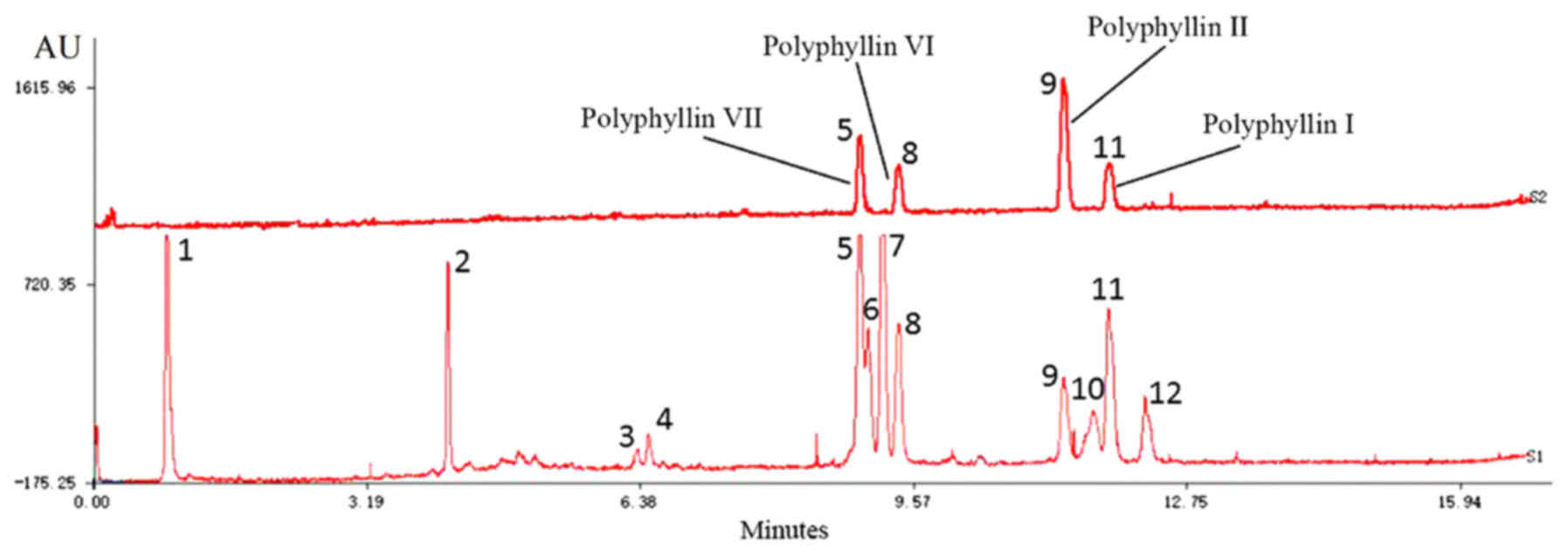
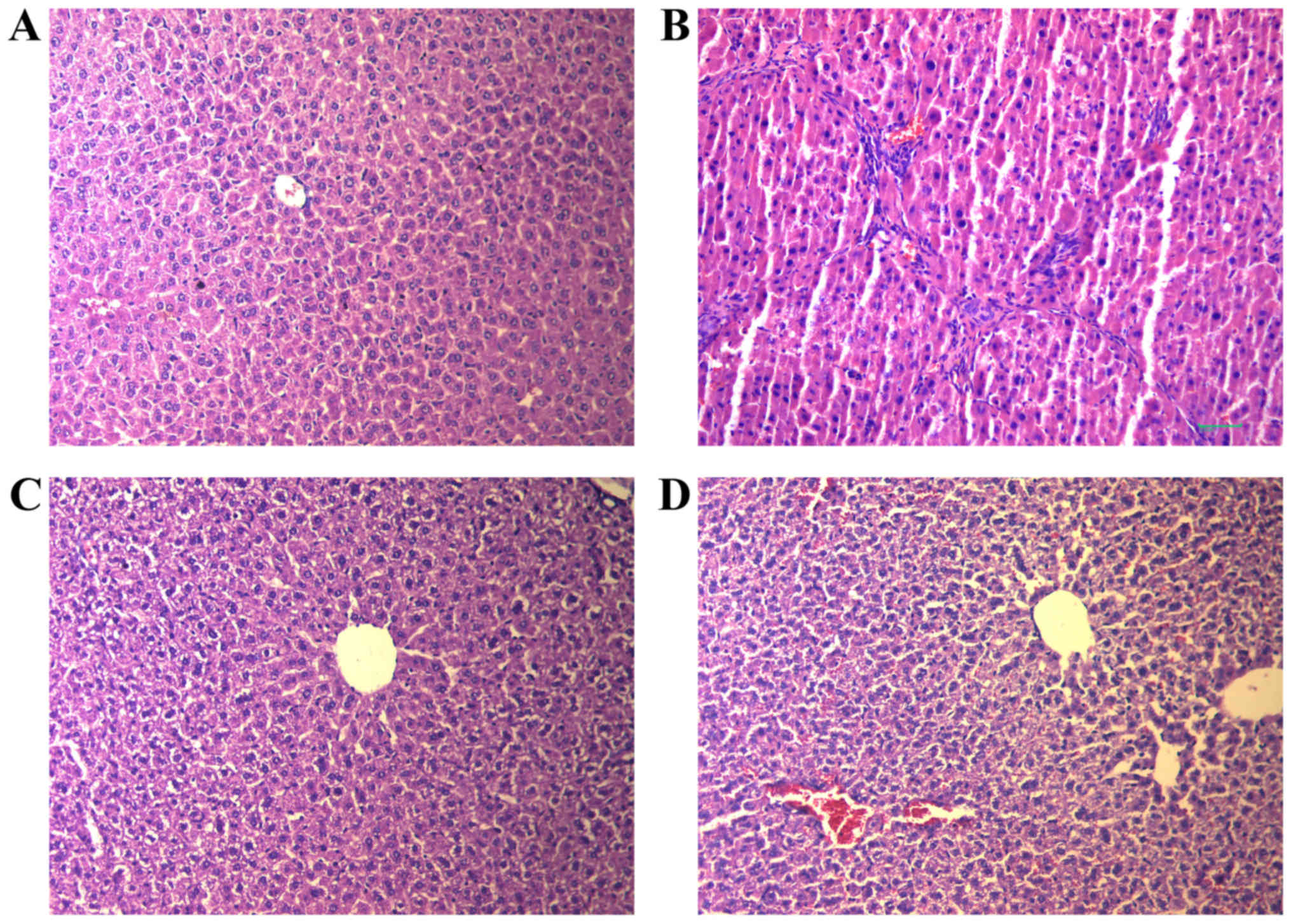
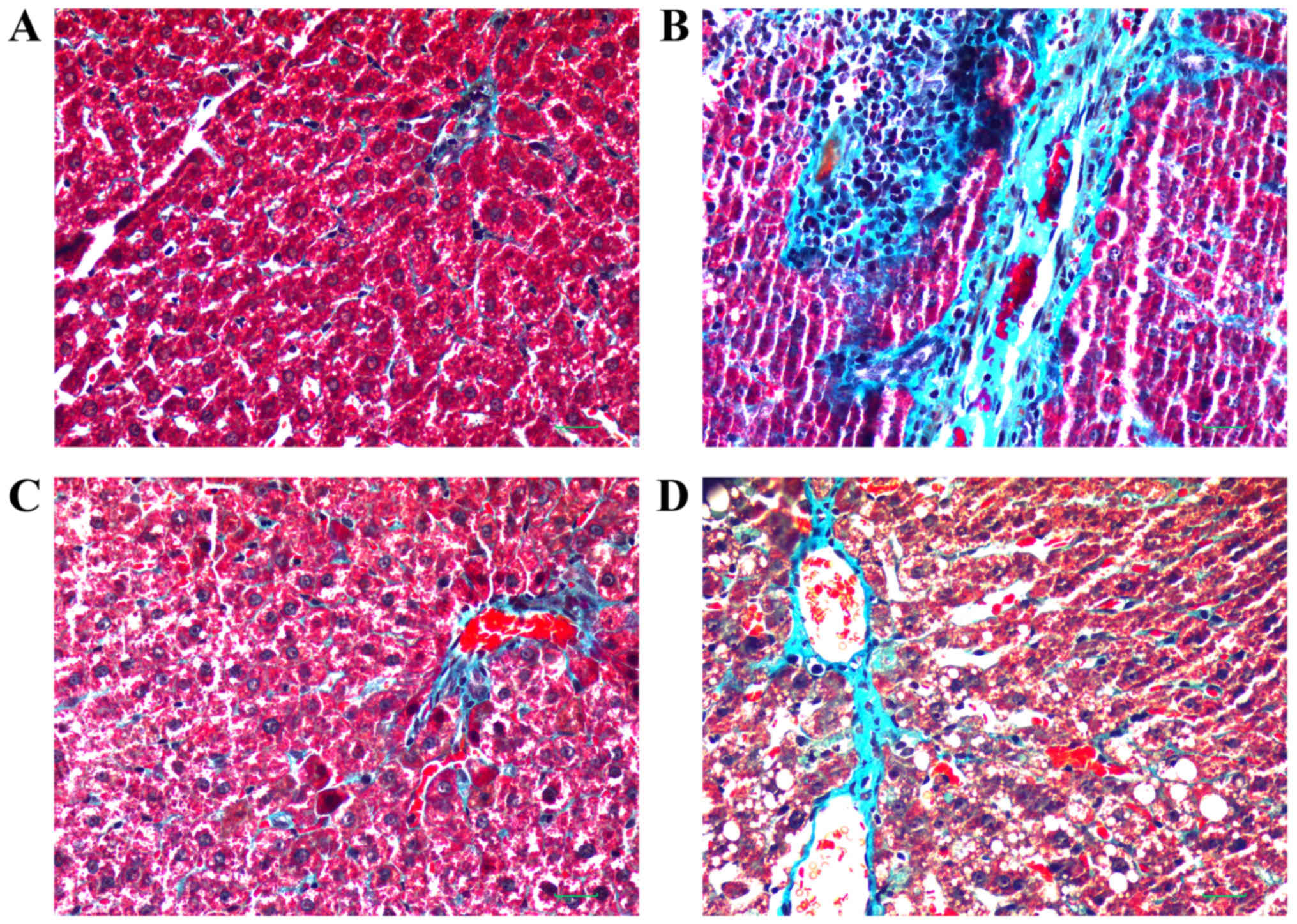
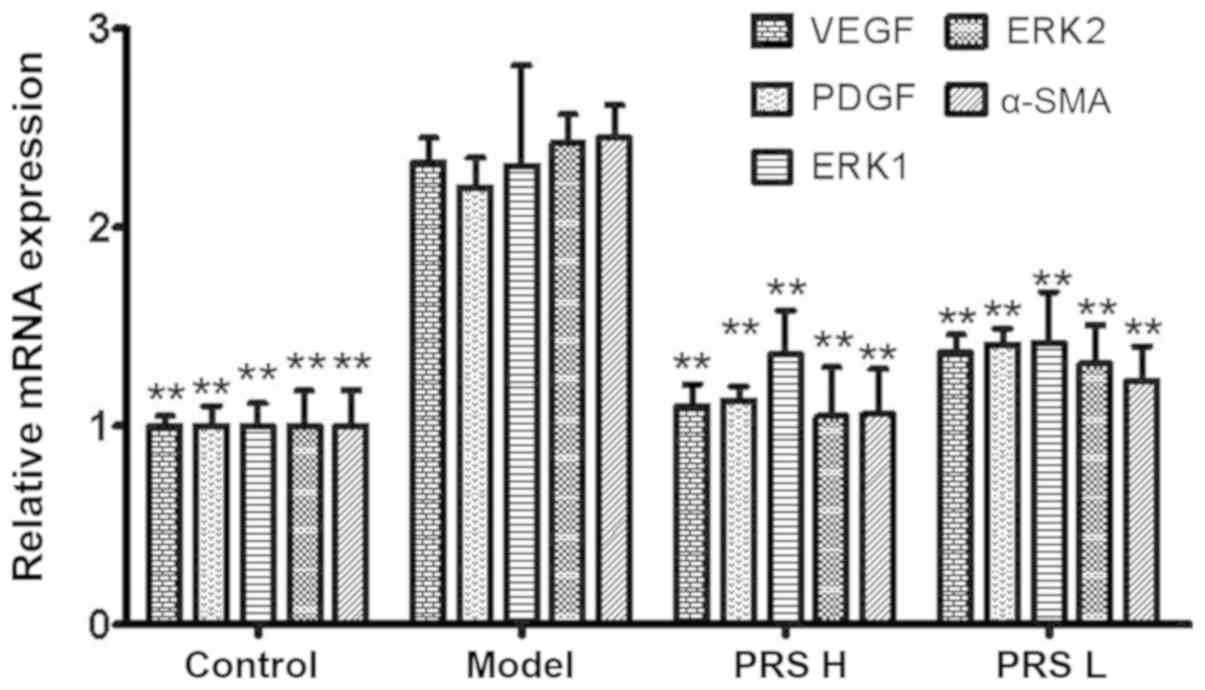
|
1
|
Zoheir KMA, Amara AA, Ahmad SF, Mohammad
MA, Ashour AE, Harisa G and Abd-Allah AR: Study of the therapeutic
effects of Lactobacillus and α-lipoic acid against
dimethylnitrosamine-induced liver fibrosis in rats. J Genet Eng
Biotechnol. 12:135–142. 2014. View Article : Google Scholar
|
|
2
|
Friedman SL: Hepatic fibrosis: Emerging
therapies. Dig Dis. 33:504–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elzamly S, Agina HA, Elbalshy AE,
Abuhashim M, Saad E and Abd Elmageed ZY: Integration of VEGF and
α-SMA expression improves the prediction accuracy of fibrosis in
chronic hepatitis C liver biopsy. Appl Immunohistochem Mol Morphol.
