Introduction
Nonalcoholic steatohepatitis (NASH) is a common
clinicopathological disease characterized by lipid deposition,
inflammatory lesions and hepatocyte damage, which may induce
varying degrees of liver cirrhosis or result in the development of
hepatocellular carcinoma (1).
Previous evidence has demonstrated that NASH can be induced by
several factors, such as lipotoxicity, oxidative stress, genetic
susceptibility, mitochondrial dysfunction, abnormal lipid
metabolism and endoplasmic reticulum stress (2,3).
Currently, the pathogenesis of NASH remains unclear, and key events
in NASH have always been targets of therapeutic management
(4). Thus, physical exercise,
weight loss and lifestyle adjustments remain the best treatment
methods for NASH. Furthermore, the absence of approved
pharmacological therapies has contributed to expanse of extensive
research into NASH treatment.
Garlic compounds have gained increasing attention
over the last few decades, owing to their diverse biological
activities, including their antioxidant, antiatherosclerotic,
antithrombotic, antidiabetic, anticarcinogenic and
anti-inflammatory effects (5–7).
Diallyl disulfide (DADS), a major component of the secondary
organosulfur compounds derived from garlic, has demonstrated
protective effects against cancer development, as well as against
the onset of allergies, arthritis, and cardiovascular,
neurological, and liver diseases (8–11).
Drug metabolism can be divided into one of two phases: The first
phase is called a biotransformation reaction, which includes
oxidation, reduction and hydrolysis reactions; and the second phase
is known as the binding reaction, where the reaction is combined
with endogenous substances. These beneficial effects may be
attributed to DADS-mediating phase I and II metabolizing enzymes
(12,13). DADS was demonstrated to inhibit
dichloroacetate-induced reactive oxygen species (ROS) production,
inflammatory factors and the expression of p50 in the nucleus in a
dose-dependent manner. Feng et al (14) revealed that DADS may be a good
candidate for the chemical prevention and therapy of Barrett's
esophagus and esophageal adenocarcinoma. In addition, DADS has
previously been reported to induce mitochondrial biogenesis to
attenuate cardiac hypertrophy via the eNOS-Nrf2-Tfam pathway in
rats (15). Previous studies have
also suggested that the use of DADS supplementation following major
hepatectomy had a positive impact on liver regeneration,
proliferation and oxidative damage (16,17).
DADS was capable of attenuating liver dysfunction, as it had been
metabolized into allylglutathione sulfide and allylmercaptan in
rats and humans (18). However,
the effects of DADS in NASH, and the associated underlying
molecular mechanisms, remain unclear.
In the present study, the effect of DADS on NASH
models induced by a methionine- and choline-deficient diet (MCD) or
a high-fat diet (HFD) was evaluated. The potential mechanisms of
the protective effects of DADS, in regard to lipid metabolism,
lipid peroxidation, lipotoxicity and inflammation, were also
further investigated.
Materials and methods
Animals and experimental groups
The experimental protocol was approved by the Animal
Ethics Committee of Tongji Medical College, Huazhong University of
Science and Technology (Wuhan, China), and performed according to
the principles of the Institutional Animal Care and Ethics
Committee guidelines (IACUC no. S619). A total of 60 male C57BL/6J
mice weighing 22 g (6–7 weeks of age) were purchased from Beijing
Huafukang Bioscience Co., Ltd., and maintained in a
temperature-controlled room (23±2°C) with a 12-h light-dark
lighting cycle.
NASH mice models were established via an MCD for 4
weeks and an HFD for 20 weeks (19). These models demonstrated increased
adipose tissue inflammation, enlarged fat cells and less oxidized
fat in their muscles. DADS (purity >75%; Sigma-Aldrich; Merck
KGaA) dissolved in corn oil was prepared immediately prior to
treatment. Following a 2-week acclimation period with standard
pellet chow and water ad libitum given prior to the
experiments, the mice were randomly divided into one of the
following 12 groups (n=5 mice per group): i) Mice fed a normal diet
with daily oral gavage of corn oil [Normal + negative control (NC)
group]; ii) mice fed a normal diet with daily oral gavage of 20
mg/kg DADS (Normal + DADS-L group); iii) mice fed a normal diet
with daily oral gavage of 50 mg/kg DADS (Normal + DADS-M group);
iv) mice fed a normal diet with daily oral gavage of 100 mg/kg DADS
(Normal + DADS-H group); v) mice fed an MCD diet with daily oral
gavage of corn oil (MCD + NC group); vi) mice fed an MCD diet with
daily oral gavage of 20 mg/kg DADS (MCD + DADS-L group); vii) mice
fed an MCD diet with daily oral gavage of 50 mg/kg DADS (MCD +
DADS-M group); viii) mice fed an MCD diet with daily oral gavage of
100 mg/kg DADS (MCD + DADS-H group); ix) mice fed an HFD diet with
daily oral gavage of corn oil (HFD + NC group); x) mice fed an HFD
diet with daily oral gavage of 20 mg/kg DADS (HFD + DADS-L group);
xi) mice fed an HFD diet with daily oral gavage of 50 mg/kg DADS
(HFD + DADS-M group); and xii) mice fed an HFD diet with daily oral
gavage of 100 mg/kg DADS (HFD + DADS-H group). The DADS
concentrations were selected based on previous studies with minor
modifications (5,17).
Following treatment with MCD for 4 weeks and HFD for
20 weeks, the mice were sacrificed following a 12-h fast via
pentobarbital sodium injection. Blood samples were collected from
the heart following euthanasia and serum was isolated via
centrifugation at 4°C for 15 min at 2,000 × g. Liver tissues were
collected and weighed following washing with physiological saline,
and then a portion of the tissue was fixed with 4% paraformaldehyde
for 24 h at room temperature to prepare for histological staining.
The remaining hepatic tissues were stored at −80°C for molecular
biological assays.
Liver histology and
immunohistochemical staining of F4/80-positive cells
Paraformaldehyde-fixed liver tissues were used for
hematoxylin and eosin (H&E) staining and oil red O staining, as
described in a previous study (20). The NASH activity score, a
histological feature scoring system, was utilized to assess the
liver lesions: Steatosis (score, 0–3), lobular inflammation (score,
0–3), and hepatocellular ballooning (score, 0–2). Samples with
scores >4 were diagnosed as ‘NASH’, samples with scores <3
were designated as ‘not NASH’, and samples with scores between 3–4
were designated as ‘possible for NASH’ (21). The expression of F4/80, a
macrophage-surface marker, was detected via immunohistochemistry
using an immunohistochemistry kit (Wuhan Boster Biological
Technology, Ltd.) with the rat anti-F4/80 monoclonal antibody
(1:3,000; cat. no. MCA497; Bio-Rad Laboratories, Inc.) at 37°C for
2 h, and subsequent incubation at 37°C for 2 h with the secondary
antibodies (horseradish peroxidase-conjugated anti-mouse IgG;
1:2,000; cat. no. SV0004; Wuhan Boster Biological Technology,
Ltd.). Detection of positive staining was performed using
3,3′-diaminobenzidine reagent (Wuhan Boster Biological Technology,
Ltd.) for 3 min at room temperature. Finally, all sections were
counterstained with 10% hematoxylin for 30 sec at room temperature,
and were then left to dry and covered with a coverslip. For every
section, 5 fields were randomly selected to evaluate the
quantification of F4/80 using Image-Pro Plus 6.0 (Media
Cybernetics, Inc.) under a light microscope (magnification,
×40).
