Introduction
Inflammatory bowel diseases (IBDs), such as Crohn’s
disease (CD) and ulcerative colitis (UC), are chronic relapsing
inflammatory disorders of the gastrointestinal tract (1). Although their precise aetiology is
unknown, they are likely to be correlated with an abnormal
exacerbated immune response to otherwise innocuous stimuli, which
is not properly abrogated by the feedback system that normally
downregulates the mucosal response to luminal factors (2). Similar to other inflammatory
processes, IBD is characterised by an upregulation of the synthesis
and release of a variety of pro-inflammatory mediators, such as
reactive oxygen species and cytokines, thus influencing mucosal
integrity and leading to excessive tissue injury (3,4).
Currently, a specific causal treatment of IBD is not available, and
the best regimen is the regulation of the oxidant/antioxidant
balance.
Oral administration of dextran sulphate sodium (DSS)
in mice induces colitis that resembles human UC (5). This model corresponds well to the
clinical signs of human UC, thus may serve as a reliable model for
studies on this disease. In this model, leukocytes, including
neutrophils, lymphocytes and macrophages, have been reported to
infiltrate inflamed tissues (6).
Simultaneously, there are numerous reactive oxygen species in the
colonic mucosa. Oxidative stress, with its dual effect of free
radical generation and enhanced lipid peroxidation, is the mainstay
of disease evolution (7). The
production and release of reactive oxygen species by immune cells
appear to play a crucial role in the pathophsyiology of UC
(8).
Flavonoids have been suggested to exert human health
benefits, which may be mediated by their antioxidant and
anti-inflammatory activities. Among the known flavonoids, myricetin
(3,3′,4′,5,5′,7-hexahydroxyflavone) is one of the major flavonoids
found in several foods, including onions, berries, grapes and red
wine (9). Myricetin has several
beneficial effects, including anti-inflammatory (10), antioxidant (11), analgesic and anticarcinogenic
effects (12,13). These pleiotropic effects render
myricetin a suitable candidate for study. The main objective of the
present study was to evaluate the anticolitis effects and possible
mechanisms of action of myricetin in a model of DSS-induced
colitis.
Materials and methods
Reagents
Myricetin was purchased from Sigma-Aldrich (St.
Louis, MO, USA) and DSS was purchased from Wako Pure Chemical
Industries Inc. (Tokyo, Japan).
Animals
Forty specific pathogen-free female BALB/c mice were
housed in standard cages with wood shavings. Eight animals/cage
were maintained in a room with a carefully controlled ambient
temperature (25°C) and artificial illumination (12 h of light from
8:00 a.m. to 8:00 p.m.). The experiments were approved by the
ethics committee of Jilin University Changchun, China.
Induction of colitis
Mice were given drinking water containing 5%
(wt/vol) DSS (mol wt 5,000 dissolved in drinking water) ad
libitum for 10 days. The normal group was given water only.
Previous results have shown that there is acute inflammation at
this stage, as indicated by the increased number of
polymorphonuclear leukocytes, multiple erosive lesions and the loss
of crypts (14).
Treatment with myricetin
The mice were treated with myricetin at 200, 100 or
50 mg/kg body weight daily, beginning on the day on which oral DSS
water was given. The normal group was administered an equal volume
of water orally.
Morphological analysis
At the end of the experiment, the body weight and
haemoglobin content were measured. After measuring the colonic
length, colon samples were fixed in 10% formalin solution (pH
7.2).
Grading of histological changes
Colon samples were embedded in paraffin. Serial
sections 4 μm thick were prepared and stained with haematoxylin and
eosin (H&E) for histological grading and evaluation. The degree
of inflammation in microscopic cross-sections of the colon was
evaluated as shown in Table I.
 | Table IDegree of inflammation in microscopic
cross-sections of the colon. |
Table I
Degree of inflammation in microscopic
cross-sections of the colon.
| No. | Ulceration | Epithelium | Infiltration | Lymphoid
follicles |
|---|
| 0 | No ulcers | Normal
morphology | No infiltrate | No lymphoid
follicles |
| 1 | 1 ulcer | Loss of goblet
cells | Infiltrate around
crypt bases | 1 lymphoid
follicle |
| 2 | 2 ulcers | Loss of goblet cells
in large areas | Infiltrate reaching
to muscularis mucosae | 2 lymphoid
follicles |
| 3 | 3 ulcers | Loss of crypts | Extensive
infiltration reaching the muscularis | 3 lymphoid
follicles |
| 4 | >3 ulcers | Loss of crypts in
large areas | Infiltration of the
submucosa | >3 lymphoid
follicles |
Assessment of myeloperoxidase (MPO)
activity in colonic tissue
MPO activity was determined by a modified method
described by Suzuki et al(15). In brief, colonic tissue was weighed
and homogenised with a homogeniser for 40 sec in ice-cold 50 mmol/l
phosphate-buffered saline (PBS; pH 6.0) containing 0.5%
hexadecyl-trimethylammonium bromide. The homogenate was thawed
three times, followed by repeated sonication for 30 sec each time.
The final MPO activity was represented as U/mg tissue.
Assessment of superoxide dismutase (SOD)
and glutathione peroxidase (GSH-Px) activity and malondialdehyde
(MDA) content in colonic tissue
After thawing, colon samples were weighed and
homogenised in 0.3 ml PBS (pH 7.2) at 4°C. The protein
concentration was determined quantitatively using the BAC 100
Protein Determination kit (Shanghai Bio-Technology Co., Ltd.,
Shanghai, China). GSH-Px activity and the MDA content in colonic
tissue were measured by chemical chromatometry using related assay
kits (Jiancheng Biotech, Nanjing, China). The final value of GSH-Px
is presented as U/mg protein, and MDA is presented as nmol/mg
protein. The SOD activity in colonic tissue was measured using the
method of Ohkawa et al(16)
to evaluate the ability of the xanthine-xanthine oxidase system to
inhibit the oxidation of oxymine. SOD is presented as U/mg
protein.
Assessment of colonic nitric oxide (NO)
content
NO content was determined by measuring its stable
metabolites, nitrite (NO2−) and nitrate
(NO3−), based on the method described by
Miranda et al(17). In
brief, 0.1 ml of colonic homogenate (20%) was added to 0.1 ml of
methylalcohol and centrifuged at 12,000 × g for 10 min. An aliquot
of the resultant supernatant (0.1 ml) was aspirated and mixed with
0.1 ml of vanadium (III) chloride. Then, 50 μl of sulphanilamide
solution and 50 μl of N-(1-naphthyl)ethylenediamine
dihydrochloride (NEDD) were added and incubated at 37°C for 30 min.
The optical density was measured at 540 nm against a blank using
the spectrophotometer UVWin 5.
