Introduction
Excessive fibrosis in the extracellular matrix (ECM)
is the most important cause of liver fibrosis, and eventually
results in liver injury (1).
Hepatic stellate cells (HSCs) have been demonstrated to be the
primary matrix-producing cells in the liver, serving a critical
role in driving liver fibrosis progression (2). It has been reported that any chronic
stimulus may lead to the activation and growth of HSCs, which
induce the accumulation of ECM proteins to trigger fibrosis and
damage the liver tissue (3,4).
Although fundamental and genomic advances have contributed towards
the understanding of the pathophysiology of the fibrosis response
in the liver, definitive therapies are not fully accessible
(5). Accordingly, there is a need
for effective therapeutic strategies aimed at novel targets.
A previous study suggested that inflammatory
cytokines are associated with fibrotic events and thus lead to the
progression of liver disease (6).
It is well established that tumor necrosis factor-α,
platelet-derived growth factor and transforming growth factor-β
(TGF-β) may mediate a variety of inflammatory reactions and drive a
progressive fibrotic response in liver disorders (7,8).
TGF-β is known to be implicated in various processes associated
with the development and progression of hepatic fibrogenesis. TGF-β
is composed of TGF-β1, β2 and β3, which are able to modulate
downstream signaling via TGF-β receptor 1 (TGFBR1) and TGF-β
receptor 2 (TGFBR2) (9,10). However, knowledge regarding the
association between TGFBR2 and hepatic fibrosis remains
limited.
An increasing number of studies have demonstrated
that miRNAs are involved in numerous molecular and cellular
processes, serving an important role in the diagnosis and treatment
of certain diseases (11).
MicroRNAs (miRNAs or miRs), a class of short non-coding RNAs of ~22
nucleotides in length, modulate gene expression post-translation or
via the induction of mRNA degradation. A number of miRNAs have been
demonstrated to be associated with fibrotic processes in liver
disorders, including miR-29, miR-150 and miR-194 (12,13).
Notably, the regulation of the expression of these miRNAs may halt
fibrogenesis in vitro and in vivo, suggesting the
significance of miRNAs as a potential target in fibrotic disease
(14). However, the role of
miR-219 in liver fibrosis remains to be completely characterized
and the precise mechanisms remain unclear.
The present study aimed to investigate the
implications of miR-219 expression during liver fibrosis in
vitro and in vivo. Furthermore, the present study
identified that miR-219 may directly target the TGFBR2 gene and
that the miR-219/TGFBR2 signaling pathway may contribute to the
diagnosis and treatment of fibrotic liver disease.
Materials and methods
Clinical samples and cell lines
Tissues were obtained from 63 patients (37 males and
26 females; mean age, 61.9±7.9 years) who were undergoing treatment
at Ningbo No. 2 Hospital (Ningbo, China), in which a diagnosis of
liver fibrosis was proven histopathologically following biopsy. The
staging of fibrosis was as follows: 31 patients in stage 0–1; 20
patients in stage 2–4; and 12 patients in stage 5–6. In addition,
12 normal subjects (seven males and five females; mean age of
58.9±6.1 years) were studied as controls. Blood samples were
collected from all subjects, which were centrifuged (1,000 × g, 10
min, 40°C) to harvest the separated serum, which was stored at
−80°C for further analysis. Written informed consent was obtained
from either subjects or their family members, and the experiment
was approved by the Ethics Committee of Ningbo No. 2 Hospital. In
addition, HSCs were isolated from Sprague-Dawley rats (n=24; male;
age, 12–16 weeks; weight, 450–550 g) purchased from the Chinese
Academy of Science (Shanghai, China). These mice were housed five
per cage under the following conditions: Constant temperature,
25°C; humidity, 40–75%; 12 h light/dark cycle; free access to food
and water. HSCs were cultured in RPMI-1640 medium (cat. no.
11875093), supplemented with 10% calf serum (both Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in a humidified incubator with
5% CO2 at 37°C. After 48 h, angiotensin II (AngII; 100 nM; R&D
Systems, Inc., Minneapolis, MN, USA) was used to stimulate HSCs
under additional incubation for 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total miRNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. cDNA was synthesized using the cDNA RT
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) at 42°C.
qPCR was performed using a SYBR-Green mix kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with a 20-µl reaction system under
conditions of 95°C for 30 sec, 95°C for 3 sec and 60°C for 30 sec
(40 cycles). Relative expression levels of miRNA and other
indicators were calculated using the 2-∆∆Cq (15) method in triplicate, and GAPDH or U6
were used as internal controls for normalization. The primer
sequences used were as follows: miR-219 forward,
5′-ACACTCCAGCTGGGTGATTGTCCAAACGCAAT-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3; TGFBR2 forward,
5′-GTAGCTCTGATGAGTGCAATGAC-3′ and reverse,
5′-CAGATATGGCAACTCCCAGTG-3′; collagen I forward,
5′-GAACCTGGGATAGCAGGACAC-3′ and reverse,
5′-CATAGTGGGTCCACAAAGACATC-3′; collagen III forward,
5′-GAACCTGGGATAGCAGGACAC-3′ and reverse,
5′-CATAGTGGGTCCACAAAGACATC-3′; α-SMA forward,
5′-CATCACGAACTGGGATGACATG-3′ and reverse,
5′-CATCTTCTCCCTGTTGGCTTTAG-3′; FSP1 forward,
5′-GAGGCTTTACTCGCACTTCG-3′ and reverse, 5′-ACCCTACGCAGACTCCCAG-3′;
GAPDH forward, 5′-AGAAGGCTGGGGCTCArTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′; and U6 reference gene forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3.
miR-219 target prediction and
luciferase reporter assay
The potential targets of miR-219 were predicted
using online programs with databases of different algorithms,
including EIMMO (http://www.mirz.unibas.ch/ElMMo3) and miRanda-mirSVR
(http://microRNA.org). For the luciferase assay,
HSCs (2.5×105 cell/well) were seeded in 6-well plates and
co-transfected with luciferase reporter plasmids
[pmiR-TGFBR2-wild-type (WT) or pmiR-TGFBR2-mutant (Mut)] (Promega
Corporation, Madison, WI, USA), along with miR-219-mimics or
controls using Lipofectamine® 2000 (Gibco; Thermo Fisher
Scientific, Inc.). Luciferase activity was analyzed using the
Dual-Luciferase Reporter system (Promega Corporation), according to
the manufacturer's protocol. The luciferase activity was determined
via comparison with Renilla luciferase activity when the cells had
been lysed with a passive lysis buffer.