Lipid and transaminase analysis
Serum aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) levels were measured using biochemical
methods according to protocols by the Nanjing Jiancheng
Bioengineering Institute. Serum triglyceride (TG) and total
cholesterol (TC) levels were measured using GPO-PAP and COD-PAP
enzymatic colorimetric assay kits (Nanjing Jiancheng Bioengineering
Institute), respectively, according to the manufacturer's
protocol.
Cytokine measurement
The plasma concentrations of malondialdehyde (MDA),
superoxide dismutase (SOD), interleukin (IL)-6 and tumor necrosis
factor (TNF)-α were measured using specific ELISA kits (BioLegend,
Inc.) according to the manufacturer's protocol. The absorbance of
the samples and standards was detected at 450 and 570 nm for the
respective kits using a microplate reader (Bio-Rad Laboratories,
Inc.).
Immunofluorescence
Paraffin-embedded liver sections (4-µm thickness)
were dewaxed with dimethyl-benzene, permeabilized with 0.3% Triton
X-100, and blocked with normal donkey serum (GeneTex, Inc.) for 60
min at room temperature. The sections were then co-incubated with
cyclic AMP-responsive element-binding protein H (CREBH) antibody
(1:100; cat. no. sc-377332; Santa Cruz Biotechnology, Inc.), and
peroxisome proliferator-activated receptor α (PPARα) antibody
(1:100; cat. no. ab8934; Abcam) or stearoyl-coenzyme A desaturase 1
(SCD-1) antibody (1:100; cat. no. 2794; Cell Signaling Technology,
Inc.) overnight at 4°C. After being washed with phosphate buffer,
the samples were incubated with secondary antibodies, including
Alexa Fluor® 488-linked (green) anti-mouse and Alexa
Fluor® 647-linked (red) anti-rabbit antibodies (1:200;
cat. nos. R37114 and A-31573; both from Thermo Fisher Scientific,
Inc.). Nuclei were stained using DAPI at room temperature for 10
min. All slides were examined under a fluorescent confocal
microscope (magnification, ×40; FV-500; Olympus Corporation).
Images were acquired using simultaneous dual-channel scanning.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA from the liver tissues was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). Next,
5 µg of RNA was used for cDNA synthesis using an M-MLV RTase cDNA
Synthesis kit (Takara Bio Inc.). Then, RT-qPCR was performed using
a SYBR Green PCR kit (Takara Bio Inc.) with the LightCycler480
sequence detector system (Roche Applied Science), using the
following reaction conditions: 95°C for 5 min, 45 cycles of 95°C
for 10 sec, 60°C for 20 sec and 70°C for 30 sec. The primer
sequences for the RT-qPCR are listed in Table I. The 2−ΔΔCq method was
used to calculate the relative mRNA expression (22), which was normalized against the
internal control gene, β-actin. Gene expression levels for each
sample were analyzed in duplicate.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR assays. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR assays.
| Genes | Forward primers
(5′-3′) | Reverse primers
(5′-3′) |
|---|
| SREBP-1c |
GCAGCCACCATCTAGCCTG |
CAGCAGTGAGTCTGCCTTGAT |
| ApoA-1 |
GCTCAAGAGCAACCCTACCTT |
GCTTTCTCGCCAAGTGTCTTC |
| CREBH |
CCTCCCGCTTCAACCTCAC |
CCGGATCTTTCTGCGGATTTT |
| FGF21 |
GTGTCAAAGCCTCTAGGTTTCTT |
GGTACACATTGTAACCGTCCTC |
| PPARα |
GTCATCACAGACACCCTCTCTCC |
TGTCCCCACATATTCGACACTC |
| SCD-1 |
CAGTTCCTACACGACCACCACTA |
GGACGGATGTCTTCTTCCAGAT |
| β-actin |
TTCTTTGCAGCTCCTTCGTTGCCG |
TGGATGGCTACGTACATGGCTGGG |
Western blot assay
Tissue lysates were prepared using RIPA lysis buffer
containing 0.1 mmol/l phenylmethylsulphonyl fluoride (PMSF;
Sigma-Aldrich; Merck KGaA). After being quantified using a Bio-Rad
protein assay kit (Bio-Rad Laboratories, Inc.), protein extracts
(50 µg/lane) were separated via 10% SDS-PAGE and transferred onto a
polyvinylidene fluoride (PVDF) membrane (EMD Millipore). Membranes
were blocked with 5% fat-free milk at room temperature for 60 min,
followed by incubation with primary antibodies overnight at 4°C.
The membranes were washed and subsequently incubated at room
temperature for 2 h in the next day with the secondary antibodies
[horseradish peroxidase-conjugated anti-rabbit IgG (1:2,000; cat.
no. GTX213110-01) and horseradish peroxidase-conjugated anti-mouse
IgG (1:2,000; cat. no. GTX213111-01); both GeneTex, Inc.].
Immunoreactive proteins were visualized using chemiluminescence
kits (Thermo Fisher Scientific, Inc.). The protein expression of
each sample was analyzed three times and normalized against the
internal reference antibody, GAPDH or tubulin.
The following primary antibodies were used:
Anti-sterol regulatory element-binding transcription factor 1
(SREBP1c; 1:1,000; cat. no. ab28481; Abcam); anti-CREBH (1:1,000;
cat. no. sc-377332; Santa Cruz Biotechnology, Inc); anti-PPARα
(1:2,000; cat. no. ab8934; Abcam); anti-SCD-1 (1:1,000; cat. no.