Assessment of interleukin (IL)-1β and
IL-6
Colon samples were weighed and homogenised in 0.3 ml
PBS (pH 7.2) containing 1% bovine serum albumin (BSA) at 4°C. The
homogenate was thawed three times and centrifuged at 12,000 × g for
10 min. Cytokine levels were assayed twice with quantitative IL-1β
and IL-6 enzyme-linked immunosorbent assay (ELISA) kits
(eBioscience, Inc., San Diego, CA, USA). IL-1β and IL-6 values are
expressed as pg/mg tissues.
Statistical analyses
The data are expressed as the mean ± SEM. The
statistical significance of the difference in each parameter among
the groups was evaluated using a one-way analysis of variance
(ANOVA) followed by the Fisher’s protected least significant
difference (PLSD) comparison tests for post hoc t-tests. P<0.05
was considered to indicate a statistically significant difference.
Scores were analysed using the Wilcoxon’s test.
Results
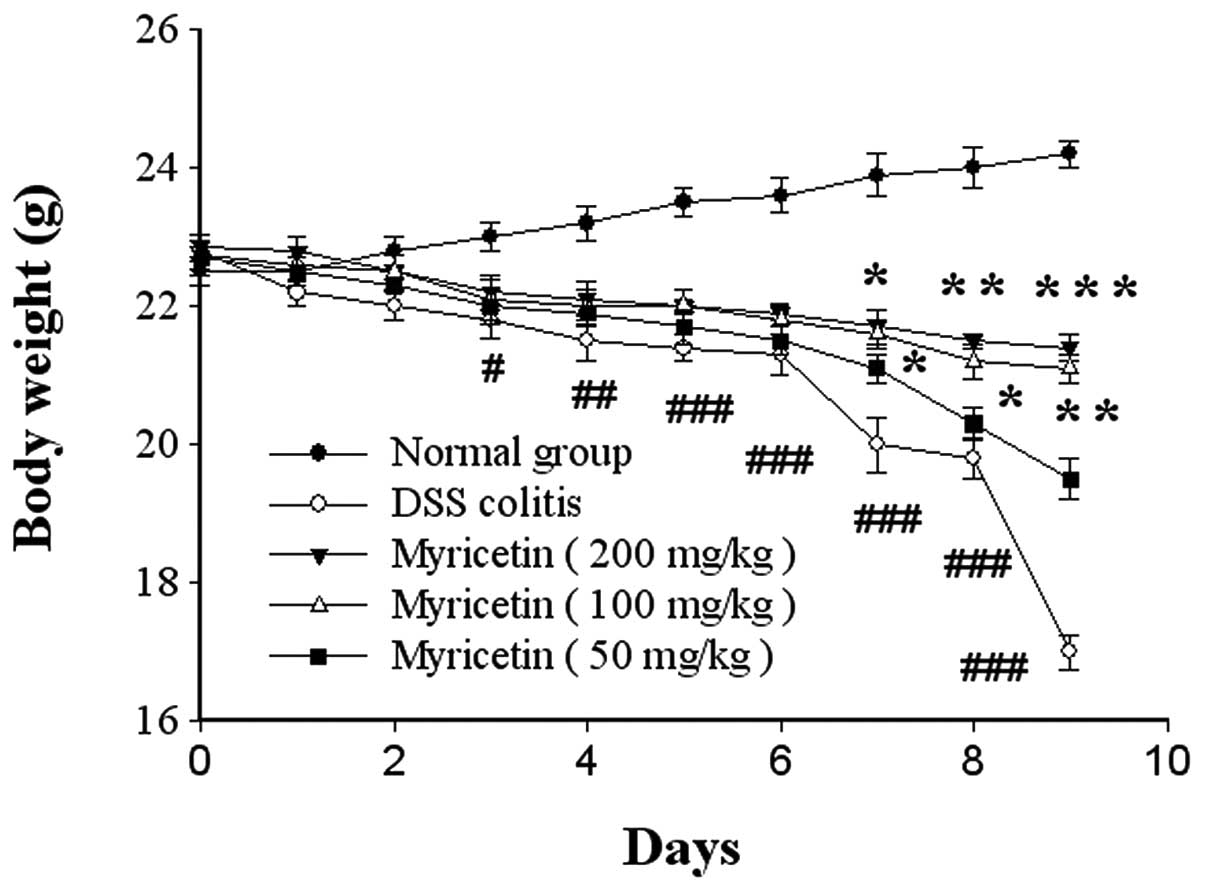
Effects on the general condition of mice
with colitis
We found that BALB/c mice subjected to the oral
administration of 5% DSS regularly developed pancolitis with severe
diarrhoea and rectal prolapse accompanied by extensive wasting
disease. In severe cases, gross blood adhering to the anus was
noted. Beginning on the 4th day subsequent to DSS administration,
the body weight began to decrease, and remained significantly
decreased compared with normal mice until the 10th day. However,
the administration of myricetin significantly reversed the loss of
body weight (Fig. 1).
