Introduction
Preeclampsia (PE), preterm labor and intrauterine
growth retardation (IUGR) are detrimental pregnancy complications
that result in significant perinatal morbidity and mortality.
Normal placental development is associated with the differentiation
and invasion of trophoblasts, the predominant cellular component of
the placenta. PE is one of the most common and serious pregnancy
complications characterized by maternal endothelial dysfunction
(1). PE pathogenesis originates
from abnormal cytotrophoblast differentiation, shallow
cytotrophoblast invasion of the uterus and decreased maternal blood
flow to the placenta (2). In
addition, it is associated with future development of
cardiovascular disease in the mother and child (3).
The molecular mechanism of PE remains unclear, but
oxidative stress is considered to have an important role in the
endothelial dysfunction and systemic vasoconstriction associated
with PE (4,5). Hydrogen peroxide
(H2O2), a key factor in the cellular
oxidative stress cascade, is also reported as an important
component in placental oxidative ischemia/reperfusion stress
(6,7). Previous study has demonstrated that
more H2O2 is produced in the maternal
circulation of patients with PE in the stage of early pregnancy
(8).
Apoptosis is critical for normal placental
development and removes superfluous or dysfunctional cells to
maintain normal tissue functions. However, apoptosis also
participates in the pathophysiology of pregnancy complications
(9). In addition, apoptosis is
important in maintaining maternal immune tolerance to the antigens
expressed on trophoblasts (10,11).
Increased trophoblast apoptosis has been observed in pregnancy
complications, including PE and IUGR (12–14),
indicating that an alteration in trophoblast apoptosis may result
in these diseases (15–17).
Lycium barbarum polysaccharides (LBP) is the
active ingredient extracted from Lycium barbarum is, a plant
species which produces the wolfberry and a traditional Chinese
medicine, and has beneficial effects, particularly in the liver,
kidney and eyes (18,19). Recent reports have demonstrated
that LBP effectively improves immune function, resists oxidative
free radicals and protects the testes from high-temperature injury
(20). In China, Lycium
barbarum is often used to treat male and female infertility in
conjunction with other medicines. However, whether LBP repairs
H2O2-induced injury in trophoblast cells
remains unknown.
In the present study, an oxidative injury model of
trophoblast cells damaged by H2O2 was
established in order to determine the protective effect of LBP on
H2O2-induced injury in trophoblast cells, and
whether thus is mediated via apoptosis pathway regulation. The
results of the present study may provide a novel strategy for the
treatment of pregnancy complications, including PE and IUGR.
Materials and methods
Cell culture and treatment
Human trophoblast HTR8/SVneo cells were obtained
from the American Type Culture Collection (Manassas, VA, USA).
Cells were cultured in Dulbecco's modified Eagle's Medium
(DMEM)/F12 nutrient mixture (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin/streptomycin in a humidified incubator containing 5%
CO2 at 37°C. Cells in the logarithmic growth phase were
used in subsequent experimentation.
LBP is the main active component of the Chinese
wolfberry (21). LBP was purchased
from Qinghai General Health Bio-science Co., LLC (Xining, China)
and the purity of LBP was >50%. HTR8/SVneo cells were treated
with different concentrations (100, 200 and 400 µg/ml) of LBP
dissolved in PBS for 6 h. H2O2 (250 µmol/l;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was subsequently
added to treat cells for 6 h. There were five experimental groups
in total: LBP1 + H2O2 (cells treated with 100
µg/ml LBP and 250 µmol/l H2O2), LBP2 +
H2O2 (cells treated with 200 µg/ml LBP and
250 µmol/l H2O2), LBP3 +
H2O2 (cells treated with 400 µg/ml LBP and
250 µmol/l H2O2), H2O2
(cells treated with 250 µmol/l H2O2) and
control (without treatment) (22–24).
Cell viability assay
HTR8/SVneo cell viability was determined following
treatment with LBP and H2O2, with Cell
Counting kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology,
Haimen, China). Cells were treated with 250 µmol/l
H2O2 for 1, 4 and 6 h. Briefly, following
cells treatments, cells were seeded into a 96-well plate at an
initial density of 5×103 cells/well and incubated in
DMEM media with FBS for 24 h at 37°C. Then cells were treated with
100 µl 250 µmol/l H2O2 for 1, 4 and 6 h. At
total of 20 µl CCK-8 reagent was added in and incubated for 1 h in
a 5% CO2 incubator at 37°C. Finally, the optical density
values were acquired with a microplate reader at 450 nm.
Reactive oxygen species (ROS)
detection
ROS were detected in different groups (Control,
H2O2, LBP1 + H2O2, LBP2
+ H2O2, LBP3 + H2O2)
with a 2′,7′-dichlorofluorescin diacetate (DCFH-DA) assay (Beyotime
Institute of Biotechnology). After 6 h of 250 µmol/l
H2O2 treatment, cells, treated with different
concentrations (100, 200 and 400 µg/ml) of LBP for 6 h, were seeded
into wells of 6-well plate. DCFH-DA (10 µmol/l) was subsequently
added into each well. After incubation for 20 min at 37°C, cells
were rinsed with PBS and analyzed by flow cytometry. ROS levels
were analyzed by CellQuest software version 5.1 (BD Biosciences,
Franklin Lakes, NJ, USA) and results were calculated relative to
the control group.
Mitochondria membrane potential (MMP)
detection
Alterations in the MMP of each group treated with
LBP (100, 200 and 400 µg/ml) for 6 h and 250 µmol/l
H2O2 for another 6 h were determined by aJC-1
assay (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China), using the
cationic dye to detect potential-dependent accumulation in
mitochondria. Briefly, 5×105 cells were collected and
resuspended in 500 µl PBS with JC-1 (10 µmol/l) for 20 min at 37°C.
MMP alterations were reflected by a fluorescence emission shift
from 550 nm (red) to 525 nm (green). Cells were analyzed by flow
cytometry with Cell Quest software version 5.1 (BD
Biosciences).
