Introduction
Glioma is the most common primary malignant tumor of
the central nervous system in adults and is associated with an
extremely poor prognosis (1). The
standard treatment for glioma includes maximal safe surgical
resection, followed by radiotherapy and chemotherapy with
temozolomide (2). However,
life-threatening tumor recurrences are inevitable in the vast
majority of patients despite the best available treatments
(3). The poor survival rate is
mainly associated with the failure of therapeutic agent delivery to
tumor regions (4). Therefore, the
development of non-viral biotechnology that allows safe and
effective gene and cell therapy in glioma is required. Human bone
marrow-derived mesenchymal stem cells (hBM-MSCs) exhibit high
anti-glioma tropism and represent a promising delivery vehicle for
targeted brain tumor therapy (4,5). The
present study aimed to test whether modified hBM-MSCs can be used
as delivery agents to improve brain tumor therapy.
MicroRNAs (miRNAs/miRs) are a class of non-coding
RNA molecules that serve crucial functions in cell senescence,
proliferation and survival by binding to target mRNAs, resulting in
mRNA translational inhibition or degradation (6). Among the miRNAs dysregulated in
cancer, the miR-34 family has received substantial attention.
miR-34a, one of the best-studied members of the family, is reported
to serve a part in p53-mediated senescence following DNA damage,
exerting an anti-tumor effect (7).
miR-34a is closely related with cellular senescence; in breast
cancer cells, it inhibits proliferation and invasion, activates
senescence and promotes sensitivity to chemotherapy (8). Recent studies have suggested that
modified MSCs can deliver miRNAs to induce a therapeutic effect in
glioma (4,9). The present study explored whether
hMSC-delivered miR-34a could induce a pro-senescent effect in
glioma cells to mediate a therapeutic effect.
The preservation of genomic integrity is an
essential process for cell homeostasis and defects in the
DNA-damage response; a complex network of proteins required for
cell-cycle checkpoint and DNA repair has been associated with
tumorigenesis (10). Targeting the
DNA damage checkpoint response in glioma cells has provided a
therapeutic model for malignant brain cancers, accompanied with the
ability to overcome radioresistance (11). miR-34a serves a part in
p53-mediated senescence following DNA damage by directly targeting
the anti-senescent protein sirtuin 1 (SIRT1) in the treatment of
breast cancer (12).
The present study investigated whether hMSCs
overexpressing miR-34a could deliver miR-34a to induce DNA damage
and subsequently cause cellular senescence in glioma cells.
Furthermore, it investigated the pro-senescent role of miR-34a
delivered by hMSCs in glioma cells, and discussed the effect of the
miR-34a/SIRT1 pathway inducing DNA damage in the glioma cells
senescence process.
Materials and methods
Cell culture and transwell co-cultures
of MSCs-U87
The hBM-MSCs were purchased from ATCC (Manassas, VA,
USA), seeded at 5,000 cells/cm2 in growth media
[Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; HyClone; GE Healthcare Life Sciences), 1% Glutamax
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 1%
penicillin-streptomycin (Beyotime Institute of Biotechnology,
Jiangsu, China)] and grown to 80–85% confluence.
The U87-MG glioblastoma of unknown origin cell line
was purchased from ATCC and maintained in DMEM supplemented with
10% FBS and 1% penicillin-streptomycin. The cells were cultured
under standard condition of 95% humidity and 5% CO2 at
37°C.
A Transwell system was used to prevent direct
contact between hMSCs and the U87 cells. hMSCs and U87 cells were
placed in the upper and lower layers of the Transwell plate,
respectively, at a density of 1×106 cells/well.
Cell transfection
Prior to miR-34a mimic and negative control (NC)
mimic transfection, hMSCs were seeded into 6-well plates at a
density of 1×105 cells per well and incubated for 12 h.
For overexpression of miR-34a, cells were transfected with 100 nM
miR-34a mimic (5′-UGGCAGUGUCUUAGCUGGUUGUAACCAGCUAAGACACUGCCAUU-3′)
or NC mimic (5′-UGUCAGCUUUGGAGCUGGUUGUAACCUAAGAUGCCACCAGCAUU-3′;
both Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
miR-34a mimic and NC mimic were transfected into hMSCs using a
transfection reagent (X-treme Transfection Reagent; Roche Applied
Science, Penzberg, Germany) according to the manufacturer's
protocol. Then, 48 h following transfection, cells were harvested
for further analysis. The transfection efficiency was analyzed by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), using U6 as the internal reference gene.
Adenoviral vectors expressing SIRT1 (Ad-SIRT1) and
control scrambled sequence (Ad-Ctrl) were designed and synthesized
by Shanghai GeneChem Co., Ltd. (Shanghai, China). U87 cells were
transfected using Lipofectamine 2000® (Invitrogen;
Thermo Fisher Scientific, Inc.) at a final concentration of 100 nM.
Subsequent experiments were performed 48 h after adenoviral
transfection.
Cell proliferation assay
Cell proliferation was assessed using the Cell
Counting Kit-8 assay (CCK-8) according to the manufacturer's
protocol (HaiGene Technology, Harbin, China). Cells in DMEM
supplemented with 10% FBS were seeded at 2×103
cells/well in 96-well plates and incubated at 37°C for 24, 48, and
72 h. CCK-8 solution (10 µl) was added to each well, and the plates
were incubated for 1 h at 37°C. The absorbance of cells at 450 nm
[Optical Density (OD) 450] was measured.