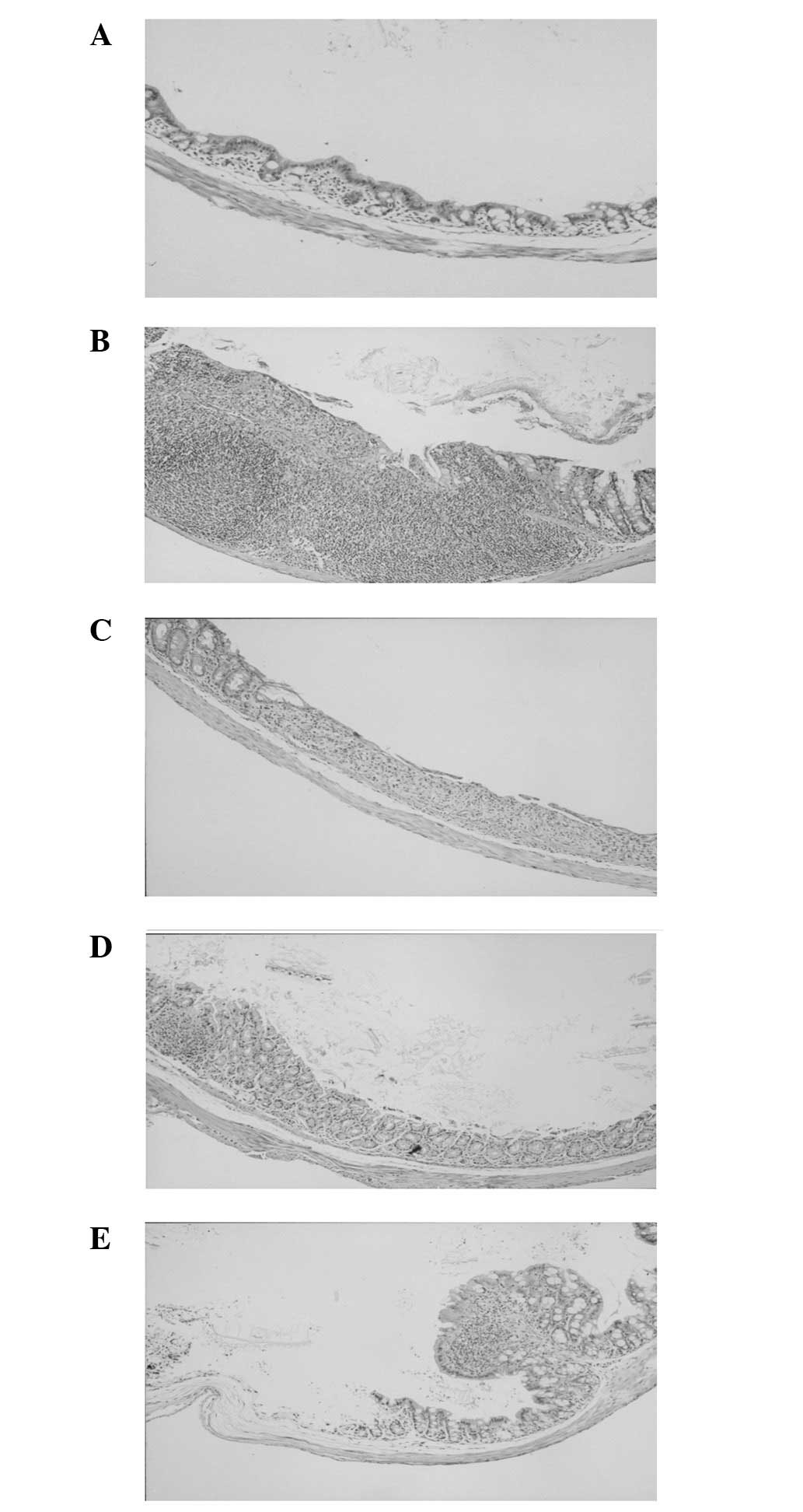
Effect on damage score and histological
evaluation
The severity of UC-like lesions was most marked in
the colon on the 10th day. Compared with normal mice, the distal
colon of DSS-treated mice showed intense inflammatory cellular
infiltration in all the layers, with polymorphonuclear leukocytes
and multiple erosions. Crypt abscesses and regenerating epithelium
were observed in the colonic mucosa. Myricetin treatment attenuated
morphological damage but showed mild cellular infiltration
(Table II, Fig. 2).
 | Table IIEffect of myricetin on damage score
and blood haemoglobin. |
Table II
Effect of myricetin on damage score
and blood haemoglobin.
| Group | Damage score | Blood haemoglobin
(g/dl) |
|---|
| Normal | 0 | 12.12±0.87 |
| DSS-induced
colitis | 11 (13-9)d | 9.04±0.61d |
| Myricetin
(mg/kg) |
| 200 | 6 (8-4)b | 10.01±0.26c |
| 100 | 8 (10-7)a | 9.88±0.32b |
| 50 | 10 (13-8) | 9.40±0.15b |
Compared with the DSS-induced colitis groups, the
damage score and the content of blood haemoglobin in the myricetin
groups were significantly lower. Mice treated with 200 and 100
mg/kg myricetin showed a significantly lower damage score and blood
haemoglobin content (P<0.01 and P<0.05, respectively),
although those treated with 50 mg/kg showed no significant
differences. The results showed a concentration-response
correlation (Table II).
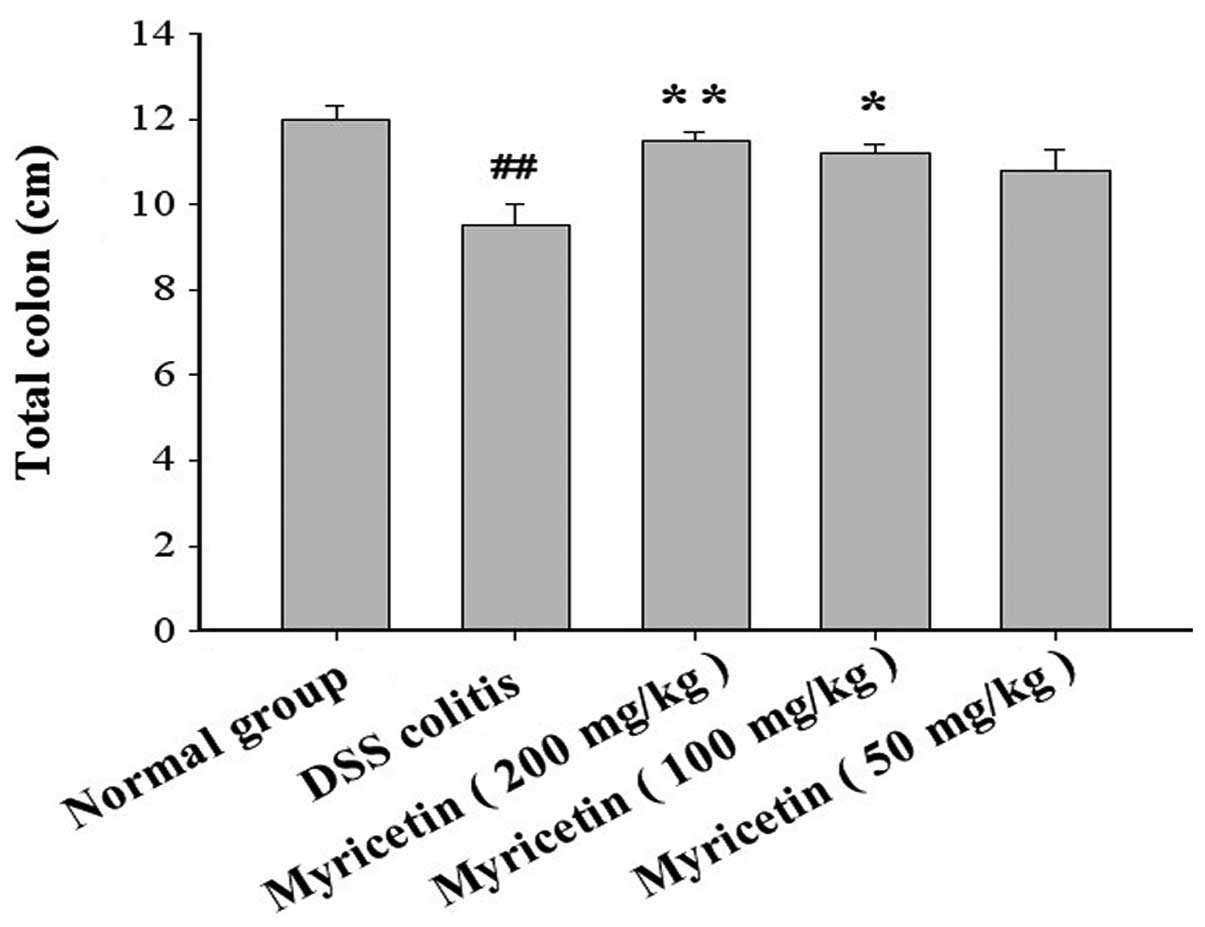
Effect on the length of the colon
The colon and caecum of the DSS-treated mice were
significantly shorter compared with those of the normal group. The
administration of myricetin significantly increased the length of
the shortened colon induced by DSS (Fig. 3).