Apoptosis detection
The cell apoptosis proportion in each group treated
with LBP (100, 200 and 400 µg/ml) for 6 h and 250 µmol/l
H2O2 for another 6 h were determined by an
Annexin-V/propidium iodide (PI) double-stain assay, according to
the manufacturer's protocol (Roche Diagnostics, Indianapolis, IN,
USA). Briefly, both floating and trypsinized adherent cells
(5×105) of the five experimental groups were collected
and resuspended in 500 µl PBS containing 0.5 µg/ml
Annexin-V-fluorescein isothiocyanate for 20 min. Subsequently, 400
µl PBS with 50 µg/ml PI was added to cells for 5 min at room
temperature in the dark. The analysis of cell apoptosis rate was
immediately conducted with a flow cytometer and CellQuest software
version 5.1 (BD Biosciences).
Superoxide dismutase (SOD) and lactate
dehydrogenase (LDH) detection
The cell supernatant of each group was collected.
The activity of the antioxidant enzyme SOD was determined using the
total SOD assay kit with WST-8 (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. During the
reaction process of SOD detection, 2-iodophenyl-3-nitrophenyl
tetrazolium chloride was catalyzed to formazin, which could be
detected by a microplate reader. The activity of LDH was detected
with a LDH assay kit (Beyotime Institute of Biotechnology),
according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of survivin, hypoxia
inducible factor 1-α (HIF1-α) and Bcl-2 apoptosis regulator
(Bcl-2), Bcl-2 associated X apoptosis regulator (Bax) in each group
was measured by RT-qPCR. Total RNA was extracted from cells using
an RNeasy kit, and 1 µg RNA was reverse transcribed to cDNA using a
Quantiscript Reverse Transcriptase kit (both Qiagen, Inc.,
Valencia, CA, USA), according to the manufacturer's protocol. PCR
amplification was performed for 15 sec at 95°C, followed by 40
cycles of denaturation at 95°C for 15 sec and annealing/extension
at 60°C for 25 sec in an ABI 7300 Thermocycler (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with Fast SYBR-Green Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.). GAPDH was
used as the reference gene; primer sequences are displayed in
Table I. The quantification was
identified by 2−ΔΔCq method (25).
 | Table I.Primers used in the reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used in the reverse
transcription-quantitative polymerase chain reaction.
| Name | Direction | Sequence
(5′-3′) | Size (base
pairs) |
|---|
| GAPDH | Forward |
CCATCTTCCAGGAGCGAGAT | 222 |
|
| Reverse |
TGCTGATGATCTTGAGGCTG |
|
| Survivin | Forward |
GGACCACCGCATCTCTACAT | 191 |
|
| Reverse |
TTGGTTTCCTTTGCATGGGG |
|
| HIF1-α | Forward |
CAGTCGACACAGCCTGGATA | 210 |
|
| Reverse |
CCACCTCTTTTGGCAAGCAT |
|
| Bax | Forward |
AACATGGAGCTGCAGAGGAT | 208 |
|
| Reverse |
AACATGGAGCTGCAGAGGAT |
|
| Bcl-2 | Forward |
TTCTTTGAGTTCGGTGGGGT | 207 |
|
| Reverse |
CTTCAGAGACAGCCAGGAGA |
|
Western blot analysis
Cells were harvested, washed twice with PBS, lysed
using lysis buffer (50 mM Tris-Cl, 150 mM NaCl, 0.02% NaN2, 100
µg/ml phenylmethanesulfonyl fluoride, 1 µg/ml aprotinin, and 1%
Triton X-100) and centrifuged at a speed of 12,000 × g for 30 min
at 4°C. The supernatant was subsequently collected from the lysate
and protein concentration was determined by a bicinchoninic acid
protein assay (Beyotime Institute of Biotechnology). A total of 10
µg proteins were boiled and separated by 10% SDS-PAGE, followed by
transfer onto polyvinylidene fluoride (PVDF) membranes. Membranes
were blocked with 5% non-fat dry milk in PBS for 1 h at 37°C and
incubated with the following primary antibodies at 4°C overnight:
Anti-survivin (cat. no. ab76424; 1:5,000), anti-HIF1-α (cat. no.
ab51608; 1:1,000), anti-Bax (cat. no. ab53154; 1:1,000), anti-Bcl-2
(cat. no. ab59348; 1:1,000), anti-GAPDH (cat. no. ab9485; 1:2,500;
all Abcam, Cambridge, MA, USA). Subsequently, proteins were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (cat. no. ab6721; 1:5,000; Abcam). PVDF
membranes were exposed to X-ray film and immunoreactive bands were
detected with an enhanced chemiluminescence detection kit (GE
Healthcare Life Sciences). Finally, protein density was detected by
Bio-Rad ChemiDoc XRS+ System with Image Lab Software version 4.1
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data is expressed as the mean ± standard deviation
of three independent experiments. Statistical analyses were
performed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) and
significance was calculated with one-way analysis of variance
followed by Dunnett's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
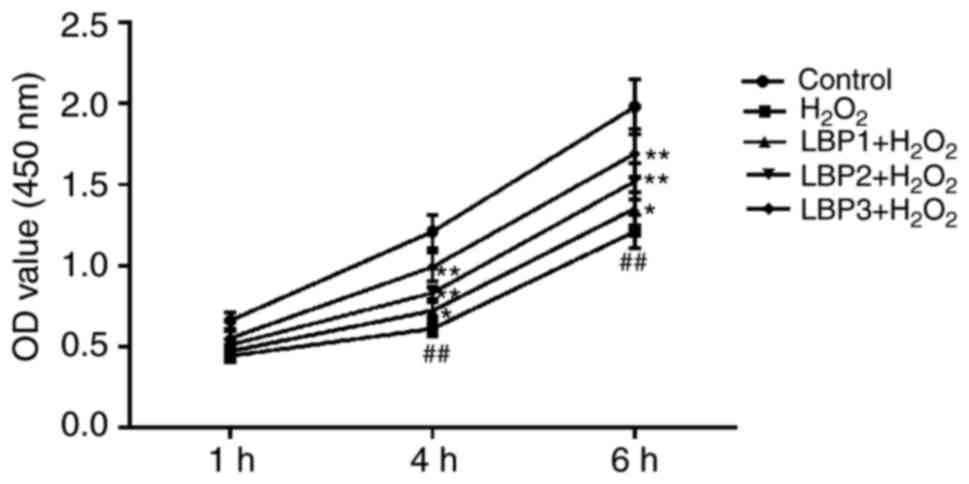
LBP protects the proliferative ability
of HTR8/SVneo cells injured with H2O2
To identify the effects of LBP on the proliferation
of HTR8/SVneo cells injured by H2O2, a CCK8
assay was conducted in each experimental group (control,
H2O2, LBP1 + H2O2, LBP2
+ H2O2 and LBP3 + H2O2
groups). The results revealed that the cell proliferation ability
of the H2O2 group was significantly reduced
by 250 µmol/l H2O2 in a time-dependent
manner, compared with the control group (P<0.01). When
pre-treated with LBP for 6 h prior to the addition of
H2O2, proliferation in the LBP1 +
H2O2, LBP2 + H2O2 and
LBP3 + H2O2 groups significantly increased in
a dose-dependent manner, compared with the
H2O2 group (P<0.05), indicating that LBP
may protect the cell proliferation ability of HTR8/SVneo cells
injured by H2O2 (Fig. 1).