Western blot analysis
Total protein was extracted from HSCs using a total
protein extraction kit (ProMab Biotechnologies, Richmond, CA, USA),
according to the manufacturer's protocol. Western blot analysis was
performed as previously described (16). Primary antibody (rabbit-anti-p21;
cat. no. ab188224, 1:500; rabbit-anti-GAPDH, cat. no. ab181603,
1:500; both Abcam, Cambridge, UK) were incubated at 4°C overnight,
and the secondary goat anti-rabbit IgG H&L (horseradish
peroxidase) antibody (cat. no. ab97051; 1:2,000; Abcam) was
incubated at room temperature for 2 h. GAPDH was used as a protein
loading control.
Immunofluorescence cytochemistry
HSCs (1.2×106 cells/ml) were respectively
transfected with 30 nM miRNA-219 mimics or negative control miRNA
mimics in 6-well plates using siPort Neo-FX (both Ambion; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The following sequences were used: miR-219 mimic agomir sense,
5′-AAAAGAATTCCCACTTCCCACTCCAGACATT-3′ and antisense,
5′-AAAGCGGCCGCCCCTCACTTCTCCGTAACCC-3′. The negative control for the
agomir was sense, 5′-UUCUUCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. Following transient transfection (24
h), the cells were synchronized in low-glucose medium (cat. no.
22320030; Thermo Fisher Scientific, Inc.) without serum for 24 h,
and stimulated with AngII, according to a previously-published
protocol (17). The expression of
α-smooth muscle actin (α-SMA; cat. no. ab5831; 1:1,000; Abcam) was
determined by immunofluorescence cytochemistry as previously
described (18).
Chronic mouse liver injury model
C57BL/6J mice (n=24; male; age, 12–16 weeks; weight,
450–550 g) were housed five per cage under the following
conditions: Constant temperature, 25°C; humidity, 40–75%; 12 h
light/dark cycle; free access to food and water. These mice were
administrated with CCl4 solution by intraperitoneal injection, and
liver injury was established as previously described (19). The mice were randomly divided into
three groups, termed the sham-operated group (8 mice), control
group (8 mice with liver injury) and observation group [8 mice with
liver injury receiving tail vein injection of miRNA-219 agonist (5
mg/kg; 200 µl, MedChemExpress, Monmouth Junction, NJ, USA)]. Mice
were sacrificed following daily administration for 14 days, and
liver tissues were collected and processed for detecting the
expression of collagen mRNA by RT-qPCR, and for
immunohistochemistry. All the procedures were performed in
