Introduction
Respiratory syncytial virus (RSV) is a globally
prevalent respiratory tract pathogen responsible for annual
epidemics of respiratory disease. RSV infections place a heavy
burden on healthcare systems and are associated with significant
morbidity and mortality (1).
Although the exact mechanism of RSV-induced airway disease remains
unclear, the inflammatory response is thought to have a central
pathogenic role (2). Reactive
oxygen species may serve as important regulators of RSV-induced
inflammatory responses (2). Acute
RSV infection induces intense neutrophilia within the airway
epithelium (3), predominantly
driven by high levels of interleukin (IL)-8, a chemoattractant and
activator of neutrophils (4,5).
Prolonged neutrophil survival contributes to mucosal inflammation,
in turn triggering a symptomatic respiratory tract infection. The
clinical manifestations range from mild upper respiratory tract
illness to severe and potentially life-threatening lower
respiratory tract disease (6).
Although the epidemiology, clinical manifestations, diagnostic
techniques and immunobiology of the disease have been well studied,
and animal models established, no safe vaccine is currently
available (6).
Grape seed proanthocyanidin extract (GSPE) is
composed of biologically active polyphenolic flavonoids, including
oligomeric proanthocyanidins. The latter compounds are naturally
found in vegetables, fruit, nuts, seeds, and bark (7). Grape seeds are a particularly rich
source of proanthocyanidins, in terms of both quantity and variety
(7). GSPE is the key
cardioprotective element of red wine (8). It has a greater antioxidant capacity
than vitamin C or E (7), and is
sold as a dietary supplement because of this activity, which is
associated with low toxicity and genotoxicity. Anticancer effects
of GSPE, attributable to the free radical-scavenging ability, have
also been reported (9,10). Some studies found that GSPE
exhibited anti-inflammatory properties (11); however, few studies have explored
the anti-inflammatory effects of GSPE on RSV-infected airway
epithelial cells.
Therefore, the present study investigated the
alterations in proinflammatory cytokine production in RSV-infected
airway epithelial A549 cells, in the presence or absence of GSPE
pretreatment. The aim of the study was to evaluate the potential of
utilizing GSPE for prevention of RSV-induced airway disease.
Materials and methods
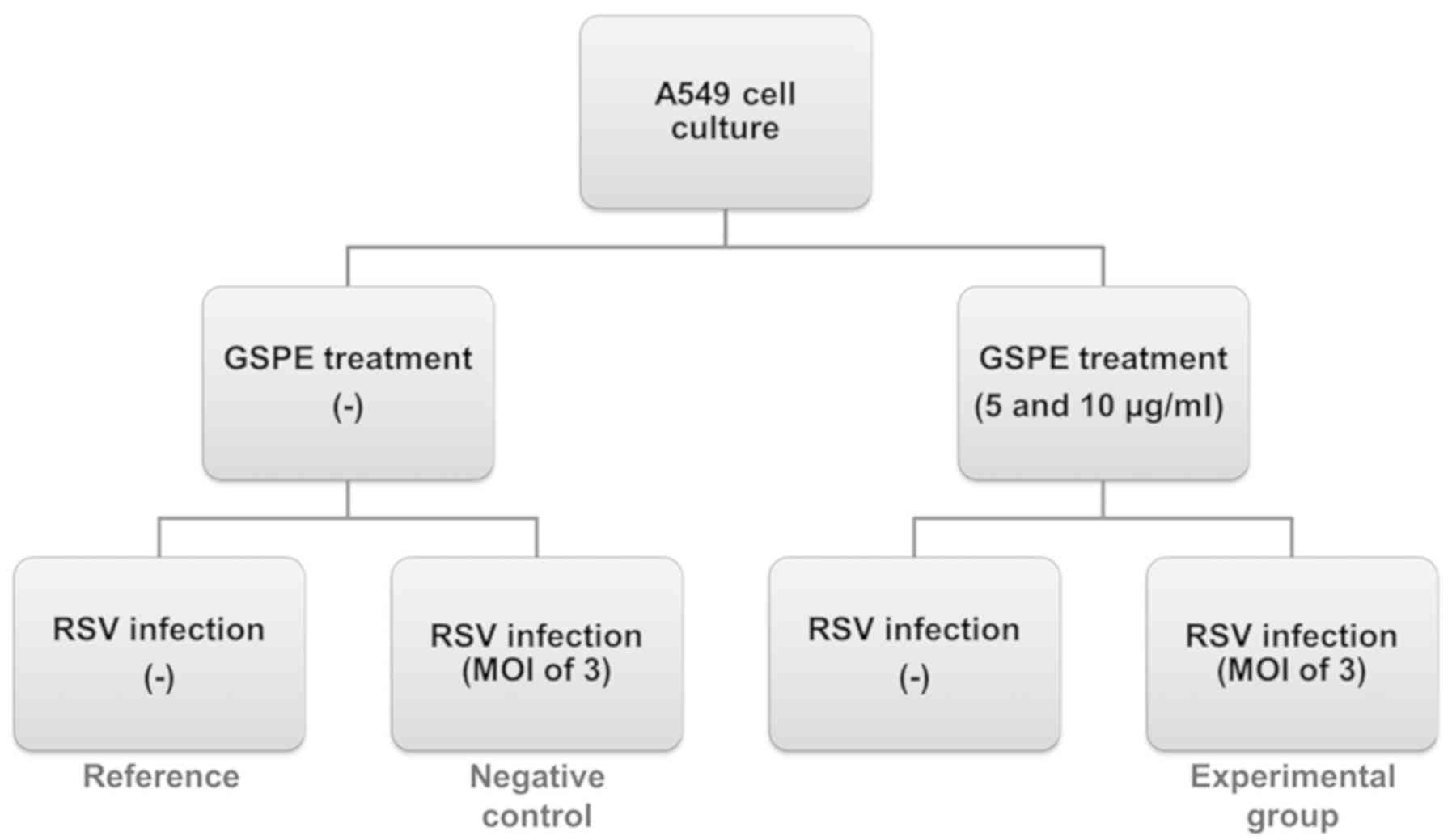
Experimental design
A549 cells were subjected to GSPE pretreatment and
infected with RSV. The experimental group underwent GSPE
pretreatment and was infected with RSV; the negative (infected)
control was not GSPE-pretreated. Another cell group received GSPE
pretreatment, but was not infected. Uninfected cells with no
pretreatment served as the reference. Fold changes in gene
expression were calculated, relative to the reference group.