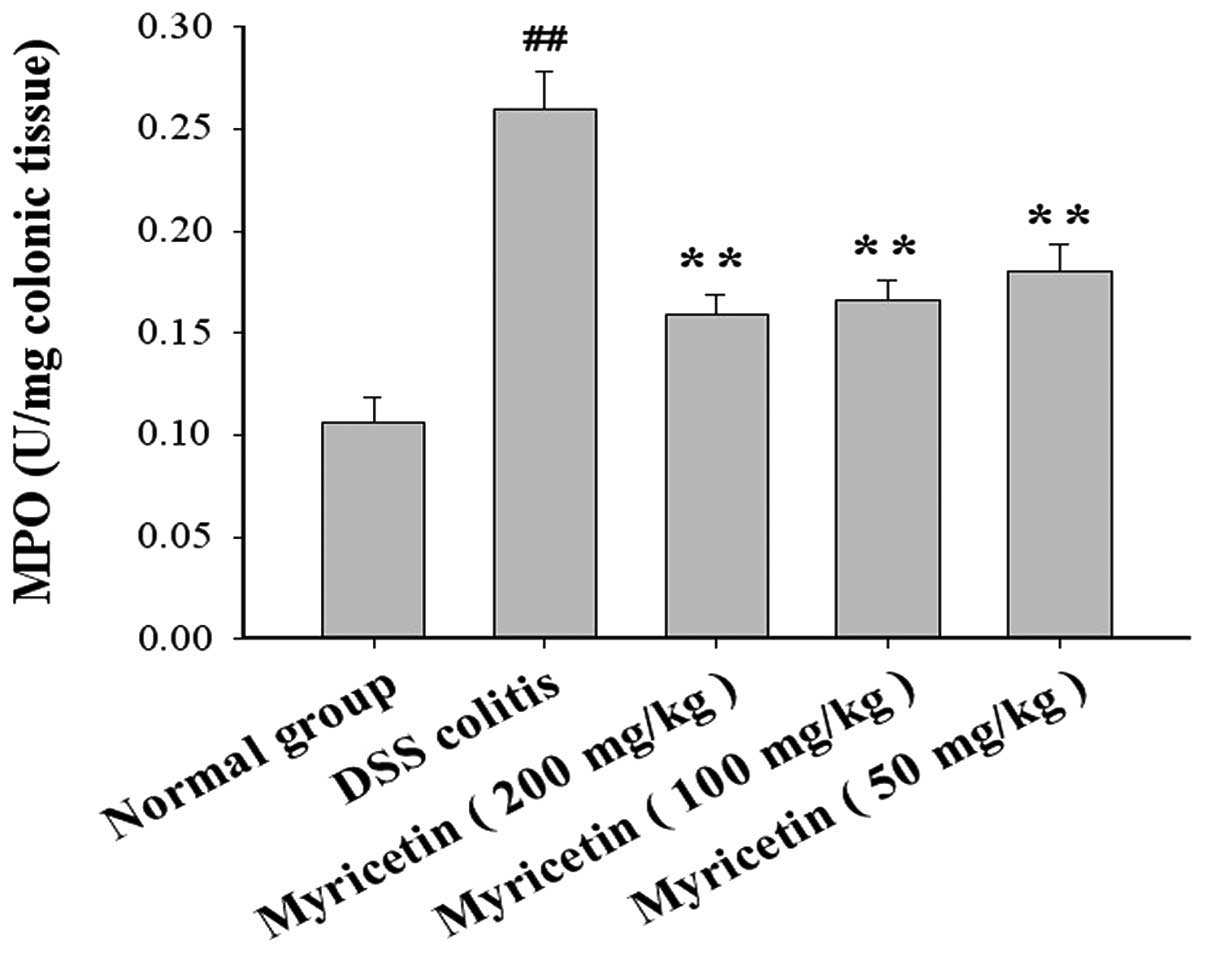
Effect on MPO, GSH-Px and SOD activity,
and MDA and NO content in colonic tissue
The MPO levels were 0.106±0.012 U/mg colonic tissue
in normal mice and 0.260±0.018 U/mg colonic tissue in mice with
DSS-induced colitis. Myricetin at 50, 100 and 200 mg/kg decreased
the MPO levels to 0.180±0.013, 0.166±0.01 and 0.159±0.018 U/mg
colonic tissue, respectively (Fig.
4).
