Introduction
In China, herbs and their products are widely used
in clinical treatment, with increasing popularity. Matrine (MT) and
oxymatrine (OMT), two types of alkaloid components in the roots of
Sophora species, have various pharmacological activities and
anti-inflammatory, anti-allergic, anti-virus, anti-fibrotic and
cardiovascular protective effects (1). At present, OMT has been widely used
in the treatment of hepatitis B and liver fibrosis in China
(2). Furthermore, OMT may exert
its anticancer activities through various channels, primarily by
inhibiting cancer cell proliferation (3), inducing cell cycle arrest (4) and differentiation (5), accelerating apoptosis (6), restraining agiogenesis (7), inhibiting metastasis and invasion
(8), and preventing or reducing
chemotherapy- and radiotherapy-induced toxicities (9). However, these previous studies are
mostly limited to observations of superficial phenomenon and lack
systematic investigation using modern molecular biology techniques.
The precise mechanism underlying the anticancer activity of OMT
remains largely unknown.
Dendritic cells (DCs) serve a critical role in
antigen capturing, processing and presentation (10). In the event of infection or
inflammation of the body, microbial infection and other factors may
promote the maturation of DCs, and thus initiate a T cell-mediated
immune response (11,12). There are a range of effector T
cells, including immunogenic cluster of differentiation
(CD)4+ T helper (Th) cells, cytotoxic CD8+ T
cells and in particular, tolerogenic regulatory T cells (Tregs),
termed the DC-Treg system. In principle, DCs are associated with
the two principal types of immunity, innate and adaptive.
Therefore, DCs may be an ideal target for the development of
immunotherapies and an adjuvant to convert their function between
tolerogenic and immunogenic may be desirable. It is important to
identify and develop strategies that may improve the efficacy of
DC-mediated antitumor immunotherapy.
The immune status of the systemic or local
microenvironment in tumor hosts may determine the responsiveness to
chemotherapy (13). The
immunomodulatory activity of OMT has been demonstrated in
rheumatoid arthritis (13),
chronic hepatitis B (14) and
colitis models by shifting the Th subsets (15). However, to the best of the authors'
knowledge, the potential effect of OMT on the DC-mediated antitumor
immune response has not yet been studied. In the present study, the
effects of OMT on DC maturation, and the subsequent simulation of
CD4+ T cell polarization and cytokine secretion in
vitro were examined. Furthermore, whether the immunomodulatory
ability of OMT may reverse drug-resistance in A549 lung cancer
cells was investigated.
Materials and methods
Subjects
Male NSCLC patients and healthy controls between the
age of 40–55 were enrolled in this study. The median age was 46.8
years (range: 40–54) in NSCLC patients (n=13), and 45.3 years
(range: 41–52) in healthy controls (n=15). Inclusion criteria for
the present study were patients with histological proven NSCLC
staging II–IV, who were primarily diagnosed in The Third Affiliated
Hospital of Sun Yat-sen University between January 2017 and
December 2017. All enrolled patients had no previous treatment with
molecular target therapy, chemotherapy or radiotherapy. Exclusion
criteria were chronic systemic diseases (including hypertension,
diabetes and coronary heart disease) or immune systemic diseases
(including HIV, organ transplantation and tumors). All subjects
joined voluntarily with informed consents. This present study
received ethical approval from the Institutional Review Board of
Sun Yat-sen University.
Isolation and culture of DCs
Blood samples (50 ml) were obtained from each
subjects. Peripheral blood mononuclear cells (PBMCs) were isolated
by Ficoll (GE Healthcare Life Sciences, Little Chalfont, UK)
density gradient centrifugation at 1,200 × g and 4°C for 20 min.
PBMCs were cultured for 2 h and non-adherent cells were removed.
The DC cell culture medium was prepared by adding recombinant human
(rh) granulocyte-macrophage colony-stimulating factor (GM-CSF; 100
ng/ml) and rh interleukin (IL)-4 (100 ng/ml) to RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA). PBMCs were incubated for 8 days at 37°C
and prepared for subsequent experiments.
Detection of DC surface markers
Cells were treated with lipopolysaccharide (LPS; 100
ng/ml), OMT (1 mg/ml) and OMT (1 mg/ml) + LPS (100 ng/ml) for 48 h.
According to preliminary experiments in the present study,
oxymatrine inhibited the proliferation of dendritic cells, and the
effect of inhibiting proliferation was observed at OMT
concentration of 0.8 mg/ml. However, the inhibitory effect was not
significantly increased with the increase of concentration, so the
concentration of OMT in this experiment was chosen to be 1.0 mg/ml
(data not shown). The cultured DCs were collected and centrifuged
at 1,000 × g for 5 min at 4°C. The supernatant was discarded and
the cells were washed three times with PBS. In total, 5 µl CD83
antigen (CD83)-phycoerythrin (PE; 556855; BD Biosciences, San Jose,
CA, USA), T-lymphocyte activation antigen CD86 (CD86)-PE (560957;
BD Biosciences), CD11 antigen-like family member C (CD11c)-PE
(555392; BD Biosciences) and major histocompatibility complex II
(MHC II)-PE(555812, BD Biosciences) were added to each tube.
Subsequent to gentle mixing, the cells were incubated at room
temperature in the dark for 30 min. The cells were washed twice
with PBS and resuspended in 100 µl PBS, and the DC surface markers
were detected using a flow cytometer (BD Accuri C6; BD
Biosciences).
Isolation and culture of
CD4+ T cells from patients with non-small
cell lung cancer (NSCLC)
A total of 10 patients with newly diagnosed NSCLC
were enrolled to collect 20 ml blood prior to any treatment.
Subsequently, PBMCs were isolated using the Ficoll density gradient
method at 1,200 × g, 4°C for 20 min. In total, 1×106
cells were used to isolate CD4+ T cells using
a magnetic cell separation system. The selected cells were cultured
in RPMI-1640 medium containing 10% FBS for subsequent
experiments.
Co-culture of DCs and T cells
The DCs were treated with control, LPS (100 ng/ml),
OMT (1 mg/ml) and OMT (1 mg/ml)+LPS (100 ng/ml) for 48 h. Cells
were subsequently centrifuged at 1,000 × g for 5 min at 4°C and the
supernatant was discarded. Subsequent to washing three times with
PBS, the DCs and peripheral blood-derived
CD4+ T cells from patients with NSCLC were
implanted into Matrigel-coated Transwell chambers; DCs were seeded
into the lower chambers and CD4+ T cells into
the upper chambers at 1:5, 1:10 and 1:20 ratios. Cells were
cultured in RPMI-1640 medium containing 10% FBS for 48 h; a
non-contact cell co-culture system was established.
qPCR for forkhead box protein P3
DC cells and T cells were co-cultured at a ratio of
1:5, 1:10 and 1:20, respectively, as described above.