25:261–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fernández M, Semela D, Bruix J, Colle I,
Pinzani M and Bosch J: Angiogenesis in liver disease. J Hepatol.
50:604–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valfrè di Bonzo L, Novo E, Cannito S,
Busletta C, Paternostro C, Povero D and Parola M: Angiogenesis and
liver fibrogenesis. Histol Histopathol. 24:1323–1341.
2009.PubMed/NCBI
|
|
6
|
Giatromanolaki A, Kotsiou S, Koukourakis
MI and Sivridis E: Angiogenic factor expression in hepatic
cirrhosis. Mediators Inflamm. 2007:671872007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang F, Li J, Zhu J, Wang D, Chen S and
Bai X: Hydroxysafflor yellow A inhibits angiogenesis of
hepatocellular carcinoma via blocking ERK/MAPK and NF-κB signaling
pathway in H22 tumor-bearing mice. Eur J Pharmacol. 754:105–114.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li DS: 45 cases clinical observation of
matrine in treatment of liver fibrosis of patients with chronic
hepatitis. China Ming Kang Med. 23:2701–2703. 2011.(In
Chinese).
|
|
10
|
Man S, Fan W, Liu Z, Gao W, Li Y, Zhang L
and Liu C: Antitumor pathway of Rhizoma Paridis saponins based on
the metabolic regulatory network alterations in H22 hepatocarcinoma
mice. Steroids. 84:17–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Man S, Gao W, Zhang Y, Jin X, Ma C, Huang
X and Li Q: Characterization of steroidal saponins in saponin
extract from Paris polyphylla by liquid chromatography tandem
multi-stage mass spectrometry. Anal Bioanal Chem. 395:495–505.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Man S, Gao W, Zhang Y, Yan L, Ma C, Liu C
and Huang L: Antitumor and antimetastatic activities of Rhizoma
Paridis saponins. Steroids. 74:1051–1056. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong Y, Han Y, Luo H, Gui J, Zhang K and
Jiang H: Effect of Chonglou saponin on markers of liver fibrosis of
hepatic fibrosis rats and correlation analysis. J Shanxi Coll Trad
Chin Med. 15:20–22+67. 2014.(In Chinese).
|
|
14
|
Hong Y, Han YQ, Wang YZ, Gao JR, Li YX,
Liu Q and Xia LZ: Paridis Rhizoma sapoinins attenuates liver
fibrosis in rats by regulating the expression of RASAL1/ERK1/2
signal pathway. J Ethnopharmacol. 192:114–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun H, Che QM, Zhao X and Pu XP:
Antifibrotic effects of chronic baicalein administration in a CCl4
liver fibrosis model in rats. Eur J Pharmacol. 631:53–60. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vatakuti S, Schoonen WG, Elferink ML,
Groothuis GM and Olinga P: Acute toxicity of CCl4 but not of
paracetamol induces a transcriptomic signature of fibrosis in
precision-cut liver slices. Toxicol In Vitro. 29:1012–1020. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Gao W, Man S, Wang J, Li N, Yin S,
Wu S and Liu C: Pharmacological evaluation of sedative-hypnotic
activity and gastro-intestinal toxicity of Rhizoma Paridis
saponins. J Ethnopharmacol. 144:67–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsushita T, Inoue SI and Tanaka R: An
assay method for determining the total lipid content of fish meat
using a 2-thiobarbituric acid reaction. J Am Oil Chem Soc.