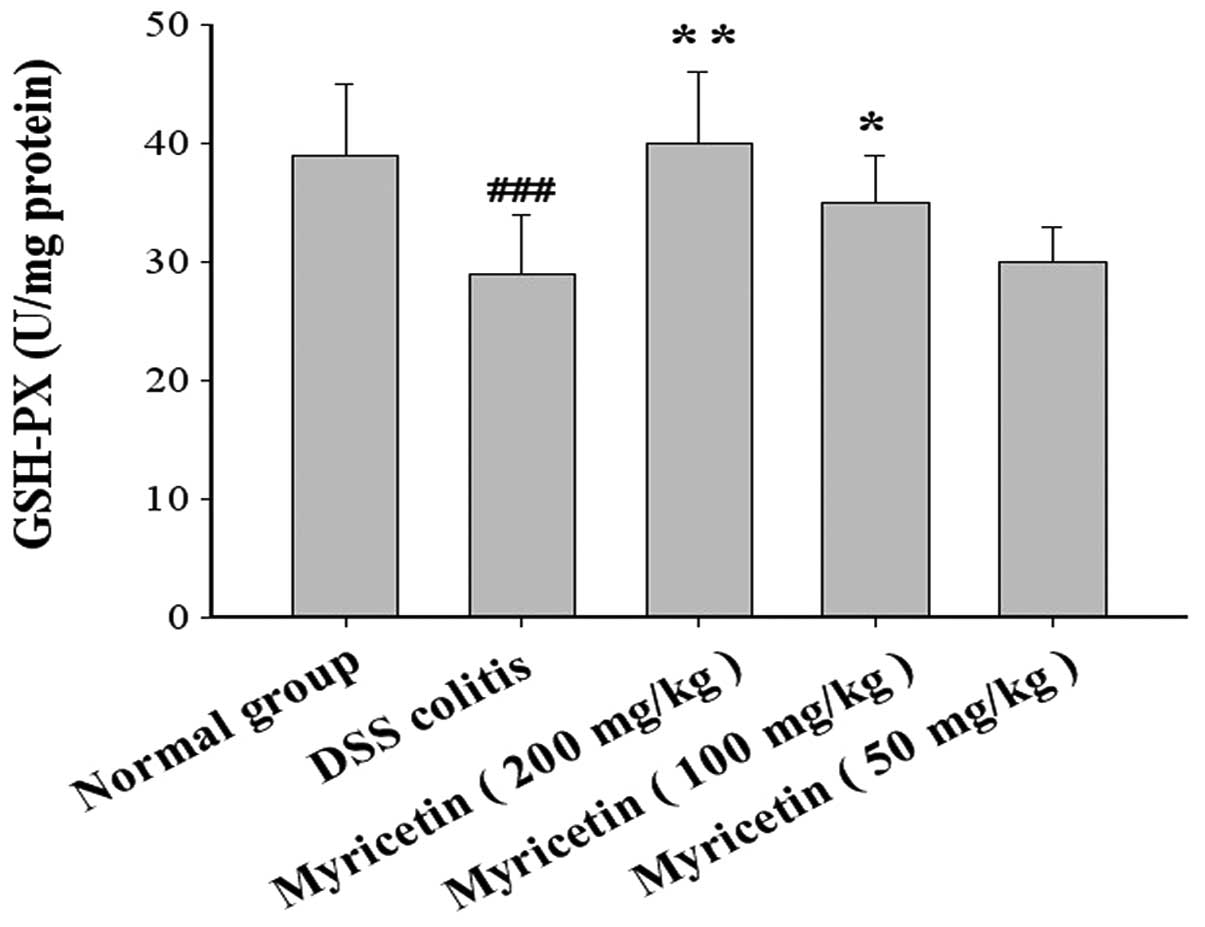
The GSH-Px activity was 39.0±6.0 U/mg colonic tissue
in normal mice and 29.0±5.0 U/mg colonic tissue in mice with
DSS-induced colitis. Myricetin at doses of 50, 100 and 200 mg/kg
increased the GSH-Px activity to 30.1±3.1, 35.0±4.2 and 40.1±6.3
U/mg colonic tissue, respectively (Fig. 5).
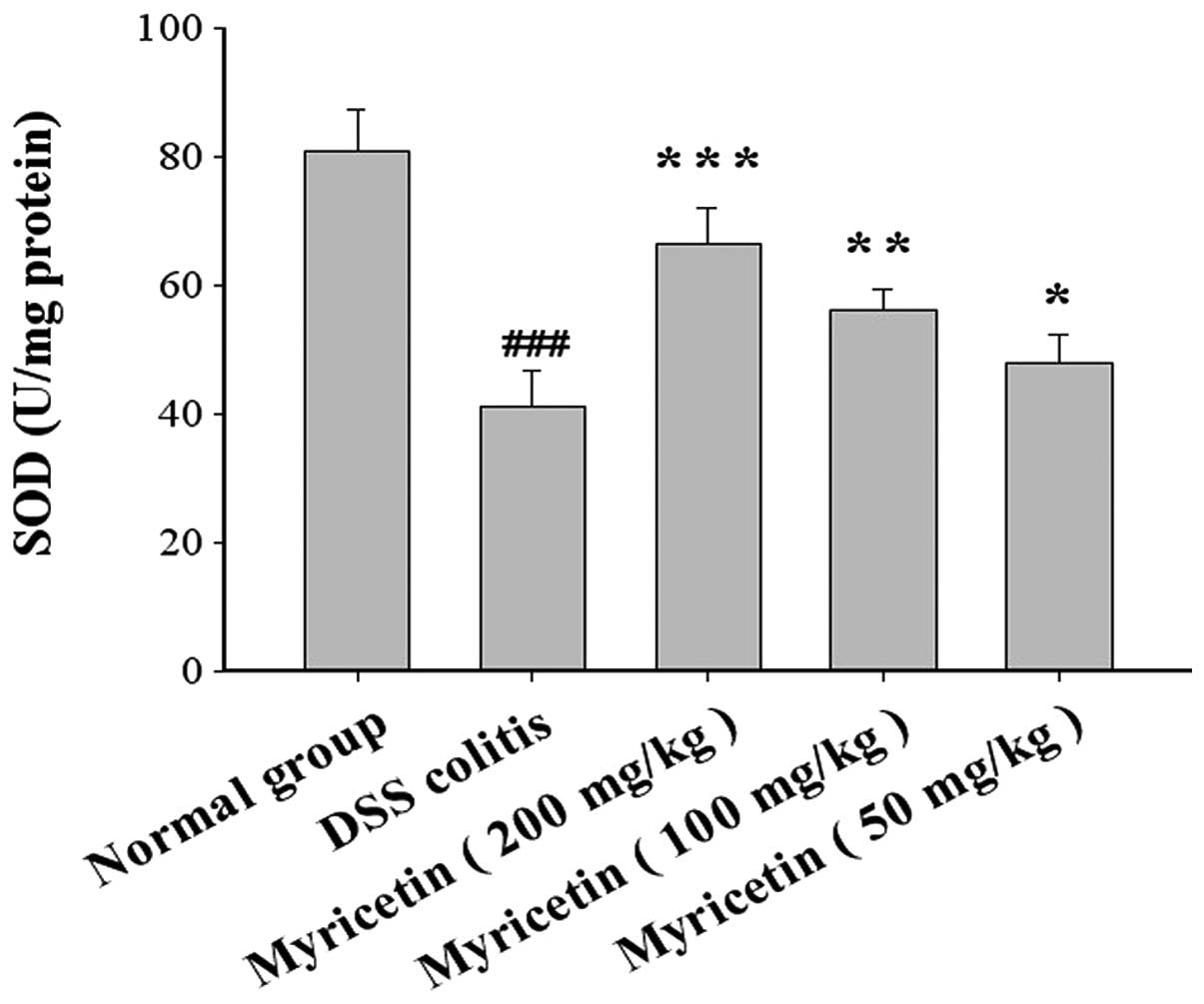
The SOD activity was 81.0±6.4 U/mg colonic tissue in
normal mice and 41.1±5.6 U/mg colonic tissue in mice with
DSS-induced colitis. Myricetin at 50, 100 and 200 mg/kg increased
the SOD activity to 48.1±4.2, 56.2±3.4 and 66.4±5.6 U/mg colonic
tissue, respectively (Fig. 6).