2794; Cell Signaling Technology, Inc.); anti-phospho (p)-nuclear
factor (NF)-κB p65 (1:2,000; cat. no. 3033; Cell Signaling
Technology, Inc.); anti-tubulin (1:2,000; cat. no. GTX76511;
GeneTex, Inc.); and anti-GAPDH (1:2,000; cat. no. GTX100118;
GeneTex, Inc.).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean (SEM). All statistical analyses were performed using SPSS
software (version 16.0; SPSS Inc.), and statistical significance
was calculated using one-way analysis of variance. Post hoc
multiple comparison testing among groups was performed using the
least significance difference test. All experiments were repeated
independently a minimum of three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
DADS ameliorated hepatic dysfunction
in mouse models of NASH
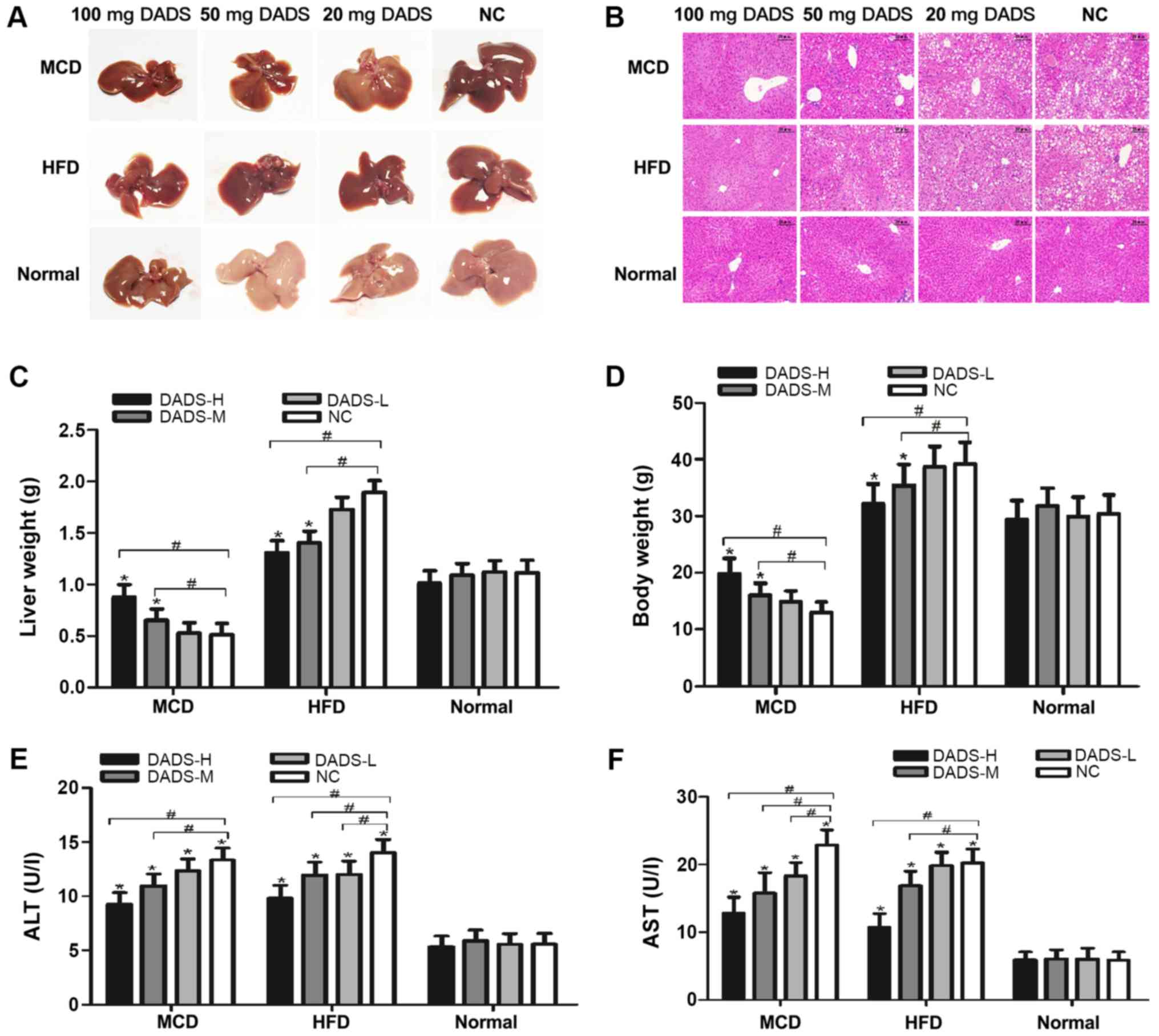
Following 4 weeks of feeding with MCD or 20 weeks of
feeding with HFD, the liver morphology and liver sections in the
mice exhibited significant vacuolated hepatocytes, marked
inflammatory cell infiltration and severe micro- and
macro-vesicular steatosis, which indicated the successful
establishment of two NASH mouse models (Fig. 1A and B). The inclusion of DADS (50
and 100 mg) in the MCD or HFD models, respectively, greatly
ameliorated the hepatic dysfunction, as determined via H&E
staining (Fig. 1B). It is worth
noting that supplementation with DADS had no effect on liver
histology in mice fed a normal diet, demonstrating its
non-hepatotoxic properties (P>0.05; Fig. 1B, E and F). Mice fed with MCD had
the lowest liver weight and mice fed with HFD had the heaviest
liver weight compared with those mice fed with a normal diet
(P<0.01; Fig. 1C). DADS (50 and
100 mg) significantly increased the liver weight in MCD-fed mice
and decreased it in HFD-fed mice, respectively (P<0.01; Fig. 1C). The trend of changes in body
weight was similar to the liver weight in mice (P<0.05; Fig. 1D). Furthermore, the serum ALT and
AST levels were significantly increased in these two NASH models,
and these levels were significantly decreased by DADS treatment in
a dose-dependent manner (P<0.05; Fig. 1E and F).