87:963–972. 2010. View Article : Google Scholar
|
|
20
|
Choi JH, Sun WJ, Kim HG, Khanal T, Hwang
YP, Lee KJ, Choi CY, Chung YC, Lee YC and Jeong HG: Platycodi Radix
attenuates dimethylnitrosamine-induced liver fibrosis in rats by
inducing Nrf2-mediated antioxidant enzymes. Food Chem Toxicol.
56:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng Y, Cheung KF, Wang N, Liu P,
Nagamatsu T and Yao T: Chinese medicines as a resource for liver
fibrosis treatment. Chin Med. 4:162009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wells RG: The role of matrix stiffness in
hepatic stellate cell activation and liver fibrosis. J Clin
Gastroenterol. 39 (Suppl 2):S158–S161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Argenio G, Mazzone G, Ribecco MT, Lembo
V, Vitaglione P, Guarino M, Morisco F, Napolitano M, Fogliano V and
Caporaso N: Garlic extract attenuating rat liver fibrosis by
inhibiting TGF-β1. Clin Nutr. 32:252–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding Q, Tian XG, Li Y, Wang QZ and Zhang
CQ: Carvedilol may attenuate liver cirrhosis by inhibiting
angiogenesis through the VEGF-Src-ERK signaling pathway. World J
Gastroenterol. 21:9566–9576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keyse SM: Protein phosphatases and the
regulation of mitogen-activated protein kinase signalling. Curr
Opin Cell Biol. 12:186–192. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sung YC, Liu YC, Chao PH, Chang CC, Jin
PR, Lin TT, Lin JA, Cheng HT, Wang J, Lai CP, et al: Combined
delivery of sorafenib and a MEK inhibitor using CXCR4-targeted
nanoparticles reduces hepatic fibrosis and prevents tumor
development. Theranostics. 8:894–905. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao Q, Gu Y, Jiang Y, Fan L, Wei Z, Jin H,
Yang X, Wang L, Li X, Tai S, et al: Long non-coding RNA Gm2199
rescues liver injury and promotes hepatocyte proliferation through
the upregulation of ERK1/2. Cell Death Dis. 9:6022018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan LH, Chen XL, Di Y and Liu ML:
CCR7/p-ERK1/2/VEGF signaling promotes retinal neovascularization in
a mouse model of oxygen-induced retinopathy. Int J Ophthalmol.
10:862–869. 2017.PubMed/NCBI
|
|
29
|
Bullard LE, Qi X and Penn JS: Role for
extracellular signal-responsive kinase-1 and −2 in retinal
angiogenesis. Invest Ophthalmol Vis Sci. 44:1722–1731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gressner AM: Transdifferentiation of
hepatic stellate cells (Ito cells) to myofibroblasts: A key event
in hepatic fibrogenesis. Kidney Int Suppl. 54:S39–S45.
1996.PubMed/NCBI
|
|
32
|
Lenz B, Klafki HW, Hillemacher T, Frieling
H, Clepce M, Gossler A, Thuerauf N, Winterer G, Kornhuber J and
Bleich S: ERK1/2 protein and mRNA levels in human blood are linked
to smoking behavior. Addict Biol. 17:1026–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonner JC: Regulation of PDGF and its
receptors in fibrotic diseases. Cytokine Growth Factor Rev.
15:255–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gallini R: A PDGFRalpha perspective on
PDGF signalling in developmental and pathological processes.
Geobiology. 7:360–372. 2014.
|
|
35
|
Chaudhary NI, Roth GJ, Hilberg F,
Müller-Quernheim J, Prasse A, Zissel G, Schnapp A and Park JE:
Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis.
Eur Respir J. 29:976–985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rosmorduc O, Wendum D, Corpechot C, Galy
B, Sebbagh N, Raleigh J, Housset C and Poupon R: Hepatocellular
hypoxia-induced vascular endothelial growth factor expression and
angiogenesis in experimental biliary cirrhosis. Am J Pathol.
155:1065–1073. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Corpechot C, Barbu V, Wendum D, Kinnman N,
Rey C, Poupon R, Housset C and Rosmorduc O: Hypoxia-induced VEGF
and collagen I expressions are associated with angiogenesis and
fibrogenesis in experimental cirrhosis. Hepatology. 35:1010–1021.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nielsen MJ, Nielsen SH, Hansen NUB,
Kristensen JH, Karsdal MA and Leeming DJ: P0525: N-Acetylated alpha
smooth muscle actin levels are increased in hepatic fibrosis but
decreased in hepatocellular carcinoma. J Hepatology. 62:S5122015.
View Article : Google Scholar
|