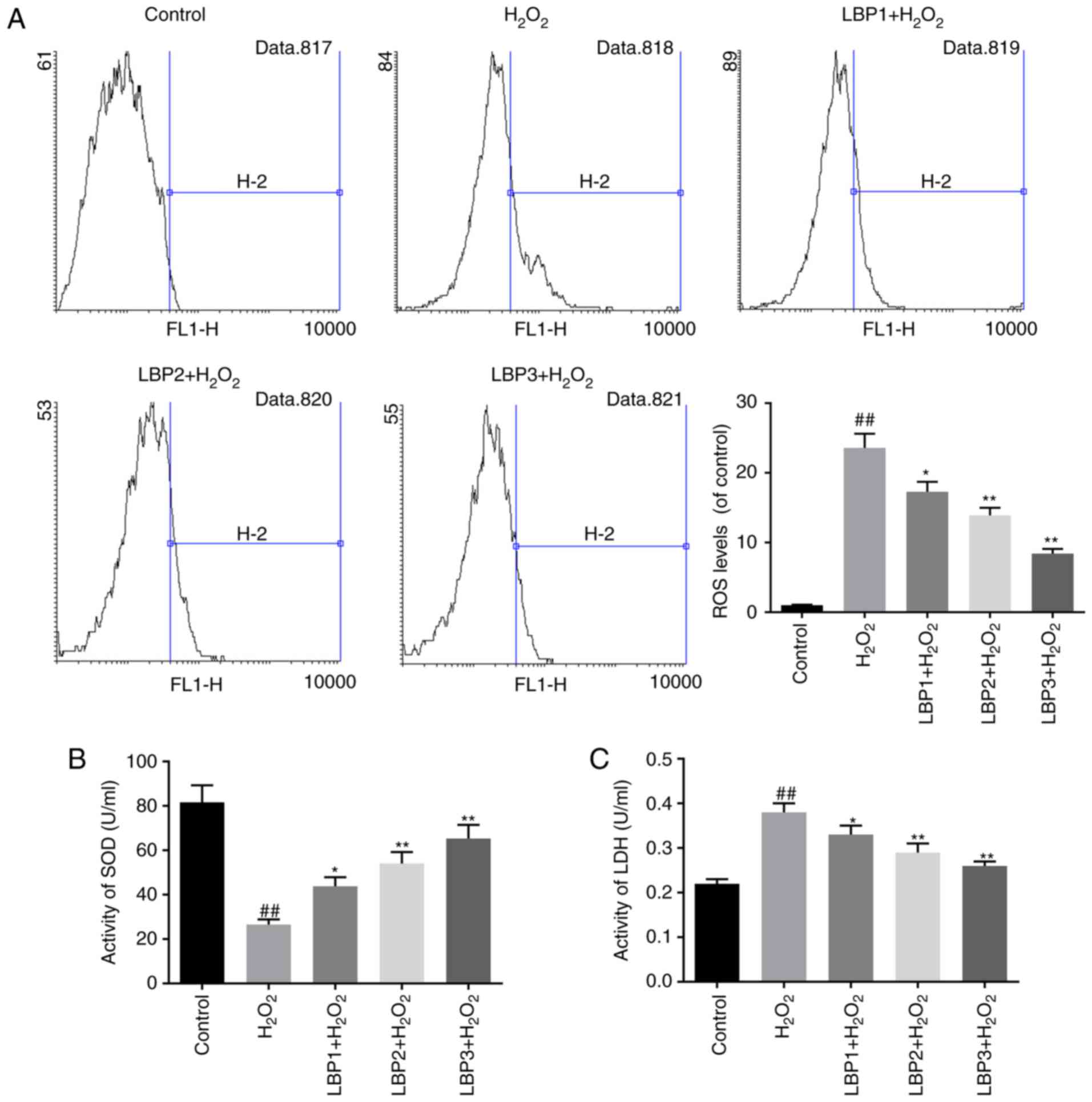
LBP reduces ROS levels in HTR8/SVneo
cells injured by H2O2
Intracellular ROS levels were measured using the
oxygen-sensitive fluorescent dye DCTH-DA. The assay results
revealed that the accumulation of ROS in HTR8/SVneo cells was
significantly increased in the H2O2 group,
compared with the control (P<0.01). Furthermore, a significant
decrease in ROS formation was detected in the LBP1 +
H2O2, LBP2 + H2O2 and
LBP3 + H2O2 groups, in a dose-dependent
manner, compared with the H2O2 group
(P<0.05; Fig. 2A).
LBP increases SOD levels and decreases
LDH levels in HTR8/SVneo cells injured by
H2O2
LDH leakage reflects cytotoxicity and mitochondrial
damage (26). Levels of
antioxidant enzyme SOD and leaked LDH in the cell supernatant were
determined by the corresponding assay kits. Decreased SOD activity
and increased LDH activity was detected in the
H2O2 group, compared with the control group
(P<0.01). LBP increased SOD activity and decreased LDH activity
in dose-dependent manner, compared with H2O2
group (P<0.05; Fig. 2B and
C).