Samples were taken after 24, 48 and 72 h of culture. All
experiments were repeated three times. The experimental design is
presented in Fig. 1.
Materials
GSPE from Vitis vinifera was supplied by
Hanlim Pharmaceutical Co., Ltd. (CAS no. 71328-22-8; Seoul, Korea).
GSPE contains proanthocyanidins (80% of all solids) and some
catechin monomers (12). GSPE was
solubilized in PBS. All chemicals were of analytical grade and were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany)
unless otherwise indicated. Dulbecco's modified Eagle's medium
(DMEM), penicillin-streptomycin and trypsin-EDTA were purchased
from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
RPMI-1640 was purchased from Corning Inc. (Corning, NY, USA) and
fetal bovine serum (FBS) was obtained from GE Healthcare (Chicago,
IL, USA). R&D Systems, Inc. (Minneapolis, MN, USA) supplied the
ELISA kits used to measure IL-1β, IL-6 and IL-8 expression. A
primary antibody against IL-1β (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and an appropriate secondary antibody (Cell
Signaling Technology, Inc., Beverly, MA, USA) were used for western
blotting.
Cell culture
The A549 human lung adenocarcinoma epithelial cell
line and HeLa derivative HEp-2 cells were purchased from the Korean
Cell Line Bank (Korean Cell Line Research Foundation, Seoul, South
Korea). A549 cells were cultured in RPMI-1640 medium supplemented
with 10% (v/v) FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and
2 mM glutamine. Cells were maintained in a 37°C humidified
incubator under 5% CO2 and 95% air (both v/v) until the
desired confluency (80%) was attained.
RSV propagation and titer
determination
RSV strain A2 was obtained from the American Type
Culture Collection (Manassas, VA, USA). The virus was grown in
HEp-2 cell monolayers with DMEM and 10% (v/v) FBS, and purified as
previously described (13,14). Viral titer and growth were
determined by quantitative plaque assays (15).
Infection of epithelial cells
A549 cells at ~80% confluence were infected with RSV
at a multiplicity of infection (MOI) of 3 for 60 min at 37°C.
Following adsorption, the viral solution was removed, cells were
rinsed twice with PBS, and incubation was continued in RPMI-1640
medium supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml
streptomycin and 2 mM glutamine for 72 h at 37°C. Samples were
taken at 24, 48 and 72 h for RNA and protein extraction; the
culture supernatants were stored at −80°C prior to ELISAs. Cells
infected with RSV served as negative controls and uninfected cells
as the reference group.
Cell viability upon GSPE
treatment
The potential effects of GSPE on A549 cell viability
were determined. Cells were grown in 6-well plates
(1×105 per well) for 24 h at 37°C, following which GSPE
was added at the indicated concentrations (5, 10, 20, 30, 40, 50
and 100 µg/ml); the control group received 0.01% (v/v) PBS. After
incubation at 37°C for 24, 48 and 72 h, cells were harvested with
trypsin-EDTA solution (JBI, Seoul, South Korea), washed once in PBS
with 5% (v/v) FBS, and counted using an ADAM-MC cell counter
(NanoEnTek, Inc., Seoul, South Korea).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression of IL-1β, IL-6 and IL-8 was
measured by RT-qPCR. Total cellular RNA was obtained with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). First-strand
cDNA was synthesized by reverse transcription in a reaction mixture
(20 µl) containing 1 mM of each dNTP, 1 µg RNA, 1X reaction buffer,
5 µM random primers and 20 units AMV reverse transcriptase (Promega
Corporation, Madison, WI, USA). cDNA synthesis was performed at
25°C for 10 min for primer annealing, 42°C for 60 min for reverse
transcription and 95°C for 5 min for transcriptase denaturation.
The sequences of the gene-specific primers used were as follows:
IL-1β forward, 5′-TGATGGCTTATTACAGTGGCAATG-3′ and reverse,
5′-GTAGTGGTGGTCGGAGATTCG-3′ (140 bp); IL-6 forward,
5′-GTCTTGCCTGCTGCCTTC-3′ and reverse, 5′-AGTGCCTCTTTGCTGCTTTC-3′
(194 bp); IL-8 forward, 5′-GACATACTCCAAACCTTTCCAC-3′ and reverse,
5′-CTTCTCCACAAACCTCTGC-3′ (160 bp); and β-actin forward,
5′-GCGAGAAGATGACCCAGATC-3′ and reverse, 5′-GGATAGCACAGCCTGGATAG-3′
(77 bp). PCR was performed in a StepOnePlusÔ Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Each reaction
mixture (20 µl) contained 1 µl cDNA, 1 µl solutions of both the
reverse and forward primers, 10 µl Power SYBR-Green PCR Master Mix,
and 7 µl PCR-grade water. The amplification protocol included
initial denaturation at 95°C for 10 min, followed by 40 cycles of
denaturation (95°C for 15 sec) and annealing (60°C for 1 min).