CD4+ T cells were collected and total RNA was
extracted using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocols. cDNA were
synthesized using PrimeScript™ RT reagent kit with gDNA Eraser
(Takara Bio, Inc., Otsu, Japan), strictly following the
manufacturer's protocols. The target genes and controls were
analyzed by RT-qPCR using SYBR-Green PCR Master Mix (Toyobo Life
Science, Osaka, Japan) and the reactions were performed on ABI 7500
system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Primers used in the study were as follows, U6 (housekeeping)
primers: 5′-CGCTTCACGAATTTGCGTGTCAT-3′ (forward) and
5′-GCTTCGGCAGCACATATACTAAAAT-3′ (reverse); FOXP3 primers:
5′-CTGACCAAGGCTTCATCTGTG-3′ (forward) and
5′-ACTCTGGGAATGTGCTGTTTC−3′ (reverse). Reagents in the reaction
included 0.5 µl of cDNA (1:20), 0.5 µl of forward primer and 0.5 µl
of reverse primer, 10 µl of PCR Master Mix and 4.0 µl
dH2O. Thermocycling conditions for PCR were 95°C for 5
min, and 40 cycles of 95°C for 15 sec, 65°C for 15 sec and 72°C for
32 sec. The average value was calculated by the relative
quantitative method, and the gene expressions in the treatment
group relative to the control group were calculated by the formula
2−ΔΔCq following Livak's method (16).
Detection of forkhead box protein P3
(FOXP3)+/CD4+ Tregs
The co-cultured CD4+ T cells
were collected and centrifuged at 1,000 × g for 5 min at 4°C; the
supernatant was discarded. Anti-Human FoxP3 Staining kit with
CD4-Fluorescein Isothiocyanate and FOXP3-PE (560132; BD
Biosciences) was used in the following experiments. The cells were
washed three times with PBS, and CD4-Fluorescein Isothiocyanate
(1:20) was added to each tube and incubated for 30 min at room
temperature in the dark. For antibodies to enter the intracellular
structure, cells were resuspended in fixation/permeabilization
buffer (00–5223/00-5123; eBioscience; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocols. Following two
washes with PBS, the cells were incubated for 30 min at room
temperature in the dark. FOXP3-PE (1:20) was added and incubated
for another 30 min in the same condition. Cells were subsequently
washed twice and resuspended with the fixation/permeabilization
buffer. The ratio of FOXP3+/CD4+
cells was detected using a flow cytometer (BD Accuri C6; BD
Biosciences).
Detection of cytokines in supernatant
of the co-culture system
The DCs were treated with control, LPS (100 ng/ml),
OMT (1 mg/ml) and OMT (1 mg/ml) + LPS (100 ng/ml), and mixed with
peripheral blood-derived CD4+ T cells from patients with
NSCLC at ratios of 1:5, 1:10 and 1:20. After 48 h, the supernatant
of the co-culture system was collected. The concentrations of
IL-10, transforming growth factor (TGF)-β, IL-35, interferon
(IFN)-γ, IL-2 and IL-12 in the supernatant were detected using
ELISA kits: IL-10 (E-EL-H0103c), TGF-β (E-EL-0110c), IL-35
(E-EL-H2443c), IFN-γ (E-EL-0108c), IL-2 (E-EL-H0099c) and IL-12
(E-EL-H0150c; all from R&D Systems, Inc., Minneapolis, MN,
USA).
Detection of A549 apoptosis following
co-culture
The induction of A549 resistant cells was performed
by gradually increasing the concentration of cisplatin from a
starting concentration of 1.0 µM, and after 4 weeks, it was
increased to 2.0 µM, and serially subcultured for ~100 passages.
Then, 6 months later, the A549/DDP resistant cell line was
established. The human lung adenocarcinoma cells A549 were
purchased from Procell Life Science & Technology Co., Ltd.
(Wuhan, China; cat. no. CL-0016). The cells were maintained in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) medium with 10%
fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences) and
1% penicillin-streptomycin solution (Beyotime Institute of
Biotechnology, Haimen, China), and cells were cultured in a
humidified atmosphere at 37°C with 5% CO2. Co-culture
systems of A549/DDP plus DC or DC-T were established. OMT (1
mg/ml)-treated DCs and the
FOXP3+/CD4+ T cells induced by
DC-T co-culture (cell ratio, 1:5) were incubated with A549/DDP
cells. The cells were seeded for 24 h in the upper and lower
chambers of Transwell plates at ratios of 1:1, 1:5, 1:10 and 1:20.
At the same time, 22 µM DDP was added to the A549/DDP medium for 24
h. The collected cells were stained using an Annexin V/Propidium
Iodide kit (BD Biosciences) according to the manufacturer's
protocol, and the apoptotic ratio was analyzed using a flow
cytometer (BD Accuri C6; BD Biosciences).
Western blotting
A549/DDP cells were lysed using
radioimmunoprecipitation assay buffer (50 mM Tris, 150 mM NaCl, 1%
NPNP-40, 1% sodium deoxycholate and 0.05% SDS; pH 7.4) following
co-culture with OMT-treated DCs/DC-T and treatment with DDP. Total
protein was extracted and subsequently quantified using a
bicinchoninic acid kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). For western blotting, protein loading was set at 40 µg and an
12% SDS-PAGE gel was used for protein separation. The proteins were
transferred to a nitrocellulose membrane and incubated with primary
antibodies against FOXP3 (1:3,000; cat. no. ab191416), apoptosis
regulator BAX, (Bax; 1:1,000; cat. no. ab32503), B cell lymphoma-2
(Bcl-2; 1:1,000; cat. no. ab32124) and β-actin (1:1,000; cat. no.
ab8224) overnight at 4°C, and subsequently incubated with Goat
Anti-Rabbit (1:1,000; cat. no. ab6702) or Anti-Mouse (1:1,000; cat.
no. ab6708) (all from Abcam, Cambridge, UK) secondary antibodies
for 1 h at room temperature. Chemiluminescence was used to develop
the color of the membrane using an ECL kit according to the
manufacturer's instructions (cat. no. FD8030; Guangzhou Fude
Biological Technology Co., Ltd., Guangzhou, China). ImageJ 8.0
software (National Institutes of Health, Bethesda, MD, USA) was
used to perform densitometric analysis.
Statistical analysis
Each experiment was repeated at least 3 times. Data
were presented as the mean ± standard deviation. Statistical
significance was determined by one-way analysis of variance with
Bonferroni Correction using SPSS software version 19.0 (IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
OMT promotes DC maturation
The monocytes were prepared from the peripheral
blood of healthy adult volunteers. To investigate the effect of
OMT, the immature DCs were cultured for 8 days containing 1 mg/ml
OMT in medium supplemented with rhGM-CSF (100 ng/ml) and rhIL-4
(100 ng/ml). Bacterial LPS is the most common ‘maturation’ signal
for DCs (17). With the
LPS-treated (100 ng/ml) group (LPS-DCs) as a positive control and
mononuclear cells as a negative control, the expression of DC
surface markers was detected by flow cytometry analysis, as
presented in Fig. 1. Compared with
the negative control group, treatment with OMT (1 mg/ml)
significantly increased the expression of CD83, CD86, CD11c and MHC
II (P<0.001), and the expression level of these markers was
higher when LPS and OMT were combined. These results demonstrated
that OMT may promote DC maturation.