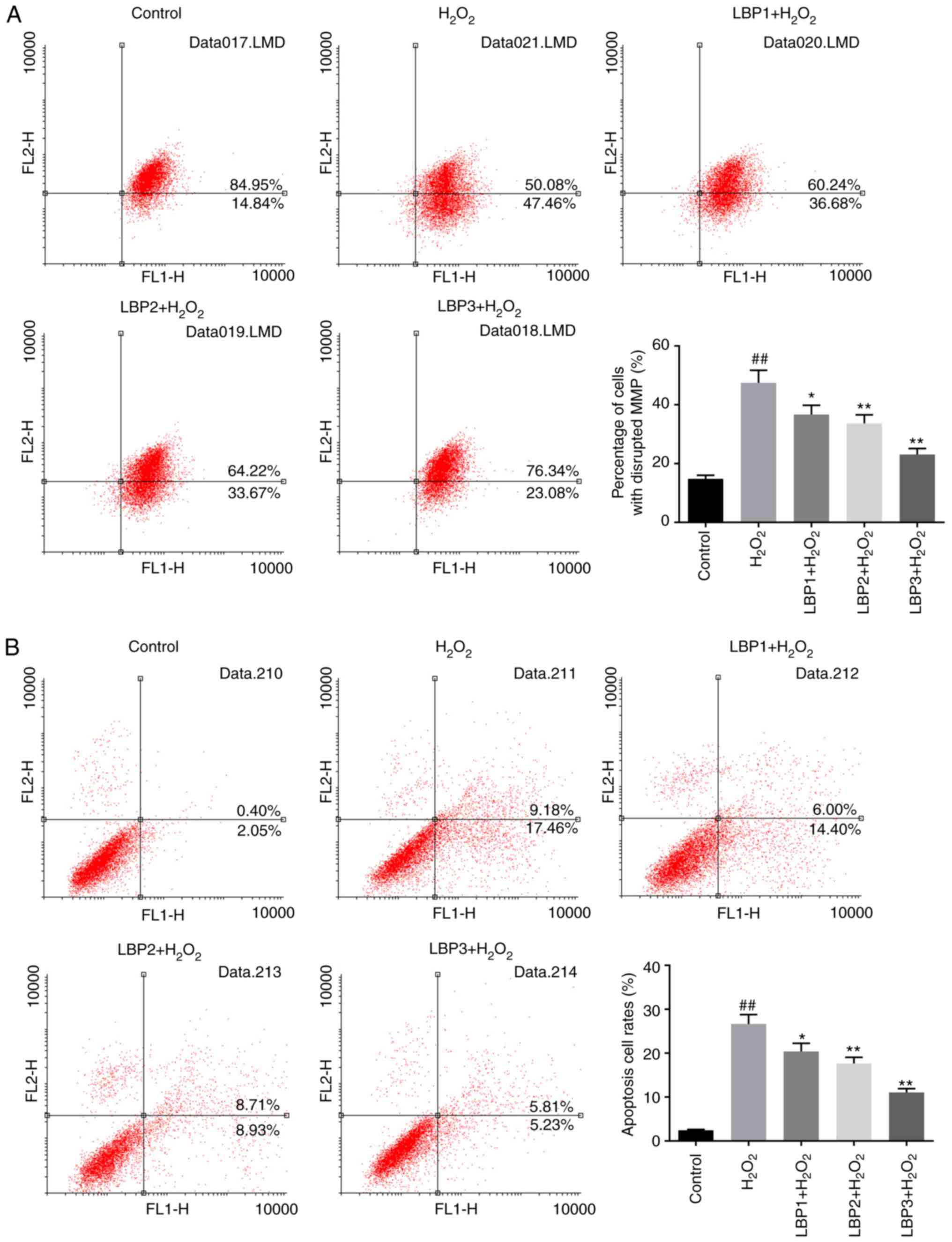
LBP reduces the percentage of cells
with MMP disruption in HTR8/SVneo cells injured by
H2O2
The percentage of cells with MMP disruption was
measured by JC-1 assay. The percentage of cells with MMP disruption
in the H2O2 group was significantly increased
compared with the control group (P<0.01). The percentage of
cells with MMP disruption in the LBP1 + H2O2,
LBP2 + H2O2 and LBP3 +
H2O2 groups decreased significantly in a
dose-dependent manner, when pretreated with different
concentrations of LBP (100, 200 and 400 µg/ml) prior to
H2O2 injury, compared with the
H2O2 group (P<0.05; Fig. 3A). These results indicate that LBP
may reduce MMP disruption in HTR8/SVneo cells with
H2O2-induced injury.
LBP inhibits cell apoptosis induced by
H2O2 in HTR8/SVneo cells
An Annexin-V/PI double-stain assay was performed to
detect the apoptosis status of each group. The apoptosis rate of
the H2O2 group significantly increased
compared with control group (P<0.01); apoptosis in the LBP1 +
H2O2, LBP2 + H2O2 and
LBP3 + H2O2 groups was significantly
decreased in a dose-dependent manner, compared with the
H2O2 group (P<0.05; Fig. 3B). This indicated that LBP may
inhibit H2O2-induced apoptosis in HTR8/SVneo
cells.
LBP regulates the expression of
apoptosis-associated factors in HTR8/SVneo cells injured by
H2O2
To detect whether the protective function of LBP on
HTR8/SVneo cells injured by H2O2 was via
regulation of apoptosis-associated factors, RT-qPCR (Fig. 4A) and western blot analysis
(Fig. 4B and C) was performed to
detect the expression of survivin, HIF1-α, Bax and Bcl-2 in
TR8/SVneo cells treated with H2O2 and/or LBP.
The results revealed that the mRNA and protein levels of survivin,
HIF1-α and Bcl-2 decreased significantly in
H2O2 group compared with control group, and
significantly increased in the LBP treated groups in a dose
dependent manner (P<0.05). The mRNA and protein levels of Bax
exhibited the opposite trend (P<0.05).
