Introduction
Dexmedetomidine (DEX) is an anesthetic agent, which,
due to its ability to produce a rapid and controlled sedative
response and few side effects, is used in medical procedures for
long-lasting sedation of patients (1–3).
Previous studies indicated that DEX can prevent brain ischemia,
lung ischemia-reperfusion injury, acute kidney injury, intestinal
ischemia-reperfusion injury and myocardial ischemia, alleviate
intracellular accumulation of reactive oxygen species (ROS) and
prevent apoptotic cell death in vivo (1–12). A
recent study demonstrated that DEX exerts its neuroprotective
effects on apoptotic, calcium and oxidative stress signaling
pathways through regulation of the transient receptor potential
cation channel, subfamily M, member 2 and transient receptor
potential cation channel subfamily V member 1 in rats (13). Neuroprotection of DEX has also been
associated with the inhibition of the pro-inflammatory nuclear
factor-κB and interleukin-1β signaling pathways (1,14).
Similarly, another in vivo study demonstrated that DEX
protects from cerebral ischemia/reperfusion injury in rats via
regulation of the phosphatidylinositol 4,5-bisphosphate
3-kinase/RAC-alpha serine/threonine-protein kinase and
extracellular signal-regulated kinase (ERK)1/2 signaling pathways
(15). However, the molecular
mechanisms underlying neuroprotective properties of DEX remain to
be elucidated.
MicroRNAs (miRs), small non-coding gene regulatory
RNAs, serve neuroprotective roles by regulating physiological or
pathological cellular processes involving cell invasion and
migration, and responses to stress and apoptosis (16–21).
Previous studies indicated that miR-223 prevents alcohol-induced
liver injury in neutrophils by inhibiting the
interleukin-6-p47phox-ROS signaling pathway (22). It has also been demonstrated that
CXCR2/miR-223-3p/miR-27b pathway protects from ischemic cerebral
injury (23). However, whether
miR-223-3p serves a role in the oxidative stress-associated brain
injury remains to be elucidated. In addition, to the best of the
authors knowledge, the association between miR-223-3p and the
neuroprotective effect of DEX has not yet been reported.
TIA1 cytotoxic granule associated RNA binding
protein like 1 (TIAL1) is a gene encoding a member of an
RNA-binding protein family that regulates a set of cellular
activities, including splicing and regulation of translation and
apoptosis. The role of TIAL1 in cytotoxicity remains to be
elucidated, but due to its apoptotic and RNA-binding properties,
the authors of the present study hypothesized that modulation of
TIAL1 can mediate the neuroprotective effects of DEX on hippocampal
cells in an miR-dependent manner.
Therefore, the present study aimed to investigate
the effect of oxidative stress on the expression of miR-223-3p in
hippocampal neurons and determine whether the neuroprotective
activity of DEX is mediated by the regulation of miR-223-3p. The
results demonstrated that miR-223-3p was significantly
downregulated as a response to oxidative injury. Furthermore,
expression of miR-223-3p was significantly upregulated following
treatment with DEX and overexpression of miR-223-3p using
miR-223-3p mimics resulted in neuroprotection. Therefore, the
results of the present study indicated that DEX-mediated
upregulation of miR-223-3p is involved in the neuroprotective
effect of DEX.
Materials and methods
Cell culture
Human Neurons-hippocampal (HN-h) primary cells (cat.
no. 1540) were purchased from ScienCell Research Laboratories, Inc.
(San Diego, CA, USA). Cells were cultured in a neuronal medium
(cat. no. 1521; ScienCell Research Laboratories, Inc.) in an
incubator at 37°C, 95% humidity and 5% CO2 for 48 h,
according to the manufacturer's protocol. A total of 2.5, 5, 10 or
20 µM DEX (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was
dissolved in 0.2% final concentration dimethyl sulfoxide (DMSO) and
incubated for 1 h. Subsequently, hydrogen peroxide
(H2O2; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was added to each sample to a 200 µM final
concentration.
Cell viability
MTT assay was used to evaluate the viability of HN-h
cells. Following a 48-h incubation, 20 µl MTT reagent (5 mg/ml) was
added to the culture and further incubated for 4 h. Supernatants
were discarded and replaced with 150 µl DMSO/well to solubilize the
formazan crystals in viable cells. The absorbance was measured at a
wavelength of 570 nm using a spectrophotometer.
Apoptosis assay
Cell apoptosis was determined using an Annexin
V/propidium iodide (PI) assay. Cells were collected by
centrifugation for 5 min at 200 × g and 4°C, rinsed twice using 1X
PBS and resuspended in 1X binding buffer (BD Biosciences, Franklin
Lakes, NJ, USA) at a concentration of 1×106 cells/ml.
Cells were incubated with a fluorescein isothiocyanate labeled
anti-Annexin V antibody (5 µl; eBioscience; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 15 min at room temperature.
Following washing with Annexin V binding buffer, cells were
resuspended in 200 µl binding buffer. PI staining solution (5 µl;
eBioscience; Thermo Fisher Scientific, Inc.) was then added for 5
min and cells were analyzed using a BD Accuri C6 flow cytometer (BD
Biosciences) within 1 h.
Lipid peroxidation assay
To evaluate the malondialdehyde (MDA) content, a
Lipid Peroxidation (MDA) Assay kit (cat. no. ab118970; Abcam,
Cambridge, UK) was used according to the manufacturer's protocol. A
total of 1×106 cells were homogenized on ice in 300 µl
MDA Lysis Buffer with 3 µl 100X butylated hydroxytoluene and
centrifuged at 13,000 × g and 4°C for 10 min to remove insoluble
material. Subsequently, 200 µl supernatant from each homogenized
sample was transferred to a microcentrifuge tube and 600 µl TBA
solution was added/sample. Following incubation at 95°C for 60 min
and cooling to room temperature using an ice bath for 10 min, the
200 µl reaction mixture was transferred into a 96-well microplate
for colorimetric analysis. The absorbance was measured at a
wavelength of 532 nm and MDA levels were calculated using standard
curve analysis according to the manufacturer's protocol.
Determination of lactate dehydrogenase
(LDH) levels
The Colorimetric Lactate Dehydrogenase (LDH)
Activity Assay kit (cat. no. ab102526; Abcam) was used to determine
LDH activity, according to the manufacturer's protocol. Cell
culture medium was centrifuged at 13,000 × g and 4°C for 10 min to
collect the supernatant for determination of cell-free LDH
activity. A total of 1×106 cells were harvested by
centrifugation at 13,000 × g and 4°C for 10 min, washed with PBS,
homogenized with three volumes of cold assay buffer and centrifuged
at 4°C at 10,000 × g for 15 min to eliminate insoluble material.