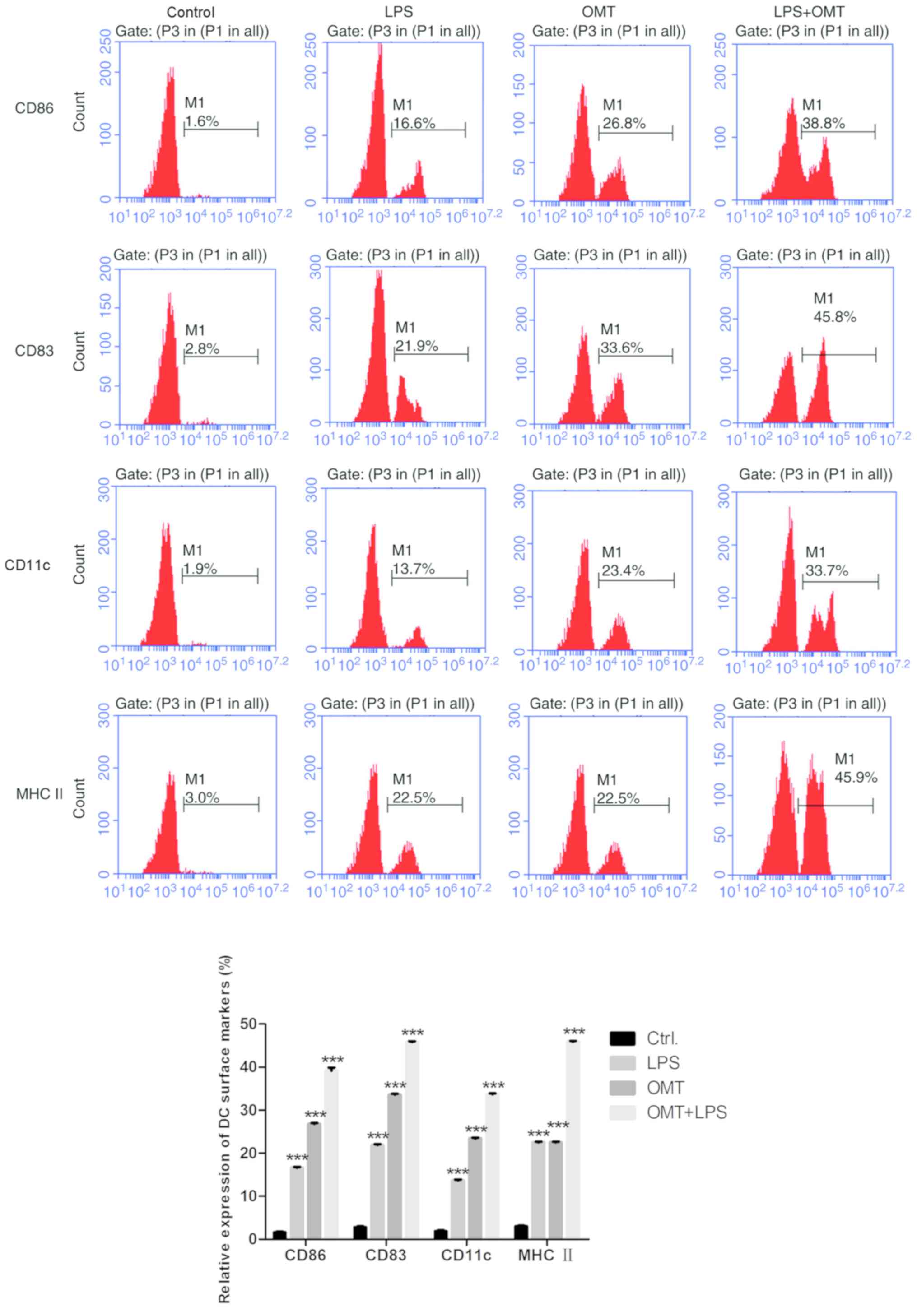 | Figure 1.OMT promotes dendritic cell
maturation. The expression levels of CD86, CD83, CD11c and MHC II
were detected by flow cytometry. Compared with the control group
and LPS group, treatment with OMT (1 mg/ml) increased the
expression level of those markers (CD86-26.8%, CD83-33.6%,
CD11c-23.4% and MHC II-22.5%), and when LPS and OMT were combined,
the expression levels were higher compared with the OMT (1 mg/ml)
group (CD86-38.8%, CD83-45.8%, CD11c-33.7% and MHC II-45.9%).
***P<0.001 vs. respective Ctrl. group OMT, oxymatrine; LPS,
lipopolysaccharide; CD86, T-lymphocyte activation antigen CD86;
CD83, CD83 antigen; CD11c, CD11 antigen-like family member C; MHC
II, major histocompatibility complex II. |
Differentiation of
FOXP3+/CD4+ Tregs induced by
OMT-DCs
To examine the response of allogeneic primary
CD4+ T cells to DC stimulation pretreated
with OMT (1 mg/ml; OMT-DC), the following experiments were
performed. CD4+ T cells were isolated from
the peripheral blood of patients with NSCLC. DCs were co-cultured
with the primary CD4+ T cells for 48 h in
Transwell plates at ratios of 1:5, 1:10 and 1:20. The expression
levels of FOXP3 in CD4+ T cells were analyzed
by flow cytometry, western blotting and qPCR.
As presented in Fig.
2, OMT-DCs stimulated the primary CD4+ T
cells to express FOXP3, and the mRNA expression level of FOXP3
decreased as the DCs were more diluted. The FOXP3 protein assay
showed that the total amount of FOXP3 was increased when LPS and
OMT were used separately or in combination (especially when used in
combination). However, in the case of the same treatment, the
dilution of DC cells had no apparent effect on FOXP3 protein amount
when groups of different DC cell dilution ratios were compared
among each other. Whether there are translation level and turnover
level factors regulating the expression of FOXP3 protein in this
case deserves further investigation. Altogether, these results
demonstrated that OMT-pretreated DCs may enhance
FOXP3+/CD4+ Treg
differentiation.