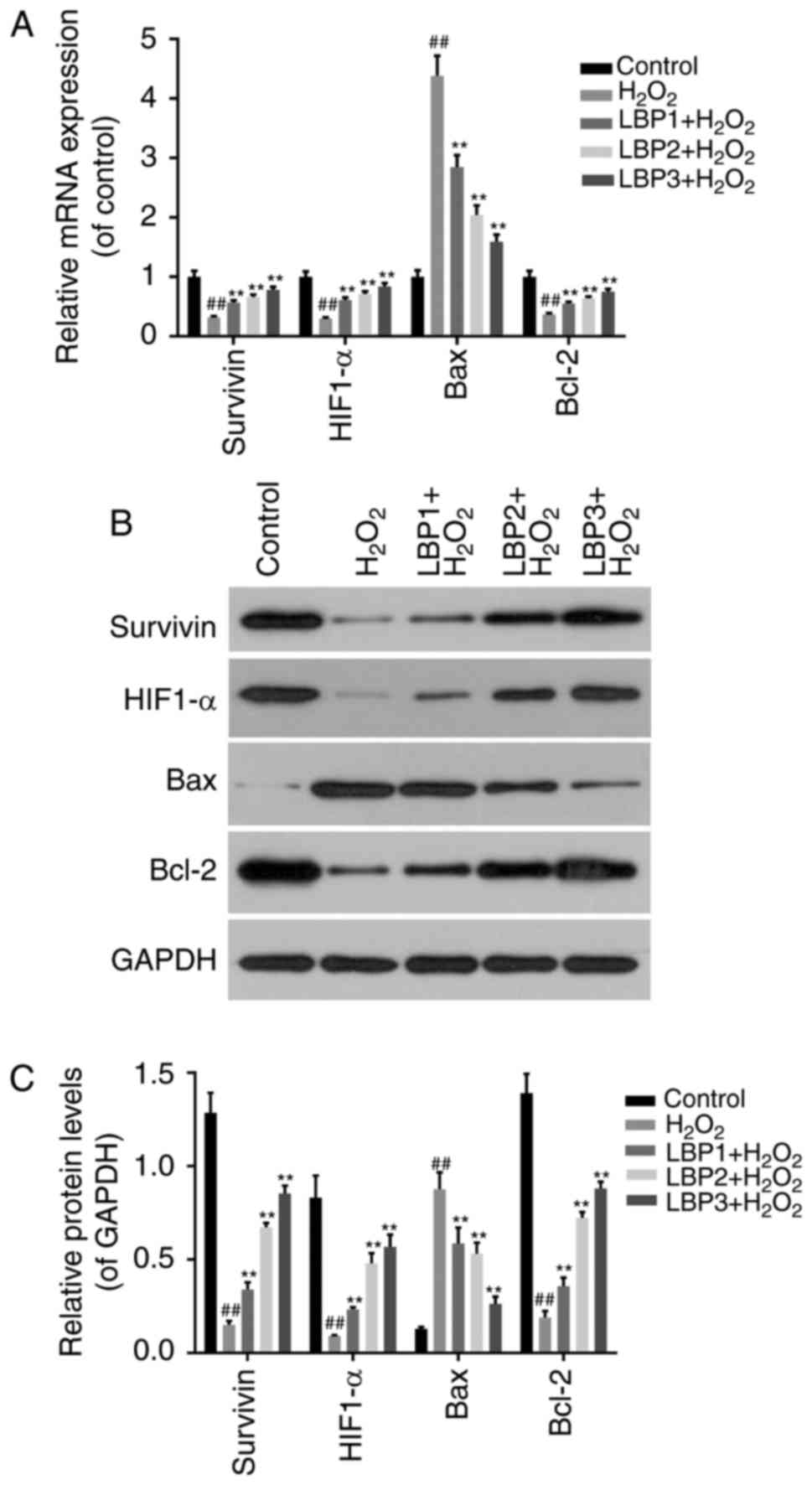 | Figure 4.LBP regulates the expression of
apoptosis-associated factors in HTR8/SVneo cells injured by
H2O2. (A) Reverse transcription-quantitative
polymerase chain reaction was performed to detect the mRNA levels
of survivin, HIF1-α, Bax and Bcl-2 in TR8/SVneo cells treated with
H2O2 and/or LBP. (B) Western blot analysis
was performed to detect the protein levels of survivin, HIF1-α, Bax
and Bcl-2 in TR8/SVneo cells treated with
H2O2 and/or LBP. (C) Relative protein levels
were determined by densitometric analysis. ##P<0.01
vs. control group; *P<0.05, **P<0.01 vs.
H2O2 group. H2O2,
hydrogen peroxide; LBP, Lycium barbarum polysaccharide;
HIF1-α, hypoxia inducible factor 1-α; Bax, Bax, Bcl-2 associated X
apoptosis regulator; Bcl-2, Bcl-2 apoptosis regulator. |
Discussion
Pregnancy complications, including PE, cause marked
damage to normal fetal development, which is associated with
abnormal cytotrophoblast differentiation and invasion. As a key
factor of the cellular oxidative stress cascade,
H2O2 is considered an important component in
placental oxidative ischemia/reperfusion stress. LBP has been
reported to protect the testes from high-temperature injury, and
improve immune ability and oxidative free radical resistance
(27). However, whether LBP
reduces H2O2-induced injury in trophoblast
cells remains unclear.
LBPs are efficient free radical scavengers in
vivo and may be useful in counteracting oxidative stress
associated with aging (28,29).
Therefore, the function of LBPs on oxidative injury in trophoblast
cells in vitro was investigated in the present study. At
present, H2O2 is the most commonly used
substance to construct a cell model of oxidative stress in various
research fields (30,31). In the present study, an oxidative
injury model was established in trophoblast cells injured by
H2O2 and the effects of LBP were examined,
including those on oxidative stress, MMP and cell apoptosis. The
proliferative ability of human trophoblast HTR8/SVneo cells
following various treatments was evaluated with aCCK-8 assay and
revealed that proliferation was reduced by
H2O2 and LBP reduced the damage caused by
H2O2 in a dose-dependent manner.
Oxidative stress is the imbalance of oxidative and
anti-oxidative factors in cells, resulting in more ROS production
than ROS elimination, causing cell and tissue damage (32). Abundant ROS accumulate when the
placenta is under conditions of ischemia hypoxia, and trophoblast
cells suffer from oxidative stress when pregnancy complications
exist (33–36). The present study demonstrated that
H2O2 treatment of cells resulted in abundant
ROS accumulation in trophoblast cells, and LBP decreased the levels
of ROS and oxidative stress induced by H2O2.
Following this, the potential effect of LBP on antioxidant levels
in H2O2 injured trophoblast HTR8/SVneo cells
was also investigated. SOD is the most important antioxidant in the
defense system of antioxidative injury (37). The latest LBP chemical component
analysis demonstrated that all glycopeptides in LBP act as the
eliminators of lipid peroxide active components (38–40).