Relative gene expression was calculated using the 2−∆∆Cq
method (16). The data were
normalized to the control group and are presented as fold changes
in mRNA expression (three experiments/group).
ELISA
A549 cells were seeded into 6-well plates at a
density of 1.5×105 cells/well and incubated in RPMI-1640
with 2% (v/v) FBS for 72 h at 37°C to avoid the confounding effect
of serum-induced cytokine expression. Cells were cultured (with or
without GSPE pretreatment) for 24 h prior to RSV infection (at a
MOI of 3) for 1 h at 37°C. After 24, 48 and 72 h of infection, the
culture media were collected and centrifuged at 2,000 × g for 10
min at 4°C to remove cell debris. The supernatants were subjected
to IL-1β (cat. no. DLB50), IL-6 (cat. no. D6050) and IL-8 (cat. no.
D8000C) (all from R&D Systems, Inc.) ELISAs, in accordance with
the manufacturer's instructions.
Western blotting
Pretreated A549 cells were infected with RSV (at a
MOI of 3) in serum-free RPMI-1640 for 60 min at 37°C, and grown
under optimal conditions for 72 h at 37°C. Next, cells were washed
with cold PBS and lysed with lysis buffer (Cell Signaling
Technology, Inc.). A bicinchoninic acid protein assay was used to
determine total protein concentration. Protein (30 µg) was mixed
with loading buffer, boiled for 5 min and loaded onto 12% (w/v)
polyacrylamide gels. After electrophoresis, proteins were
transferred onto polyvinylidene difluoride membranes. Membranes
were blocked in 5% (w/v) non-fat milk powder solution for 1 h at
room temperature, and subsequently incubated overnight at 4°C in
Tris-buffered saline with 0.05% (v/v) Tween-20 (TBS-T) containing
primary antibodies against IL-1β (1:250; cat. no. sc-7884; Santa
Cruz Biotechnology, Inc.) and β-actin (1:1,000; cat. no. 4970S;
Cell Signaling Technology, Inc.). Following washing with TBS-T, the
membranes were soaked in TBS-T containing goat anti-rabbit IgG
horseradish peroxidase-conjugated secondary antibodies (1:2,500;
cat. no. 7074; Cell Signaling Technology, Inc.) for 1 h at room
temperature. Following a further wash with TBS-T, target protein
bands were visualized using an Enhanced Chemiluminescence kit
(Thermo Fisher Scientific, Inc.) and a Davinch-Chemi™
Chemiluminescence Imaging system (Davinch-K Co., Ltd., Seoul, South
Korea). Densitometric analysis of scanned immunoblot images was
performed using ImageJ software version 1.49 (National Institutes
of Health, Bethesda, MD, USA).
Statistical analysis
All values are expressed as the mean ± standard
deviation. SPSS version 23.0 (IBM Corp., Armonk, NY, USA) was used
for statistical analyses. SigmaPlot version 10 (Systat Software,
Inc., Chicago, IL, USA) was used for plotting graphs. Two-way
analysis of variance followed by Bonferroni's post-hoc test was
used to determine the statistical significance of differences
between GSPE pretreatment groups and groups subjected to RSV
infection alone. In all analyses, P<0.05 was considered to
indicate a statistically significant difference.
Results
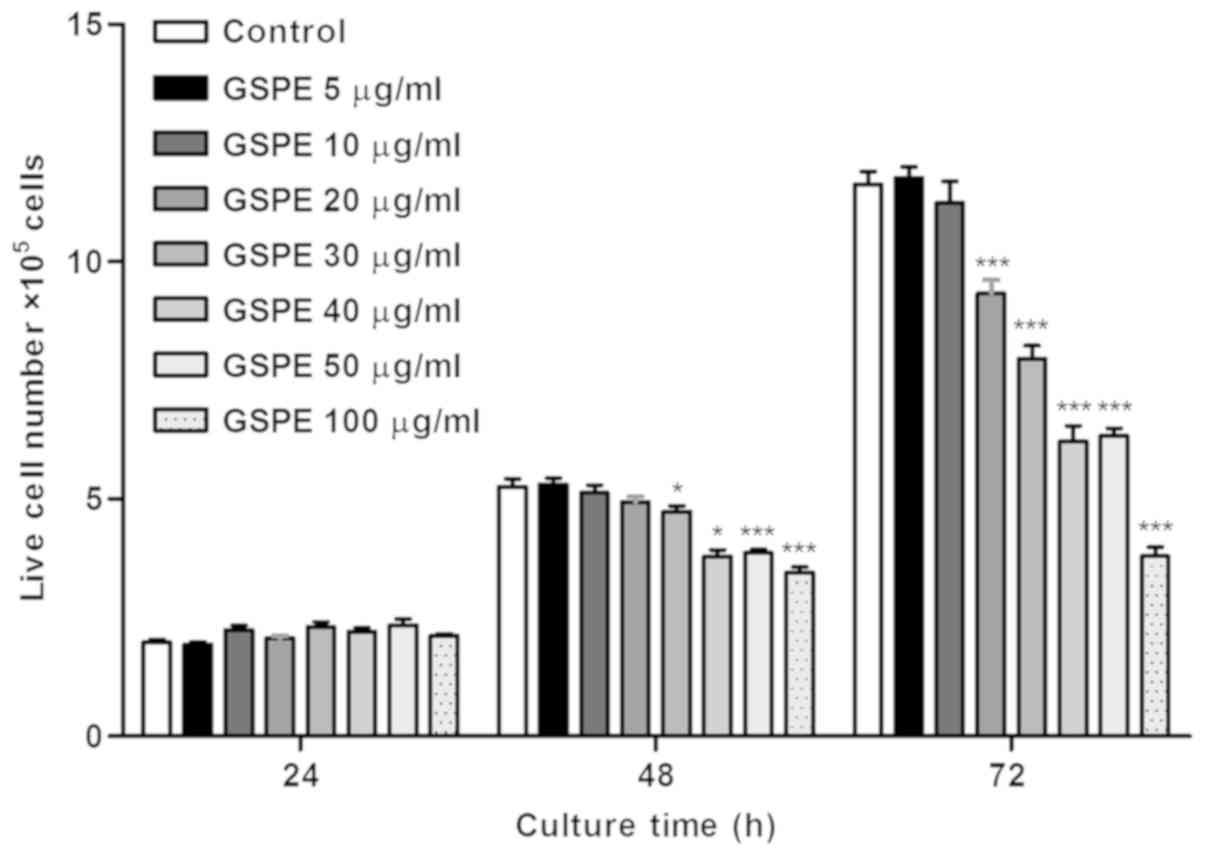
GSPE treatment does not affect A549
cell viability at 5 or 10 µg/ml
The optimal concentration for GSPE treatment was
evaluated by exposing A549 cells to varying concentrations (5, 10,
20, 30, 40, 50 and 100 µg/ml) of GSPE for 24, 48 and 72 h. The data
are expressed as cell numbers compared with those in the control
group (Fig. 2). It was determined
that GSPE was not toxic at either 5 or 10 µg/ml; therefore, these
concentrations used in all subsequent experiments.