The absorbance was measured at a wavelength of 450 nm. LDH activity
was estimated using a standard curve with NADH as a reference and
LDH leakage was expressed as a ratio of cell-free LDH
activity/total LDH activity.
Detection of ROS levels
Following seeding, control,
H2O2 and DEX-treated cells were rinsed three
times using PBS, trypsinized and centrifuged at 13,000 × g and 4°C
for 10 min. Subsequently, cells were harvested and incubated with
diluted Dihydroethidium (Reactive Oxygen Detection kit; cat. no.
S0033; Beyotime Institute of Biotechnology, Haimen, China)
according to the manufacturer's protocol. Finally, the levels of
ROS were evaluated by flow cytometry analysis using the BD Accuri™
C6 flow cytometer (BD Biosciences) for measurement of fluorescence
levels in the FL2 channel.
Measurement of intracellular
Ca2+
For the measurement of intercellular
Ca2+, cells were incubated with Fluo 3-AM reagent
(GeneCopoeia, Inc., Rockville, MD, USA) according to the
manufacturer's protocol. Subsequently, Ca2+-dependent
fluorescence intensity was measured by flow cytometry using the BD
Accuri™ C6 Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA)
and 10,000 cells/sample were acquired and analyzed.
Transfection
HN-h cells (5×104 cells/cm2)
were transfected with 2 nM mir-223-3p mimic (MISSION microRNA
Mimic; 5′-UGUCAGUUUGUCAAAUACCCCA-3′), miR-223-3p inhibitor (MISSION
Synthetic microRNA Inhibitor; 5′-UGGGGUAUUUGACAAACUGACA-3′) or
their scrambled controls (all Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The expression vector pPM-C-HA-TIAL1 (Applied Biological
Materials, Inc., Richmond, BC, Canada) was used for expression of
TIAL1 and an empty pPM-C-HA vector served as a control.
Transfection of these vectors was performed using DNAfectin Plus
reagent (Applied Biological Materials, Inc.) according to the
manufacturer's protocol. Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) was used to evaluate the
transfection efficiency 48 h after transfection prior to subsequent
experiments.
Bioinformatics
The online tool TargetScan (www.targetscan.org/vert_71/) was used to predict TIAL1
mRNA 3′-untranslated region (UTR) as a target for Homo
sapiens (has)-miR-223-3p.
Luciferase assay
Cells were transfected with human
TIAL1-3′UTR-luciferase reporter plasmid (Promega Corporation,
Madison, WI, USA) using Lipofectamine® 2000 transfection
reagent (Thermo Fisher Scientific, Inc.) and cultured for 48 h.
Luciferase assays were performed using a Dual-Luciferase Reporter
Assay system (Promega Corporation, Madison, WI, USA) according to
the manufacturer's protocol. Renilla luciferase was used for
normalization (Promega Corporation). Each experiment was repeated
three times in duplicate.
RT-qPCR analysis
Total RNA was extracted from cultured cells using
the miRVana miRNA Isolation kit (Thermo Fisher Scientific, Inc.).
Isolated RNA was reverse-transcribed into cDNA using the TaqMan
MicroRNA Reverse Transcription kit (Thermo Fisher Scientific,
Inc.). Subsequently, qPCR experiments were performed using
hsa-miR-223-3p-specific primers (Sigma-Aldrich; Merck KGaA) and the
CFX96 Touch™ Real-Time PCR Detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) according to the manufacturer's protocol.
The following thermocycling conditions were used for the PCR: 30
sec at 95°C; 45 cycles of 95°C for 5 sec and 58°C for 34 sec. The
forward (F) and reverse (R) primers used for amplification were as
follows: F, 5′-AATTCAGAAGAGGCATACCTTAGA-3′ and R,
5′-TTCAGGCTTGGGTTGTGTGT-3′ for TIAL1; F, 5′-GCAACTAGGATGGTGTGGCT-3′
and R, 5′-TCCCATTCCCCAGCTCTCATA-3′ for GAPDH; F,
5′-CCACGCTCCGTGTATTTGAC-3′ and R, 5′-cCGCACTTGGGGTATTTGAC-3′ for
hsa-miR-223-3p; and F, 5′-CTCGCTTCGGCAGCACA-3′ and R,
5′-AACGCTTCACGAATTTGCGT-3′ for U6. Relative expression of a
hsa-miR-223-3p was evaluated using the 2−∆∆Cq method
(24) with U6 small nuclear RNA
used for normalization for hsa-miR-223-3p and GAPDH used for
normalization of TIAL1.
Western blotting
Total protein was extracted from cultured HN-h cells
using the radioimmunoprecipitation assay buffer (Sigma-Aldrich;
Merck KGaA). Protein concentration was determined using a
Bicinchoninic Acid kit. A total of 50 µg protein was loaded per
lane and purified by 10% SDS-PAGE, transferred to a polyvinylidene
fluoride membrane and blocked with 5% skimmed milk in 0.1 M PBS for
2 h at room temperature. The membranes were incubated overnight in
the dark at 4°C with the following primary antibodies at 1:1,000
dilution: Anti-TIAL1 (cat. no. ab129499),
anti-Ca2+/calmodulin-dependent protein kinase II
(CaMKII; cat. no. ab22609), anti-CaMKII (phospho T286; cat. no.
ab171095) and anti-GAPDH (cat. no. ab37168; all Abcam). The
membranes were subsequently washed with PBS and incubated with
horseradish peroxidase conjugated rabbit-anti-goat secondary
antibodies (1:40,000; cat. no. PI-1000; Vector Laboratories, Inc.,
Burlingame, CA, USA) for 120 min at room temperature. Finally, the
membranes were washed three times in PBS and incubated with an
enhanced chemiluminescence kit (Roche Diagnostics, Basel,
Switzerland) for 5 min for visualization. Densitometry
quantification was performed using Image J software (version 1.51
k; National Institutes of Health, Bethesda, MD, USA) and relative
protein expression levels were calculated using GAPDH as a
control.