DADS decreased hepatic lipid
accumulation in mouse models of NASH
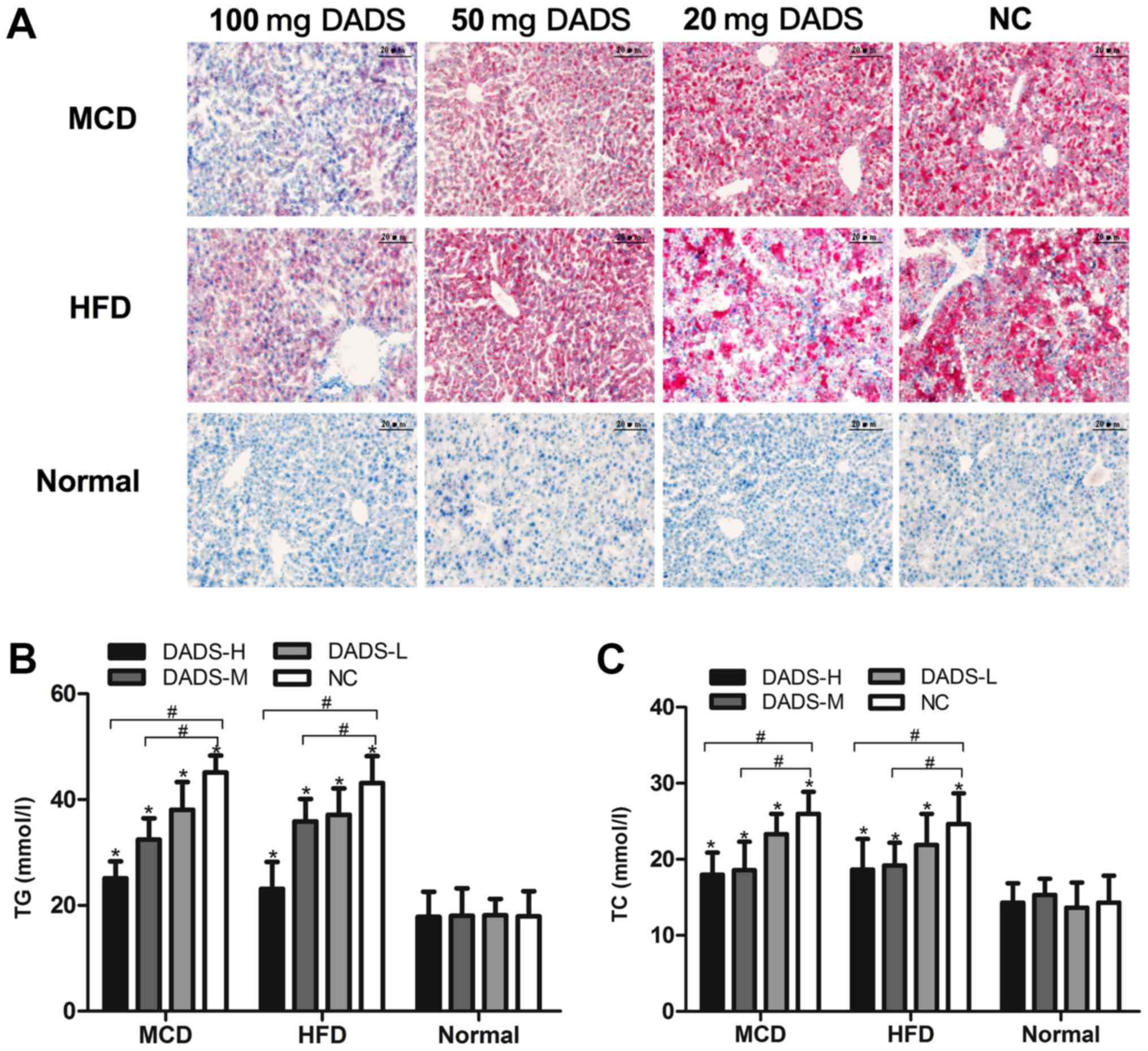
Oil Red O staining was performed in liver sections
in order to investigate the effect of DADS on lipid deposition and
lipid droplet accumulation. As presented in Fig. 2A, the hepatic lipid accumulation
was greater in mouse models of NASH than in the mice fed with a
normal diet (P<0.05). DADS treatment caused a marked decrease in
the number and size of lipid droplets (P<0.01; Fig. 2A). The liver TG and TC contents in
MCD-fed mice were elevated by 2.51- and 1.81-fold in MCD-fed mice,
respectively, compared with the mice fed a normal diet (P<0.05;
Fig. 2B and C). These contents
were also increased by 2.42- and 1.72-fold in HFD-fed mice,
respectively, when compared with those mice fed a normal diet
(P<0.05; Fig. 2B and C).
Interestingly, DADS treatment significantly decreased MCD-induced
or HFD-induced elevations in the liver TG and TC contents in a
dose-dependent manner (P<0.05; Fig.
2B and C). These results clearly indicated the anti-steatotic
role of DADS on MCD- or HFD-induced NASH. Since the high-dose DADS
group displayed the best hepatoprotective effects, 100 mg/kg DADS
was chosen for the following experiments.
DADS ameliorated hepatic steatosis by
regulating key regulators of lipid metabolism
To investigate the molecular mechanisms behind DADS
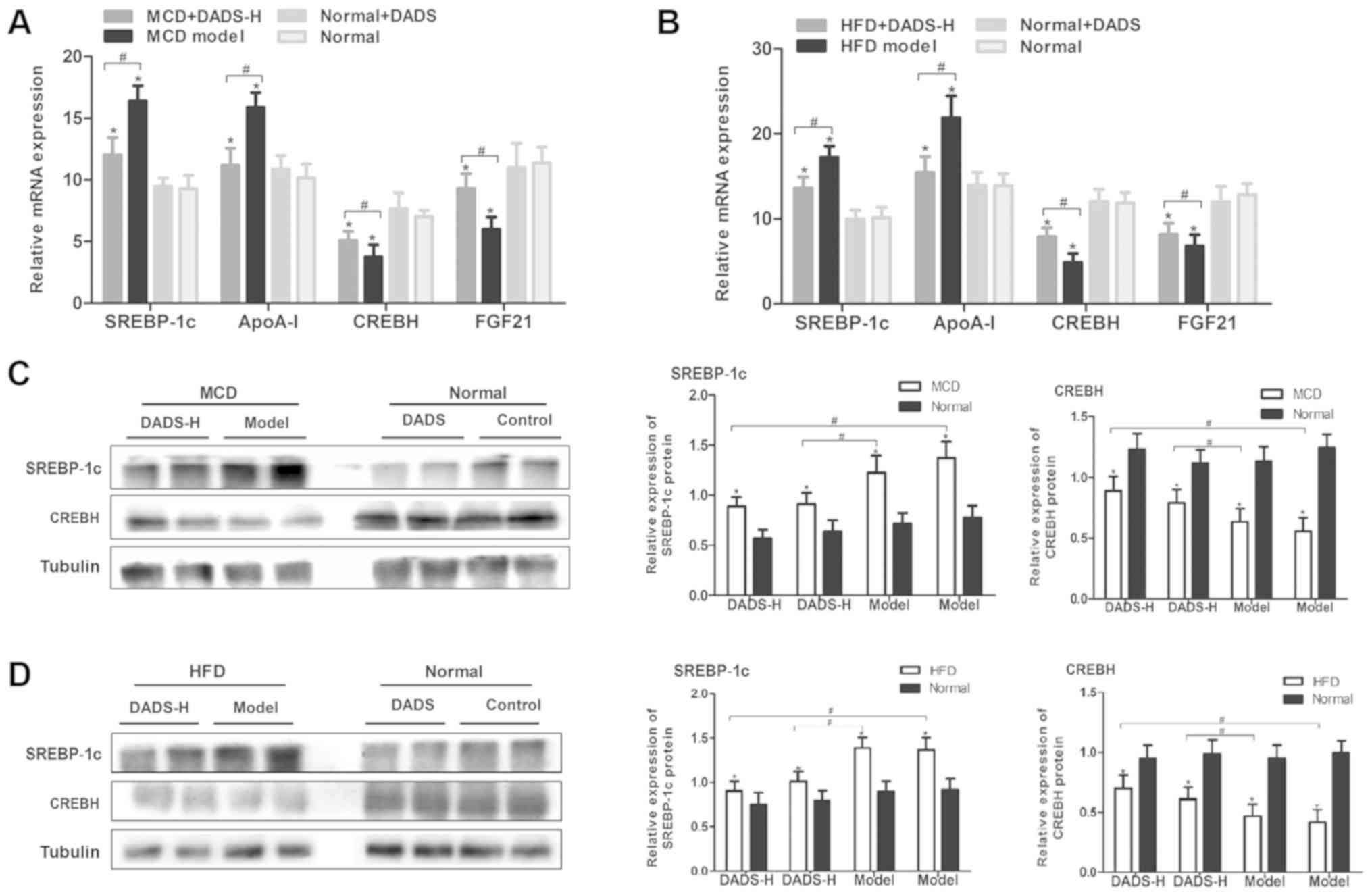
in ameliorating hepatic steatosis, the mRNA levels of key lipid
metabolism-associated genes were examined. MCD or HFD caused a
significant increase in the mRNA levels of SREBP-1c and
apolipoprotein A1 (ApoA-I), while they led to a significant
decrease in the mRNA levels of CREBH and fibroblast growth factor
(FGF)21 (P<0.05; Fig. 3A and
B). In addition, DADS treatment reversed the effect of MCD or
HFD on the expression of these genes (P<0.05; Fig. 3A and B). As the major regulators of
lipid biosynthesis, SREBP-1c and CREBH regulate the expression of
several genes associated with TG and fatty acid (FA) synthesis.