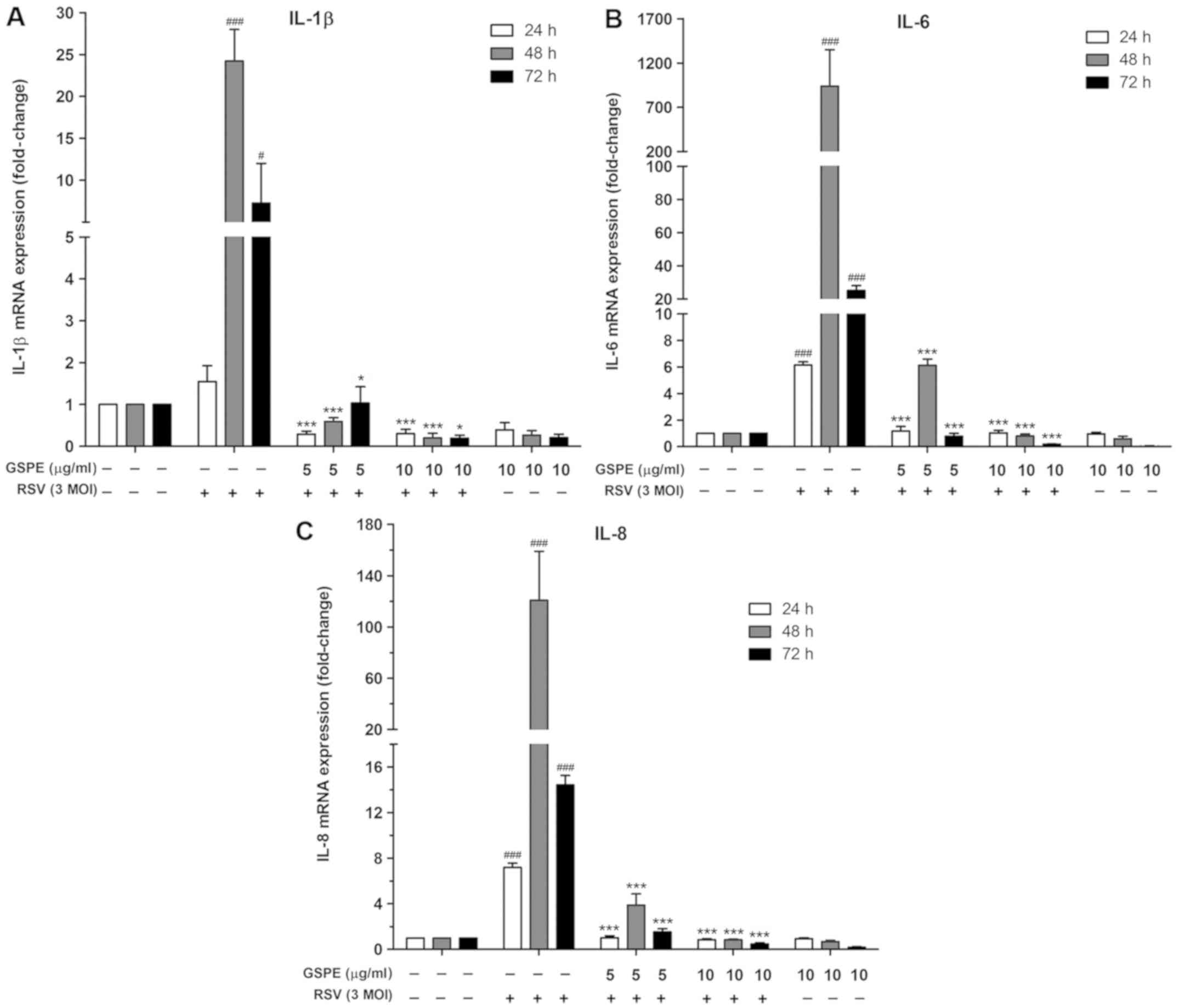
GSPE decreases proinflammatory
cytokine gene expression
To evaluate the effects of GSPE pretreatment on
RSV-infected A549 cells, the mRNA expression of IL-1β, IL-6 and
IL-8 was determined by RT-qPCR. RSV infection significantly
increased the mRNA expression of IL-1β, IL-6 and IL-8, compared
with the control group. However, GPSE pretreatment significantly
decreased the expression of IL-1β mRNA (from 24.23- to 0.59-fold at
5 µg/ml, and to 0.20-fold at 10 µg/ml after 48 h; Fig. 3A). In addition, the mRNA expression
of IL-6 significantly decreased (from 939.04- to 6.13-fold at 5
µg/ml, and to 0.80-fold at 10 µg/ml after 48 h; Fig. 3B). Similarly, the expression of
IL-8 mRNA decreased (from 120.85- to 3.87-fold at 5 µg/ml, and to
0.84-fold at 10 µg/ml; Fig. 3C).