Statistical analysis
Statistical analyses were performed using one-way or
two-way analysis of variance followed by the Bonferroni post-hoc
test in GraphPad prism software (version 6.01; GraphPad Software,
Inc., La Jolla, CA, USA). Data were presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
DEX protects HN-h cells against
H2O2-induced cytotoxicity
To investigate the neuroprotective effect of DEX on
HN-h cells, cells were treated with different concentrations of
H2O2 in the presence or absence of serial
concentrations of DEX. MTT assay (Fig.
1A) indicated that H2O2 significantly
inhibited the viability of HN-h cells in a dose dependent manner.
DEX reversed the adverse effects of H2O2,
also in a dose-dependent manner. Flow cytometry analysis of cell
apoptosis (Fig. 1B) confirmed that
H2O2 induced apoptotic cell death. Treatment
of H2O2-pretreated cells with DEX
significantly inhibited cell apoptosis. Levels of cytotoxic
markers, including LDH leakage and MDA content (Fig. 1C and D, respectively) were
significantly increased in H2O2-treated
cells, compared with the untreated cells. Treatment with DEX
inhibited H2O2-mediated increase in LDH
leakage and MDA content, in a dose dependent manner (Fig. 1C and 1D). Similarly, treatment with
H2O2 increased ROS levels which decreased
following treatment with DEX, in a dose-dependent manner (Fig. 1E). Level of intracellular calcium
in HN-h cells was significantly increased in response to treatment
with H2O2 while DEX inhibited and
significantly attenuated H2O2-induced
Ca2+ increase, in a dose dependent manner (Fig. 1F). Western blot analysis indicated
that phosphorylation of CAMII was inhibited following treatment
with DEX (Fig. 1G and H). The
aforementioned results indicated that DEX protects HN-h cells from
H2O2 induced neurotoxicity.
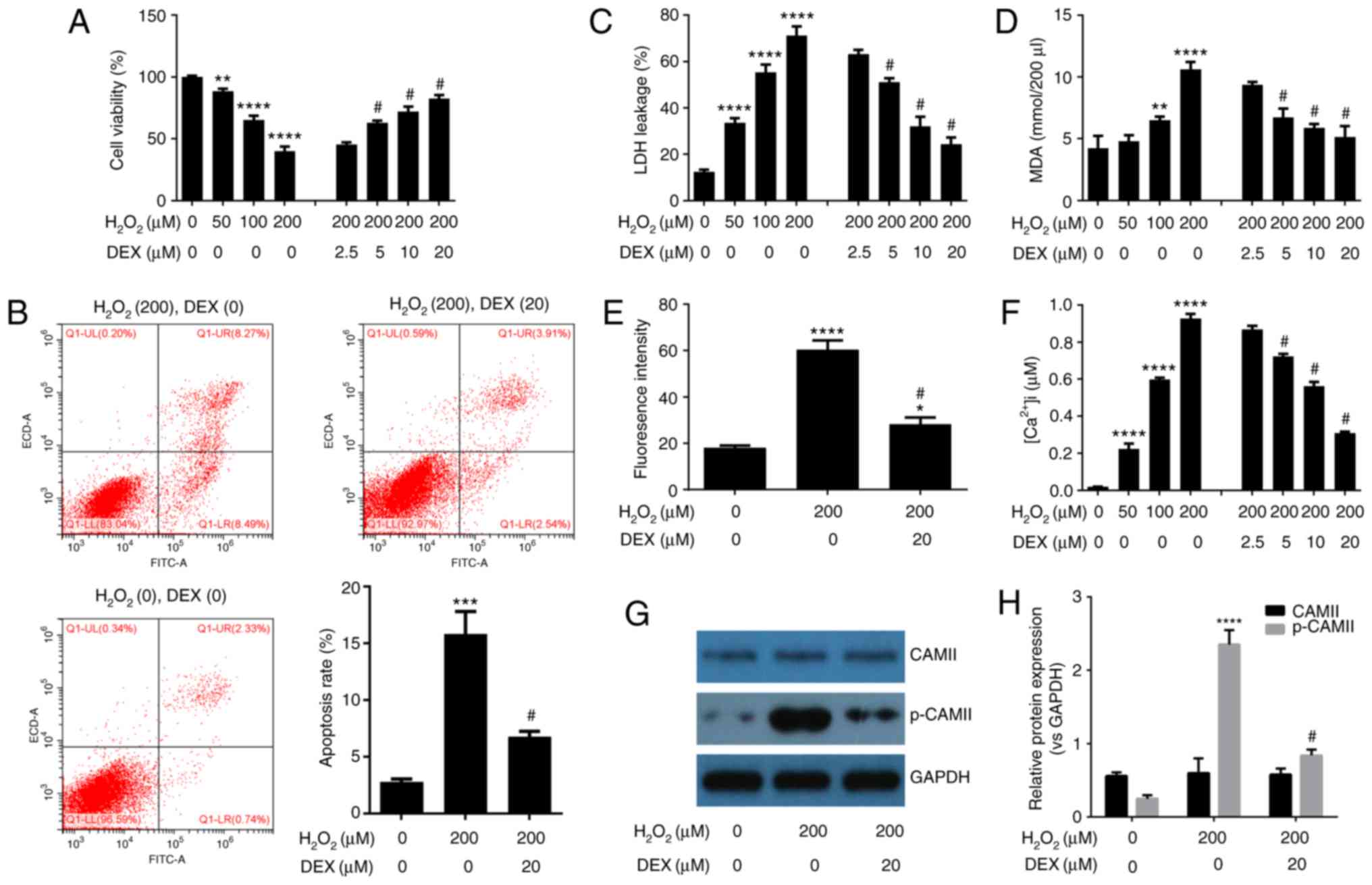 | Figure 1.DEX protects HN-h cells against
H2O2-induced cytotoxicity. (A) MTT assay
indicated that DEX prevents H2O2-induced cell
death. (B) Flow cytometry analysis of cell apoptosis indicated that
DEX inhibits H2O2-induced cell apoptosis. DEX
inhibits H2O2-induced (C) release of LDH, (D)
production of MDA, (E) production of ROS and (F) production of
intracellular Ca2+. (G) Western blot analysis indicated
that DEX inhibits H2O2-induced
phosphorylation of CAMII. (H) Densitometry analysis of bands