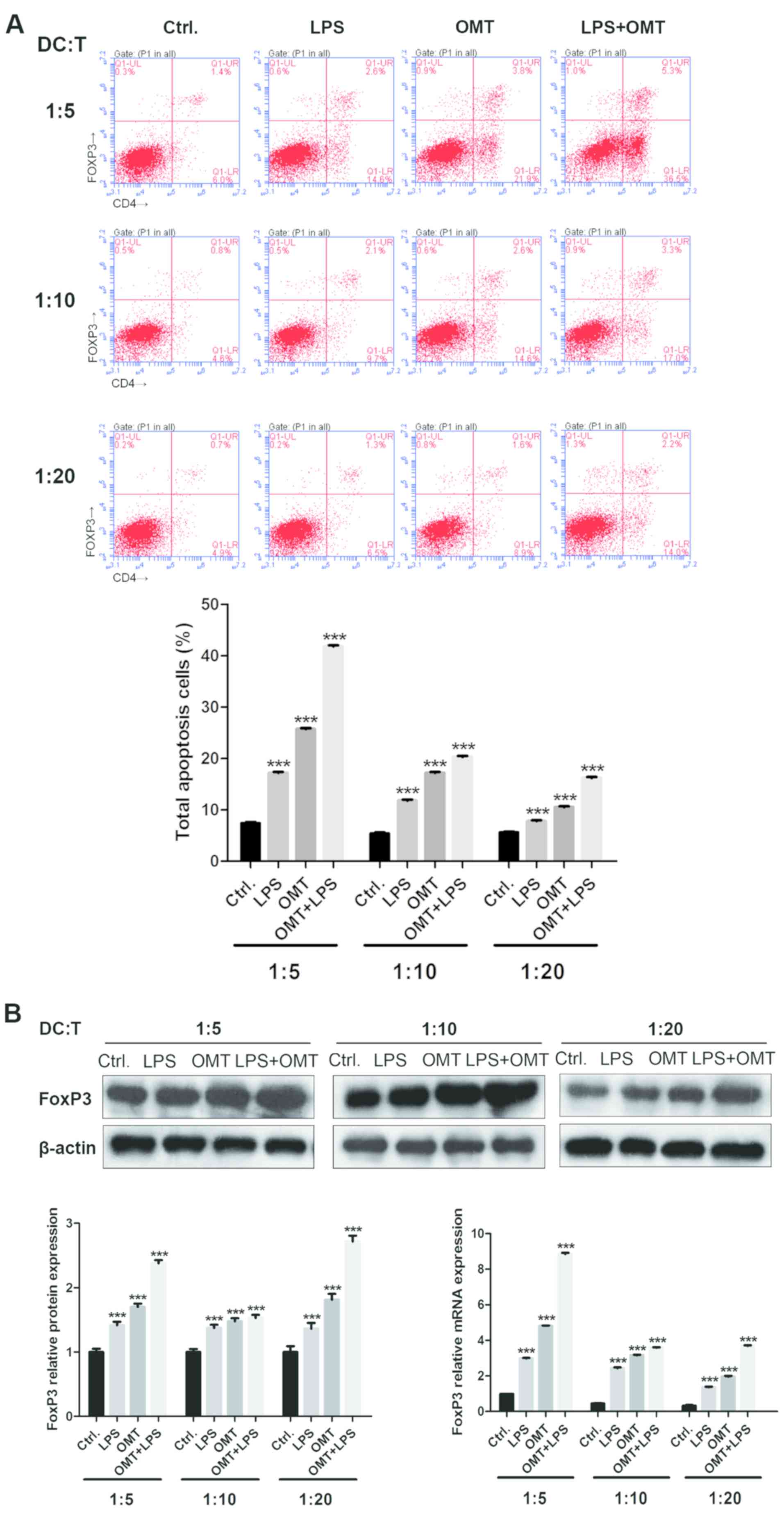 | Figure 2.Differentiation of
FOXP3+/CD4+ regulatory T cells
induced by OMT-DCs. (A) Compared with the control group and LPS
group, OMT-DCs stimulated the primary CD4+ T
cells to express FOXP3, and the expression level of FOXP3 decreased
with the decrease of co-culture cell ratio (1:5-3.8, 1:10-2.6 and
1:20-1.6%). When LPS and OMT were combined, the expression of FOXP3
was higher compared with the OMT-DCs group (1:5-5.3, 1:10-3.3 and
1:20-2.2%). (B) Expression of FOXP3 detected by western blotting
and quantitative polymerase chain reaction presented similar
results to the flow cytometry assay. ***P<0.001 vs. respective
Ctrl. group. FOXP3, forkhead box protein P3; OMT, oxymatrine; DCs,
dendritic cells; LPS, lipopolysaccharide; Ctrl., control; CD,
cluster of differentiation. |
Cytokine secretion stimulated by
OMT-pretreated DCs
Inflammatory factors serve an important role in the
immune response. ELISA was used to quantitatively analyze
inflammatory factors in the co-culture supernatants. When OMT-DCs
were co-cultured with T cells, a large number of anti-inflammatory
factors, including IL-10, TGF-β and IL-35, and pro-inflammatory
cytokines, including IFN-γ, IL-12 and IL-2, were secreted.
Furthermore, the expression levels of those inflammatory factors
decreased as the DCs were more diluted with T cells (Fig. 3). When LPS-DCs were co-cultured
with T cells, it additionally promoted the secretion of
inflammatory factors, in a similar manner to OMT-DCs. When LPS and
OMT were applied simultaneously, the secretion of inflammatory
factors reached the highest concentration. These results suggested
that OMT-pretreated DCs may promote the primary T cells to polarize
into FOXP3+/CD4+ Tregs, and enhance the
anti-inflammatory and pro-inflammatory cytokines secretion. Based
on these results, the co-culture ratio of DCs to CD4+ T
cells was set at 1:5 for subsequent experiments.
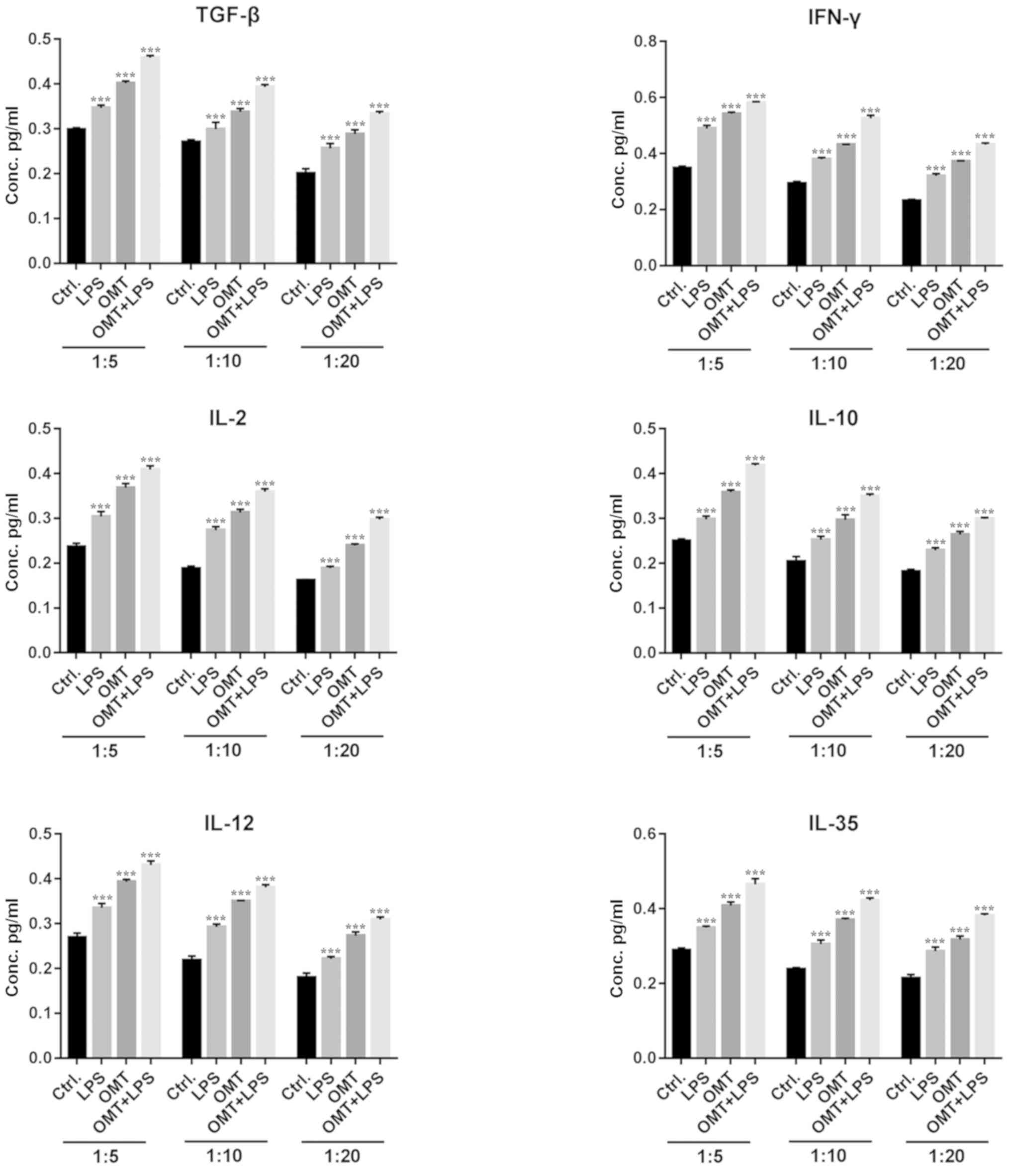 | Figure 3.Cytokine secretion stimulated by
OMT-pretreated DCs. At different co-culture cell ratios, compared
with the control group and LPS group, OMT-DCs stimulated the
primary CD4+ T cell to secrete cytokines,
including IL-10, TGF-β and IL-35, and pro-inflammatory cytokines,
including IFN-γ, IL-12 and IL-2. When LPS and OMT were combined,
the cytokine secretion was higher compared with the OMT-DC group.