RT-qPCR
The total RNA was isolated by TRIzol®
reagent (Life Technologies; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The first-strand cDNA was
synthesized through a cDNA synthesis kit (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
qPCR was carried out using the FastStart Universal SYBR Master
(Roche Diagnostics GmbH, Mannheim, Germany) and fluorescence
quantitative PCR system. The thermocycling conditions were as
follows: 40 cycles of 95°C for 15 sec, 63°C for 20 sec and 71°C for
25 sec. The experiments were repeated three times. Quantification
was performed relative to the levels of the housekeeping gene
GAPDH. The data analysis was performed using the 2−ΔΔCq
method (13). The primer sequences
are listed in the Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Sequences |
|---|
| miR-34a | F:
5′-CAGAGCATCACACGCAAGC-3′ |
|
| R:
5′-CAGGAAACAGAAACCCCAGC-3′ |
| p53 | F:
5′-TTCCTCTTCCTGCAGTACTC-3′ |
|
| R:
5′-ACCCTGGGCAACCAGCCCTGT-3′ |
| Cdkn1a | F:
5′-TCACTGTCTTGTACCCTTGTGC-3′ |
|
| R:
5′-GGCGTTTGGAGTGGTAGAAA-3′ |
| Cdkn2c | F:
5′-CGGGAGGTTCTTGTTCTG-3′ |
|
| R:
5′-TTTGTTGGCTTGCTTGAC-3′ |
| SIRT1 | F:
5′-TGGAGGAAGGGTGTTTGTCC-3′ |
|
| R:
5′-CAAGGCAGATGGTGGCTGA-3′ |
| Telomere
length | F:
5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′ |
|
| R:
5′-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′ |
| ERCC1 | F:
5′-GGGAATTTGGCGACGTAATC-3′ |
|
| R:
5′-GCGGAGGCTGAGGAACG-3′ |
| ERCC5 | F:
5′-GAAGCAATGCCAGAGGAG-3′ |
|
| R:
5′-CCACTCTCCTTGACTCTACC-3′ |
| GAPDH | F:
5′-GGGTGGAGCCAAACGGGTC-3′ |
|
| R:
5′-GGAGTTGCTGTTGAAGTCGCA-3′ |
| U6 | F:
5′-AAGGCCACGGATAGGTCCATA-3′ |
|
| R:
5′-CGCTTTGGTGGTTCTGAAAGG-3′ |
Western blotting
Cells were lysed in ice-cold lysis buffer to obtain
total protein (Beyotime Institute of Biotechnology). Protein
concentrations were measured using a BCA Protein Assay kit
(Beyotime Institute of Biotechnology). Equal amounts of total
protein (20 µg/lane) were separated by 10% SDS-PAGE and transferred
onto nitrocellulose membranes. Membranes were blocked with 5%
nonfat dry milk at 37°C for 1 h, and then incubated with primary
antibodies against SIRT1 (1:1,000; cat. no. 3931), γ-H2AX (1:500;
cat. no. 9718) and β-actin (1:2,000; cat. no. 4970; all Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight.
Subsequently, the membranes were incubated with goat anti-rabbit
immunoglobulin G, horseradish peroxidase-linked secondary antibody
(1:2,000; cat. no. 7074; Cell Signaling Technology, Inc.) at 37°C
for 1 h. Proteins were detected using the BeyoECL Plus kit
(Beyotime Institute of Biotechnology). The stained protein bands
were visualized on a Bio-Rad ChemiDoc XRS equipment, and analyzed
using Quantity One software version 4.5.2 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Senescence-associated β-galactosidase
(SA-β-gal) assay
Cellular senescence was measured using SA-β-gal
assay (Cell Signaling Technology, Inc.). Briefly, the cells at the
density of 2×104 were washed with PBS, fixed with 2%
paraformaldehyde for 30 min at room temperature and incubated with
a fresh SA-β-gal staining solution as previously described
(14). The results are presented
as a ratio of the SA-β-gal-positive cells to the total cells for at
least 100 cells per treatment per experiment.
Relative telomere length
measurement
Relative telomere length quantification in U87 cells
was performed using qPCR as previously described (15,16),
using GAPDH as the normalizing gene. The primer pairs used to
detect the telomere length are listed in Table I.
Relative telomerase activity
measurement
Telomerase activity of whole cell lysate was
measured by a TeloTAGGG™ telomerase PCR ELISA PLUS kit
(cat. no. 12013789001; Roche Diagnostics GmbH, Penzberg, Germany).