accordance with national (D.L.n.26; March 4th, 2014) and
international laws and policies (directive 2010/63/EU) (20). All experiments involving animals
were approved by the Ethics Committee of Zhejiang Chinese Medical
University.
Morphological alterations
Alterations in liver morphology were examined by
Masson and hematoxylin and eosin (H&E) staining. The liver
tissues were fixed in 10% neutral formalin liquid for 24 h at room
temperature, followed by fully-automatic dehydration,
paraffin-embedding and slicing into tissue sections (4 mm). Serial
liver slices were stained with Masson's trichrome stain (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) for
10–20 min at room temperature, and H&E for 45 min at room
temperature.
Statistical analysis
Data were acquired from three independent
experiments and are presented as the mean ± standard deviation.
Comparisons between two groups were performed using the Student's
t-test, and a paired Student's t-test was used to analyze paired
data. Comparisons among three or more groups were performed using
one-way analysis of variance. Comparisons among three or more
groups were performed with the Bonferroni correction used as a post
hoc test. P<0.05 was considered to indicate a statistically
significant difference. The statistical analysis was performed
using SPSS software (SPSS for Windows 17.0; SPSS Inc., Chicago, IL,
USA).
Results
miRNA-219 is downregulated in patient
serum
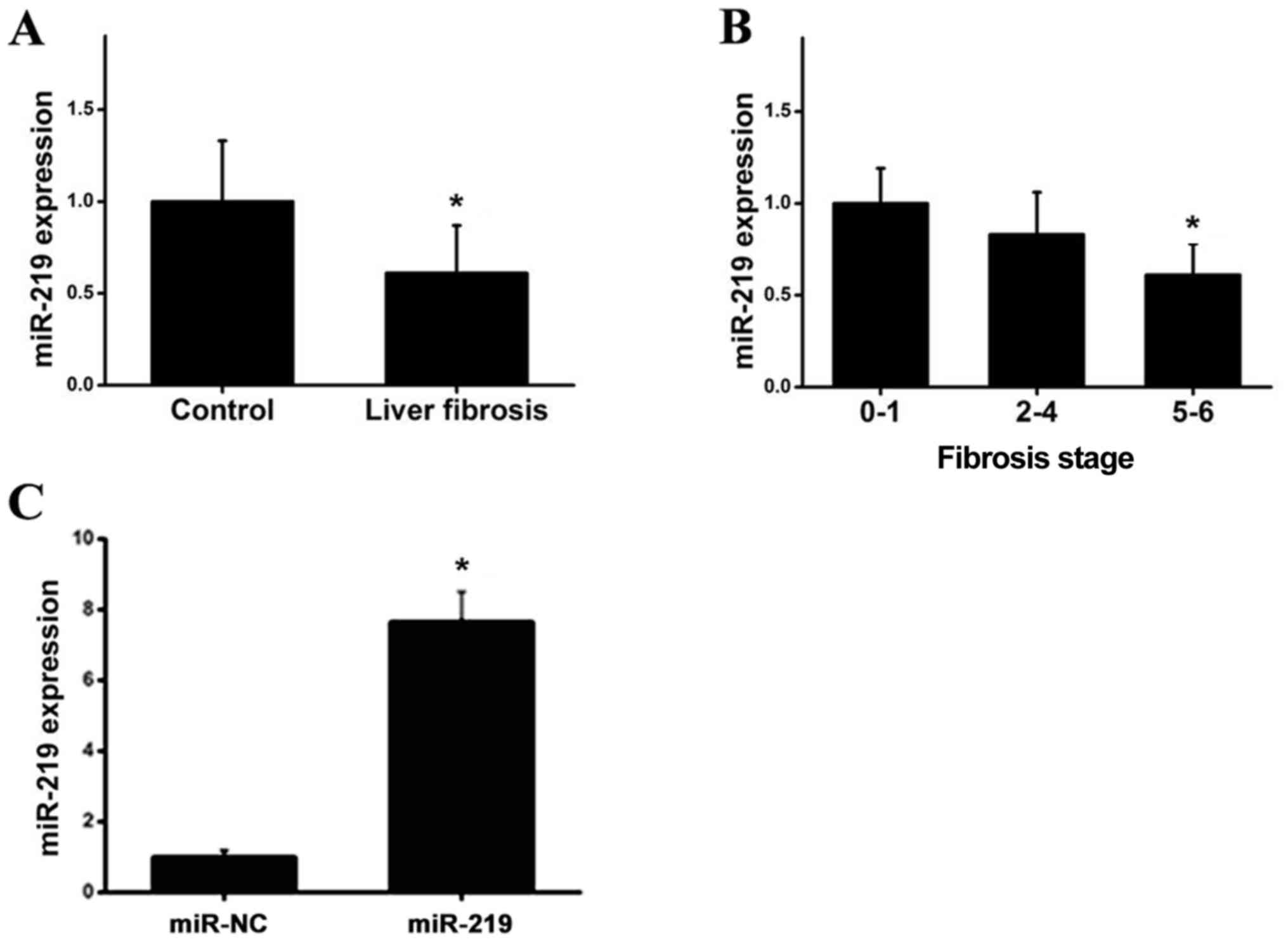
To investigate whether miRNA-219 serves a role in
liver fibrosis, miRNA-219 expression in clinical blood samples was
detected using RT-qPCR. As demonstrated in Fig. 1A, the expression of miR-219 was
significantly decreased in the serum of patients when compared with
the controls. To obtain further insight into the clinical
relevance, the expression levels of miR-219 were compared in the
blood samples of patients with various clinical pathological
features. Notably, miR-219 was negatively associated with clinical
stage, as demonstrated in Fig. 1B,
and the transfection with miR-219 was successful (Fig. 1C). In summary, these data suggested
that miRNA-219 may be involved in the development and progression
of liver fibrotic responses.
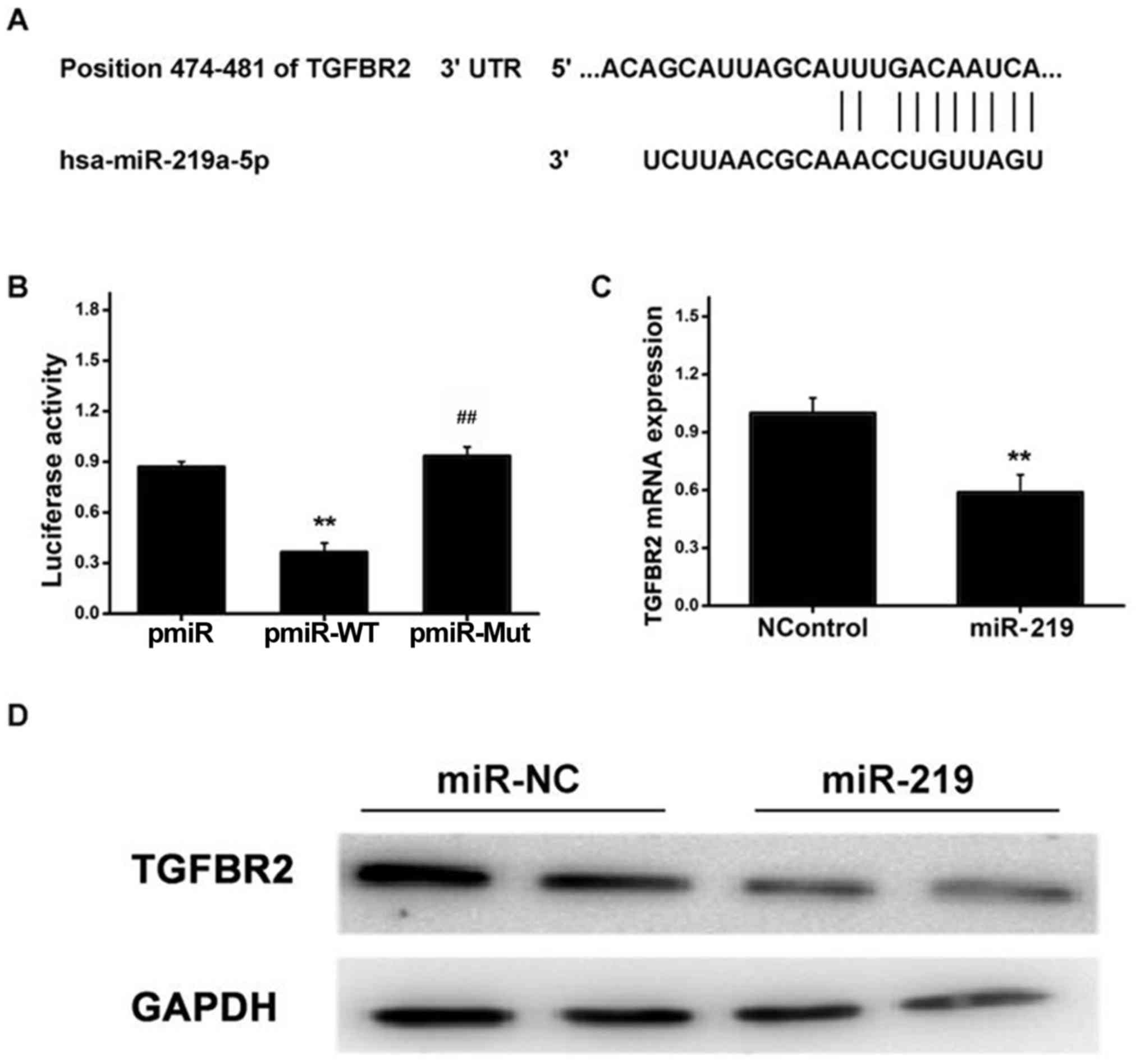
TGFBR2 is verified as a direct binding
target of miR-219
It has been reported that the TGFBR gene serves as a
useful indictor associated with fibrotic events (21,22).