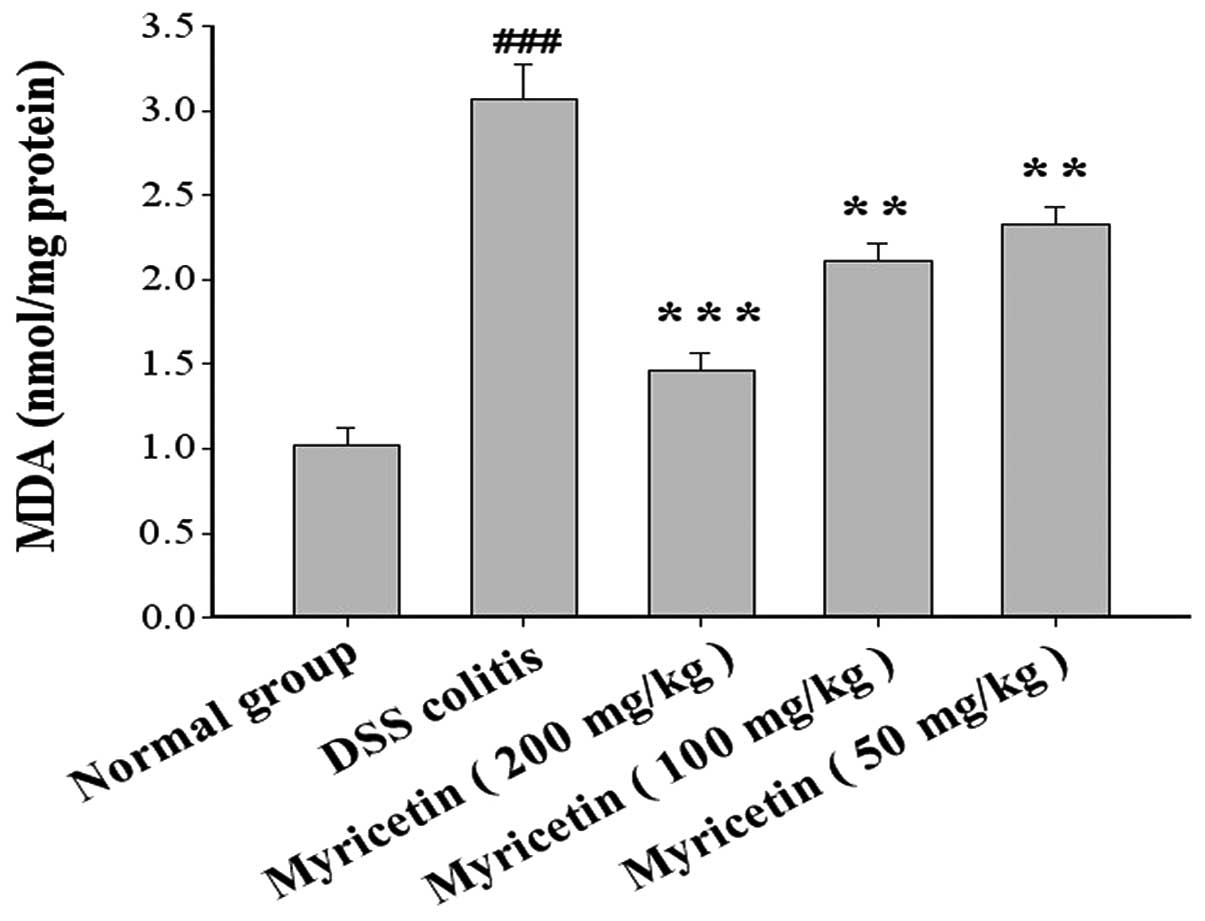
The MDA content was 1.02±0.10 nmol/mg protein in
normal mice and 3.07±0.20 nmol/mg protein in mice with DSS-induced
colitis. Myricetin at 50, 100 and 200 mg/kg decreased the MDA
content to 2.33±0.10, 2.11±0.11 and 1.46±0.11 nmol/mg protein,
respectively (Fig. 7).
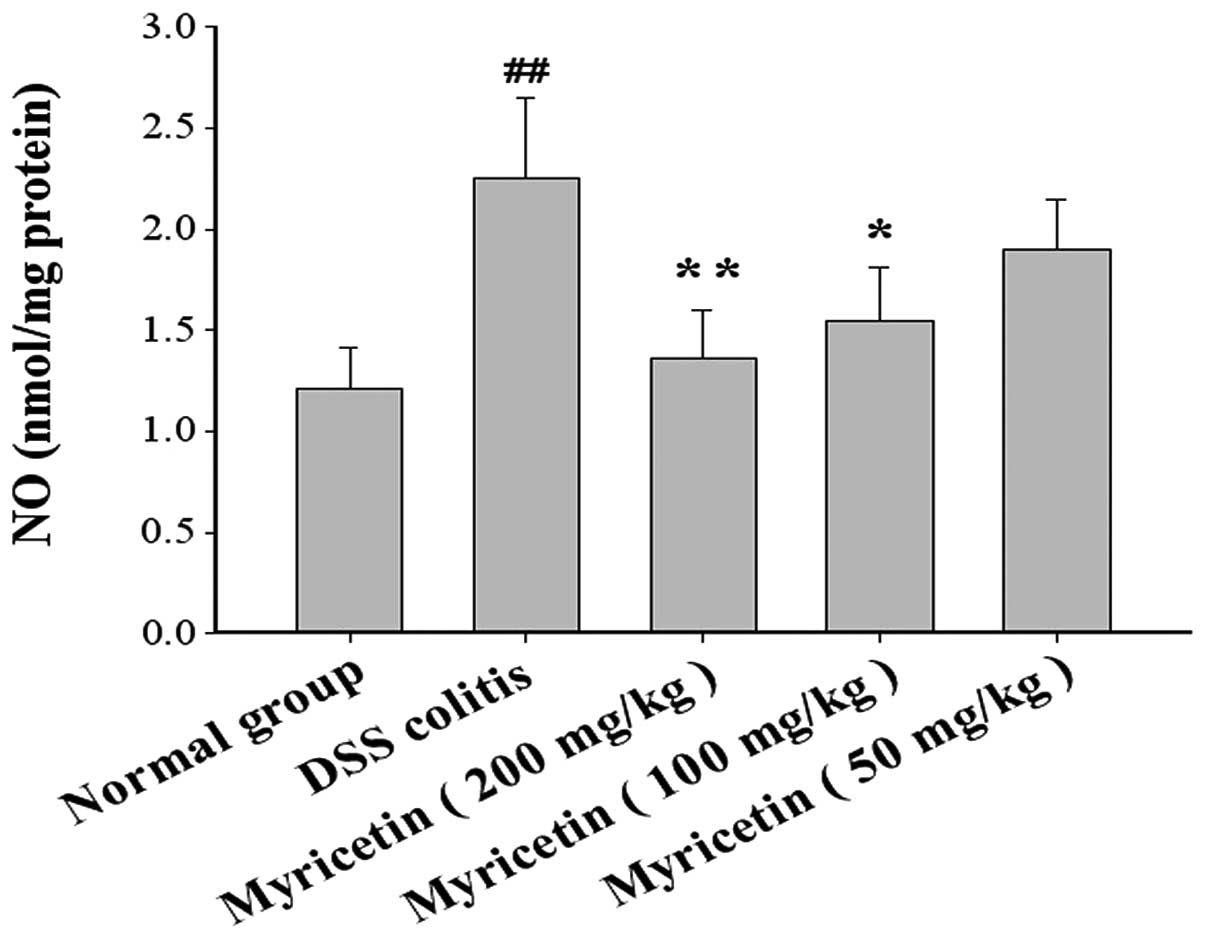
The NO content was 1.21±0.20 μmol/mg protein in
normal mice and 2.25±0.40 μmol/mg protein in mice with DSS-induced
colitis. Myricetin at 50, 100 and 200 mg/kg decreased the NO
content to 1.90±0.25, 1.55±0.26 and 1.36±0.24 μmol/mg protein,
respectively (Fig. 8).