Furthermore, the expression levels of the inflammatory factors
decreased as the DCs were more dilute. ***P<0.001 vs. respective
Ctrl. group. OMT, oxymatrine; DCs, dendritic cells; CD, cluster of
differentiation; LPS, lipopolysaccharide; IL, interleukin; TGF-β,
transforming growth factor-β; IFN-γ, interferon-γ; Ctrl., control;
Conc., concentration. |
Reversal of DDP-resistance in A549/DDP
by OMT-DC/OMT-DC-T
A DDP-resistant cell line, A549/DDP, was
established. As presented in Fig.
4A, A549/DDP has a half maximal inhibitory concentration of 22
µM for DDP. In order to investigate the effect of OMT-pretreated
DCs and OMT-DCs induced CD4+ T cells on the sensitivity
of A549/DDP to antitumor drugs, OMT-stimulated DCs (OMT-DC) and
OMT-DC-stimulated primary CD4+ T cells (OMT-DCs-T) were
co-cultured with the A549/DDP cells at ratios of 1:1, 1:5, 1:10 or
1:20 for 24 h. At the same time, the A549/DDP cells were treated
with 22 µM DDP for 24 h. Annexin V/PI staining was used to analyze
the apoptosis of the A549/DDP cells.
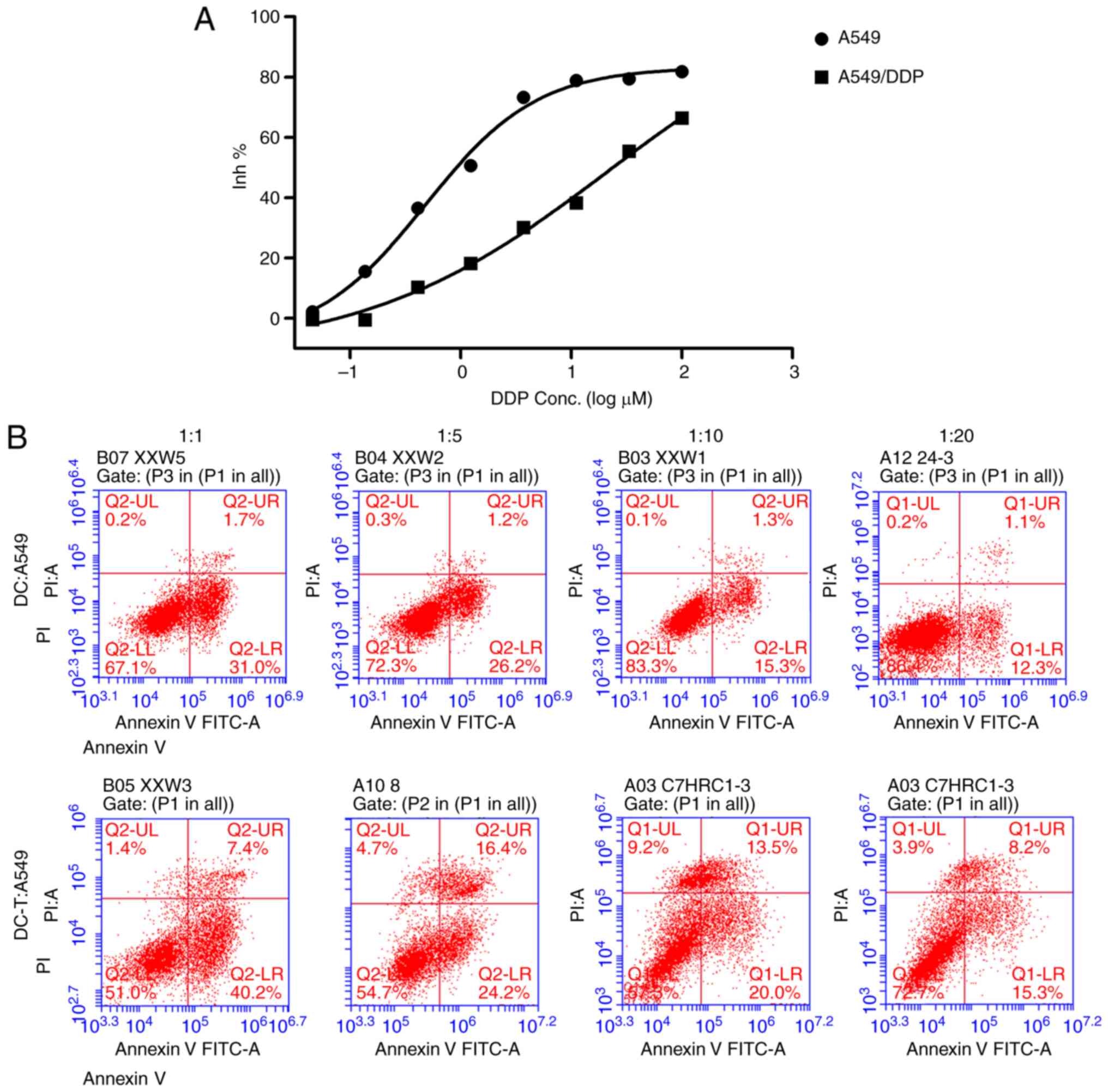 | Figure 4.Reversal of DDP-resistance in
A549/DDP by OMT-DCs/OMT-DCs-T. (A) A549/DDP has a half maximal
inhibitory concentration of 22 µM for DDP. (B) At the same cell
co-culture ratio, OMT-DCs and OMT-DCs-T may increase the percentage
of apoptotic cells in A549/DDP, and the apoptotic rate decreased
accompanied by a gradual decrease in the co-culture ratio of
OMT-DCs/OMT-DCs-T and A549/DDP, detected by flow cytometry. DDP,
cisplatin; A549/DDP, DDP-resistant A549 cells; OMT, oxymatrine;
DCs, dendritic cells; Inh, inhibition; PI, propidium iodide; FITC,
fluorescein isothiocyanate; Q, quadrant; UL, upper left; UR, upper
right; LL, lower left; LR, lower right; T, cluster of
differentiation 4+ T cells. |
As presented in Fig.
4B, OMT-DC and OMT-DC-T may increase the percentage of
apoptotic cells in A549/DDP, and the apoptotic rate decreased
accompanied by the gradual dilution of the OMT-DCs/OMT-DCs-T with
A549/DDP.