Thus, the protein levels of SREBP-1c and CREBH were detected and
the results revealed that DADS decreased the protein expression
levels of SREBP-1c and increased the protein expression levels of
CREBH (P<0.05; Fig. 3C and
D).
DADS attenuated lipotoxicity and lipid
peroxidation in HFD-fed mice
To investigate the role of DADS in lipotoxicity and
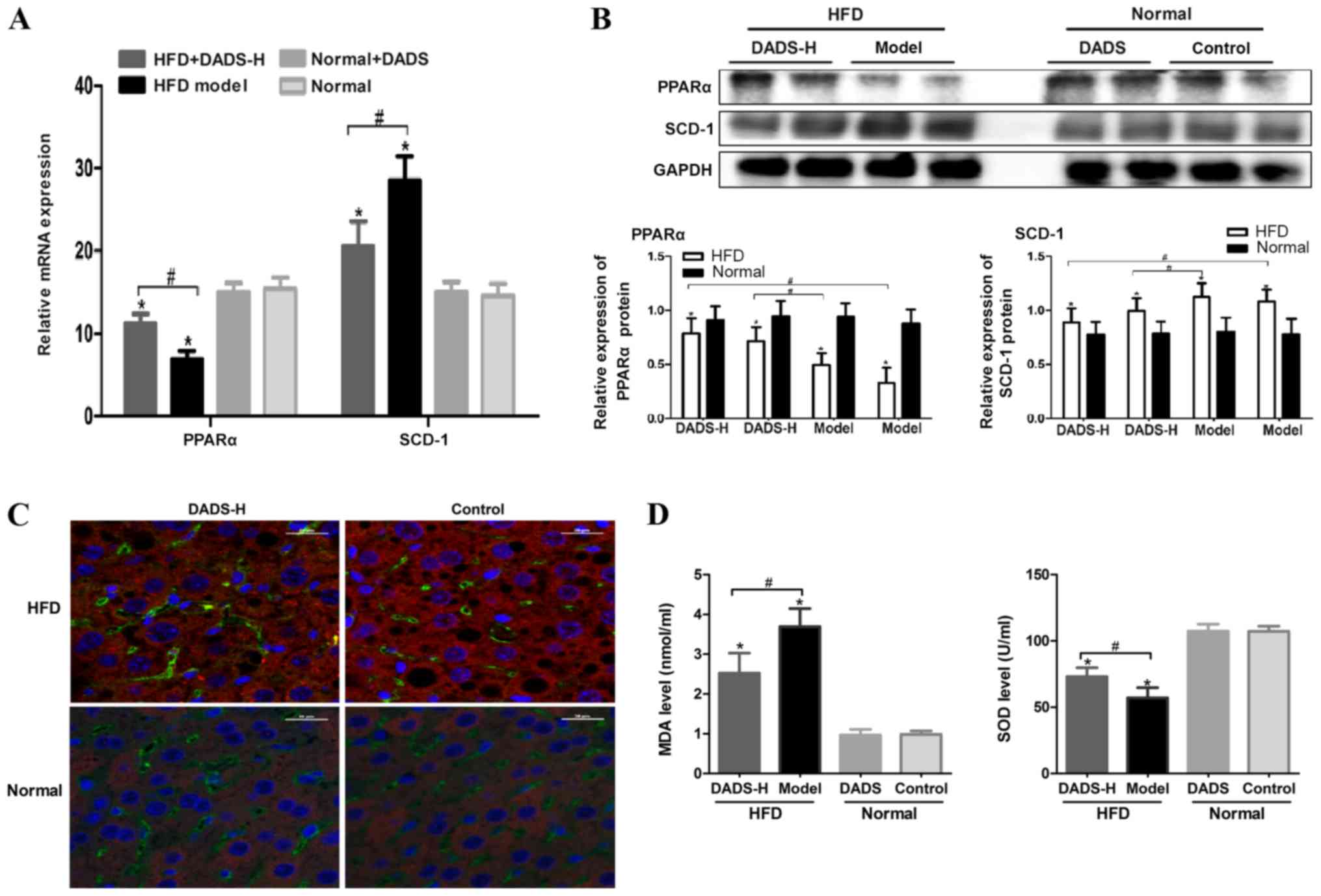
lipid peroxidation in HFD-fed mice, the expression levels of PPARα
and SCD-1 were detected in the present study. As presented in
Fig. 4A and B, the expression
levels of PPARα or SCD-1 were significantly decreased or increased,
respectively, in HFD-fed mice when compared with the normal control
group (P<0.05). DADS treatment led to an increase in PPARα
expression and a decrease in SCD-1 expression in HFD-fed mice
(P<0.05; Fig. 4A and B). These
results were further verified via an immunofluorescence assay,
demonstrating the expression levels of PPARα and SCD-1 (Fig. 4C). The abnormalities in MDA and SOD
levels in the plasma could also be alleviated by DADS treatment in
HFD-fed mice (P<0.05; Fig.
4D).