obtained from western blot analysis. The experiments were performed
three times in duplicate. *P<0.05, **P<0.01, ***P<0.001,
****P<0.0001 vs. the control group (DEX=0 µM,
H2O2=0 µM); #P<0.05 vs. the 200
µM H2O2 treatment group. DEX,
dexmedetomidine; LDH, lactate dehydrogenase; MDA, malondialdehyde;
ROS, reactive oxygen species; CAMII, calmodulin-2; p,
phosphorylated; ns, not significant. |
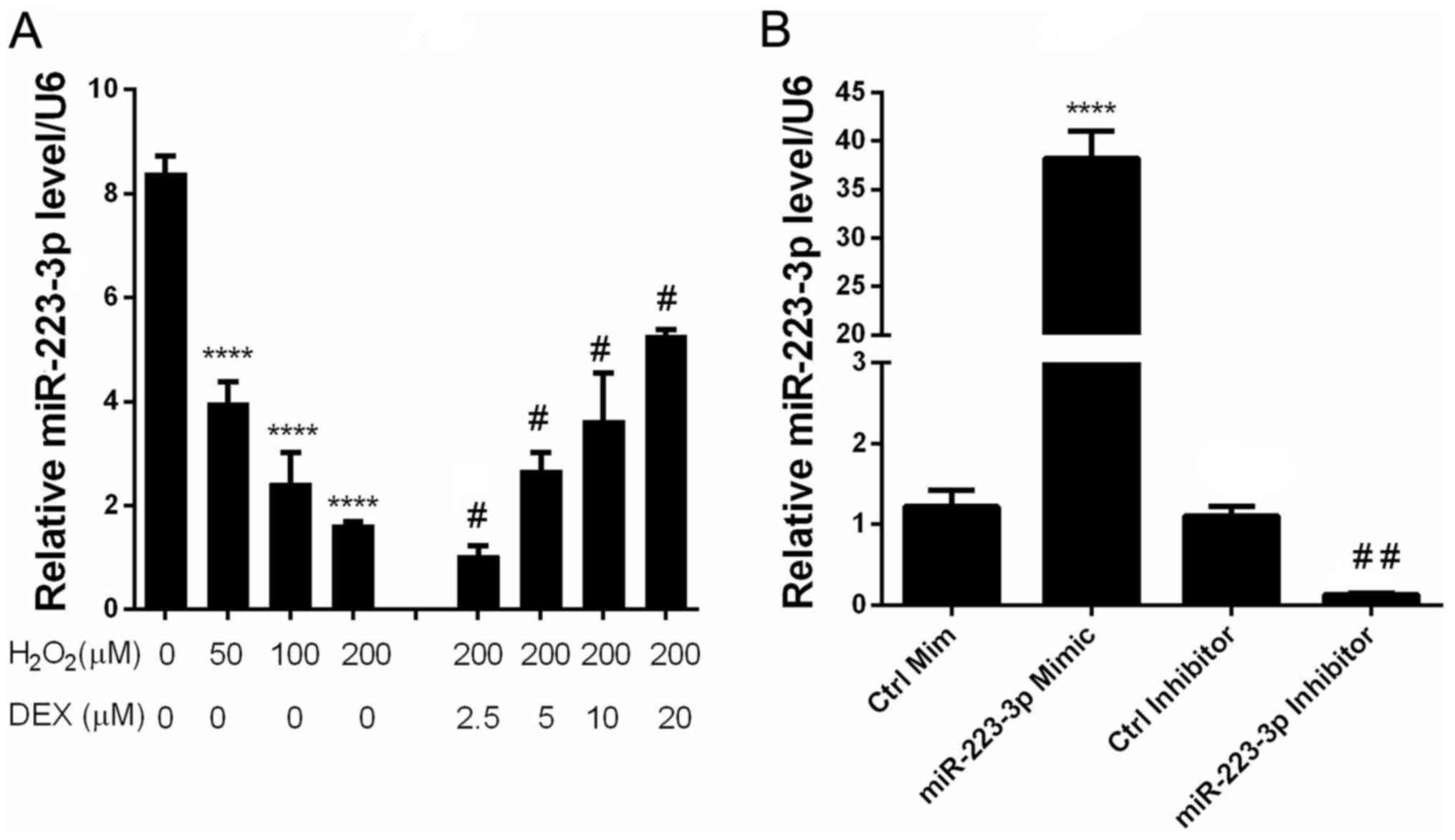
DEX prevents
H2O2-mediated downregulation of miR-223-3p
expression in HN-h cells
In order to elucidate the involvement of miR-223-3p
in the neuroprotective effect of DEX, the expression of miR-223-3p
was measured in cells treated with different concentrations of DEX
and H2O2. The results indicated that
H2O2 and DEX inhibited and enhanced the
expression of miR-223-3p, respectively, in a dose-dependent manner
(Fig. 2). These observations
suggested that miR-223-3p may be involved in the neuroprotective
effect of DEX.
Upregulation of miR-223-3p expression
is a mechanism involved in the neuroprotective effect of DEX
against H2O2 induced-cytotoxicity
To identify how miR-223-3p expression is involved in
DEX-mediated protection of HN-H cells against
H2O2-induced cytotoxicity, HN-H cells were
transiently transfected with a miR-223-3p mimic or miR-223-3p
inhibitor or their corresponding scrambled controls and treated or
untreated with H2O2. Using RT-qPCR to detect
the expression efficiency, it was observed that transfection with
the miR-223-3p mimic effectively led to the overexpression of
mir-223-3p whereas the transfection with mir-223-3p inhibitor led
to significantly downregulated expression of mir-223-3p (Fig. 2B). The results of MTT and flow
cytometry assays demonstrated that miR-223-3p inhibitor promoted
H2O2-induced HN-H cell death and apoptosis
(Fig. 3A and B). By contrast, HN-H
cell death and apoptosis were markedly inhibited following
transfection with miR-223-3p mimics (Fig. 3A and B). No significant differences
were identified between the negative controls. Similarly, the
results indicated that miR-223-3p mimics significantly inhibited
the LDH leakage, MDA release, ROS production, Ca2+
levels and CAMII phosphorylation (Fig.
3C-H, respectively). The aforementioned results suggested that
activation of miR-223-3p led to neuroprotective effects.