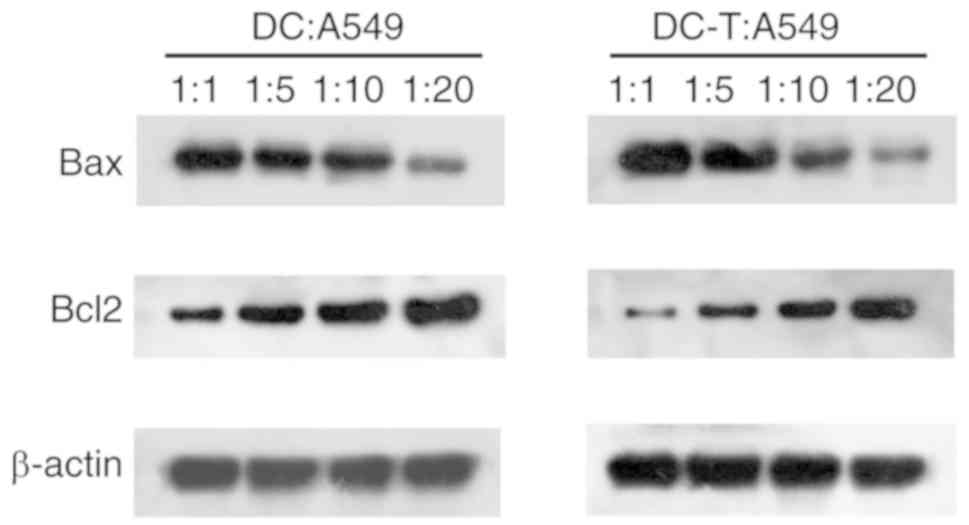
The western blotting results (Fig. 5) demonstrated that the expression
of apoptosis-associated protein Bcl-2 increased as the A549/DDP
cell apoptosis decreased; however, the protein expression level of
Bax decreased. These results suggested that OMT-pretreated DCs and
DC-induced T cells may reverse DDP-resistance in a non-contactable
co-culture cell model.
Discussion
Carcinogenesis is a multistep process; agents that
are able to target one or more of these processes maybe ideal
cancer chemopreventive agents. According to previous studies
(18,19), OMT may exert anticancer activities
through various channels. Notably, it was observed that all of
these functions were a result of direct contact of OMT with tumor
cells. The present study is, to the best of the authors' knowledge,
the first study to demonstrate that the therapeutic efficacy of OMT
may be indirectly achieved by activating the DC-mediated antitumor
immune response in circulation and/or in the tumor
microenvironment.
Monocyte-derived mature DCs serve a key role in the
immune response. Activated DCs may release various cytokines that
mediate T cell activation and efficiently present a subset of
antigens to T cells (20). At
present, the potential impact of OMT on the maturation and function
of DCs has not yet been fully investigated. In the present study,
it was identified that OMT may enhance DC maturation in the process
of differentiation of mononuclear cells derived from human
peripheral blood. The positive effect of OMT on proliferation was
significant at 1 mg/ml, and the surface markers of DCs, including
CD83, CD86, CD11c and MHC II, were increased, suggesting that OMT
may promote DC maturation. Immature DCs facilitate tolerance toward
cancer cells, whereas, fully mature DCs may strongly promote
anticancer immunity (21). The
immune inhibitory microenvironment of the tumor inhibits DC
differentiation and maturation (22,23).
Efficiency and the success of such an interaction is dependent on
the maturation status of the DCs (24). These data provide insight to
support the use of OMT-mediated anticancer immunotherapy in human
cancer.
DCs are key regulators of adaptive immunity with the
potential to induce T cell activation/immunity or T cell
suppression/tolerance. DCs are capable of differentiating naïve T
cells into a range of effector cells, including immunogenic
CD4+ Th cells, cytotoxic CD8+ T
cells and tolerogenic Tregs (25).
In the present study, similar to LPS, OMT-activated DCs were able
to subsequently promote the differentiation of T cells into
FOXP3+/CD4+ Treg cells in
vitro. In agreement with the present results, Ma et al
(26) demonstrated that OMT
significantly upregulated FOXP3 and downregulated nuclear receptor
ROR-γ expression, thereby inducing Treg/Th17 imbalance and
inhibiting inflammation of rheumatoid arthritis. In contrast, it
was identified that treatment with OMT triggers an increase in Th1
cytokines and a decrease in Th2 cytokines, which enhances the
immune response and improves inhibitory activities to hepatitis B
virus (14). Accordingly, OMT may
promote the immune response to remove viruses and relieve
hyperinflammation to protect organ function in autoimmune disorders
(26). Therefore, it was
hypothesized that OMT may have bilateral modulation on DC-T
interaction, which may be switched mutually, according to the
specific pathophysiological setting.
It is generally accepted that
FOXP3+/CD4+ Treg infiltration in
tumor tissue serves a role in inhibiting antitumor immunity and
contributing to immune tolerance (27–29),
thus promoting the occurrence and progression of tumors. However, a
number of previous studies demonstrated that
FOXP3+/CD4+ Treg infiltration was
positively associated with good prognosis in colorectal, head and
neck cancer, NSCLC and breast cancer (30–33).
Immunosuppression or immune escape has been implicated as a primary
mechanism in the initial establishment of tumors. Along with the
secondary infection and cytotoxic drug usage in advanced stages,
the resultant chronic systemic inflammation may alter the antigenic
landscape of tumor cells, rewire oncogenic signaling networks,
protect against cell death and reprogramme immune cell functions
(34). It represents a shared
resistance mechanism to different therapy (35,36).
At this stage, a higher proportion of
FOXP3+/CD4+ Tregs may relieve
hyperinflammation and therapy-induced injury, thereby contributing
to improve the overall prognosis of patients. Therefore, the DC-T
response to treatment with OMT may vary at each stage of tumor
progression.
The supernatant of the DC-T co-culture medium
contained anti-inflammatory factors (including IL-10, TGF-β and
IL-35) and pro-inflammatory cytokines (IFN-γ, IL-12 and IL-2).
Furthermore, pro-inflammatory and anti-inflammatory factor
secretion decreased when the co-cultured DCs were more diluted with
the primitive T cells. This suggested that the DC-T co-culture
system may affect the final anti-/pro-inflammatory concentration in
the tumor microenvironment. The therapeutic effect of OMT is partly
achieved by immune modulation. The protection of OMT is associated
with inhibiting pro-inflammatory cytokines in acute lung injury
(37), pulmonary fibrosis
(38), rheumatoid arthritis
(13) and organic
ischemia/reperfusion injury (39,40).
However, Ning et al (41)
demonstrated that OMT was able to directly induce antiviral
cytokine secretions in the peripheral lymphocytes isolated from
patients with chronic hepatitis B. Therefore, further study is
required to clarify the specific pattern of OMT regulating DC-T
interaction, and determining the ultimate immune balance seems to
be particularly critical.
In the past 5 years, MT and OMT have been
extensively studied for their cancer chemopreventive potential
against various cancer types. However, only a number of previous
studies have investigated the underlying mechanisms of
drug-resistance reversion. It was demonstrated that OMT and its
derivatives may reverse DDP resistance by inhibiting multidrug
resistance-associated protein 1 expression and the downregulation
of Bax/Bcl-2 in the human nasopharyngeal carcinoma cell line HONE1
(42). Wang et al (43) observed that MT induced
mitochondrial apoptosis in DDP-resistant NSCLC cells via
suppression of β-catenin/survivin signaling (43). In addition, OMT may additionally
reverse taxol resistance in NCI-H520/TAX25 human lung cancer cells
(44), whereas, MT was able to
induce apoptotic effects in doxorubicin resistant K562 cells
(45). Therefore, OMT and its
derivatives maybe used as ideal adjuncts for cancer
chemotherapy.