Cell lysates were centrifuged at 12,000 × g for 20 min at 4°C and 3
µl of cell extract was used for each telomeric repeat PCR
amplification reaction and 3 µl of inactivated cell lysate were
used for Telomeric Repeat Amplification Protocol (TRAP) reaction
according to the manufacturer's recommendations. Using the ELISA
method, the amplified products were immobilized on
streptavidin-coated microtiter plates via biotin-streptavidin
interaction. Thereafter, anti-digoxigenin horseradish peroxidase
solution was added to detect the amplifications. Following addition
of the peroxidase substrate (3,3′,5,5′-tetramethylbenzidine), the
amount of TRAP products was determined by measurement of absorbance
at 450 nm using a microplate reader.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Differences among three groups or more were tested by
one-way analysis of variance followed by Tukey's post hoc test, and
comparisons between two groups were evaluated by Student's t-tests
using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Introduction of cellular senescence in
glioma cells following co-culture with hMSCs overexpressing
miR-34a
As a well-known tumor suppressor miRNA, miR-34 is
closely associated with cellular senescence (17). The present study further examined
whether delivery of miR-34a by hMSCs can induce cellular senescence
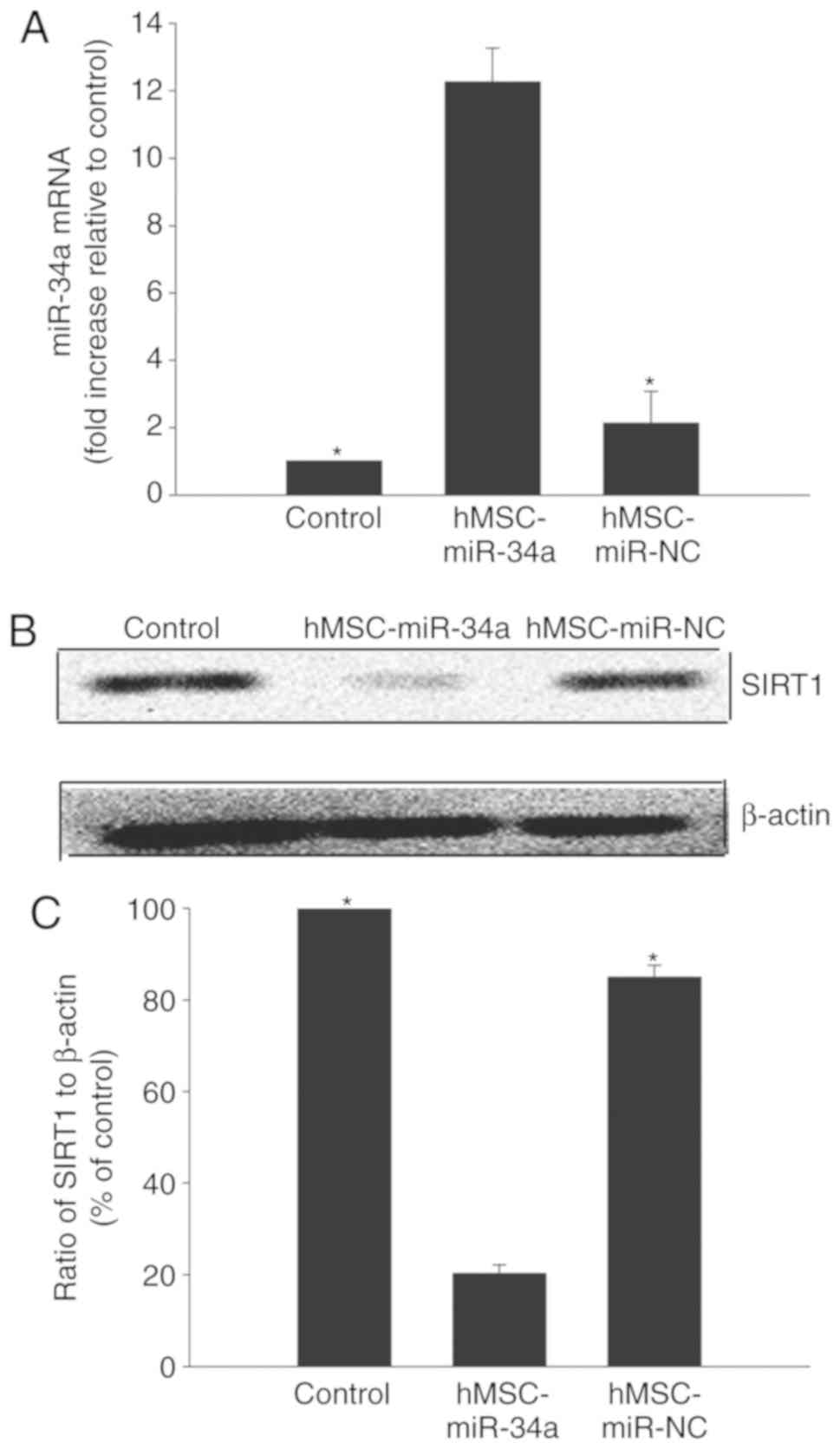
in U87 glioma cells. As presented in Fig. 1A, when hMSCs were transfected with
miR-34a mimic, the expression of miR-34a significantly increased.
Next, a Transwell culture system was used: Co-culturing with MSCs
overexpressing miR-34a for 24 h impaired U87 glioma cell
proliferation (Fig. 1B).
Additionally, it induced expression of senescence-associated genes
p53 (Fig. 1C), Cdkn1a (Fig. 1D) and Cdkn2c (Fig. 1E). As expected, this result was
corroborated by an increase in the percentage of cells positively
marked with the senescence-associated β-galactosidase (Fig. 1F and G).
