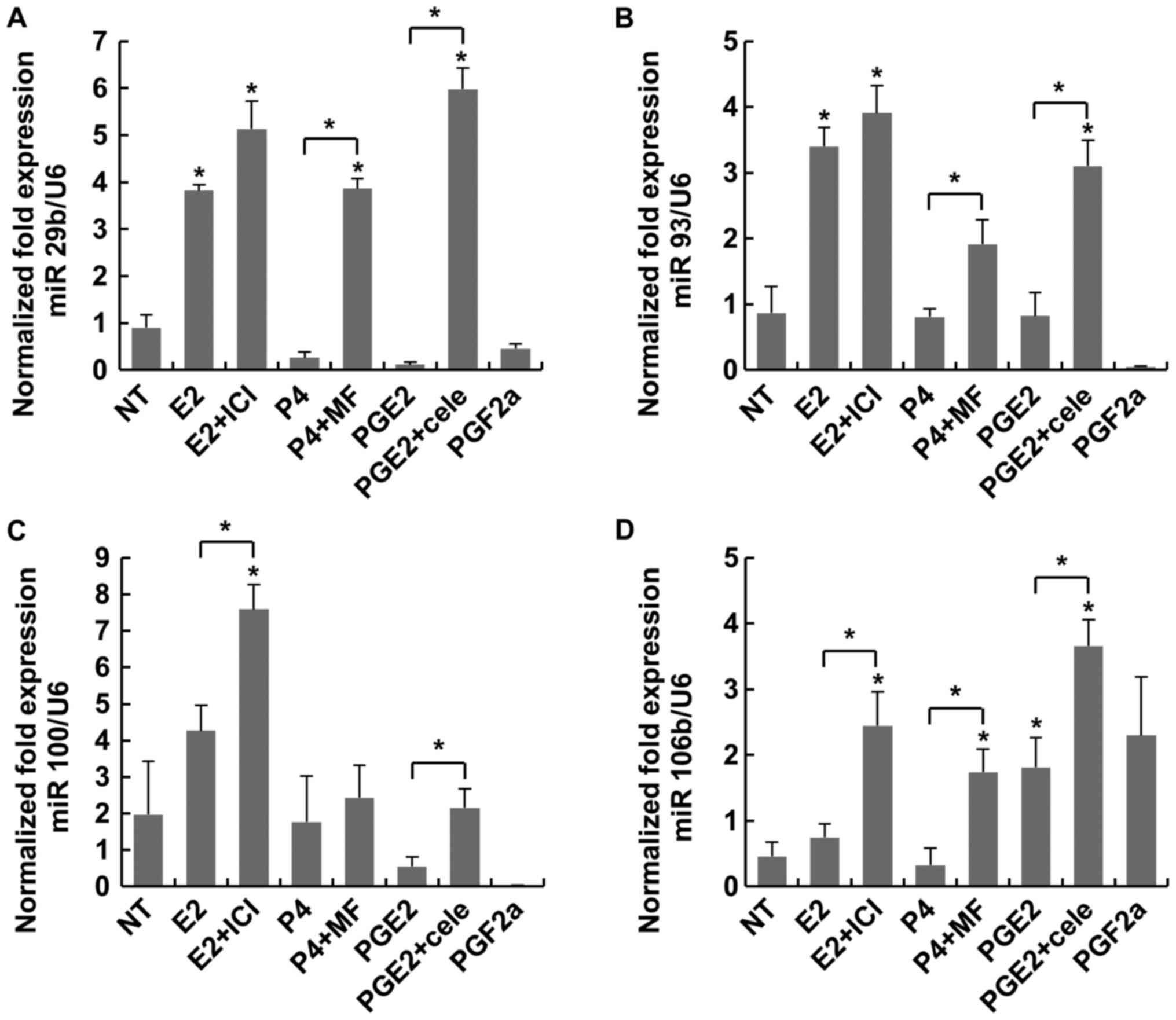 | Figure 4.Expression of the miRNAs miR-29b,
miR-93, miR-100 and miR-106b was considered to regulate
inflammation in MC. (A) P4 plus MF and PGE2 plus cele induced
significantly higher expression of miR-29b than P4 and PGE2 alone,
respectively. (B) PGE2 plus cele induced a higher expression of
miR-93 than NT and PGE2 alone. (C) There were no significant
differences between treatment groups except for PGF2α. (D) PGE2
plus cele induced a higher expression when compared with NT.
*P<0.05 vs. NT or indicated bracket. MC, myometrial cells; NT,
non-treated; E2, estradiol; ICI, fulvestrant; P4, progesterone; MF,
mifepristone; PG, prostaglandin; Cele, celecoxib. |
Discussion
Despite the high prevalence of ULs, their specific
etiology remains largely unknown. Although the exact etiology of
this transforming event is currently unknown, the neoplastic change
of a myometrial cell is likely to be due to a cellular insult
(11). Regardless of the cellular
insult, a common primary characteristic of ULs is their
responsiveness to steroid hormones. E2 and P4 lead to tumor growth
by stimulating a modest rate of cell proliferation and the
production of abundant amounts of extracellular collagen
matrix.
There is a 3-fold increase in PG levels in the
endometrium from the follicular to the luteal phase, with a further
increase during menstruation (12). Most of the PG release during
menstruation occurs during the first 48 h, which corresponds to the
greatest intensity of the symptoms. However, the effects of PGs on
UL growth have not been adequately investigated to date. In this
study, we found that PGs may promote the growth of cultured LC as a
result of the miRNA expression fold ratio to E2. However, further
in vivo studies are required to validate the effects of PGs
in the future.
Since the introduction of miRNAs, accumulating
evidence of abnormal miRNA expression has revealed their function
in normal biological activities, and has provided a novel insight
into their potential functional significance in a wide range of
common human diseases, such as malignancy, cardiac disease,
diabetes and insulin resistance (13–15).