Using publicly accessible databases, the present study identified
the putative miR-219 target sites in the 3′-UTR region of TGFBR2,
as demonstrated in Fig. 2A. To
further demonstrate whether TGFBR2 is a direct target of miR-219,
the two putative miR-219 target sites (WT or Mut miR-219 target
sequences) were fused into a luciferase reporter gene.
Subsequently, HSCs were co-transfected with pmiR-WT or pmiR-Mut
reporter and miR-219 mimics or controls, and cultured for 36 h,
prior to a luciferase reporter assay being performed. As
demonstrated in Fig. 2B, cells
transfected with miR-219 mimics exhibited significantly decreased
luciferase activity from the WT 3′-UTR reporter gene, while
treatment with miR-219 mimics failed to affect the luciferase
activity of the mutant reporter gene. These observations indicated
that miR-219 is able to directly bind to the 3′-UTR of TGFBR2. In
order to further investigate whether miR-219 is able to
successfully modulate TGFBR2 expression in HSCs, the subsequent
experiments were performed to determine TGFBR2 expression in cells
transfected with miR-219 mimics or controls. As demonstrated in
Fig. 2C, the RT-qPCR results
revealed that treatment with miR-219-mimics strongly attenuated
TGFBR2 expression at the mRNA level compared with the controls. In
line with this, western blot analysis indicated that cells
transfected with miR-219 mimics exhibited significantly decreased
TGFBR2 protein expression compared with the controls (Fig. 2D). These observations suggested
that miR-219 may repress TGFBR2 expression at the mRNA and protein
levels in HSCs by directly targeting the 3′-UTR of TGFBR2.
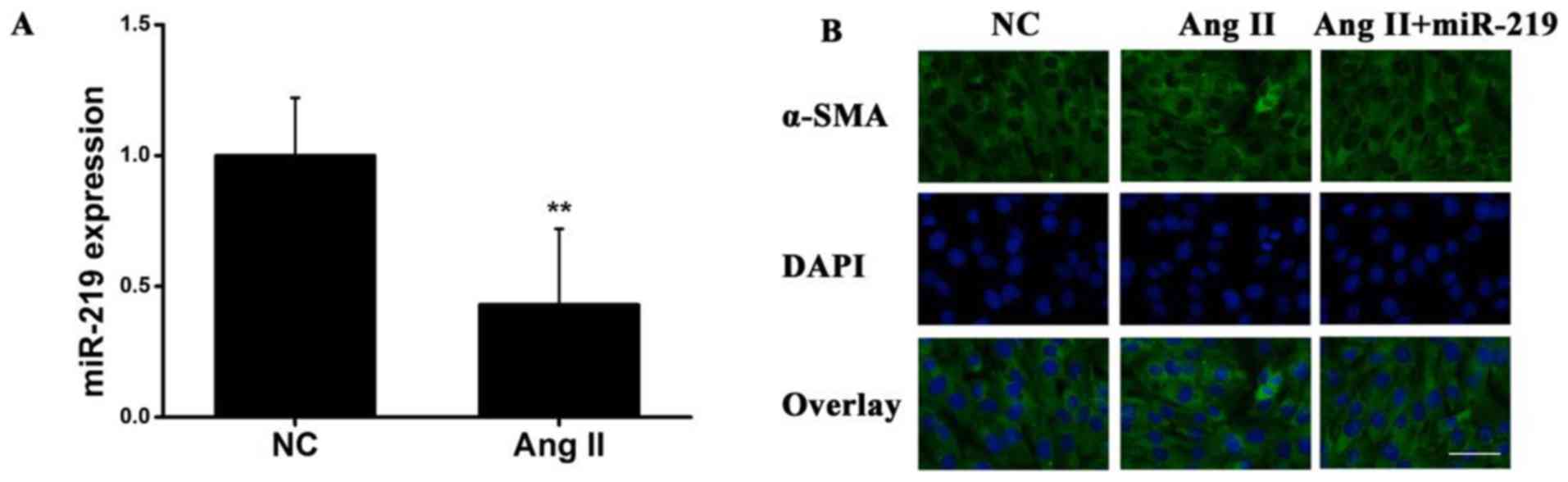
miRNA-219 represses α-SMA expression
in vitro
Next, transfected HSCs were used to elucidate the
functional significance of miRNA-219 in liver fibrosis. Ang II has
been demonstrated to promote hepatic fibrogenesis and to upregulate
miRNA expression in HSCs (23). To
identify whether AngII exerts an effect on miR-219 expression
during liver fibrotic disease, HSCs were exposed to AngII for 48 h.
RT-qPCR was used to examine miR-219 expression at the mRNA level,
and the results revealed that miR-219 exhibited significantly
decreased expression in response to AngII compared with the
controls (Fig. 3A). Additionally,
using immunofluorescence cytochemistry, α-SMA expression was
revealed to be increased following the activation of AngII in HSCs,
whereas this response was reversed by treatment with miR-219
(Fig. 3B). These data indicated
that miR-219 was able to downregulate AngII-induced α-SMA
expression in liver fibrosis.