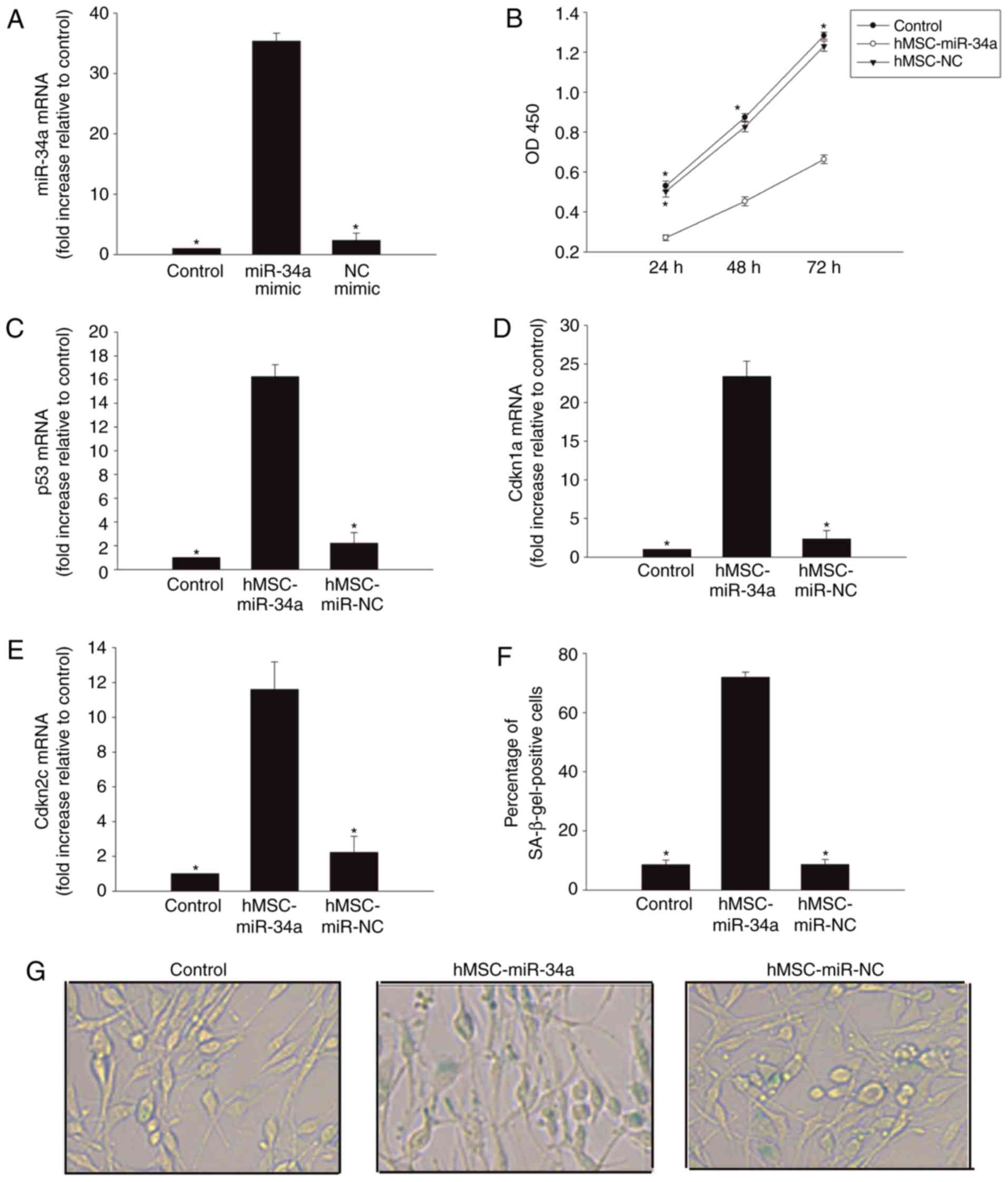 | Figure 1.Introduction of cellular senescence
in glioma cells following co-culturing with hMSCs overexpressing
miR-34a. (A) RT-qPCR analysis of miR-34a mRNA levels in hMSCs,
hMSCs transfected with miR-34a mimic, or NC mimic. *P<0.05 vs.
miR-34a mimic. To explore the role of the pro-senescence effect of
hMSCs overexpressing miR-34a on U87 cells, we used a Transwell
co-culture system. In the U87 cells co-cultured with hMSCs
overexpressing miR-34a, or with hMSCs transfected NC mimic 24 h,
(B) cell proliferation was determined by CCK-8 assay and (C) p53,
(D) Cdkn1a, and (E) Cdkn2c mRNA levels were analyzed by RT-qPCR.
(F) The percentage of β-gal-positive cells. (G) Representative
images of the SA-β-gal staining (magnification, ×40). Data
represents the mean ± standard deviation from three independent
experiments; *P<0.05 vs. hMSC-miR-34a. hMSCs, human mesenchymal
stem cells; miR, microRNAs; NC, negative control; β-gal,
β-galactosidase; SA, senescence-associated; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Downregulation of SIRT1 expression in
glioma cells by hMSC-derived miR-34a
To detect the delivery of miR-34a to U87 glioma
cells, the expression of miR-34a in the U87 glioma cells was
examined. As shown in Fig. 2A,
intracellular miR-34a expression in U87 glioma cells increased when
co-cultured with hMSCs overexpressing miR-34a. However, the
coculture of U87 cells with hMSCs transfected with NC mimic did not
induce miR-34a expression.