Thus, it was speculated that as an anti-oxidant, LBP may decrease
trophoblast cell injury induced by oxygen free radicals and protect
cell development and differentiation. The results of the present
study demonstrated that LBP significantly increased SOD activity
and reduced ROS levels simultaneously, to protect cells from
oxidative injury. Disruption of the cell membrane induced by
oxidative stress or apoptosis may mediate the release of enzymes
from the cytoplasm into the culture media, including LDH, which is
relatively stable. By detecting the amount of LDH leakage into
culture media, the cytomembrane integrity was measured. The results
indicated that LDH leakage was attenuated by LBP treatment in
H2O2 injured trophoblast cells.
Oxidative stress is one of the main causes of cell
apoptosis, through the mitochondria-dependent or independent
pathways (41). Mitochondrial
dysfunction caused by damage to structural integrity induces
oxidative stress and the release of ROS (42). In addition, the apoptosis rates may
reflect the degree of oxidative stress (43–45).
The present research demonstrated an increased percentage of cells
with MMP disruption and apoptosis in trophoblast cells injured by
H2O2, whereas LBP could markedly alleviate
these effects. Furthermore, it was revealed that the function of
LBP in protecting trophoblast cells from
H2O2-induced injury may be via regulation of
apoptosis-associated factors, including survivin, HIF1-α, Bax and
Bcl-2.
Survivin is an important member of the inhibitor of
apoptosis protein family that regulates apoptosis and cell division
(46). Previous research has
demonstrated that the expression of survivin in embryonic
development promotes tissue stability and differentiation (47,48).
The inhibition of survivin expression in the early stage of
embryonic development results in embryonic deformities (49). HIF1-α is highly expressed in
anaerobic conditions, and promotes cell proliferation and tumor
angiogenesis in cancer (50,51).
Previous studies demonstrated that HIF1-α overexpression is
associated with tumor aggressiveness in human neoplasms (52,53).
HIF1-α knockdown promotes cell apoptosis by downregulating
surviving, and upregulating caspase-3 and other apoptosis-promoting
factors (54). Bcl-2 family
members directly regulate death signals, or act indirectly through
the intrinsic pathways of apoptosis, including regulation of
pro-apoptotic factor release from the mitochondria. Aberrant excess
expression of Bcl-2 inhibits cell apoptosis and induces tumor
development, including in gastric, lung, papillary thyroid and
ovarian cancer (55–58). Bax interacts with Bcl-2 to suppress
its apoptosis-inhibiting effects. The data of the present study
indicated that H2O2 may suppress the mRNA and
protein expression of survivin, HIF1-α and Bcl-2, and facilitate
Bax expression to promote apoptosis and oxidative stress. LBP
pre-treatment promoted the expression of survivin, HIF1-α and
Bcl-2, and decreased Bax expression to inhibit apoptosis and
oxidative stress. Taken together, the results of the present study
suggest that LBP alleviated H2O2-induced
oxidative stress through altering MMP, inhibiting cell apoptosis
and regulating the expression of apoptosis-associated factors in
HTR8/SVneo cells.
In summary, an H2O2-injured
HTR8/SVneo trophoblast cell model was constructed and LBP was
demonstrated to reduce oxidative stress and apoptosis via
regulating the expression of apoptosis-associated factors. Future
research should focus on elucidating the mechanism of LBP in
oxidative stress alleviation in trophoblast cells. Increased
trophoblast cell protection from oxidative stress and apoptosis in
pregnancy may provide improved treatment outcomes for individuals
with pregnancy complications. In the future, the authors of the
present study will examine the effects of LBP from a mechanistic
view, in order to identify the receptors and downstream signals
involved.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
JL designed the study; JL and ZD performed the flow
cytometry to investigate the role of LBP on ROS, mitochondria and
apoptosis regulation; YY detected cell viability; BM and YW did the
RT-qPCR and western blotting assays; XX analyzed and interpreted
all the experiments' data, drafted the manuscript and revised
it.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim YM, Chaiworapongsa T, Gomez R, Bujold
E, Yoon BH, Rotmensch S, Thaler HT and Romero R: Failure of
physiologic transformation of the spiral arteries in the placental
bed in preterm premature rupture of membranes. Am J Obstet Gynecol.
187:1137–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pardi G, Marconi AM and Cetin I:
Pathophysiology of intrauterine growth retardation: Role of the
placenta. Acta Paediatr Suppl. 423:170–172. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hauspurg A, Ying W, Hubel CA, Michos ED
and Ouyang P: Adverse pregnancy outcomes and future maternal
cardiovascular disease. Clin Cardiol. 41:239–246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hannan NJ, Binder NK, Beard S, Nguyen TV,
Kaitu'u-Lino TJ and Tong S: Melatonin enhances antioxidant
molecules in the placenta, reduces secretion of soluble fms-like
tyrosine kinase 1 (sFLT) from primary trophoblast but does not
rescue endothelial dysfunction: An evaluation of its potential to
treat preeclampsia. PLoS One. 13:e01870822018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wan J, Hu Z, Zeng K, Yin Y, Zhao M, Chen M
and Chen Q: The reduction in circulating levels of estrogen and
progesterone in women with preeclampsia. Pregnancy Hypertens.
11:18–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagai R, Watanabe K, Wakatsuki A, Hamada
F, Shinohara K, Hayashi Y, Imamura R and Fukaya T: Melatonin
preserves fetal growth in rats by protecting against
ischemia/reperfusion-induced oxidative/nitrosative mitochondrial
damage in the placenta. J Pineal Res. 45:271–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okatani Y, Wakatsuki A, Shinohara K,
Taniguchi K and Fukaya T: Melatonin protects against oxidative
mitochondrial damage induced in rat placenta by ischemia and
reperfusion. J Pineal Res. 31:173–178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aris A, Benali S, Ouellet A, Moutquin JM
and Leblanc S: Potential biomarkers of preeclampsia: Inverse
correlation between hydrogen peroxide and nitric oxide early in
maternal circulation and at term in placenta of women with
preeclampsia. Placenta. 30:342–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Straszewski-Chavez SL, Abrahams VM and Mor
G: The role of apoptosis in the regulation of trophoblast survival
and differentiation during pregnancy. Endocr Rev. 26:877–897. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukushima K, Miyamoto S, Tsukimori K,
Kobayashi H, Seki H, Takeda S, Kensuke E, Ohtani K, Shibuya M and
Nakano H: Tumor necrosis factor and vascular endothelial growth
factor induce endothelial integrin repertories, regulating
endovascular differentiation and apoptosis in a human extravillous
trophoblast cell line. Biol Reprod. 73:172–179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huppertz B, Frank HG, Kingdom JC, Reister
F and Kaufmann P: Villous cytotrophoblast regulation of the
syncytial apoptotic cascade in the human placenta. Histochem Cell
Biol. 110:495–508. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lewis MP, Clements M, Takeda S, Kirby PL,
Seki H, Lonsdale LB, Sullivan MH, Elder MG and White JO: Partial
characterization of an immortalized human trophoblast cell-line,
TCL-1, which possesses a CSF-1 autocrine loop. Placenta.