In contrast to a previous study (46), it was demonstrated that
OMT-pretreated DCs and DCs-T coculture may enhance apoptosis of
DDP/A549 cells in vitro, without direct contact of OMT with
tumor cells. Therefore, it was hypothesized that OMT-induced DC
maturation and activation may be key in promoting DDP/A549
apoptosis. At present, it is well documented that mature DCs are
able to induce strong antitumor responses mediated by effective
candidates, including cytotoxic CD8+ T cells and large
numbers of cytokines, to enhance sensitivity of tumor cells to
various chemotherapeutic drugs (47). Immunogenic cell death of tumor
cells starts from the interaction between dendritic cells and
cancer cells. Cytokines released during apoptosis of tumor cells
promote the maturation of dendritic cells. Mature dendritic cells
activate tumor-specific cytotoxic T cells, which shows the
antitumor effect (48). Further
investigations on the molecular mechanisms of DC-mediated antitumor
capacity following OMT administration are required.
As DCs are largely responsible for the ability of
the immune response to recognize and eliminate cancer cells, DCs
have attracted interest for antitumor immunotherapy in recent
decades (49). In order for
DC-based immunotherapy to elicit potent antitumor immune responses,
an immune stimulatory response, as opposed to a tolerogenic immune
response, is required following the administration of DCs. At
present, research has focused on identifying the key factors
responsible for therapeutic success/failure to determine the full
clinical potential use of DCs in cancer immunotherapy. Due to the
low toxicity and wide range of biological activities, OMT and
specific natural products, which have therapeutic selectivity or
may preferentially kill cancer cells without significant toxicity
to normal cells, are being considered for future cancer
therapy.
In conclusion, the multi-targeted chemopreventive
efficacy of OMT was demonstrated in the present study, focusing on
its immunoregulatory capacity. OMT may increase the apoptosis of
drug-resistant tumor cells by activating DC differentiation and
function, thereby enhancing the antitumor immune response. The
present data suggested that OMT is a promising reagent for the
development of more effective DC-based immunotherapy or cancer
vaccines. The results of the present study are not only applicable
to patients with tumors; however, additionally have reference value
for infectious and immune diseases, and provide a theoretical basis
for the use of OMT in the clinical setting.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from the
China National Natural Science Funds (grant no. 81472760) and the
Science and Technology Planning Project of Guangdong Province,
China (grant no. 2014A020212078).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL designed all the experiments, applied for project
funding and wrote the final version of the paper. MZ performed the
in vitro cell culture, including isolation and culture of
DCs and CD4+ T cells, as well as co-culture
of DCs and T cells. PL performed flow cytometeric detection,
including detection of surface markers of DCs and the ratio of
FOXP3+ Tregs/CD4+. HW enrolled and
collected the baseline data for the NSCLC patients and healthy
controls. HW completed the detection of cytokines in supernatants
using ELISA kits. XL completed the qPCR and western blotting in
overall experiments, and completed the detection of A549 apoptosis.
JY performed the statistical analysis and wrote the paper in
cooperation with HL. JY and HL dealt with editorial queries and the
overall submission. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All participants provided written consent, and the
study was approved by the Institutional Review Board of Sun Yat-sen
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Antitumor activities of matrine and oxymatrine: Literature
review. Tumour Biol. 35:5111–5119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parvez MK, Arbab AH, Al-Dosari MS and
Al-Rehaily AJ: Antiviral natural products against chronic hepatitis
B: Recent developments. Curr Pharm Des. 22:286–293. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang CZ, Zhang JK, Shi Z, Liu B, Shen CQ
and Tao HM: Matrine induces caspase-dependent apoptosis in human
osteosarcoma cells in vitro and in vivo through the upregulation of
Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother
Pharmacol. 69:317–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao B, Li B, Bai S, Shen L, Ren R, Jonas
JB, Xu X, Lu Q and Liu Q: Effects of matrine on proliferation and
apoptosis of cultured retinoblastoma cells. Graefes Arch Clin Exp
Ophthalmol. 250:897–905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xing Y, Yan F, Liu Y, Liu Y and Zhao Y:
Matrine inhibits 3T3-L1 preadipocyte differentiation associated
with suppression of ERK1/2 phosphorylation. Biochem Biophys Res
Commun. 396:691–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J,
Zhang S, Wu J, Yu K and Han Y: Matrine induces apoptosis in human
acute myeloid leukemia cells via the mitochondrial pathway and Akt
inactivation. PLoS One. 7:e468532012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen H, Zhang J, Luo J, Lai F, Wang Z,
Tong H, Lu D, Bu H, Zhang R and Lin S: Antiangiogenic effects of
oxymatrine on pancreatic cancer by inhibition of the NF-κB-mediated
VEGF signaling pathway. Oncol Rep. 30:589–595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu H, Zhang H, Li D, Zhang X, Xue H and
Zhao S: Matrine inhibits matrix metalloproteinase-9 expression and
invasion of human hepatocellular carcinoma cells. J Asian Nat Prod
Res. 13:242–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun Q, Ma W, Gao Y, Zheng W, Zhang B and
Peng Y: Meta-analysis: Therapeutic effect of transcatheter arterial
chemoembolization combined with compound kushen injection in
hepatocellular carcinoma. Afr J Tradit Complement Altern Med.
9:178–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Villadangos JA, Schnorrer P and Wilson NS:
Control of MHC class II antigen presentation in dendritic cells: A
balance between creative and destructive forces. Immunol Rev.
207:191–205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Merad M, Sathe P, Helft J, Miller J and
Mortha A: The dendritic cell lineage: Ontogeny and function of
dendritic cells and their subsets in the steady state and the
inflamed setting. Annu Rev Immunol. 31:563–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sallusto F and Lanzavecchia A: Efficient
presentation of soluble antigen by cultured human dendritic cells
is maintained by granulocyte/macrophage colony-stimulating factor
plus interleukin 4 and downregulated by tumor necrosis factor
alpha. J Exp Med. 179:1109–1118. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghiringhelli F and Apetoh L: The interplay
between the immune system and chemotherapy: emerging methods for
optimizing therapy. Expert Rev Clin Immunol. 10:19–30. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Y, Xi H, Yu Y, Wang Q, Jiang K and Li
L: Effects of oxymatrine on the serum levels of T helper cell 1 and
2 cytokines and the expression of the S gene in hepatitis B virus S
gene transgenic mice: A study on the anti-hepatitis B virus
mechanism of oxymatrine. J Gastroenterol Hepatol. 17:1299–1306.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Wang HL, Zhao WC, Chen Y and Deng
HZ: Total alkaloids of Sophora alopecuroides increases the
expression of CD4+ CD25+ Tregs and
IL-10 in rats with experimental colitis. Am J Chin Med. 38:265–277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stein J, Steven S, Bros M, Sudowe S,
Hausding M, Oelze M, Münzel T, Grabbe S, Reske-Kunz A and Daiber A:
Role of protein kinase C and Nox2-derived reactive oxygen species
formation in the activation and maturation of dendritic cells by
phorbol ester and lipopolysaccharide. Oxid Med Cell Longev.