As a well-known target of miR-34a, SIRT1 was
selected for further study. To ensure that miR-34a downregulates
SIRT1 in U87 cells, its expression was detected with western blot
analysis in U87 cells. The results confirmed that the level of
SIRT1 decreased in U87 cells co-cultured with hMSCs overexpressing
miR-34a, while NC mimic transfection had no observable effect
(Fig. 2B and C).
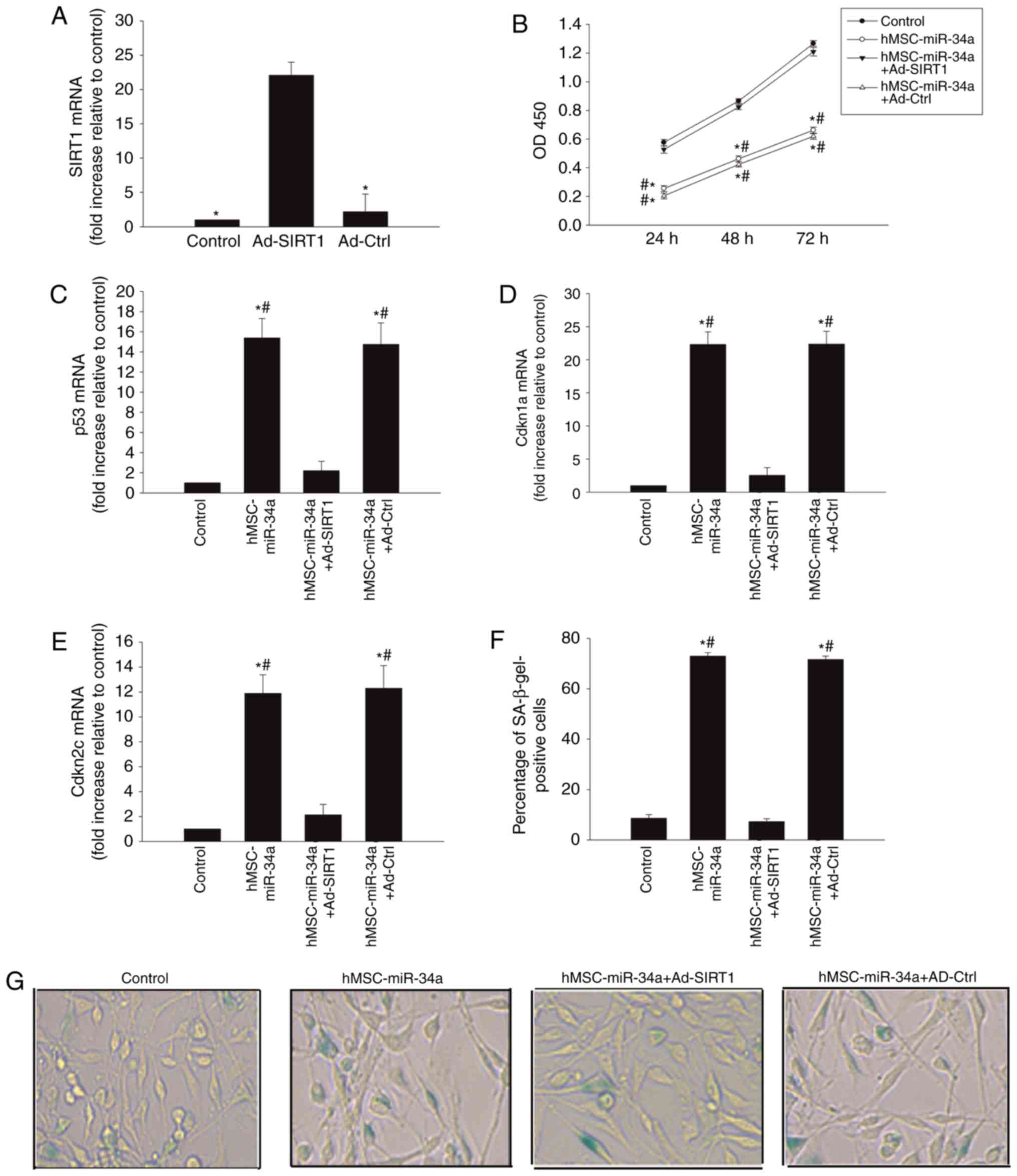
Modulation of SIRT1 weakens
pro-senescent effect of miR-34a derived from hMSCs
To further detect the miR-34a/SIRT1 signaling
pathway in the induction of U87 cellular senescence, SIRT1 was
overexpressed using Ad-SIRT1 transfection. As shown in Fig. 3A, Ad-SIRT1 transfection induced
high SIRT1 expression in the U87 cells, compared with the control
group.
The results revealed that U87 cells, transfected
with Ad-SIRT1, recovered cellular proliferation (Fig. 3B), decreased senescent related gene
p53 expression (Fig. 3C), as well
as the expression of cell cycle inhibitors Cdkn1a (Fig. 3D) and Cdkn2c (Fig. 3E). It also decreased
senescence-associated β-galactosidase positive cells (Fig. 3F and G).