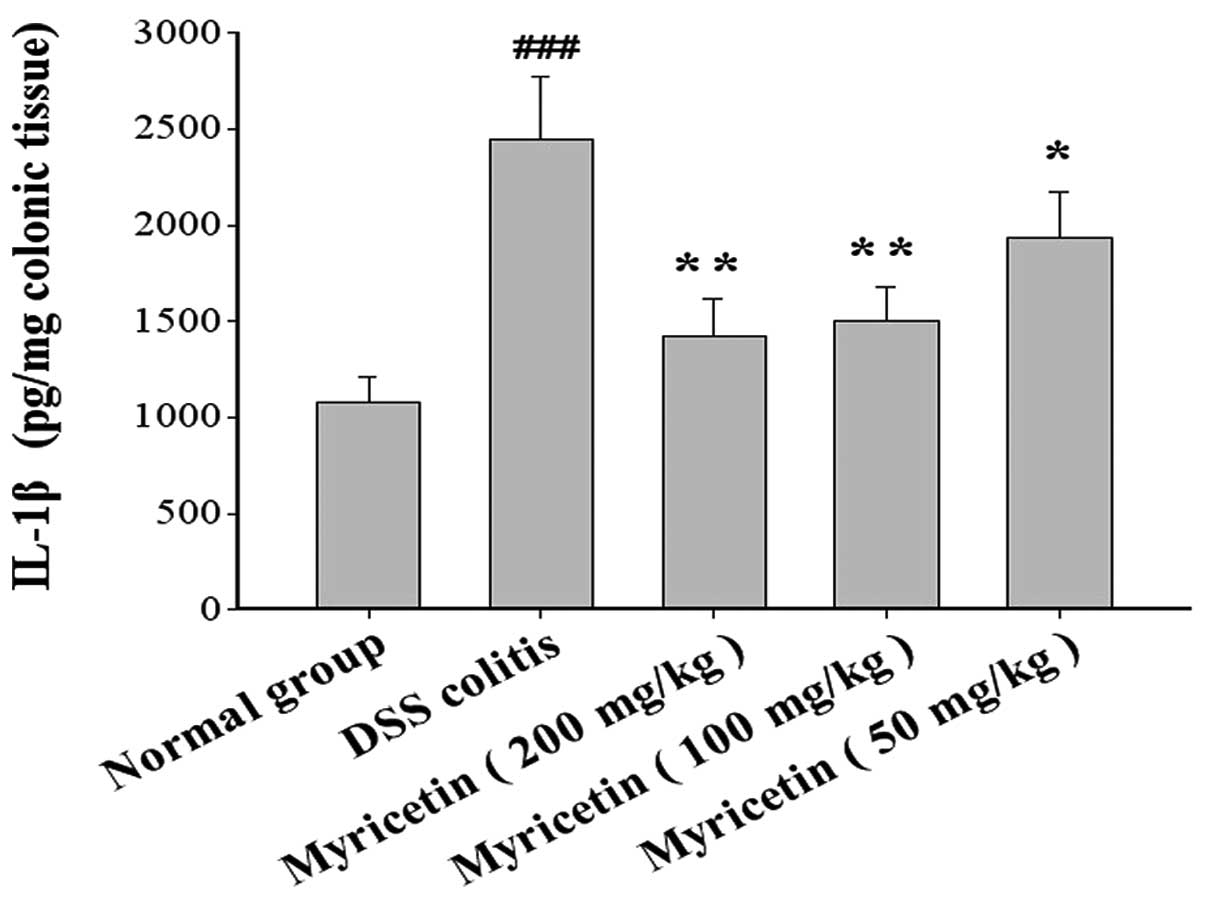
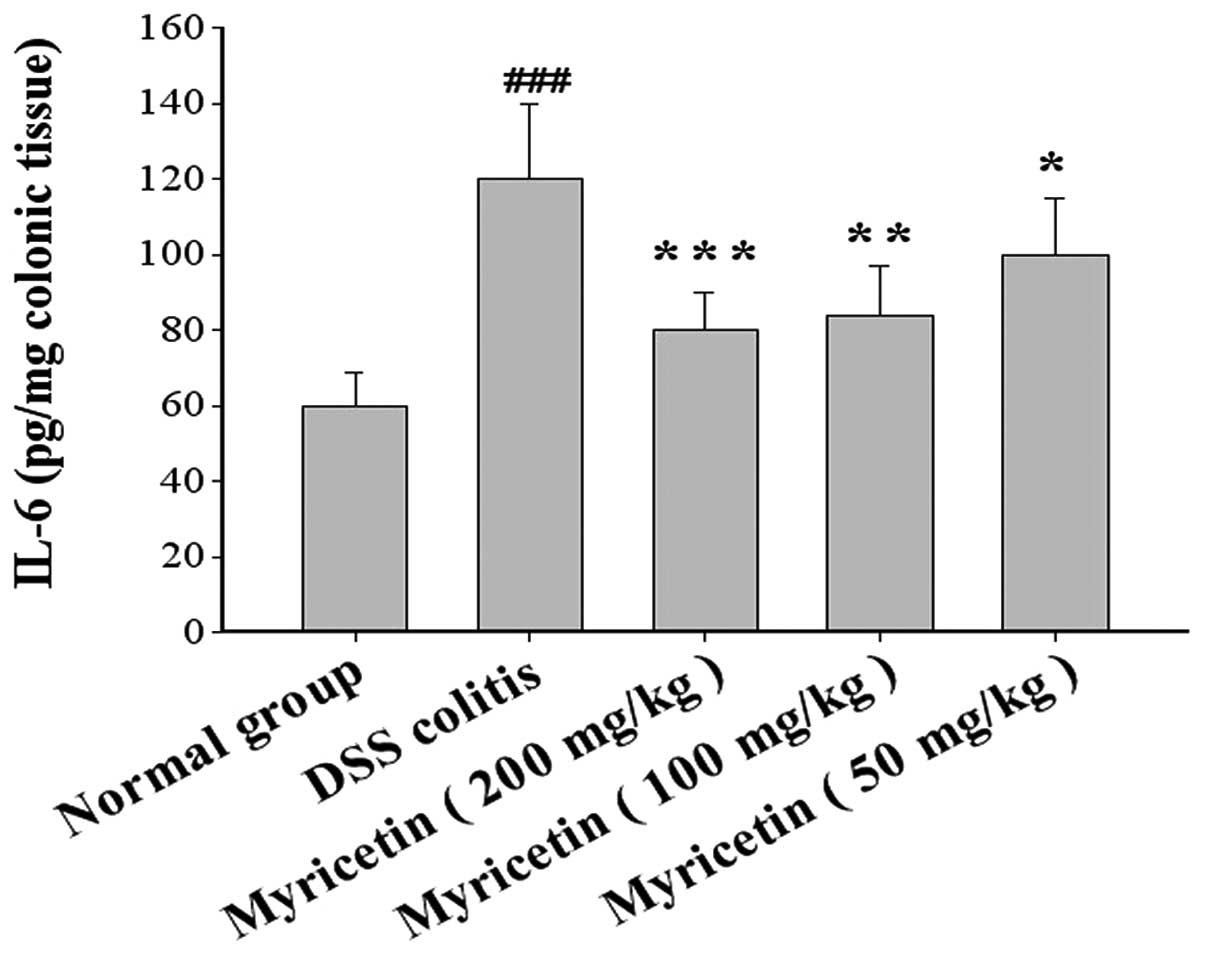
Effect on IL-1β and IL-6 in colonic
tissue
DSS-induced colitis was accompanied by a disturbance
in cytokine levels. The effects of myricetin on IL-1β and IL-6
production in colonic tissue were evaluated. The administration of
myricetin to the DSS-induced colitis group resulted in lower levels
of IL-1β and IL-6 (Figs. 9 and
10).
Discussion
In this study, the oral administration of myricetin
for 10 days markedly prevented the loss of body weight and reduced
the damage score in the colonic mucosa of DSS-induced colitis, and
histological analysis demonstrated that myricetin ameliorated the
pathological manifestations of DSS colitis, including inflammatory
cell infiltration, crypt abscesses and regenerating epithelium, in
the colonic mucosa. Mucosal lesions in UC are characterised by a
dense inflammatory cell infiltrate that primarily contains
neutrophils, macrophages and lymphocytes. During chronic
inflammation, whereby the sustained production of reactive oxygen
species (ROS) occurs, antioxidant defences may weaken, resulting in
oxidative stress. Increased ROS levels in the intestinal epithelial
cell membrane may lead to oxidative damage mediated by free radical
attacks and lipid peroxidation, which may be an early critical
event in the model of experimental IBD. Acute attacks of UC lead to
an inflamed colonic mucosa, which contributes to an excess
production of reactive oxygen metabolites by lipid peroxidation
(18). Inflammatory tissue injury
may further stimulate ROS production and reactive oxygen
metabolites, thus forming a positive feedback loop and rendering
the antioxidant system insufficient (19). DSS-induced colitis is a
well-established experimental model with several of the signs and
symptoms of human UC. ROS are highly reactive, and their levels are
significantly increased in the colonic mucosa (20).
Myricetin treatment was found to significantly lower
MPO activity, MDA and NO content and increase the activity of SOD
and GSH-Px. MPO and NO are known sources of free radicals and are
able to induce the reduction of ferritin (Fe3+) to free
Fe2+, contributing to oxidative damage (21,22).
Moreover, increased MDA activity leads to lipid peroxidation, which
causes the cross-linking of protein and nucleic acid molecules and
cell toxicity. MDA is an essential co-factor of GSH-Px and SOD and