DADS inhibits inflammation through the
NF-κB pathway in MCD-fed mice
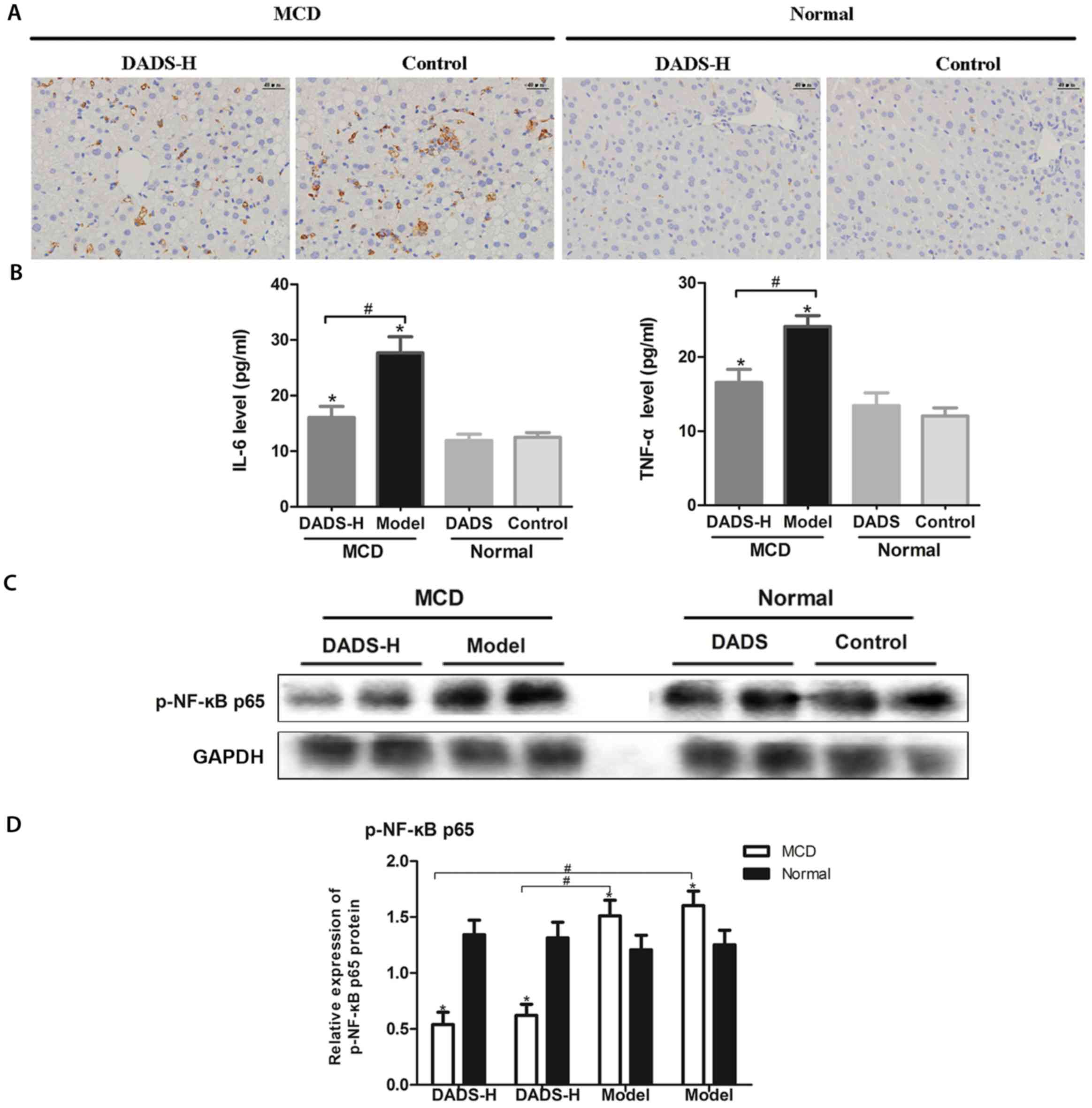
The results of immunohistochemical staining of
F4/80-positive cells revealed that the number of macrophage cells
had increased in the liver in MCD-fed group, while it had decreased
following DADS treatment (P<0.05; Fig. 5A). TNF-α and IL-6 are the two most
common inflammatory cytokines. TNF-α is the earliest and most
important inflammatory mediator in the inflammatory process; it
activates neutrophils and lymphocytes and allows them to pass
through the vascular endothelial cells. In addition, TNF-α
regulates other metabolic activities in the tissues and promotes
the synthesis and release of other cytokines. IL-6 induces B-cell
differentiation and produces antibodies. It also induces the
activation, proliferation and differentiation of sputum cells, and
participates in the immune response of the body. IL-6 is a trigger
for inflammatory reactions. Therefore, these two most
representative inflammatory factors were chosen to assess the
therapeutic effects of DADS in the present study. The plasma levels
of IL-6 and TNF-α were higher in the MCD-fed mice than in the mice
that were fed a normal diet (P<0.05; Fig. 5B). In addition, DADS treatment in
MCD-fed mice effectively reversed these elevations (P<0.05;
Fig. 5B). We then detected the
expression of the NF-κB pathway, which was revealed to play a
pivotal role in the pathogenesis of NASH. As presented in Fig. 5C and D, MCD led to an increase in
the protein expression levels of p-NF-κB p65 in the liver of
MCD-fed mice, and DADS treatment blocked this increase
(P<0.05).
Discussion
The global incidence of NASH increases constantly
with the improvement of living standards. However, the medications
that have been proven to be most effective for NASH are limited,
and treatment for end-stage NASH is confined to liver
transplantation only (23,24). Thus, discovering effective drugs
for NASH and elucidating its underlying molecular mechanism is
urgently required. Herein, the results from the present study
demonstrated the apparent hepatoprotective effect of DADS that was
involved in regulating the key regulators of hepatic steatosis,
lipotoxicity, lipid peroxidation and inflammation. Diversified
animal models of NASH have been established to understand its
etiology and to discover efficient therapies. Two mouse models of
NASH induced by MCD or HFD were established, which were associated
with decreased very-low-density lipoprotein secretion or increased
FA levels associated with the liver, respectively (25). Consistent with a previous study,
the results from the present study revealed that both MCD and HFD
induced the histological features of NASH in mice, and included
significant vacuolated hepatocytes, marked inflammatory cell
infiltration and severe micro- and macro-vesicular steatosis
(Fig. 1A and B) (19). Mice fed with an MCD had the lowest
liver weight when compared with that of the mice fed a normal diet
(Fig. 1C). The trend of changes
with body weight was similar to the liver weight in mice
(P<0.05; Fig. 1D). Furthermore,
the serum ALT and AST levels, which are sensitive markers of liver
damage, were also significantly increased in these two NASH models
(Fig. 1E and F). In addition to
the hepatic dysfunction, hepatic lipid accumulation was observed in
the MCD-fed and HFD-fed mice (Fig.
2A). The two mouse models of NASH induced by MCD and HFD were
associated with reduced very-low-density lipoprotein secretion and
increased FA delivery to the liver, respectively. MCD and HFD
induced the histological features of NASH in mice, including
significant vacuolated hepatocytes, marked inflammatory cell
infiltration and severe micro- and macro-vesicular steatosis.
Previous studies have suggested that DADS has potential as a
treatment option for metabolic syndrome and acute liver injury due
to its hepatoprotective and antioxidant effects (5,17).