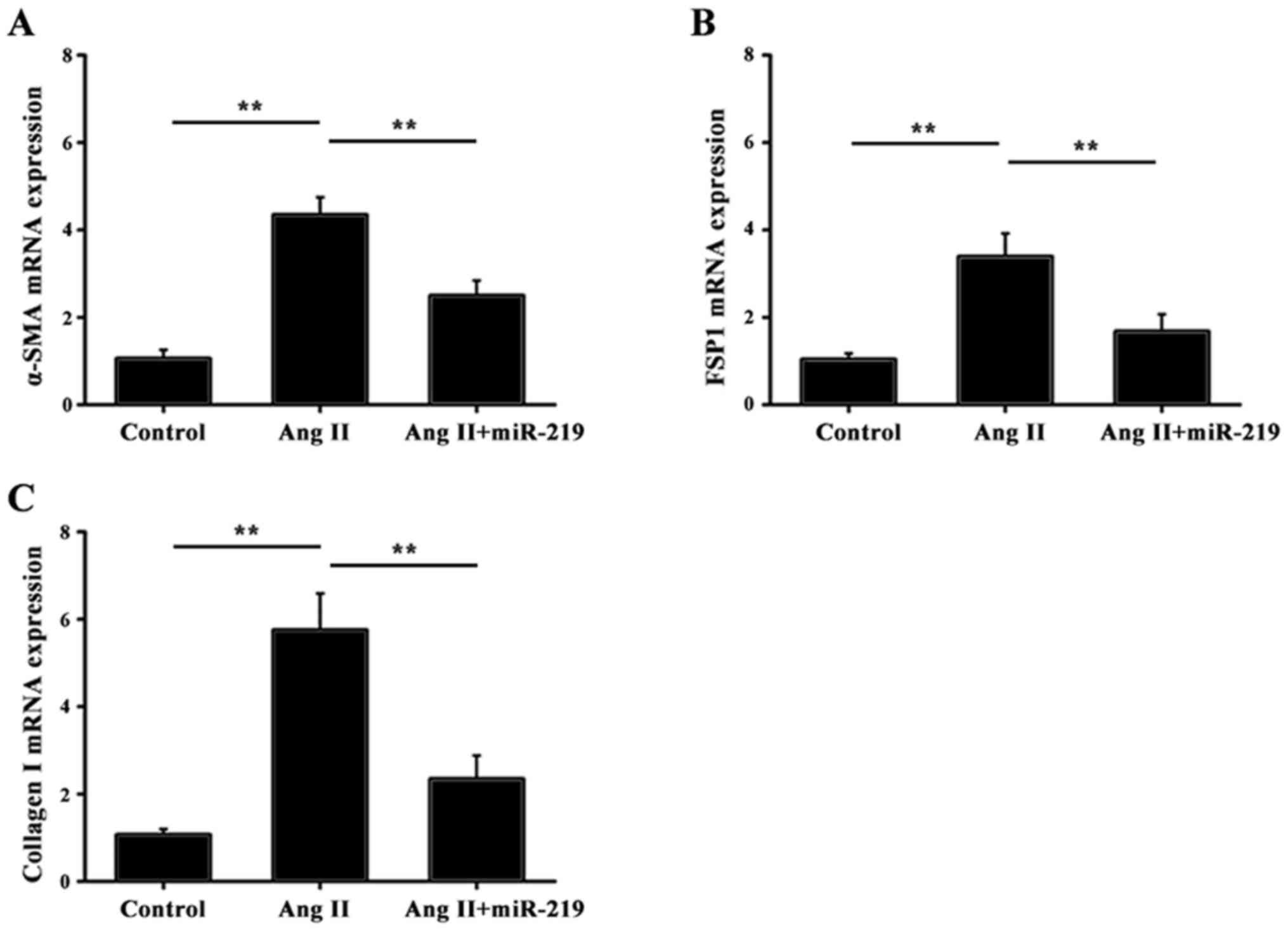
miRNA-219 inhibits liver fibrosis by
downregulation of pro-fibrotic markers
It is well known that the accumulation of α-SMA,
FSP1 and collagen can contribute toward fibrotic diseases (24,25).
To investigate the functional role of miR-219 in the liver fibrotic
response, RT-qPCR was performed to determine the expression of
α-SMA, FSP1 and collagen I in response to miR-219. As demonstrated
in Fig. 4, transfection with
miRNA-219-mimics significantly attenuated the mRNA expression of
α-SMA, FSP1 and collagen I, compared with treatment with AngII
alone. These data revealed that miR-219 exhibited an inhibitory
effect in liver fibrosis via downregulation of pro-fibrotic
markers.
miR-219 serves an important role in a
mouse chronic liver injury model
An in vivo study was performed to obtain
insight into the implications of miR-219 in the regulation of liver
fibrosis in a mouse model. miRNA expression analysis was conducted
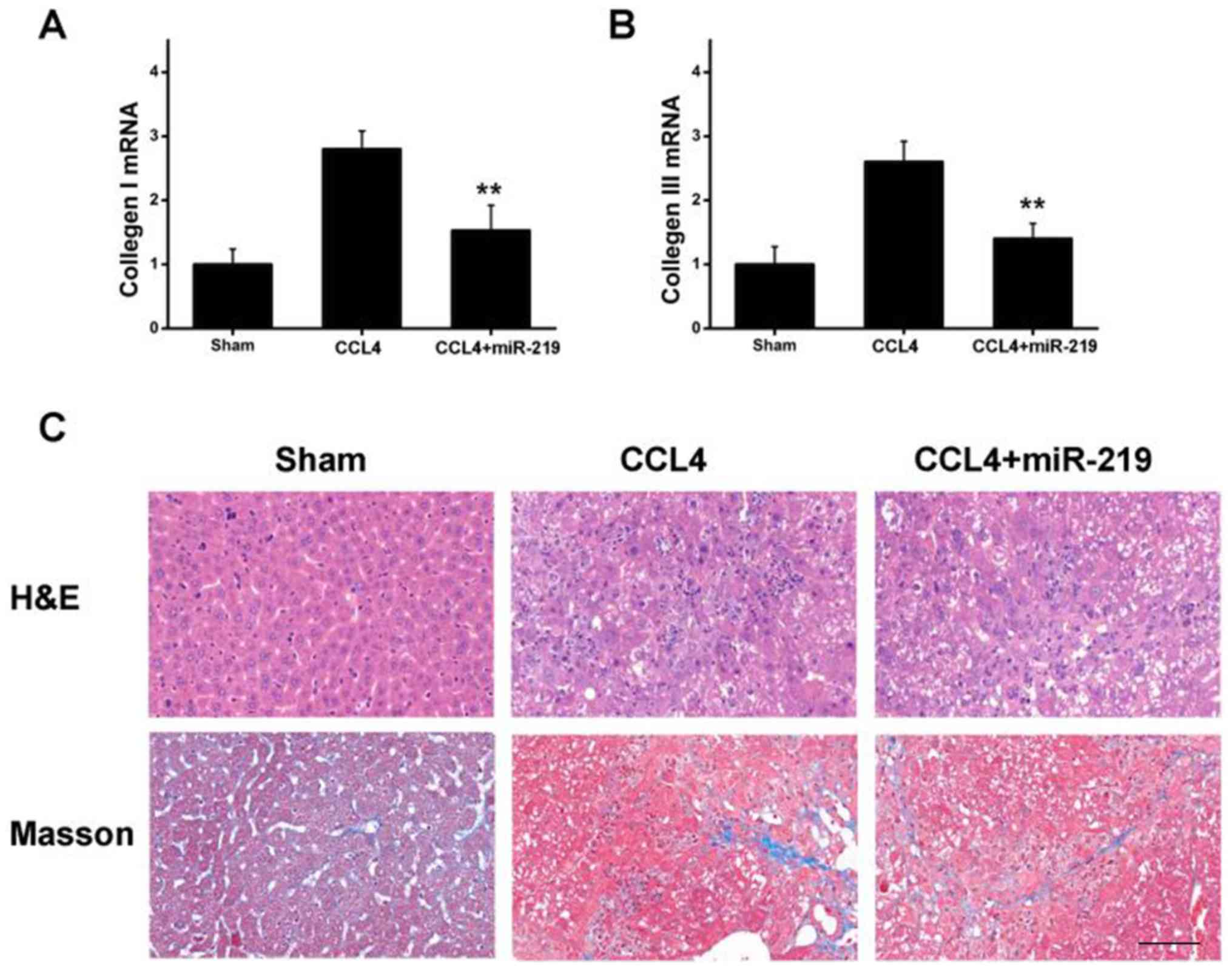
from the liver tissues collected, and the results indicated that
the mRNA expression of collagen type I and III was increased in
mice treated with CCl4 (Fig. 5A and
B). By contrast, miR-219 treatment markedly decreased the
expression of the aforementioned pro-fibrotic indictors in a
chronic mouse liver injury model (Fig.
5A and B), which was consistent with the in vitro study.
Furthermore, histological examination revealed that treatment with
CCl4 led to apparent lymphocyte infiltration and collagen
deposition in liver tissues, whereas attenuation was identified in
mice injected with miR-219, as presented in Fig. 5C. Taken together, these results
suggested that miR-219 serves as an important agent in the control
of collagen deposition during liver fibrosis in vivo.
Discussion
Hepatic fibrosis, which is regarded as a scarring
process, is associated with an accumulated and altered deposition
of ECM in the liver. This progressive fibrotic response was
characterized by cellular activation of HSCs, and aberrant
expression of cellular factors and downstream molecule regulators
(26). Recent studies have
demonstrated that a certain number of miRNAs are involved in
numerous cellular processes, including proliferation, migration,
apoptosis, differentiation, the cell cycle and tumorigenesis
(27). An increasing volume of
evidence has revealed that a number of miRNAs function as critical
regulators in alcoholic liver disease and fibrotic disorders.
However, the role of miR-219 in liver fibrosis remains largely
unknown. The present study reported on the functional relevance of
miR-219 in liver fibrotic responses in vitro and in
vivo. Notably, the present study provided evidence of the
mechanism by which miR-219 may regulate pro-fibrotic markers
implicated in liver fibrogenesis. Accordingly, it was demonstrated