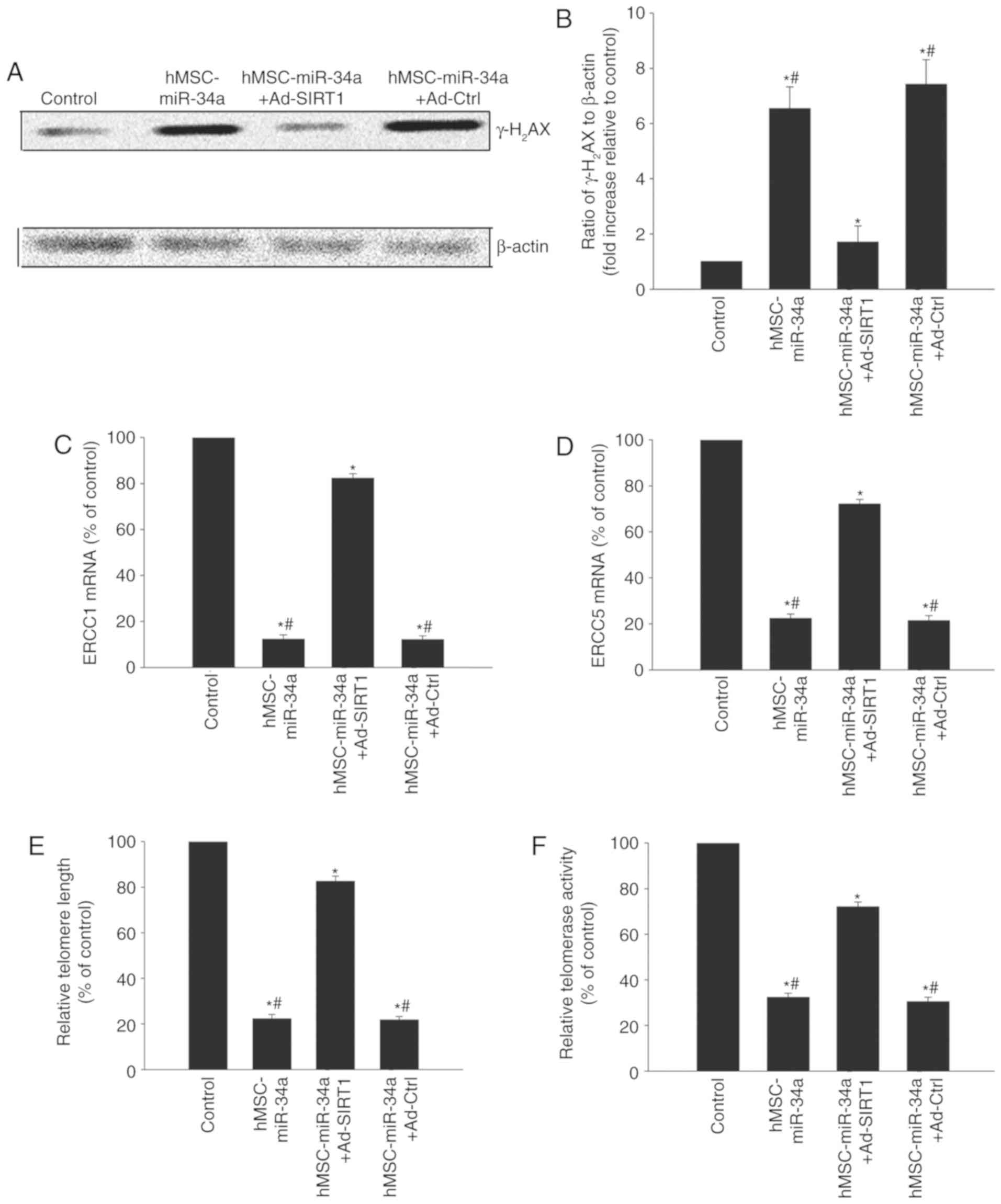
hMSC-derived miR-34a trigger the
DNA-damage response and impair telomeres
DNA damage and telomere dysfunction leads to
cellular senescence, particularly in tumors (18). Immunoblotting was used to detect
DNA damage-related protein γ-H2AX expression. Following
co-culturing with hMSCs overexpressing miR-34a, γ-H2AX
expression in U87 cells was increased (Fig. 4A and B) while DNA repair related
genes ERCC1 and ERCC5 were decreased (Fig. 4C and D). In addition, it was
identified that co-culture with hMSCs overexpressing miR-34a
decreased the telomere length and impaired the telomerase activity
(Fig. 4E and F). However, SIRT1
overexpression in the U87 cells rebalanced the DNA-damage and
repair, lengthened the telomere length and recovered the telomerase
activity, but Ad-Ctrl did not (Fig.
4).
Discussion
Gliomas are primary brain tumors derived from the
malignant transformation of oligodendrocytes and astrocytes
(19). Gliomas typically exhibit a
poor prognosis irrespective of treatment, with the most common
form, glioblastoma multiforme (GBM), with a 5-year survival rate of
only 5% (20). Current treatment
regimens for GBM include surgery, radiation therapy and
chemotherapy (21). Unfortunately,
due to the diffuse nature of malignant gliomas, complete surgical
resection is almost impossible. In addition, due to their
resistance to chemotherapy and radiotherapy, the post-surgical
treatment of GBM also requires improvement (22). One of the reasons for the poor
survival rate is the tumor microenvironment, which is not only
hypoxic and acidic but also is surrounded by high interstitial
pressure, which acts as a pathologic barrier to drug delivery into
tumors (23). One efficient method
of targeted delivery to glioma is the use of adult stem cells as a
delivery agent that can carry a therapeutic agent (24), with hMSCs among the most attractive
candidates for cell-based therapy. hMSCs can cross the blood-brain
barrier and have been shown to possess innate tumor tropism and low
immunogenicity (25). The present
study identified that hMSCs overexpressing miR-34a delivered the
miR to glioma cells.