Thus, amelioration of hepatic dysfunction by DADS was thought not
to be due to direct interactions with either the MCD or HFD diet.
Excessive TG and TC accumulation in the liver has been proven to be
an early event in NASH, which may be attributed to the enhancement
of FA synthesis and to the impairment of FA oxidation (26,27).
It was revealed that there were significantly greater liver TG and
TC contents in MCD-fed and HFD-fed mice when compared with those
mice fed a normal diet (Fig. 2B and
C).
DADS is one of the most abundant, oil-soluble
organosulfur compounds derived from garlic. It has been
demonstrated to have protective effects against several diseases
(9). In the present study, it was
revealed that DADS treatment ameliorated hepatic dysfunction and
reduced hepatic lipid accumulation in MCD-fed and HFD-fed mice
(Figs. 1 and 2). Previous studies have also suggested
that DADS demonstrated potential in the treatment of metabolic
syndrome and acute liver injury (5,17,28).
Lee et al (28) revealed
the hepatoprotective and antioxidant effects of DADS on carbon
tetrachloride-induced hepatic oxidative damage and the inflammatory
response in rats. To elucidate the underlying molecular mechanisms
of DADS in ameliorating hepatic steatosis, the expression of key
lipid metabolism-associated genes was examined. It was revealed
that DADS decreased the expression levels of SREBP-1c and ApoA-I
and increased the expression levels of CREBH and FGF21 (Fig. 3A and B). Lipid
metabolism-associated SREBP-1c has been indicated to regulate
several crucial genes associated with TG synthesis, including
ApoA-I and SCD-1, thus playing a pivotal role in hepatic steatosis
(29). Furthermore, CREBH can
modulate the expression levels of genes involved in lipogenesis,
sterol metabolism, lipid peroxidation, lipotoxicity, inflammation
and gluconeogenesis (30,31). These data indicated that DADS
ameliorated hepatic steatosis by regulating SREBP-1c and CREBH, the
key regulators of lipid metabolism (Fig. 3C and D).
In addition, data from the present study also
revealed that the expression levels of the genes involved in FA
oxidation (including PPARα and SCD-1) and lipid peroxidation
(including MDA and SOD) were reversed by DADS in HFD-fed mice
(Fig. 4). Decreased PPARα
expression may result in the reduction of FA oxidation and lead to
an acceleration of lipid accumulation in the liver, thereby
contributing to the development of NASH. MDA is a marker for
oxidative stress, which can lead to lipid peroxidation. The
anti-oxidant enzyme SOD is capable of eliminating ROS, which leads
to hepatic apoptosis. Therefore, upregulated PPARα, decreased MDA
and increased SOD induced by DADS indicated its attenuated effect
on the lipotoxicity and lipid peroxidation in HFD-fed mice.
In addition to lipid peroxidation, the inflammatory
response was also a striking feature of NASH. The inflammatory
response in the NASH model induced by MCD was investigated in the
present study, which could induce high-grade hepatic inflammatory
cell infiltration. It was revealed that DADS dramatically reduced
the number of macrophage cells in the liver (Fig. 5A). Furthermore, DADS also decreased
the expression levels of TNF-α and IL-6 (Fig. 5B), which is consistent with results
from previous studies (32,33).
The activity of NF-κB was decreased by DADS treatment in MCD-fed
mice (Fig. 5C and D). It has been
reported that DADS was effective in reducing the levels of oxidized
low-density lipoprotein, lipid peroxidation, as well as NF-κB
activity, revealing good anti-inflammatory and antioxidant
properties (34). The results from
the present study demonstrated that the therapeutic effects of DADS
on NASH were associated with decreased inflammatory cell
infiltration and downregulated pro-inflammatory cytokines in
MCD-fed mice.
There were certain limitations to the present study.
The results of the study indicated that DADS exerted beneficial
effects on MCD- or HFD-induced NASH by suppressing key regulators
of lipid metabolism, lipid peroxidation and inflammation; however,
insulin resistance and abnormal sterol metabolism are also involved
in the pathogenesis of NASH. Whether DADS improves insulin
resistance and sterol metabolism abnormalities requires further
investigation. Previous research has demonstrated that DADS
inhibited the proliferation and transdifferentiation of lung
fibroblasts via the induction of cyclooxygenase and the synthesis
of prostaglandin E2 (35), suggesting that DADS may also have
an important role in the pathogenesis of hepatic fibrosis. It will
serve as the direction for future research.
In conclusion, DADS effectively attenuated hepatic
steatosis, lipotoxicity, lipid peroxidation and inflammation in
NASH mouse models. These results suggest that DADS may be a
potential therapeutic agent in the prevention and treatment of
NASH, given its high efficacy and low risk of side effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81670515).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
NZ and YW conceived and designed the study. NZ, JZ,
BL, SX and GL performed the experiments. NZ and YW analyzed the
data and drafted the manuscript. KX conceived the study and gave
final approval of the version to be published. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the Animal
Ethics Committee of Tongji Medical College, Huazhong University of
Science and Technology (Wuhan, China), and performed according to
the principles of the Institutional Animal Care and Ethics
Committee guidelines (IACUC no. S619).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Italian Association for the Study of the
Liver (AISF), . AISF position paper on nonalcoholic fatty liver
disease (NAFLD): Updates and future directions. Dig Liver Dis.
49:471–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caligiuri A, Gentilini A and Marra F:
Molecular pathogenesis of NASH. Int J Mol Sci. 17(pii): E15752016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tilg H and Moschen AR: Evolution of
inflammation in nonalcoholic fatty liver disease: The multiple
parallel hits hypothesis. Hepatology. 52:1836–1846. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lassailly G, Caiazzo R, Pattou F and
Mathurin P: Perspectives on treatment for nonalcoholic
steatohepatitis. Gastroenterology. 150:1835–1848. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lai YS, Chen WC, Ho CT, Lu KH, Lin SH,
Tseng HC, Lin SY and Sheen LY: Garlic essential oil protects
against obesity-triggered nonalcoholic fatty liver disease through
modulation of lipid metabolism and oxidative stress. J Agric Food
Chem. 62:5897–5906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yun HM, Ban JO, Park KR, Lee CK, Jeong HS,
Han SB and Hong JT: Potential therapeutic effects of functionally
active compounds isolated from garlic. Pharmacol Ther. 142:183–195.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sheen LY, Wu CC, Lii CK and Tsai SJ:
Effect of diallyl sulfide and diallyl disulfide, the active
principles of garlic, on the aflatoxin B(1)-induced DNA damage in
primary rat hepatocytes. Toxicol Lett. 122:45–52. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Li A, Feng X, Sun X, Zhu X and Zhao
Z: Pharmacological investigation of the anti-inflammation and
anti-oxidation activities of diallyl disulfide in a rat emphysema
model induced by cigarette smoke extract. Nutrients. 10(pii):
E792018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ko JW, Park SH, Shin NR, Shin JY, Kim JW,
Shin IS, Moon C, Heo JD, Kim JC and Lee IC: Protective effect and
mechanism of action of diallyl disulfide against
acetaminophen-induced acute hepatotoxicity. Food Chem Toxicol.