Increased expression of a specific miRNA causes the suppression of
translation of the targeted mRNA, whereas downregulation of the
miRNA exerts the opposite effect. These translational modification
processes induce distinct protein expression profiles, causing
various cellular and tissue changes. The functional significance of
differential miRNA expression under normal and disease conditions
is not always as apparent as may be expected. We herein
investigated eight miRNAs known to regulate the cellular events of
apoptosis or inflammation, based on previous studies (2,16,17).
Dysregulated expression of miRNAs such as let-7, miR-21, miR-92,
miR-106b and miR-200 has been reported to be associated with the
development of leiomyomas (17).
In this study, we tried to evaluate the potential biologic effects
of PGs from menstruating uterus through expression ratios of the
eight miRNAs comparing with those of sex steroid hormones.
The expression patterns of miRNAs in this experiment
were not consistent with previous studies. For example, in a
previous study, it was reported that E2 reduced the expression of
miR-21 and miR-26a, and the pattern was reversed by treatment with
the anti-estrogen compound fulvestrant in MC and LC (9). However, co-treatment with E2 and
fulvestrant decreased the expression of miR-21 and miR-26a in the
present experiment. These opposite results may be attributed to the
fact that the present study was performed under different in
vitro culture conditions. Moreover, although >1,000 miRNAs
have been identified in humans recently, their detailed functional
role in gene regulation has not been adequately investigated thus
far. The intracellular anti-apoptotic or anti-inflammatory
functional roles of the eight miRNAs examined in the present study
were based on previous studies. It is considered that such a
functional analysis of miRNAs may be helpful in understanding the
role of miRNAs, as the effects of E and P4 on the cell are well
known. Furthermore, as translational research into the application
of miRNAs is progressing, diagnostic and therapeutic biomarkers may
have the potential to change the standard of care of ULs.
In conclusion, PGE2, a key inflammatory mediator,
which is produced in copious amounts during menstruation, may be a
potential promoter of UL growth. Therefore, reducing the amount or
antagonizing the action of PGE2 produced by the menstruating uterus
may be considered as an alternative therapeutic strategy.
Acknowledgements
Not applicable.
Funding
This study was supported by Research Institute for
Convergence of Biomedical Science and Technology (30-2016-000),
Pusan National University Yangsan Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HK and YC conceived and designed the experiments. GJ
and SP performed cell culture, RNA isolation and RT-qPCR. YC and MH
recruited the patients who had provided their extirpated uterus for
this study. MH analyzed the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients (age range, 40–50) at the Department of Obstetrics and
Gynecology, Dong-A University Hospital (Busan, Republic of Korea)
that participated in the study, and the study was approved by the
Ethics Committee of Dong-A University.
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Myers ER, Barber MD, Gustilo-Ashby T,
Couchman G, Matchar DB and McCrory DC: Management of uterine
leiomyomata: What do we really know? Obstet Gynecol. 100:8–17.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karmon AE, Cardozo ER, Rueda BR and Styer
AK: MicroRNAs in the development and pathobiology of uterine
leiomyomata: Does evidence support future strategies for clinical
intervention? Hum Reprod Update. 20:670–687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park SB, Jee BC, Kim SH, Cho YJ and Han M:
Cyclooxygenase-2 inhibitor, celecoxib, inhibits leiomyoma cell
proliferation through the nuclear factor κB pathway. Reprod Sci.
21:1187–1195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Georgieva B, Milev I, Minkov I, Dimitrova
I, Bradford AP and Baev V: Characterization of the uterine
leiomyoma microRNAome by deep sequencing. Genomics. 99:275–281.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carletti MZ and Christenson LK: MicroRNA
in the ovary and female reproductive tract. J Anim Sci. 87 14
Suppl:E29–E38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marsh EE, Lin Z, Yin P, Milad M,
Chakravarti D and Bulun SE: Differential expression of microRNA
species in human uterine leiomyoma versus normal myometrium. Fertil
Steril. 89:1771–1776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan Q, Luo X and Chegini N: Differential
expression of microRNAs in myometrium and leiomyomas and regulation
by ovarian steroids. J Cell Mol Med. 12:227–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moravek MB, Yin P, Ono M, Coon JS V, Dyson
MT, Navarro A, Marsh EE, Chakravarti D, Kim JJ, Wei JJ and Bulun
SE: Ovarian steroids, stem cells and uterine leiomyoma: Therapeutic
implications. Hum Reprod Update. 21:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eldering JA, Nay MG, Hoberg LM, Longcope C
and McCracken JA: Hormonal regulation of prostaglandin production
by rhesus monkey endometrium. J Clin Endocrinol Metab. 71:596–604.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu H, Xiao Z, Wang K, Liu W and Hao Q:
MiR-145 is downregulated in human ovarian cancer and modulates cell
growth and invasion by targeting p70S6K1 and MUC1. Biochem Biophys
Res Commun. 441:693–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thum T and Mayr M: Review focus on the
role of microRNA in cardiovascular biology and disease. Cardiovasc
Res. 93:543–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu H, Shyh-Chang N, Segrè AV, Shinoda G,
Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG,
et al: The Lin28/let-7 axis regulates glucose metabolism. Cell.
147:81–94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chuang TD, Luo X, Panda H and Chegini N:
miR-93/106b and their host gene, MCM7, are differentially expressed
in leiomyomas and functionally target F3 and IL-8. Mol Endocrinol.
26:1028–1042. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chuang TD, Panda H, Luo X and Chegini N:
miR-200c is aberrantly expressed in leiomyomas in an
ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5.
Endocr Relat Cancer. 19:541–556. 2012. View Article : Google Scholar : PubMed/NCBI
|


