In just over two decades since the discovery of the
first miRNA, the field of miRNA biology has been considerably
explored. Insights into the roles of miRNAs in development of
diseases, particularly in cancers, have made miRNAs attractive
tools and targets for novel therapeutic approaches (17). Several studies have demonstrated
that loss of miR-34a has a causative role in lung, breast and
several other cancers (12,26).
Acute miR-34 overexpression elicits various p53 downstream effects,
including cell cycle arrest, apoptosis, and senescence (27,28).
This was consistent with the results of the present study, which
identified that MSCs overexpressing miR-34a delivered miR-34a to
the U87 cells, resulting in lower cell proliferation, increased p53
expression, depression of the cell cycle and induction of cellular
senescence.
One of the important factors involved in miR-34a
inhibition is SIRT1 in the glioma (29); miR-34a indirectly promotes cellular
senescence by targeting and repressing levels of SIRT1 (30). A previous study revealed that SIRT1
is an effective anti-senescent factor, and serves protective roles
in several neurodegenerative diseases (31). In glioma, inhibition of Sirt1
expression induces sensitization to radiotherapy (32). The results of the present study
suggested that miR-34a derived from MSCs inhibited the expression
of SIRT1 in the U87 cells. To further confirm the role of SIRT1 in
the senescence of glioma cells, Ad-SIRT1 transfection was use to
overexpress SIRT1 in U87 cells, and it was identified that the
pro-senescent effect induced by miR-34a delivered by hMSCs, was
completely reversed by SIRT1 overexpression.
DNA damage is a network of signaling pathways that
is able to induce cellular impairment in glioma (11). The activation of DNA damage also
modulates senescence-related cellular processes, including cell
cycle checkpoint regulation and programmed cell death (33). DNA damage induces cell cycle arrest
to provoke cellular senescence (34). The present study identified that
inducing DNA damage was accompanied with glioma cell senescence,
and γ-H2AX expression increased. A previous study
reported that miR-34a impaired cardiac contractile function during
ageing, by inducing DNA damage responses and telomere attrition
(35). A previous study also
showed that transient introduction of miR-34a into HCC cell lines
inhibited telomere length, which induced senescence-like phenotypes
and affected cellular viability (36). In agreement with previous results,
the present study revealed that miR-34a delivered by hMSCs induced
DNA damage, shortened telomere length, and impaired telomerase
activity, resulting in cellular senescence.
In conclusion, the results of the present study
indicated that miR-34a delivered by MSCs induced glioma U87 cell
senescence by directly inhibiting SIRT1, resulting in increased DNA
damage, suggesting that hMSCs delivered miR-34a may serve as a
novel therapeutic target in glioma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Provincial Natural Science Foundation of China (grant no.
LQ17C060002), the Medical Scientific Research foundation of
Zhejiang Province of China (grant no. WKJ-ZJ-1525) and the Wenzhou
Science and Technology Bureau Foundation (grant no. Y20170085).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL and CDW made substantial contributions to the
acquisition of data. LC, JLL and ZZZ analyzed and interpreted the
data. CYW was involved in data analysis and drafting the
manuscript. ZPS and XHL were involved in the conception and design
of the study, and revised it critically for important intellectual
content.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Osuka S and Van Meir EG: Overcoming
therapeutic resistance in glioblastoma: The way forward. J Clin
Invest. 127:415–426. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hingtgen S, Figueiredo JL, Farrar C,
Duebgen M, Martinez-Quintanilla J, Bhere D and Shah K: Real-time
multi-modality imaging of glioblastoma tumor resection and
recurrence. J Neurooncol. 111:153–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharif S, Ghahremani MH and Soleimani M:
Delivery of exogenous miR-124 to glioblastoma multiform cells by
Wharton's Jelly mesenchymal stem cells decreases cell proliferation
and migration, and confers chemosensitivity. Stem Cell Rev.
14:236–246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mangraviti A, Tzeng SY, Gullotti D,
Kozielski KL, Kim JE, Seng M, Abbadi S, Schiapparelli P,
Sarabia-Estrada R, Vescovi A, et al: Non-virally engineered human
adipose mesenchymal stem cells produce BMP4, target brain tumors,
and extend survival. Biomaterials. 100:53–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okada N, Lin CP, Ribeiro MC, Biton A, Lai
G, He X, Bu P, Vogel H, Jablons DM, Keller AC, et al: A positive
feedback between p53 and miR-34 miRNAs mediates tumor suppression.