that miR-219 overexpression may contribute toward the diagnosis and
treatment of liver fibrosis.
Little is known regarding the expression of
miRNA-219 in liver fibrosis. A previous study demonstrated that
miR-219 was potentially involved in the progression and metastasis
of gastric cancer (28). miR-219
has also been demonstrated to be markedly attenuated in
hepatocellular carcinoma, displaying a tumor-inhibitory role in
hepatic carcinogenesis in HCC cell lines and tissues (29). Additionally, miR-219 suppressed the
proliferation and growth of a variety of cells (30,31).
Notably, the present study identified an association between the
expression of miR-219 and liver fibrosis. By analyzing the
expression levels of serum miR-219 in clinical blood samples, it
was revealed that the downregulation of miR-219 primarily occurred
in patients with liver fibrosis, and that its expression is
negatively associated with clinical stage, suggesting that miR-219
may be associated with the development and progression of liver
fibrotic disease. Therefore, miR-219 was introduced for further
investigation due to its expression signature.
It has been widely accepted that AngII is implicated
in the pathogenesis of fibrotic response in kidneys, lungs and
livers (32). Numerous studies
have reported on AngII-mediated cardiac fibrosis through miRNA
regulation. Siddesha et al (33) reported that AngII drives the
migration of cardiac fibroblasts and thereby leads to cardiac
fibrosis by inhibiting the expression of reversion inducing
cysteine rich protein with kazal motifs, which is regarded as a
critical agent for cardiac fibroblast migration (34). AngII also represses pro-apoptotic
phosphatase and tensin homolog, and activates matrix
metallopeptidase 2 (35,36). In addition, a study undertaken by
Ning et al (37) identified
that treatment with AngII resulted in increased expression of
miR-224 in adult rat cardiac fibroblasts. Ning et al
(25) highlighted that AngII
induced the NLR family pyrin domain containing 3
inflammasome/interleukin-1β axis and thus promoted HSC activation
via the miR-21/sprouty RTK signaling antagonist 1/extracellular
signal-regulated kinase/nuclear-κB pathway. Based on these findings
in fibrotic events, the present study investigated whether AngII
may promote liver fibrosis via regulation of miR-219 and the
precise mechanism involved in this. The present study revealed that
AngII treatment decreased the expression of miR-219 in HSCs.
However, overexpression of miR-219 decreased α-SMA expression
induced by AngII, suggesting that miR-219 exerted an important
effect in HSCs via downregulation of this pro-fibrotic marker.
This notion was further supported by the results of
in vitro and in vivo studies. Fibrotic markers,
including α-SMA, FSP1 and collagen, may be induced to participate
in the signaling conduction pathway in order to promote fibrosis.