All changes were significant compared with the RSV infection-alone
group.
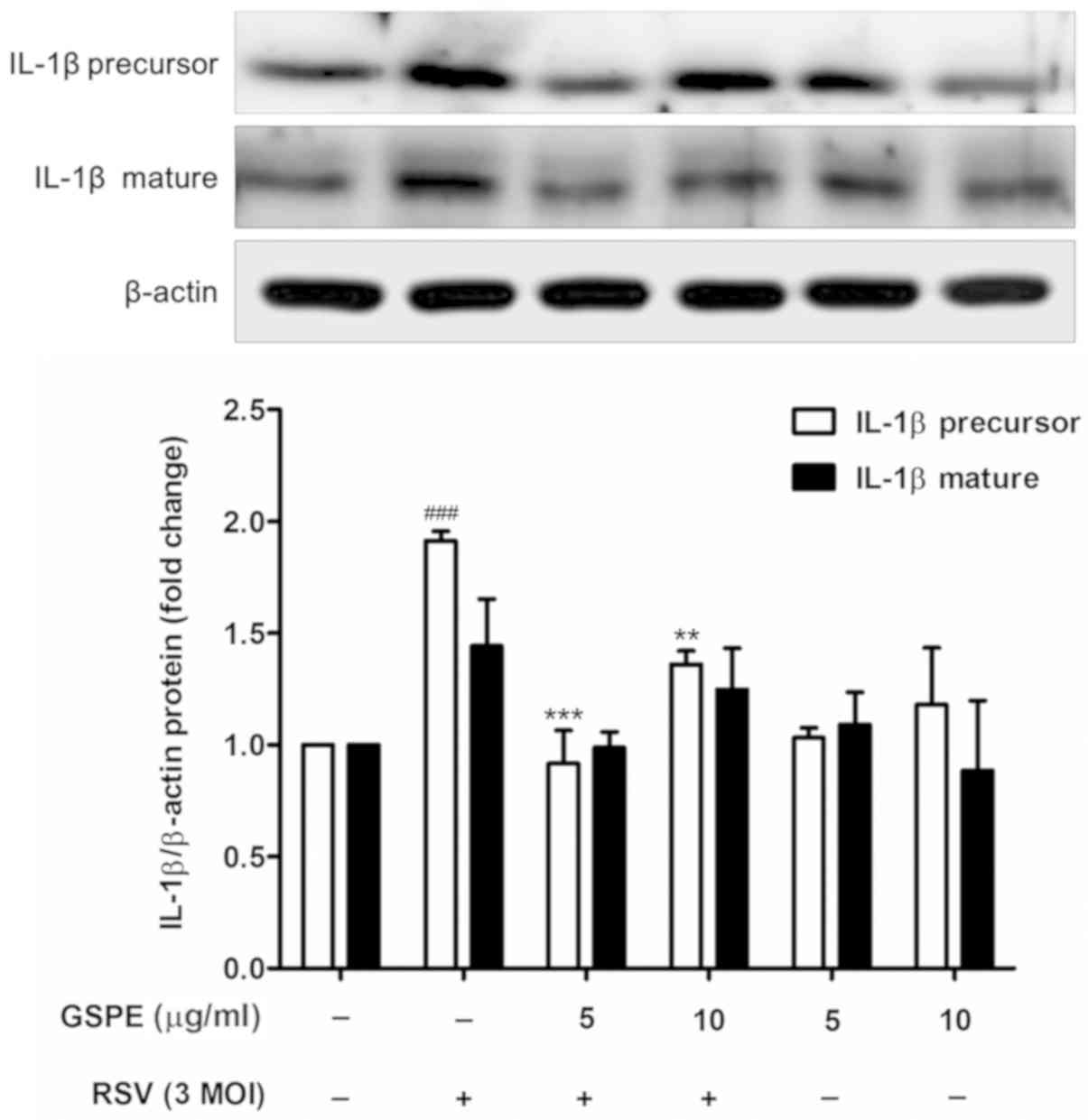
GSPE decreased proinflammatory
cytokine protein expression
ELISAs were performed to confirm the effects of GSPE
on proinflammatory cytokine protein expression. However, the ELISA
did not detect IL-1β protein expression; thus additional western
blot analysis was performed, using a polyclonal antibody against
IL-1β. Negligible IL-1β expression was detected at baseline, but
expression increased upon RSV infection (at an MOI of 3). GSPE
pretreatment (5 or 10 µg/ml) significantly reduced IL-1β expression
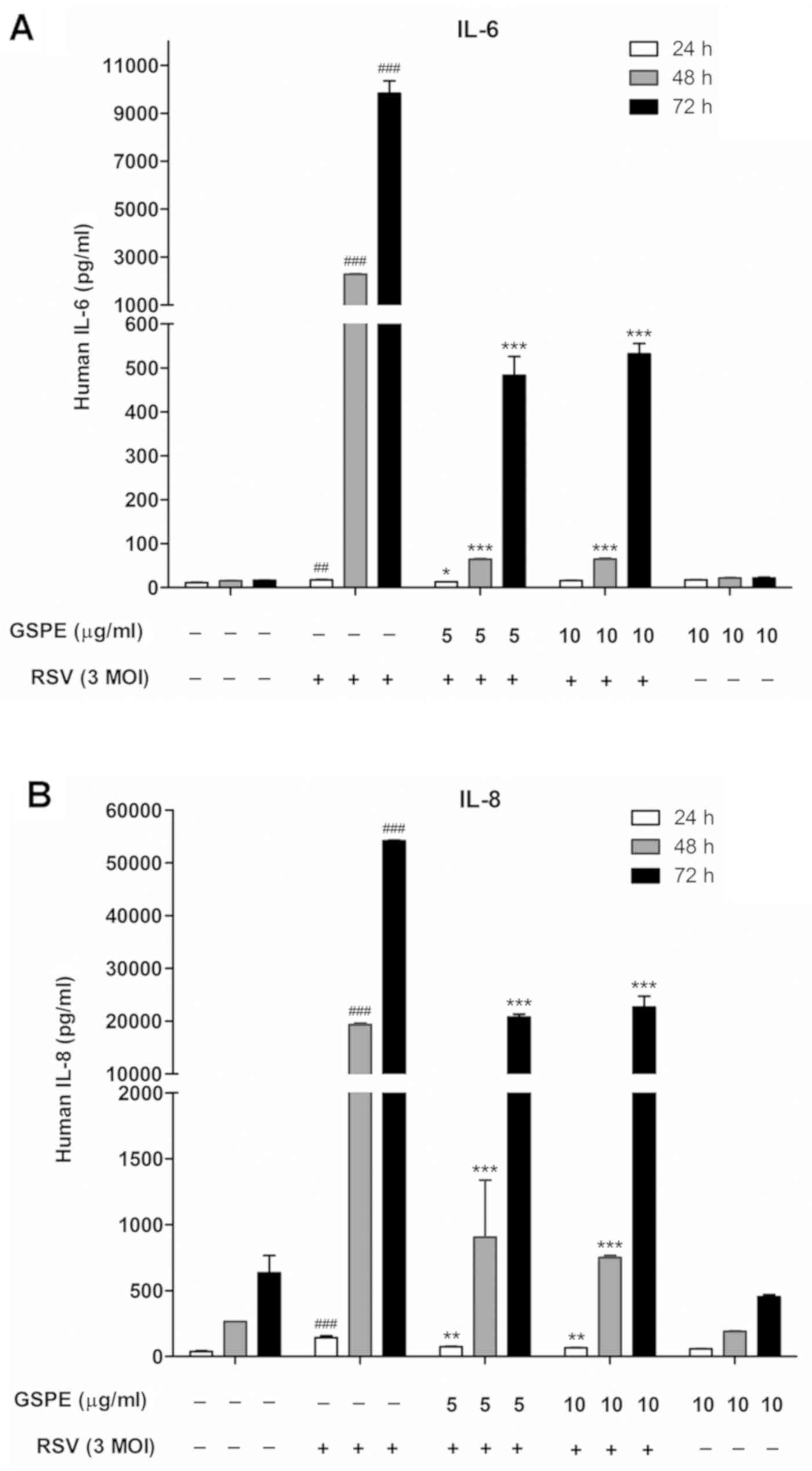
(Fig. 4). The levels of IL-6
(Fig. 5A) and IL-8 (Fig. 5B), as determined by ELISAs,
significantly increased upon RSV infection (at an MOI of 3)
compared with the control, whereas GSPE pretreatment significantly
reduced the production of these factors.
Discussion
The present study examined whether GSPE suppressed
RSV-induced inflammation in the human respiratory epithelial cell
line A549. To the best of our knowledge, this is the first study to
have investigated the potential preventative effects of GSPE in the
context of RSV-induced airway disease. It was demonstrated that
GSPE pretreatment suppressed RSV-induced proinflammatory cytokine
production, specifically that of IL-1β, IL-6 and IL-8, in
RSV-infected A549 cells. This suggested that GSPE may be a valuable
preventative agent when used to treat airway inflammation caused by
RSV infection.
Although the mechanisms of RSV-induced airway
disease remain incompletely understood, inflammatory responses
likely serve fundamental roles in its pathogenesis (2). A bronchoalveolar lavage (BAL) study