2017:41572132017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Zhang Y, Liu Q, Zhao Q, Xu L, Huang
S, Huang S and Wei X: Oxymatrine inhibits proliferation of human
bladder cancer T24 cells by inducing apoptosis and cell cycle
arrest. Oncol Lett. 13:4453–4458. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin B, Li D and Zhang L: Oxymatrine
mediates Bax and Bcl-2 expression in human breast cancer MCF-7
cells. Pharmazie. 71:154–157. 2016.PubMed/NCBI
|
|
20
|
Sarhan D, Palma M, Mao Y, Adamson L,
Kiessling R, Mellstedt H, Österborg A and Lundqvist A: Dendritic
cell regulation of NK-cell responses involves lymphotoxin-α, IL-12,
and TGF-β. Eur J Immunol. 45:1783–1793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dudek AM, Martin S, Garg AD and Agostinis
P: Immature, semi-mature, and fully mature dendritic cells: Toward
a DC-cancer cells interface that augments anticancer immunity.
Front Immunol. 4:4382013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Menetrier-Caux C, Montmain G, Dieu MC,
Bain C, Favrot MC, Caux C and Blay JY: Inhibition of the
differentiation of dendritic cells from CD34(+) progenitors by
tumor cells: Rrole of interleukin-6 and macrophage
colony-stimulating factor. Blood. 92:4778–4791. 1998.PubMed/NCBI
|
|
23
|
Bell D, Chomarat P, Broyles D, Netto G,
Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA and
Banchereau J: In breast carcinoma tissue, immature dendritic cells
reside within the tumor, whereas mature dendritic cells are located
in peritumoral areas. J Exp Med. 190:1417–1426. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sittig S, de Vries I and Schreibelt G:
Primary human blood dendritic cells for cancer
immunotherapy-tailoring the immune response by dendritic cell
maturation. Biomedicines. 3:282–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Joffre O, Nolte MA, Spörri R and Reis e
Sousa C: Inflammatory signals in dendritic cell activation and the
induction of adaptive immunity. Immunol Rev. 227:234–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma A, Yang Y, Wang Q, Yin W, Jing W and
Zhang Y: Anti-inflammatory effects of oxymatrine on rheumatoid
arthritis in rats via regulating the imbalance between Treg and
Th17 cells. Mol Med Rep. 15:3615–3622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao Y, Poschke I and Kiessling R:
Tumour-induced immune suppression: Role of inflammatory mediators
released by myelomonocytic cells. J Intern Med. 276:154–170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:492014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jandus C, Bioley G, Speiser D and Romero
P: Selective accumulation of differentiated FOXP3(+) CD4 (+) T
cells in metastatic tumor lesions from melanoma patients compared
to peripheral blood. Cancer Immunol Immunother. 57:1795–1805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeong J, Thike AA, Lim JC, Lee B, Li H,
Wong SC, Hue SS, Tan PH and Iqbal J: Higher densities of
Foxp3+ regulatory T cells are associated with better
prognosis in triple-negative breast cancer. Breast Cancer Res
Treat. 163:21–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jackute J, Zemaitis M, Pranys D,
Sitkauskiene B, Miliauskas S, Bajoriunas V, Lavinskiene S and
Sakalauskas R: The prognostic influence of tumor infiltrating
Foxp3(+)CD4(+), CD4(+) and CD8(+) T cells in resected non-small
cell lung cancer. J Inflamm (Lond). 12:632015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
West NR, Kost SE, Martin SD, Milne K,
Deleeuw RJ, Nelson BH and Watson PH: Tumour-infiltrating FOXP3(+)
lymphocytes are associated with cytotoxic immune responses and good
clinical outcome in oestrogen receptor-negative breast cancer. Br J
Cancer. 108:155–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Badoual C, Sandoval F, Pere H, Hans S, Gey
A, Merillon N, Van Ryswick C, Quintin-Colonna F, Bruneval P, Brasnu
D, et al: Better understanding tumor-host interaction in head and
neck cancer to improve the design and development of
immunotherapeutic strategies. Head Neck. 32:946–958.
2010.PubMed/NCBI
|
|
34
|
Musolino C, Allegra A, Pioggia G and
Gangemi S: Immature myeloid-derived suppressor cells: A bridge
between inflammation and cancer. Oncol Rep. 37:671–683. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao F, Liang B, Reddy S, Farias-Eisner R
and Su X: Role of inflammation-associated microenvironment in
tumorigenesis and metastasis. Curr Cancer Drug Targets. 14:30–45.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beatty GL: Overcoming therapeutic
resistance by targeting cancer inflammation. Am Soc Clin Oncol Educ
Book. 35:e168–e173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu G, Yao L, Rao S, Gong Z, Zhang S and Yu
S: Attenuation of acute lung injury in mice by oxymatrine is
associated with inhibition of phosphorylated p38 mitogen-activated
protein kinase. J Ethnopharmacol. 98:177–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen X, Sun R, Hu J, Mo Z, Yang Z, Liao D
and Zhong N: Attenuation of bleomycin-induced lung fibrosis by
oxymatrine is associated with regulation of fibroblast
proliferation and collagen production in primary culture. Basic
Clin Pharmacol Toxicol. 103:278–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu ST, Tang Y, Shen YF, Ao HH, Bai J, Wang
YL and Yang YJ: Protective effect of oxymatrine on chronic rat
heart failure. J Physiol Sci. 61:363–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ning Y and Xin W: Effect on Toll-like
receptor 7 signaling pathway in PBMCs of chronic hepatitis B
patients by oxymatrine in vitro. Chin Arch Trad Chin Med.
36:1196–1199. 2018.
|
|
42
|
Zhang J and Tang A: Reversal effect of
matrine on drug resistance of human nasopharyngeal carcinoma cell
line. Zhong Yao Cai. 35:1989–1994. 2012.(In Chinese). PubMed/NCBI
|
|
43
|
Wang HQ, Jin JJ and Wang J: Matrine
induces mitochondrial apoptosis in cisplatin-resistant non-small
cell lung cancer cells via suppression of β-catenin/survivin
signaling. Oncol Rep. 33:2561–2566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luo SX, Deng WY, Wang XF, Lü HF, Han LL,
Chen BB, Chen XB and Li N: Molecular mechanism of
indirubin-3′-monoxime and Matrine in the reversal of paclitaxel
resistance in NCI-H520/TAX25 cell line. Chin Med J (Eng).
126:925–929. 2013.
|
|
45
|
Chai S, To KK and Lin G: Circumvention of
multi-drug resistance of cancer cells by Chinese herbal medicines.
Chin Med. 5:262010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang B, Han Q and Zhu Y: Oxymatrine
inhibited cell proliferation by inducing apoptosis in human lung
cancer A549 cells. Biomed Mater Eng. 26 (Suppl 1):S165–S172.
2015.PubMed/NCBI
|
|
47
|
Truxova I, Hensler M, Skapa P, Halaska MJ,
Laco J, Ryska A, Spisek R and Fucikova J: Rationale for the
combination of dendritic cell-based vaccination approaches with
chemotherapy agents. Int Rev Cell Mol Biol. 330:115–156. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Di Blasio S, Wortel IM, van Bladel DA, de
Vries LE, Duiveman-de Boer T, Worah K, de Haas N, Buschow SI, de
Vries IJ, Figdor CG and Hato SV: Human CD1c(+) DCs are critical
cellular mediators of immune responses induced by immunogenic cell
death. Oncoimmunology. 5:e11927392016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View Article : Google Scholar : PubMed/NCBI
|