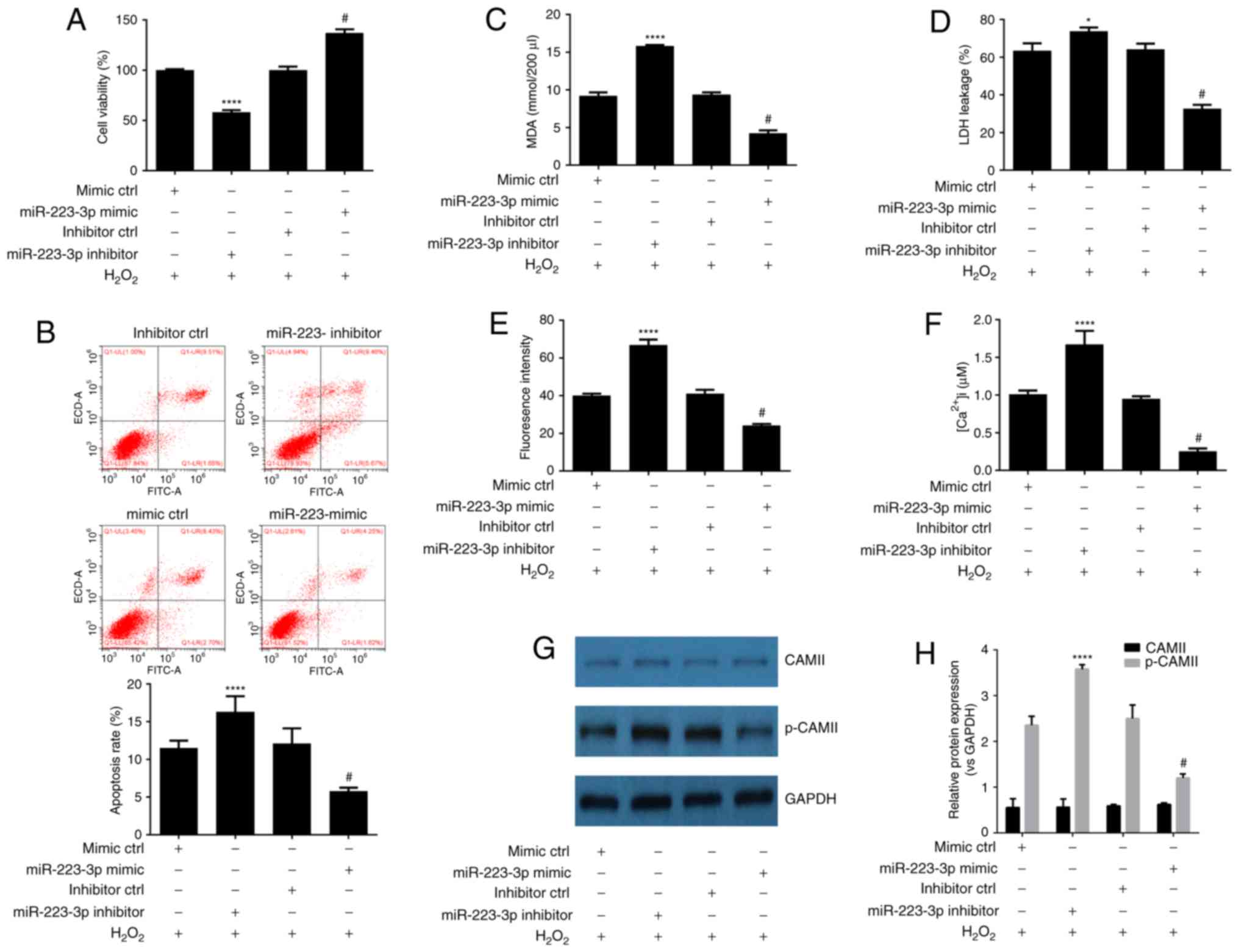 | Figure 3.Upregulation of miR-223-3p expression
is a mechanism involved in the neuroprotective effect of DEX
against H2O2 induced-cytotoxicity. (A) MTT
assay indicated that mir-223-3p overexpression reverses
H2O2-induced cell death. (B) Flow cytometry
analysis of cell apoptosis indicated that miR-223-3p overexpression
inhibits H2O2-induced cell apoptosis.
miR-223-3p overexpression inhibits
H2O2-induced (C) production of MDA, (D)
release of LDH, (E) production of ROS and (F) production of
intracellular Ca2+. (G) Western blot analysis indicated
that miR-223-3p overexpression inhibits
H2O2-induced phosphorylation of CAMII. (H)
Densitometry analysis of bands obtained from western blot analysis.
Experiments were performed three times in duplicate. *P<0.05,
****P<0.001 vs. the inhibitor control group
(H2O2=200 µM); #P<0.05 vs. the
mimic control group (200 µM H2O2). DEX,
dexmedetomidine; LDH, lactate dehydrogenase; MDA, malondialdehyde;
ROS, reactive oxygen species; CAMII, calmodulin-2; miR, microRNA;
ns, not significant. |
TIAL1 is a direct target gene for
mir-223-3p in HN-H cells
Bioinformatics analysis indicated TIAL1 as a
potential target gene for miR-223-3p (Fig. 4A). Due to its involvement in
cytotoxicity, the authors of the present study selected TIAL1 for
further investigation. The protein expression level of TIAL1 was
determined in HN-H cells following treatment with different
concentrations of H2O2 and DEX by western
blot analysis. Expression of TIAL1 was significantly enhanced by
H2O2 and inhibited by treatment with DEX in a
dose-dependent manner (Fig. 4B).
To determine whether TIAL1 is a target gene of miR-223-3p in HN-H
cells, the effect of mir-223-3p mimic or inhibitor was
investigated. miR-223-3p mimics significantly inhibited the
expression of TIAL1 while miR-223-3p inhibitor increased expression
levels of TIAL1 in cultured HN-h cells (Fig. 4C). The aforementioned results
suggested that TIAL1 was positively regulated by miR-223-3p. To
confirm the direct binding of miR-223-3p to TIAL1, a luciferase
reporter assay was performed. miR-223-3p mimic significantly
decreased luciferase activity, while treatment with miR-223-3p
inhibitor led to increased luciferase activity in cells transfected
with a wild type TIAL1 3′-UTR vector. No significant differences
were recorded between cells cotransfected with TIAL1 3′-UTR MUT
vector and miR-223-3p mimic, inhibitor or control miRs (Fig. 4D). The aforementioned results
indicated that TIAL1 mRNA 3′-UTR is a direct target of
miR-223-3p.