reported that neutrophils were the most abundant cell type
identified in patients with RSV infections, constituting up to 85%
of all cells (17). Inflammation
dominated by intense neutrophilia in the upper and lower airways is
the principal mechanism of RSV infection (18). Neutrophil products including
myeloperoxidase and neutrophil elastase make significant
contributions to mucosal inflammation, increasing airway secretion,
coughing, and sneezing (4).
Therefore, neutrophils may be critically involved in symptom
causation; they also likely contribute to viral transmission by
increasing mucus levels, thus enhancing the production of infected
respiratory droplets (19).
Neutrophilia is driven by cytokines such as IL-6 and
IL-8, that are produced in response to RSV; these cytokines
stimulate airway neutrophil generation and recruitment (18). Previous studies have demonstrated
that the RSV F protein activates innate immunity via Toll-like
receptor 4 and CD14+ monocytes, stimulating the
production of proinflammatory cytokines (such as IL-1β, IL-6 and
IL-8) by promoting nuclear translocation of the transcription
factor NF-κB (20,21). Reactive oxygen species may serve as
important regulators of RSV-induced cellular signaling, which in
turn triggers the expression of proinflammatory cytokines (2). These proinflammatory cytokines have
critical function in neutrophil and macrophage chemotaxis and
activation during RSV infection (21). Neutrophil numbers positively
correlate with IL-8 expression, and both of these factors are
associated with symptom severity (22). Therefore, inhibition of
proinflammatory cytokine synthesis prior to symptomatic RSV
infection is an important strategy for preventing mucosal
inflammation.