17:137–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith SC, Baker PN and Symonds EM:
Increased placental apoptosis in intrauterine growth restriction.
Am J Obstet Gynecol. 177:1395–1401. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crocker IP, Cooper S, Ong SC and Baker PN:
Differences in apoptotic susceptibility of cytotrophoblasts and
syncytiotrophoblasts in normal pregnancy to those complicated with
preeclampsia and intrauterine growth restriction. Am J Pathol.
162:637–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishihara N, Matsuo H, Murakoshi H,
Laoag-Fernandez JB, Samoto T and Maruo T: Increased apoptosis in
the syncytiotrophoblast in human term placentas complicated by
either preeclampsia or intrauterine growth retardation. Am J Obstet
Gynecol. 186:158–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DiFederico E, Genbacev O and Fisher SJ:
Preeclampsia is associated with widespread apoptosis of placental
cytotrophoblasts within the uterine wall. Am J Pathol. 155:293–301.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moindjie H, Santos ED, Gouesse RJ,
Swierkowski-Blanchard N, Serazin V, Barnea ER, Vialard F and
Dieudonne MN: Preimplantation factor is an anti-apoptotic effector
in human trophoblasts involving p53 signaling pathway. Cell Death
Dis. 7:e25042016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Liu Y, Sun Y, Mou Q, Wang B, Zhang
Y and Huang L: Structural characterization of LbGp1 from the fruits
of Lycium barbarum L. Food Chem. 159:137–142. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varoni MV, Gadau SD, Pasciu V, Baralla E,
Serra E, Palomba D and Demontis MP: Investigation of the effects of
Lycium barbarum polysaccharides against cadmium induced
damage in testis. Exp Mol Pathol. 103:26–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Po KK, Leung JW, Chan JN, Fung TK,
Sánchez-Vidaña DI, Sin EL, So KF, Lau BW and Siu AM: Protective
effect of Lycium Barbarum polysaccharides on
dextromethorphan-induced mood impairment and neurogenesis
suppression. Brain Res Bull. 134:10–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu WJ, Jiang HF, Rehman FU, Zhang JW,
Chang Y, Jing L and Zhang JZ: Lycium barbarum
polysaccharides decrease hyperglycemia-aggravated ischemic brain
injury through maintaining mitochondrial fission and fusion
balance. Int J Biol Sci. 13:901–910. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Lao W, Ji QS, Yang ZH, Yu GC and
Zhong JX: Lycium barbarum polysaccharides protected human
retinal pigment epithelial cells against oxidative stress-induced
apoptosis. Int J Ophthalmol. 8:11–16. 2015.PubMed/NCBI
|
|
23
|
Zhang XJ, Yu HY, Cai YJ and Ke M:
Lycium barbarum polysaccharides inhibit proliferation and
migration of bladder cancer cell lines BIU87 by suppressing
Pi3K/AKT pathway. Oncotarget. 8:5936–5942. 2017.PubMed/NCBI
|
|
24
|
Tian JY, Chen WW, Cui J, Wang H, Chao C,
Lu ZY and Bi YY: Effect of Lycium bararum polysaccharides on
methylmercury-induced abnormal differentiation of hippocampal stem
cells. Exp Ther Med. 12:683–689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Datta S and Chakrabarti N: Age related
rise in lactate and its correlation with lactate dehydrogenase
(LDH) status in post-mitochondrial fractions isolated from
different regions of brain in mice. Neurochem Int. 118:23–33. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shan T, Shan T, Liu F, Zheng H and Li G:
Effects of Lycium barbarum polysaccharides on the damage to
human endometrial stromal cells induced by hydrogen peroxide. Mol
Med Rep. 15:879–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao Y, Wei Y, Wang Y, Gao F and Chen Z:
Lycium barbarum: A traditional chinese herb and a promising
anti-aging agent. Aging Dis. 8:778–791. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin CL, Wang CC, Chang SC, Inbaraj BS and
Chen BH: Antioxidative activity of polysaccharide fractions
isolated from Lycium barbarum Linnaeus. Int J Biol Macromol.
45:146–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bittner L, Wyck S, Herrera C, Siuda M,
Wrenzycki C, van Loon B and Bollwein H: Negative effects of
oxidative stress in bovine spermatozoa on in vitro development and
DNA integrity of embryos. Reprod Fertil Dev. May 1–2018.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Che X, Zhao Q and Li D: Expression of
thioredoxin-2 in human lens epithelial cells with oxidative damage
and its significance. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
43:253–259. 2018.(In Chinese). PubMed/NCBI
|
|
32
|
Kupsco A and Schlenk D: Oxidative stress,
unfolded protein response, and apoptosis in developmental toxicity.
Int Rev Cell Mol Biol. 317:1–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Poston L, Igosheva N, Mistry HD, Seed PT,
Shennan AH, Rana S, Karumanchi SA and Chappell LC: Role of
oxidative stress and antioxidant supplementation in pregnancy
disorders. Am J Clin Nutr. 94 6 Suppl:1980S–1985S. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deepa D, Jayakumari N and Thomas SV:
Oxidative stress is increased in women with epilepsy: Is it a
potential mechanism of anti-epileptic drug-induced teratogenesis?