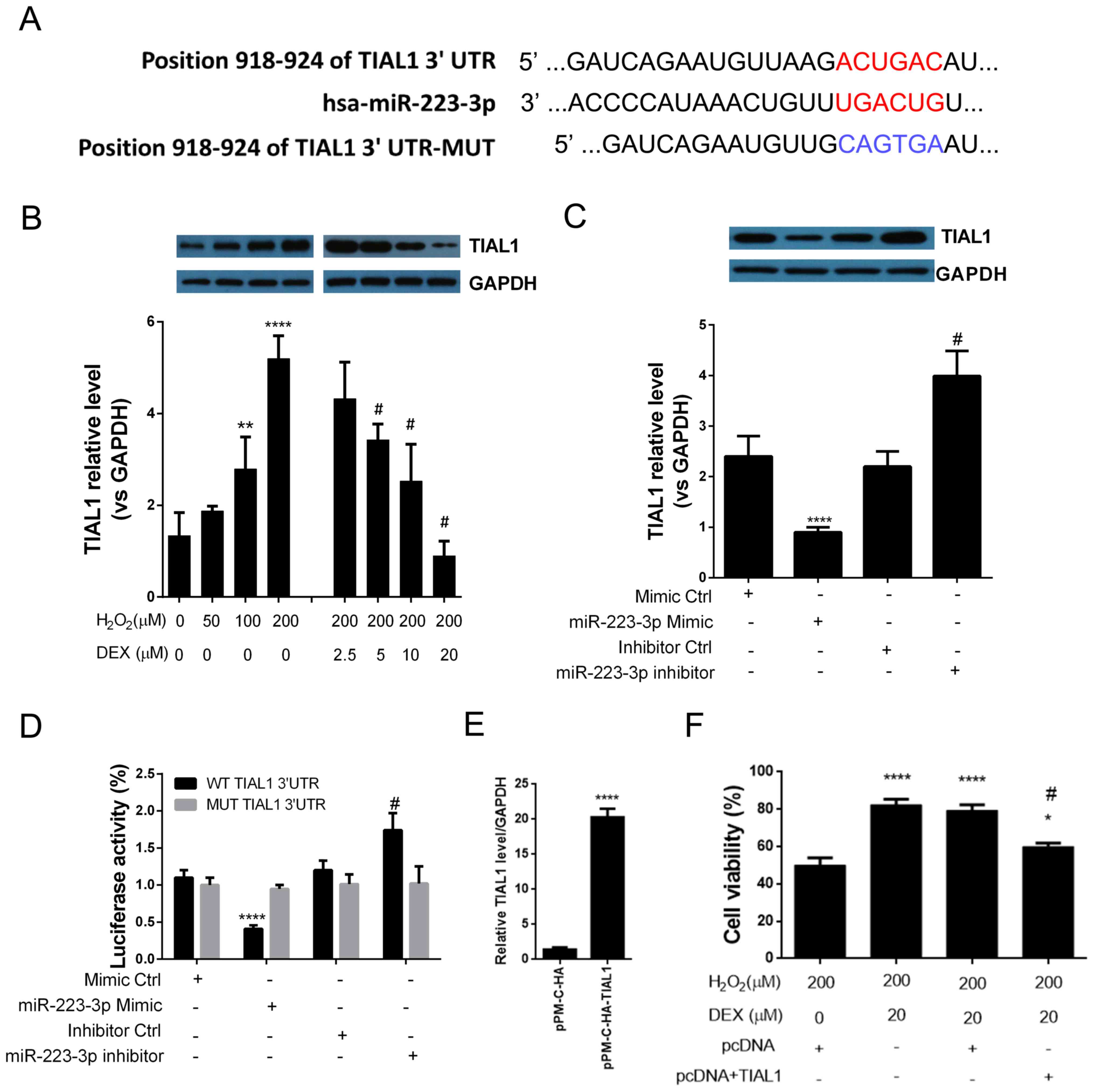 | Figure 4.TIAL1 is a direct target of
miR-223-3p in HN-H cells. (A) Bioinformatics analysis predicted the
3′-UTR of TIAL1 to be a putative seed sequence of miR-223-3p. (B)
Western blot analysis indicated that DEX inhibited
H2O2-induced TIAL1 expression. **P<0.01,
****P<0.0001 vs. control untreated cells (DEX=0 µM,
H2O2=0 µM); #P<0.05 vs. the 200
µM H2O2 treatment group. (C) miR-223-3p
overexpression inhibits TIAL1 expression. ****P<0.0001 vs.
control mimic; #P<0.05 vs. control inhibitor group.
(D) Luciferase assay indicated that TIAL1 is a direct target of
mir-223-3p. ****P<0.0001 vs. control mimic;
#P<0.05 vs. control inhibitor group. (E) Transfection
efficiency assessment. ****P<0.0001 vs. control vector. (F)
Overexpression of TIAL1 abrogated the effect of DEX on cell
viability. *P<0.05, ****P<0.0001 vs. control cells (DEX=0 µM,
H2O2=200 µM, pcDNA vector);
#P<0.05 vs. the TIAL1 vector group (DEX=0 µM,
H2O2=200 µM, pcDNA+TIAL1 vector). The
experiments were performed three times in duplicate. DEX,
dexmedetomidine; TIAL1, TIA1 cytotoxic granule associated RNA
binding protein like 1; miR, microRNA; UTR, untranslated region;
NS, not significant; miR, microRNA. |
Overexpression of TIAL1 abrogated the
neuroprotective effect of DEX
To investigate whether DEX-induced TIAL1 inhibition
mediates the neuroprotective effect, TIAL1 was overexpressed in
HN-h cells and cell viability was measured following treatment with
H2O2 and DEX. The transfection of TIAL1
vector allowed the effective overexpression of TIAL1 compared with
control vector (Fig. 4E). The
results indicated that overexpression of TIAL1 significantly
decreased the effect of DEX on H2O2-induced
cell death (Fig. 4F). The results
of the present study indicated that DEX exerts neuroprotective
effects via upregulation of miR-223-3p and subsequent
downregulation of TIAL1.
Discussion
Anesthetics are vital for protection of the central
nervous system during surgery. Administration of neuroprotective
anesthetic drugs is a relevant approach for improving overall
neurological outcomes of surgical procedures. Therefore,
understanding of the protective properties of various anesthetic
agents and elucidating the underlying molecular mechanisms is
crucial for adequate selection of appropriate anesthetic
treatments.