GSPE is a known antioxidant exerting beneficial
effects in patients with oxidative stress-associated diseases
(7). Along with the many other
beneficial effects of GSPE, its anti-inflammatory effect has been
studied (11,23–25).
A previous in vitro study showed that GSPE inhibits the
production of nitric oxide and prostaglandin E2, and
suppresses inducible nitric oxide synthase and NF-κB expression,
thereby exerting anti-inflammatory effects (23). Others have reported that GSPE may
be beneficial when used to treat low-grade inflammatory diseases;
such effects may be associated with inhibition of proinflammatory
cytokines and stimulation of anti-inflammatory adiponectin
production (11,24). GSPE attenuates not only the
production of inflammation-associated adipokines, but also that of
reactive oxygen species in experiments involving the coculture of
adipocytes and macrophages; implying that GSPE has
anti-inflammatory effects via its antioxidant properties (26). Although not GSPE, the
administration of antioxidants to RSV-infected mice revealed that
antioxidant treatment reduced RSV-induced oxidative stress,
RSV-induced lung inflammation, airway hyper-reactivity and clinical
illness (27). Based on such
findings, it was hypothesize that GSPE may protect against airway
epithelial cell inflammation following RSV infection.
In the present study, GSPE pretreatment of airway
epithelial cells infected with RSV evidently resulted in the
suppression of proinflammatory cytokine expression, including
IL-1β, IL-6 and IL-8. Notably, IL-1β was detected by western blot
analysis, but not ELISA. This could be due to the low levels of
secreted IL-1β, which was not sufficient to be measured by ELISA.
Inflammatory stimuli, such as RSV infection, induce excessive
cytokine production, which enhances the immune response and
subsequent inflammation (6).
Therefore, anti-inflammatory therapies often target proinflammatory
cytokines (28). It is therefore
of note that GSPE exerted its anti-inflammatory effects by
inhibiting synthesis of the IL-1β, IL-6 and IL-8 protein. The
antioxidant action of GSPE may also serve an anti-inflammatory role
by eliminating reactive oxygen species; these significantly affect
the pathogenesis of RSV infection (2). GSPE may reduce RSV-induced oxidative
stress and inhibit the production of inflammatory mediators such as
cytokines and chemokines, and, consequentially reduce RSV-induced
lung inflammation (27). This
suggests that GSPE may be useful to prevent and treat RSV-induced
airway disease.
In the present study, cells were pretreated with
GSPE at 5 and 10 µg/ml. In preliminary experiments, cells were
exposed to GPSE at concentrations between 5–100 µg/ml. GSPE at ≥20
µg/ml was associated with significant cytotoxicity. Thus, GSPE at 5
and 10 µg/ml was selected. Earlier investigators performed toxicity
studies to evaluate the safety of GSPE, which demonstrated that
GSPE was safe at higher concentrations in vivo and in
vitro (7).
The present study had several limitations. This was
a preliminary work that evaluated the anti-inflammatory effects of
GSPE in RSV-induced airway disease. A549 human lung adenocarcinoma
epithelial cells were used, which are not physiologically
representative of the human airway epithelium. The expression of
only three proinflammatory cytokines was examined and the
underlying signaling pathways were not investigated. In addition,
the levels of reactive oxygen species were not evaluated. These
limitations will be addressed in future research.
In conclusion, GSPE pretreatment may have regulated
the immune response by reducing RSV infection-induced transcription
of proinflammatory cytokines in airway epithelial cells. GSPE
pretreatment significantly suppressed IL-1β, IL-6 and IL-8
expression, suggesting that GSPE may serve as a preventative agent
in RSV-induced airway disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SWK designed the study. SJK and JWL performed the
experiments, and analyzed and interpreted the data. SJK wrote the
manuscript. YGE, KHL, SGY and SWK were involved in the
interpretation of data and revised the manuscript for important
intellectual content. All authors have read and approved of the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bont L, Checchia PA, Fauroux B,
Figueras-Aloy J, Manzoni P, Paes B, Simões EA and Carbonell-Estrany
X: Defining the epidemiology and burden of severe respiratory
syncytial virus infection among infants and children in Western
countries. Infect Dis Ther. 5:271–298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garofalo RP, Kolli D and Casola A:
Respiratory syncytial virus infection: Mechanisms of redox control
and novel therapeutic opportunities. Antioxid Redox Signal.
18:186–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith PK, Wang SZ, Dowling KD and Forsyth
KD: Leucocyte populations in respiratory syncytial virus-induced
bronchiolitis. J Paediatr Child Health. 37:146–151. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abu-Harb M, Bell F, Finn A, Rao WH, Nixon
L, Shale D and Everard ML: IL-8 and neutrophil elastase levels in
the respiratory tract of infants with RSV bronchiolitis. Eur Respir
J. 14:139–143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones A, Qui JM, Bataki E, Elphick H,
Ritson S, Evans GS and Everard ML: Neutrophil survival is prolonged
in the airways of healthy infants and infants with RSV
bronchiolitis. Eur Respir J. 20:651–657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borchers AT, Chang C, Gershwin ME and
Gershwin LJ: Respiratory syncytial virus-a comprehensive review.