Furthermore, a previous study (38) reported that TGFβ1 enhanced α-SMA,
COL1A1 and COL3A1 levels in order to drive renal fibrosis mediated
by miR-433. Tian et al (39) reported that the apoptosis of
hepatocytes induced by miR-34a markedly increased the expression of
α-SMA, TGFβ1 and collagen I at the mRNA and protein levels. The
present study used RT-qPCR to determine the mRNA expression levels
of specific liver fibrosis-associated molecules, including α-SMA,
FSP1 and collagen I in HSCs. Notably, it was demonstrated that
miR-219 significantly downregulated the expression of α-SMA, FSP1
and collagen I. Furthermore, a CCl4-triggered mouse liver fibrosis
model was proposed to validate the functional relevance of miR-219
in liver fibrosis. Histological examination indicated that CCl4
induced apparent lymphocyte infiltration and collagen deposition in
liver tissues, whereas miR-219 notably reversed this response by
downregulating mRNA expression of collagen type I and III. These
results emphasized the significance of miR-219 as a protective
agent in liver fibrosis.
As described earlier, TGF-β ligands contribute
toward multiple biological processes, including cell proliferation,
apoptosis, hypertrophy and mesangial cell fibrosis. TGF-β ligand
binding is known to promote the formation of TGFB2R dimers, and
phosphorylation of threonine and serine residues activates TGFB1R.
The activated TGF-β receptor, which recruits the downstream
signaling mediator, sequentially initiates a signal transduction
that eventually modifies gene expression, thereby resulting in a
fibrotic response (40). With
regards to hepatic fibrogenesis, TGF-β serves a key role in the
differentiation and progression of HSCs, which may increase the
accumulation and deposition of collagen, leading to progressive
fibrosis and organ dysfunction (41). However, few studies have
investigated the role of TGFB2R in liver fibrosis. To the best of
our knowledge, the present study was the first to demonstrate that
TGFB2R is regulated by miR-219 in liver fibrosis. A luciferase
reporter assay indicated that miR-219 was able to directly bind to
the 3′-UTR of TGFBR2, and further experiments demonstrated that
transfection of miR-219 downregulated the expression of TGFBR2 at
the mRNA and protein levels, suggesting TGFBR2 as a potential
target of miR-219 implicated in fibrotic liver disease.
In conclusion, the present study provided a novel
insight into the functional implications of miR-219 expression in
the development and progression of liver fibrosis. It was also
demonstrated that miR-219 overexpression served an inhibitory role
in liver fibrosis by directly targeting TGFBR2. Therefore, the
study provides a rationale for miR-219 as a promising biomarker for
diagnosis and therapy of liver fibrosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LM designed the study, performed experiments,
analyzed the data and wrote the manuscript. JM and H-LO performed
the experiments. LM and H-LO analyzed the data and drafted the
manuscript, designed and supervised the study, and edited the
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from subjects,
and the study was approved by the Ethics Committee of Ningbo No. 2
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roderburg C, Urban GW, Bettermann K, Vucur
M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi
M, et al: Micro-RNA profiling reveals a role for miR-29 in human
and murine liver fibrosis. Hepatology. 53:209–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elsharkawy AM, Oakley F and Mann DA: The
role and regulation of hepatic stellate cell apoptosis in reversal
of liver fibrosis. Apoptosis. 10:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piscaglia F, Dudás J, Knittel T, et al:
Expression of ECM proteins fibulin-1 and −2 in acute and chronic
liver disease and in cultured rat liver cells. Cell Tissue Res.
337:449–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan Y, Han Q, Li S, Tian Z and Zhang J:
Wnt2b attenuates HSCs activation and liver fibrosis through
negative regulating TLR4 signaling. Sci Rep. 7:39522017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Henderson N and Iredale J: Liver fibrosis:
Cellular mechanisms of progression and resolution. Clin Sci (Lond).
112:265–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He Y, Huang C, Zhang SP, Sun X, Long XR
and Li J: The potential of microRNAs in liver fibrosis. Cell
Signal. 24:2268–2272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kakino S, Ohki T..Nakayama H, Yuan X,
Otabe S, Hashinaga T, Wada N, Kurita Y, Tanaka K, Hara K, et al:
Pivotal role of TNF-α in the development and progression of
nonalcoholic fatty liver disease in a murine model. Horm Metab Res.
50:80–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogawa S, Ochi T, Shimada H, Inagaki K,
Fujita I, Nii A, Moffat MA, Katragadda M, Violand BN, Arch RH and
Masferrer JL: Anti-PDGF-B monoclonal antibody reduces liver
fibrosis development. Hepatol Res. 40:1128–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czochra P, Klopcic B, Meyer E, Herkel J,
Garcia-Lazaro JF, Thieringer F, Schirmacher P, Biesterfeld S, Galle
PR, Lohse AW and Kanzler S: Liver fibrosis induced by hepatic
overexpression of PDGF-B in transgenic mice. J Hepatol. 45:419–428.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ueberham E, Lã WR, Ueberham U, Schönig K,
Bujard H and Gebhardt R: Conditional tetracycline-regulated
expression of TGF-beta1 in liver of transgenic mice leads to
reversible intermediary fibrosis. Hepatology. 37:1067–1078. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
George J, Roulot D, Koteliansky VE and
Bissell DM: In vivo inhibition of rat stellate cell activation by
soluble transforming growth factor beta type II receptor: A
potential new therapy for hepatic fibrosis. Proc Natl Acad Sci USA.
96:12719–12724. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kato M and Natarajan R: MicroRNAs in
diabetic nephropathy: Functions, biomarkers, and therapeutic
targets. Ann N Y Acad Sci 1353. 72–88. 2015. View Article : Google Scholar
|
|
13
|
Zhong X, Chung AC, Chen HY, Dong Y, Meng
XM, Li R, Yang W, Hou FF and Lan HY: miR-21 is a key therapeutic
target for renal injury in a mouse model of type 2 diabetes.
Diabetologia. 56:663–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Y, He Y and Li J: MicroRNA-21: A
central regulator of fibrotic diseases via various targets. Curr
Pharm Des. 21:2236–2242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zavadil J, Narasimhan M, Blumenberg M and
Schneider RJ: Transforming growth factor-beta and microRNA: mRNA
regulatory networks in epithelial plasticity. Cells Tissues Organs.