109:28–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raghu R, Liu CT, Tsai MH, Tang X, Kalari
KR, Subramanian S and Sheen LY: Transcriptome analysis of
garlic-induced hepatoprotection against alcoholic fatty liver. J
Agric Food Chem. 60:11104–11119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimada M, Liu L, Nussler N, Jonas S,
Langrehr JM, Ogawa T, Kaminishi M, Neuhaus P and Nussler AK: Human
hepatocytes are protected from ethanol-induced cytotoxicity by DADS
via CYP2E1 inhibition. Toxicol Lett. 163:242–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeong HG and Lee YW: Protective effects of
diallyl sulfide on N-nitrosodimethylamine-induced immunosuppression
in mice. Cancer Lett. 134:73–79. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guyonnet D, Siess MH, Le Bon AM and
Suschetet M: Modulation of phase II enzymes by organosulfur
compounds from allium vegetables in rat tissues. Toxicol Appl
Pharmacol. 154:50–58. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng C, Luo Y, Nian Y, Liu D, Yin X, Wu J,
Di J, Zhang R and Zhang J: Diallyl disulfide suppresses the
inflammation and apoptosis resistance induced by DCA through ROS
and the NF-κB signaling pathway in human barrett's epithelial
cells. Inflammation. 40:818–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khatua TN, Dinda AK, Putcha UK and
Banerjee SK: Diallyl disulfide ameliorates isoproterenol induced
cardiac hypertrophy activating mitochondrial biogenesis via
eNOS-Nrf2-Tfam pathway in rats. Biochem Biophys Rep. 5:77–88.
2015.PubMed/NCBI
|
|
16
|
Battal M, Kartal A, Citgez B, Yilmaz B,
Akcakaya A and Karatepe O: Impact of allyl disulfide on oxidative
damage and liver regeneration in an experimental hepatectomy model.
Chirurgia (Bucur). 110:117–122. 2015.PubMed/NCBI
|
|
17
|
Lee IC, Kim SH, Baek HS, Moon C, Kim SH,
Kim YB, Yun WK, Kim HC and Kim JC: Protective effects of diallyl
disulfide on carbon tetrachloride-induced hepatotoxicity through
activation of Nrf2. Environ Toxicol. 30:538–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Germain E, Chevalier J, Siess MH and
Teyssier C: Hepatic metabolism of diallyl disulphide in rat and
man. Xenobiotica. 33:1185–1199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan JG and Qiao L: Commonly used animal
models of non-alcoholic steatohepatitis. Hepatobiliary Pancreat Dis
Int. 8:233–240. 2009.PubMed/NCBI
|
|
20
|
Zhang J, Zhang H, Deng X, Zhang N, Liu B,
Xin S, Li G and Xu K: Baicalin attenuates non-alcoholic
steatohepatitis by suppressing key regulators of lipid metabolism,
inflammation and fibrosis in mice. Life Sci. 192:46–54. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kleiner DE, Brunt EM, Van Natta M, Behling
C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS,
Unalp-Arida A, et al: Design and validation of a histological
scoring system for nonalcoholic fatty liver disease. Hepatology.
41:1313–1321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bellentani S: The epidemiology of
non-alcoholic fatty liver disease. Liver Int. 37 (Suppl 1):S81–S84.
2017. View Article : Google Scholar
|
|
24
|
Musso G, Cassader M and Gambino R:
Non-alcoholic steatohepatitis: Emerging molecular targets and
therapeutic strategies. Nat Rev Drug Discov. 15:249–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Imajo K, Yoneda M, Kessoku T, Ogawa Y,
Maeda S, Sumida Y, Hyogo H, Eguchi Y, Wada K and Nakajima A: Rodent
models of nonalcoholic fatty liver disease/nonalcoholic
steatohepatitis. Int J Mol Sci. 14:21833–21857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Banini BA and Sanyal AJ: Nonalcoholic
fatty liver disease: Epidemiology, pathogenesis, natural history,
diagnosis, and current treatment options. Clin Med Insights Ther.
8:75–84. 2016.PubMed/NCBI
|
|
27
|
Benedict M and Zhang X: Non-alcoholic
fatty liver disease: An expanded review. World J Hepatol.
9:715–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee IC, Kim SH, Baek HS, Moon C, Kang SS,
Kim SH, Kim YB, Shin IS and Kim JC: The involvement of Nrf2 in the
protective effects of diallyl disulfide on carbon
tetrachloride-induced hepatic oxidative damage and inflammatory
response in rats. Food Chem Toxicol. 63:174–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagaya T, Tanaka N, Suzuki T, Sano K,
Horiuchi A, Komatsu M, Nakajima T, Nishizawa T, Joshita S, Umemura
T, et al: Down-regulation of SREBP-1c is associated with the
development of burned-out NASH. J Hepatol. 53:724–731. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Del Campo JA, Gallego-Durán R, Gallego P
and Grande L: Genetic and epigenetic regulation in nonalcoholic
fatty liver disease (NAFLD). Int J Mol Sci. 19(pii): E9112018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JH, Giannikopoulos P, Duncan SA, Wang
J, Johansen CT, Brown JD, Plutzky J, Hegele RA, Glimcher LH and Lee
AH: The transcription factor cyclic AMP-responsive element-binding
protein H regulates triglyceride metabolism. Nat Med. 17:812–815.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee IC, Baek HS, Kim SH, Moon C, Park SH,
Kim SH, Shin IS, Park SC and Kim JC: Effect of diallyl disulfide on
acute gastric mucosal damage induced by alcohol in rats. Hum Exp
Toxicol. 34:227–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bauer D, Mazzio E, Soliman KF, Taka E,
Oriaku E, Womble T and Darling-Reed S: Diallyl disulfide inhibits
TNFα-induced CCL2 release by MDA-MB-231 cells. Anticancer Res.
34:2763–2770. 2014.PubMed/NCBI
|
|
34
|
Rai SK, Sharma M and Tiwari M: Inhibitory
effect of novel diallyldisulfide analogs on HMG-CoA reductase
expression in hypercholesterolemic rats: CREB as a potential
upstream target. Life Sci. 85:211–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Cao R, Wei B, Chai X, Sun D, Guan
Y and Liu XM: Diallyl disulfide inhibits proliferation and
transdifferentiation of lung fibroblasts through induction of
cyclooxygenase and synthesis of prostaglandin E2. Mol
Cell Biochem. 393:77–87. 2014. View Article : Google Scholar : PubMed/NCBI
|