Genes Dev. 28:438–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adams BD, Wali VB, Cheng CJ, Inukai S,
Booth CJ, Agarwal S, Rimm DL, Győrffy B, Santarpia L, Pusztai L, et
al: miR-34a silences c-SRC to attenuate tumor growth in
triple-negative breast cancer. Cancer Res. 76:927–939. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lang FM, Hossain A, Gumin J, Momin EN,
Shimizu Y, Ledbetter D, Shahar T, Yamashita S, Parker Kerrigan B,
Fueyo J, et al: Mesenchymal stem cells as natural biofactories for
exosomes carrying miR-124a in the treatment of gliomas. Neuro
Oncol. 20:380–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Squatrito M, Brennan CW, Helmy K, Huse JT,
Petrini JH and Holland EC: Loss of ATM/Chk2/p53 pathway components
accelerates tumor development and contributes to radiation
resistance in gliomas. Cancer Cell. 18:619–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Yuan L, Luo J, Gao J, Guo J and Xie
X: MiR-34a inhibits proliferation and migration of breast cancer
through down-regulation of Bcl-2 and SIRT1. Clin Exp Med.
13:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Filippi-Chiela EC, Bueno e Silva MM, Thomé
MP and Lenz G: Single-cell analysis challenges the connection
between autophagy and senescence induced by DNA damage. Autophagy.
11:1099–1113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crepin T, Carron C, Roubiou C, Gaugler B,
Gaiffe E, Simula-Faivre D, Ferrand C, Tiberghien P, Chalopin JM,
Moulin B, et al: ATG-induced accelerated immune senescence:
Clinical implications in renal transplant recipients. Am J
Transplant. 15:1028–1038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cawthon RM: Telomere measurement by
quantitative PCR. Nucleic Acids Res. 30:e472002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Micco R: Sensing the breaks: Cytosolic
chromatin in senescence and cancer. Trends Mol Med. 23:1067–1070.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dolecek TA, Propp JM, Stroup NE and
Kruchko C: CBTRUS statistical report: Primary brain and central
nervous system tumors diagnosed in the United States in 2005–2009.
Neuro Oncol. 14 Suppl 5:v1–v49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furnari FB, Fenton T, Bachoo RM, Mukasa A,
Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al:
Malignant astrocytic glioma: Genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang T, Kim CK, Alvarez AA, Pangeni RP,
Wan X, Song X, Shi T, Yang Y, Sastry N, Horbinski CM, et al: MST4
phosphorylation of ATG4B regulates autophagic activity,
tumorigenicity, and radioresistance in glioblastoma. Cancer Cell.
32:840–855.e8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JS, Kim IK, Han S, Park I, Kim C, Bae
J, Oh SJ, Lee S, Kim JH, Woo DC, et al: Normalization of tumor
vessels by Tie2 activation and Ang2 inhibition enhances drug
delivery and produces a favorable tumor microenvironment. Cancer
Cell. 30:953–967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Menon LG, Shi VJ and Carroll RS:
Mesenchymal stromal cells as a drug delivery system. StemBook
[Internet]. Harvard Stem Cell Institute; Cambridge, MA:
2008-2009
|
|
25
|
Reagan MR and Kaplan DL: Concise review:
Mesenchymal stem cell tumor-homing: Detection methods in disease
model systems. Stem Cells. 29:920–927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim NH, Kim HS, Li XY, Lee I, Choi HS,
Kang SE, Cha SY, Ryu JK, Yoon D, Fearon ER, et al: A p53/miRNA-34
axis regulates Snail1-dependent cancer cell epithelial-mesenchymal
transition. J Cell Biol. 195:417–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang TC, Wentzel EA, Kent OA,
Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M,
Ferlito M, Lowenstein CJ, et al: Transactivation of miR-34a by p53
broadly influences gene expression and promotes apoptosis. Mol
Cell. 26:745–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong X, Jin Z, Chen Y, Xu H, Ma C, Hong X,
Li Y and Zhao G: Knockdown of long non-coding RNA ANRIL inhibits
proliferation, migration, and invasion but promotes apoptosis of
human glioma cells by upregulation of miR-34a. J Cell Biochem.
119:2708–2718. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang F, Cui J, Liu X, Lv B, Liu X, Xie Z
and Yu B: Roles of microRNA-34a targeting SIRT1 in mesenchymal stem
cells. Stem Cell Res Ther. 6:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Herskovits AZ and Guarente L: SIRT1 in
neurodevelopment and brain senescence. Neuron. 81:471–483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li T, Ma J, Han X, Jia Y, Yuan H, Shui S
and Guo D: MicroRNA-320 enhances radiosensitivity of glioma through
down-regulation of sirtuin type 1 by directly targeting forkhead
box protein M1. Transl Oncol. 11:205–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ciccia A and Elledge SJ: The DNA damage
response: Making it safe to play with knives. Mol Cell. 40:179–204.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sulli G, Rommel A, Wang X, Kolar MJ, Puca
F, Saghatelian A, Plikus MV, Verma IM and Panda S: Pharmacological
activation of REV-ERBs is lethal in cancer and oncogene-induced
senescence. Nature. 553:351–355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boon RA, Iekushi K, Lechner S, Seeger T,
Fischer A, Heydt S, Kaluza D, Tréguer K, Carmona G, Bonauer A, et
al: MicroRNA-34a regulates cardiac ageing and function. Nature.
495:107–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu X, Chen W, Miao R, Zhou Y, Wang Z,
Zhang L, Wan Y, Dong Y, Qu K and Liu C: miR-34a induces cellular
senescence via modulation of telomerase activity in human
hepatocellular carcinoma by targeting FoxM1/c-Myc pathway.
Oncotarget. 6:3988–4004. 2015.PubMed/NCBI
|