185:157–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen M, Men R, Liu X and Yang L:
Involvement of miR-30c in hepatic stellate cell activation through
the repression of plasminogen activator inhibitor-1. Life Sci.
155:21–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hyun J, Choi SS, Diehl AM and Jung Y:
Potential role of Hedgehog signaling and microRNA-29 in liver
fibrosis of IKKβ-deficient mouse. J Mol Histol. 45:103–112. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Venugopal SK, Jiang J, Kim TH, Li Y, Wang
SS, Torok NJ, Wu J and Zern MA: Liver fibrosis causes
downregulation of miRNA-150 and miRNA-194 in hepatic stellate
cells, and their overexpression causes decreased stellate cell
activation. Am J Physiol Gastrointest Liver Physiol. 298:G101–G106.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hartung T: Comparative analysis of the
revised directive 2010/63/EU for the protection of laboratory
animals with its predecessor 86/609/EEC-a t4 report. ALTEX.
27:285–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Müllenbach R, Fund N, Hall R, Dooley S and
Lammert F: Genotype to phenotype: Modelling the impact of natural
TGFbR2 expression variation on fibrosis initiation in vivo. Z
Gastroenterol. 49:2011. View Article : Google Scholar
|
|
22
|
Zhang Y, Zhou X, Long YI, Peng S, Zhang Q
and Mantian MI: Dihydromyricetin attenuates activation of hepatic
stellate cells through TGF-β1/Smad signaling pathway. J Third Mil
Med Univ. 40:282–289. 2018.
|
|
23
|
García-Sánchez O, López-Hernández FJ and
López-Novoa JM: An integrative view on the role of TGF-beta in the
progressive tubular deletion associated with chronic kidney
disease. Kidney Int. 77:950–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Border WA and Noble NA: Transforming
growth factor beta in tissue fibrosis. N Engl J Med. 331:1286–1292.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ning ZW, Luo XY, Wang GZ, Li Y, Pan MX,
Yang RQ, Ling XG, Huang S, Ma XX, Jin SY, et al: MicroRNA-21
mediates angiotensin II-induced liver fibrosis by activating NLRP3
Inflammasome/IL-1β axis via targeting Smad7 and Spry1. Antioxid
Redox Signal. 27:1–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Simms R, Coward WR, Pang L and Knox AJ;
Carol Feghali-Bostwick, : Identification of the sources of lung
myofibroblasts using FSP1 And ±-SMA As markers in idiopathic
pulmonary fibrosis. Am J Respir Crit Care Med. 181:A11172010.
|
|
27
|
Ding H, Yang Q, Wang Z, et al: Effects of
sulfotanshinone IIA sodium on murine renal interstitial fibrosis
and CTGF level. Immunol J. 27:398–397. 2011.
|
|
28
|
Gressner AM and Weiskirchen R: Modern
pathogenetic concepts of liver fibrosis suggest stellate cells and
TGF-beta as major players and therapeutic targets. J Cell Mol Med.
10:76–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kota J, Chivukula RR, O'Donnell KA,
Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P,
Torbenson M, Clark KR, et al: Therapeutic microRNA delivery
suppresses tumorigenesis in a murine liver cancer model. Cell.
137:1005–1017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lei H, Zou D, Li Z, Luo M, Dong L, Wang B,
Yin H, Ma Y, Liu C, Wang F, et al: MicroRNA-219-2-3p functions as a
tumor suppressor in gastric cancer and is regulated by DNA
methylation. PLoS One. 8:e603692013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang N, Lin J, Ruan J, Su N, Qing R, Liu
F, He B, Lv C, Zheng D and Luo R: MiR-219-5p inhibits
hepatocellular carcinoma cell proliferation by targeting
glypican-3. FEBS Lett. 586:884–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong TS, Liu XB, Wong YH, Ng RW, Yuen AP
and Wei WI: Mature miR-184 as potential oncogenic microRNA of
squamous cell carcinoma of tongue. Clin Cancer Res. 14:2588–2592.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siddesha JM, Valente AJ, Yoshida T,
Sakamuri SS, Delafontaine P, Iba H, Noda M and Chandrasekar B:
Docosahexaenoic acid reverses angiotensin II-induced RECK
suppression and cardiac fibroblast migration. Cell Signal.
26:933–941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dugas JC, Cuellar TL, Scholze A, Ason B,
Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT and Barres BA:
Dicer1 and miR-219 are required for normal oligodendrocyte
differentiation and myelination. Neuron. 65:597–611. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia Y, Jin X, Yan J, Entman ML and Wang Y:
CXCR6 plays a critical role in angiotensin II-induced renal injury
and fibrosis. Arterioscler Thromb Vasc Biol. 34:1422–1428. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maegdefessel L, Azuma J, Toh R, Deng A,
Merk DR, Raiesdana A, Leeper NJ, Raaz U, Schoelmerich AM, McConnell
MV, et al: MicroRNA-21 blocks abdominal aortic aneurysm development
and nicotine-augmented expansion. Sci Transl Med. 4:122ra222012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ning Q and Jiang X: Angiotensin II
upregulated the expression of microRNA-224 but not microRNA-21 in
adult rat cardiac fibroblasts. Biomed Rep. 1:776–780. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lorenzen JM, Schauerte C, Hübner A,
Kölling M, Martino F, Scherf K, Batkai S, Zimmer K, Foinquinos A,
Kaucsar T, et al: Osteopontin is indispensible for AP1-mediated
angiotensin II-related miR-21 transcription during cardiac
fibrosis. Eur Heart J. 36:2184–2196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tian XF, Ji FJ, Zang HL and Cao H:
Activation of the miR-34a/SIRT1/p53 signaling pathway contributes
to the progress of liver fibrosis via inducing apoptosis in
hepatocytes but not in HSCs. PLoS One. 11:e01586572016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Santibañez JF, Quintanilla M and Bernabeu
C: TGF-β/TGF-β receptor system and its role in physiological and
pathological conditions. Clin Sci (Lond). 121:233–251. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Y: Epithelial to mesenchymal
transition in renal fibrogenesis: Pathologic significance,
molecular mechanism, and therapeutic intervention. J Am Soc
Nephrol. 15:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|