Ann Indian Acad Neurol. 15:281–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kovacic P and Somanathan R: Mechanism of
teratogenesis: Electron transfer, reactive oxygen species, and
antioxidants. Birth Defects Res C Embryo Today. 78:308–325. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burton GJ and Jauniaux E: Placental
oxidative stress: From miscarriage to preeclampsia. J Soc Gynecol
Investig. 11:342–352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su Y, Han W and Cao Y: Association between
activities of SOD, MDA and Na+-K+-ATPase in peripheral blood of
patients with acute myocardial infarction and the complication of
varying degrees of arrhythmia. Hellenic J Cardiol. Apr
24–2018.(Epub ahead of print).
|
|
38
|
Schoppet M, Tailhades J, Kulkarni K and
Cryle MJ: Precursor Manipulation in Glycopeptide Antibiotic
Biosynthesis: Are β-Amino Acids Compatible with the Oxidative
Cyclization Cascade? J Org Chem. Apr 30–2018.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen L, Li W, Qi D and Wang D: Lycium
barbarum polysaccharide protects against LPS-induced ARDS by
inhibiting apoptosis, oxidative stress, and inflammation in
pulmonary endothelial cells. Free Radic Res. 52:480–490. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Varoni MV, Pasciu V, Gadau SD, Baralla E,
Serra E, Palomba D and Demontis MP: Possible antioxidant effect of
Lycium barbarum polysaccharides on hepatic cadmium-induced
oxidative stress in rats. Environ Sci Pollut Res Int. 24:2946–2955.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen S, Lv X, Hu B, Zhao L, Li S, Li Z,
Qing X, Liu H, Xu J and Shao Z: Critical contribution of RIPK1
mediated mitochondrial dysfunction and oxidative stress to
compression-induced rat nucleus pulposus cells necroptosis and
apoptosis. Apoptosis. Apr 28–2018.(Epub ahead of print). View Article : Google Scholar
|
|
43
|
Ahmadian E, Khosroushahi AY, Eftekhari A,
Farajnia S, Babaei H and Eghbal MA: Novel angiotensin receptor
blocker, azilsartan induces oxidative stress and NFkB-mediated
apoptosis in hepatocellular carcinoma cell line HepG2. Biomed
Pharmacother. 99:939–946. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liang J, Wu S, Xie W and He H: Ketamine
ameliorates oxidative stress-induced apoptosis in experimental
traumatic brain injury via the Nrf2 pathway. Drug Des Devel Ther.
12:845–853. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sarvestani Namazi N, Firouzi Saberi S,
Falak R, Karimi MY, Gholami Davoodzadeh M, Rangbar A and Hosseini
A: Phosphodiesterase 4 and 7 inhibitors produce protective effects
against high glucose-induced neurotoxicity in PC12 cells via
modulation of the oxidative stress, apoptosis and inflammation
pathways. Metab Brain Dis. Apr 30–2018.(Epub ahead of print).
|
|
46
|
Zhang Z, Wang H, Jin Z, Cai X, Gao N, Cui
X, Liu P, Zhang J, Yang S and Yang X: Downregulation of survivin
regulates adult hippocampal neurogenesis and apoptosis, and
inhibits spatial learning and memory following traumatic brain
injury. Neuroscience. 300:219–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kawasaki H, Altieri DC, Lu CD, Toyoda M,
Tenjo T and Tanigawa N: Inhibition of apoptosis by survivin
predicts shorter survival rates in colorectal cancer. Cancer Res.
58:5071–5074. 1998.PubMed/NCBI
|
|
49
|
Adida C, Crotty PL, McGrath J, Berrebi D,
Diebold J and Altieri DC: Developmentally regulated expression of
the novel cancer anti-apoptosis gene survivin in human and mouse
differentiation. Am J Pathol. 152:43–49. 1998.PubMed/NCBI
|
|
50
|
Magnon C, Opolon P, Ricard M, Connault E,
Ardouin P, Galaup A, Métivier D, Bidart JM, Germain S, Perricaudet
M and Schlumberger M: Radiation and inhibition of angiogenesis by
canstatin synergize to induce HIF-1alpha-mediated tumor apoptotic
switch. J Clin Invest. 117:1844–1855. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fujiwara S, Nakagawa K, Harada H, Nagato
S, Furukawa K, Teraoka M, Seno T, Oka K, Iwata S and Ohnishi T:
Silencing hypoxia-inducible factor-1alpha inhibits cell migration
and invasion under hypoxic environment in malignant gliomas. Int J
Oncol. 30:793–802. 2007.PubMed/NCBI
|
|
52
|
Unruh A, Ressel A, Mohamed HG, Johnson RS,
Nadrowitz R, Richter E, Katschinski DM and Wenger RH: The
hypoxia-inducible factor-1 alpha is a negative factor for tumor
therapy. Oncogene. 22:3213–3220. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zagzag D, Zhong H, Scalzitti JM, Laughner
E, Simons JW and Semenza GL: Expression of hypoxia-inducible factor
1alpha in brain tumors: Association with angiogenesis, invasion,
and progression. Cancer. 88:2606–2618. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li X, Liu X, Xu Y, Liu J, Xie M, Ni W and
Chen S: KLF5 promotes hypoxia-induced survival and inhibits
apoptosis in non-small cell lung cancer cells via HIF-1α. Int J
Oncol. 45:1507–1514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mitselou A, Peschos D, Dallas P,
Charalabopoulos K, Agnantis NJ and Vougiouklakis T:
Immunohistochemical analysis of expression of bcl-2 protein in
papillary carcinomas and papillary microcarcinomas of the thyroid
gland. Exp Oncol. 26:282–286. 2004.PubMed/NCBI
|
|
56
|
Basolo F, Pollina L, Fontanini G, Fiore L,
Pacini F and Baldanzi A: Apoptosis and proliferation in thyroid
carcinoma: Correlation with bcl-2 and p53 protein expression. Br J
Cancer. 75:537–541. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yoo NJ, Kim MS and Lee SH: Expression and
mutation analyses of Fas, FLIP and Bcl-2 in granulosa cell tumor of
ovary. Tumori. 98:118e–121e. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yildirim M, Suren D, Goktas S, Dilli UD,
Kaya C, Copuroglu R, Yildiz M and Sezer C: The predictive role of
Bcl-2 expression in operable locally advanced or metastatic gastric
carcinoma. J BUON. 17:106–109. 2012.PubMed/NCBI
|