In the present study, DEX protected HN-h cells
against H2O2-induced neurotoxicity by
inhibiting cell apoptosis, decreasing LDH activity, MDA content,
ROS production and calcium levels as indicated by the decrease in
intracellular production of Ca2+ and phosphorylation of
CAMII. The results of the present study are consistent with
previous studies that demonstrated the neuroprotective effects of
DEX. A previous study demonstrated that DEX exhibits
neuroprotective effects on ischemia reperfusion by inhibiting
apoptosis (25). In vitro
data indicated that DEX prevents cultured hippocampal neurons from
propofol-induced neurotoxicity by modulating the cyclic
AMP-responsive element-binding protein, apoptosis regulator B cell
lymphoma-2 and brain-derived neurotrophic factor (BDNF) signaling
pathways (26). DEX has been
reported to protect from focal cerebral ischemia reperfusion damage
by inhibiting the nuclear factor-kB signaling pathway and to
protect against traumatic brain injury (1,27).
Previous reports have demonstrated that DEX induces neuroprotective
effects by promoting BDNF expression in astrocytes via regulation
of the ERK signaling pathway (28). However, the molecular mechanism
underlying the neuroprotective effect of DEX remains to be
elucidated.
miRs in the nervous system are associated with
neuronal survival and control of the accumulation of toxic proteins
associated with neurodegeneration (29–34).
miR-223 is a regulatory factor involved in differentiation of
immature neurons (35).
Furthermore, a previous study has demonstrated that miR-223 exerts
neuroprotective effects through glutamate receptors GluR2 and NR2B
(36). However, whether the
neuroprotective effect of DEX is mediated by regulation of
miR-223-3p remains to be elucidated. In the present study,
treatment with H2O2 induced miR-223-3p
downregulation in hippocampal neurons in a dose-dependent manner.
Treatment of H2O2-pretreated HN-h cells with
DEX markedly increased miR-223-3p expression in a dose-dependent
manner. The results of the present study indicated that
upregulation of miR-223-3p expression represents a mechanism
involved in the neuroprotective effect of DEX against
H2O2 induced-neurotoxicity. The present study
also demonstrated that overexpression of miR-223-3p resulted in
inhibition of H2O2-induced apoptosis of HN-h
cells. The luciferase reporter assay demonstrated that TIAL1 gene
is a direct target of miR-223-3p in HN-h cells. Overexpression of
TIAL1 abrogated the neuroprotective effect of DEX on
H2O2-induced neurotoxicity. To the best of
the authors knowledge, the present study is the first to
demonstrate that DEX exerts neuroprotective effects by
downregulating TIAL1 via activation of miR-223-3p in hippocampal
neuronal cells in vitro.
Apoptosis, an autonomous, genetically controlled
cell death, contributes to selective elimination of cells in
physiological and pathological conditions. In the present study,
DEX and miR-223-3p demonstrated a protective effect against
H2O2-induced apoptosis in HN-H cells. The
results of the present study suggested that apoptosis was involved
in the neuroprotective mechanism of DEX and that the effect of DEX
may have been mediated by the miR-223-3p/TIAL1 interaction.
Calcium is an important signal transducer in nerve
cells. The present study aimed to determine whether intracellular
calcium homeostasis could be influenced by treatment with DEX and
associated upregulation of miR-223-3p.
H2O2-induced increase in Ca2+
levels was significantly decreased by treatment with DEX and
overexpression of miR-223-3p. The aforementioned results indicated
that DEX may disrupt intracellular calcium levels by increasing
Ca2+ levels in hippocampal cells and that calcium
signaling may serve an important role in apoptosis.
In conclusion, the present study demonstrated that
DEX exerts neuroprotective effects on hippocampal neuronal cells
in vitro by downregulating TIAL1 via activation of
miR-223-3p. It was also demonstrated that, in the present study,
apoptosis and ROS and calcium overload were regulated by treatment
with DEX. The results of the present study contribute to the
understanding of the molecular mechanism underlying DEX-mediated
neuroprotection. Manipulation of miR-223-3p/TAL1 interaction can in
the future influence application of DEX in anesthesia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QW, HY, HMY, MM, YM and RL all participated in the
conception and design of the study. QW and HY performed the
experiments. QW, HY, HMY, MM and YM analyzed the data. QW wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang L, Liu H, Zhang L, Wang G, Zhang M
and Yu Y: Neuroprotection of dexmedetomidine against cerebral
ischemia-reperfusion injury in rats: Involved in inhibition of
NF-κB and inflammation response. Biomol Ther (Seoul). 25:383–389.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fu C, Dai X, Yang Y, Lin M, Cai Y and Cai
S: Dexmedetomidine attenuates lipopolysaccharide-induced acute lung
injury by inhibiting oxidative stress, mitochondrial dysfunction
and apoptosis in rats. Mol Med Rep. 15:131–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Küçükebe ÖB, Özzeybek D, Abdullayev R,
Ustaoğlu A, Tekmen I and Küme T: Effect of dexmedetomidine on acute
lung injury in experimental ischemia-reperfusion model. Rev Bras
Anestesiol. 67:139–146. 2017.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen J and Fu G, Jiang L, Xu J, Li L and
Fu G: Effect of dexmedetomidine pretreatment on lung injury
following intestinal ischemia-reperfusion. Exp Ther Med.
6:1359–1364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie C, Li Y, Liang J, Xiao J, Zhao Z and
Li T: The effect of dexmedetomidine on autophagy and apoptosis in
intestinal ischemia reperfusion-induced lung injury. Zhonghua Jie
He He Hu Xi Za Zhi. 38:761–764. 2015.(In Chinese). PubMed/NCBI
|
|
6
|
Ammar AS, Mahmoud KM, Kasemy ZA and Helwa
MA: Cardiac and renal protective effects of dexmedetomidine in
cardiac surgeries: A randomized controlled trial. Saudi J Anaesth.
10:395–401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cakir M, Polat A, Tekin S, Vardi N,
Taslidere E, Rumeysa Duran Z and Tanbek K: The effect of
dexmedetomidine against oxidative and tubular damage induced by
renal ischemia reperfusion in rats. Ren Fail. 37:704–708. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Carvalho AL, Vital RB, Kakuda CM, Braz
JR, Castiglia YM, Braz LG, Módolo MP, Ribeiro OR, Domingues MA and
Módolo NS: Dexmedetomidine on renal ischemia-reperfusion injury in
rats: Assessment by means of NGAL and histology. Ren Fail.
37:526–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu YE, Tong CC, Zhang YB, Jin HX, Gao Y
and Hou MX: Effect of dexmedetomidine on rats with renal
ischemia-reperfusion injury and the expression of tight junction
protein in kidney. Int J Clin Exp Med. 8:18751–18757.