Clin Rev Allergy Immunol. 45:331–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray
SD, Kuszynski CA, Joshi SS and Pruess HG: Free radicals and grape
seed proanthocyanidin extract: Importance in human health and
disease prevention. Toxicology. 148:187–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bagchi D, Sen CK, Ray SD, Das DK, Bagchi
M, Preuss HG and Vinson JA: Molecular mechanisms of
cardioprotection by a novel grape seed proanthocyanidin extract.
Mutat Res. 523:87–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akhtar S, Meeran SM, Katiyar N and Katiyar
SK: Grape seed proanthocyanidins inhibit the growth of human
non-small cell lung cancer xenografts by targeting insulin-like
growth factor binding protein-3, tumor cell proliferation, and
angiogenic factors. Clin Cancer Res. 15:821–831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bagchi D, Swaroop A, Preuss HG and Bagchi
M: Free radical scavenging, antioxidant and cancer chemoprevention
by grape seed proanthocyanidin: An overview. Mutat Res. 768:69–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chacón MR, Ceperuelo-Mallafré V,
Maymó-Masip E, Mateo-Sanz JM, Arola L, Guitiérrez C, Fernandez-Real
JM, Ardèvol A, Simón I and Vendrell J: Grape-seed procyanidins
modulate inflammation on human differentiated adipocytes in vitro.
Cytokine. 47:137–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gabetta B, Fuzzati N, Griffini A, Lolla E,
Pace R, Ruffilli T and Peterlongo F: Characterization of
proanthocyanidins from grape seeds. Fitoterapia. 71:162–175. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dakhama A, Park JW, Taube C, Joetham A,
Balhorn A, Miyahara N, Takeda K and Gelfand EW: The enhancement or
prevention of airway hyperresponsiveness during reinfection with
respiratory syncytial virus is critically dependent on the age at
first infection and IL-13 production. J Immunol. 175:1876–1883.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
LeVine AM, Elliott J, Whitsett JA,
Srikiatkhachorn A, Crouch E, DeSilva N and Korfhagen T: Surfactant
protein-d enhances phagocytosis and pulmonary clearance of
respiratory syncytial virus. Am J Respir Cell Mol Biol. 31:193–199.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McKimm-Breschkin JL: A simplified plaque
assay for respiratory syncytial virus-direct visualization of
plaques without immunostaining. J Virol Methods. 120:113–117. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heidema J, Lukens MV, van Maren WW, van
Dijk ME, Otten HG, van Vught AJ, van der Werff DB, van Gestel SJ,
Semple MG, Smyth RL, et al: CD8+ T cell responses in
bronchoalveolar lavage fluid and peripheral blood mononuclear cells
of infants with severe primary respiratory syncytial virus
infections. J Immunol. 179:8410–8417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Russell CD, Unger SA, Walton M and
Schwarze J: The human immune response to respiratory syncytial
virus infection. Clin Microbiol Rev. 30:481–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ugonna K, Douros K, Bingle CD and Everard
ML: Cytokine responses in primary and secondary respiratory
syncytial virus infections. Pediatr Res. 79:946–950. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurt-Jones EA, Popova L, Kwinn L, Haynes
LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT,
Anderson LJ and Finberg RW: Pattern recognition receptors TLR4 and
CD14 mediate response to respiratory syncytial virus. Nat Immunol.
1:398–401. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polack FP, Irusta PM, Hoffman SJ, Schiatti
MP, Melendi GA, Delgado MF, Laham FR, Thumar B, Hendry RM, Melero
JA, et al: The cysteine-rich region of respiratory syncytial virus
attachment protein inhibits innate immunity elicited by the virus
and endotoxin. Proc Natl Acad Sci USA. 102:8996–9001. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Henriquez KM, Hayney MS, Xie Y, Zhang Z
and Barrett B: Association of interleukin-8 and neutrophils with
nasal symptom severity during acute respiratory infection. J Med
Virol. 87:330–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Terra X, Valls J, Vitrac X, Mérrillon JM,
Arola L, Ardèvol A, Bladé C, Fernandez-Larrea J, Pujadas G, Salvadó
J and Blay M: Grape-seed procyanidins act as antiinflammatory
agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting
NFkB signaling pathway. J Agric Food Chem. 55:4357–4365. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terra X, Montagut G, Bustos M, Llopiz N,
Ardèvol A, Bladé C, Fernández-Larrea J, Pujadas G, Salvadó J, Arola
L and Blay M: Grape-seed procyanidins prevent low-grade
inflammation by modulating cytokine expression in rats fed a
high-fat diet. J Nutr Biochem. 20:210–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim H, Kim JY, Song HS, Park KU, Mun KC
and Ha E: Grape seed proanthocyanidin extract inhibits
interleukin-17-induced interleukin-6 production via MAPK pathway in
human pulmonary epithelial cells. Naunyn Schmiedebergs Arch
Pharmacol. 383:555–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakurai T, Kitadate K, Nishioka H, Fujii
H, Kizaki T, Kondoh Y, Izawa T, Ishida H, Radák Z and Ohno H:
Oligomerized grape seed polyphenols attenuate inflammatory changes
due to antioxidative properties in coculture of adipocytes and
macrophages. J Nutr Biochem. 21:47–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Castro SM, Guerrero-Plata A, Suarez-Real
G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP and Casola A:
Antioxidant treatment ameliorates respiratory syncytial
virus-induced disease and lung inflammation. Am J Respir Crit Care
Med. 174:1361–1369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bellik Y, Boukraâ L, Alzahrani HA,
Bakhotmah BA, Abdellah F, Hammoudi SM and Iguer-Ouada M: Molecular
mechanism underlying anti-inflammatory and anti-allergic activities
of phytochemicals: An update. Molecules. 18:322–353. 2012.
View Article : Google Scholar : PubMed/NCBI
|