2015.PubMed/NCBI
|
|
10
|
Si YN, Bao HG, Xu L, Wang XL, Shen Y, Wang
JS and Yang XB: Dexmedetomidine protects against
ischemia/reperfusion injury in rat kidney. Eur Rev Med Pharmacol
Sci. 18:1843–1851. 2014.PubMed/NCBI
|
|
11
|
Zhang XK, Zhou XP, Zhang Q and Zhu F: The
preventive effects of dexmedetomidine against intestinal
ischemia-reperfusion injury in Wistar rats. Iran J Basic Med Sci.
18:604–609. 2015.PubMed/NCBI
|
|
12
|
Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao
X, Huang WQ and Liu KX: Dexmedetomidine administration before, but
not after, ischemia attenuates intestinal injury induced by
intestinal ischemia-reperfusion in rats. Anesthesiology.
116:1035–1046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akpınar H, Nazıroğlu M, Övey İS, Çiğ B and
Akpınar O: The neuroprotective action of dexmedetomidine on
apoptosis, calcium entry and oxidative stress in cerebral
ischemia-induced rats: Contribution of TRPM2 and TRPV1 channels.
Sci Rep. 6:371962016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanabe K, Matsushima-Nishiwaki R, Kozawa O
and Iida H: Dexmedetomidine suppresses interleukin-1β-induced
interleukin-6 synthesis in rat glial cells. Int J Mol Med.
34:1032–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu YM, Wang CC, Chen L, Qian LB, Ma LL,
Yu J, Zhu MH, Wen CY, Yu LN and Yan M: Both PI3K/Akt and ERK1/2
pathways participate in the protection by dexmedetomidine against
transient focal cerebral ischemia/reperfusion injury in rats. Brain
Res. 1494:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaudhuri AD, Choi DC, Kabaria S, Tran A
and Junn E: MicroRNA-7 regulates the function of mitochondrial
permeability transition pore by targeting VDAC1 expression. J Biol
Chem. 291:6483–6493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma Q, Dasgupta C, Li Y, Bajwa NM, Xiong F,
Harding B, Hartman R and Zhang L: Inhibition of microRNA-210
provides neuroprotection in hypoxic-ischemic brain injury in
neonatal rats. Neurobiol Dis. 89:202–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quinlan S and Jimenez-Mateos EM: Can we
protect the brain via preconditioning? Role of microRNAs in
neuroprotection. Neural Regen Res. 11:388–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren L, Zhu R and Li X: Silencing miR-181a
produces neuroprotection against hippocampus neuron cell apoptosis
post-status epilepticus in a rat model and in children with
temporal lobe epilepsy. Genet Mol Res. 15:2016. View Article : Google Scholar :
|
|
20
|
Sinoy S, Fayaz SM, Charles KD, Suvanish
VK, Kapfhammer JP and Rajanikant GK: Amikacin inhibits miR-497
maturation and exerts post-ischemic neuroprotection. Mol Neurobiol.
54:3683–3694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou X, Su S, Li S, Pang X, Chen C, Li J
and Liu J: MicroRNA-146a down-regulation correlates with
neuroprotection and targets pro-apoptotic genes in cerebral
ischemic injury in vitro. Brain Res. 1648:136–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao
Y, Ross RA, Cao H, Cai Y, Xu M, et al: MicroRNA-223 ameliorates
alcoholic liver injury by inhibiting the
IL-6-p47phox-oxidative stress pathway in neutrophils.
Gut. 2016.
|
|
23
|
Shin JH, Park YM, Kim DH, Moon GJ, Bang
OY, Ohn T and Kim HH: Ischemic brain extract increases SDF-1
expression in astrocytes through the CXCR2/miR-223/miR-27b pathway.
Biochim Biophys Acta. 1839:826–836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu GJ, Chen JT, Tsai HC, Chen TL, Liu SH
and Chen RM: Protection of dexmedetomidine against
ischemia/reperfusion-induced apoptotic insults to neuronal cells
occurs via an intrinsic mitochondria-dependent pathway. J Cell
Biochem. 118:2635–2644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei Y, Hu J, Liang Y, Zhong Y, He D, Qin
Y, Li L, Chen J, Xiao Q and Xie Y: Dexmedetomidine pretreatment
attenuates propofol-induced neurotoxicity in neuronal cultures from
the rat hippocampus. Mol Med Rep. 14:3413–3420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schoeler M, Loetscher PD, Rossaint R,
Fahlenkamp AV, Eberhardt G, Rex S, Weis J and Coburn M:
Dexmedetomidine is neuroprotective in an in vitro model for
traumatic brain injury. BMC Neurol. 12:202012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Degos V, Charpentier TL, Chhor V, Brissaud
O, Lebon S, Schwendimann L, Bednareck N, Passemard S, Mantz J and
Gressens P: Neuroprotective effects of dexmedetomidine against
glutamate agonist-induced neuronal cell death are related to
increased astrocyte brain-derived neurotrophic factor expression.
Anesthesiology. 118:1123–1132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eacker SM, Dawson TM and Dawson VL:
Understanding microRNAs in neurodegeneration. Nat Rev Neurosci.
10:837–841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh SK: miRNAs: From neurogeneration to
neurodegeneration. Pharmacogenomics. 8:971–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bushati N and Cohen SM: MicroRNAs in
neurodegeneration. Curr Opin Neurobiol. 18:292–296. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karnati HK, Panigrahi MK, Gutti RK, Greig
NH and Tamargo IA: miRNAs: Key players in neurodegenerative
disorders and epilepsy. J Alzheimers Dis. 48:563–580. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kamal MA, Mushtaq G and Greig NH: Current
update on synopsis of miRNA dysregulation in neurological
disorders. CNS Neurol Disord Drug Targets. 14:492–501. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goodall EF, Heath PR, Bandmann O, Kirby J
and Shaw PJ: Neuronal dark matter: The emerging role of microRNAs
in neurodegeneration. Front Cell Neurosci. 7:1782013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harraz MM, Xu JC, Guiberson N, Dawson TM
and Dawson VL: MiR-223 regulates the differentiation of immature
neurons. Mol Cell Ther. 2:182014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harraz MM, Eacker SM, Wang X, Dawson TM
and Dawson VL: MicroRNA-223 is neuroprotective by targeting
glutamate receptors. Proc Natl Acad Sci USA. 109:18962–18967. 2012.
View Article : Google Scholar : PubMed/NCBI
|