plays a significant antioxidative role by binding to the active
site of GSH-Px. SOD protects cells against ROS-induced damage by
removing these molecules. GSH-Px detoxifies organic peroxides and
H2O2 and permits the regeneration of cellular
lipid molecules through reacylation in the cell membrane.
Furthermore, our data showed that myricetin was able
to decrease the production of IL-1β and IL-6. Inhibiting the action
of endogenous IL-1β or IL-6 attenuates acute and chronic
experimental colitis and their systemic complications (23–25).
Previous studies have shown that the anti-inflammatory mechanisms
of myricetin are most likely associated with the inhibition of
antioxidant activity (26).
Therefore, myricetin may exert its effects in DSS colitis through
its antioxidant effect.
In conclusion, myricetin may ameliorate inflammation
in mice treated with DSS. The mechanism is likely due to its
antioxidant effect. Myricetin may be used as a novel therapeutic
agent of colitis.
References
|
1
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar
|
|
2
|
Torres MI and Rios A: Current view of the
immunopathogenesis in inflammatory bowel disease and its
implications for therapy. World J Gastroenterol. 14:1972–1980.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carroll IM, Andrus JM, Bruno-Bárcena JM,
Klaenhammer TR, Hassan HM and Threadgill DS: Anti-inflammatory
properties of Lactobacillus gasseri expressing manganese
superoxide dismutase using the interleukin 10-deficient mouse model
of colitis. Am J Physiol Gastrointest Liver Physiol. 293:G729–G738.
2007.PubMed/NCBI
|
|
4
|
Dutra RC, Claudino RF, Bento AF, Marcon R,
Schmidt EC, Bouzon ZL, Pianowski LF and Calixto JB: Preventive and
therapeutic euphol treatment attenuates experimental colitis in
mice. PLoS One. 6:e271222011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990.PubMed/NCBI
|
|
6
|
Tokoi S, Ohkusa T, Okayasu I and Nakamura
K: Population changes in immunoglobulin-containing mononuclear
cells in dextran sulfate sodium-induced coltitis. J Gastroenterol.
31:182–188. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rezaie A, Parker RD and Abdollahi M:
Oxidative stress and pathogenesis of inflammatory bowel disease: an
epiphenomenon or the cause? Dig Dis Sci. 52:2015–2021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Damiani CR, Benetton CA, Stoffel C,
Bardini KC, Cardoso VH, Di Giunta G, Pinho RA, Dal-Pizzol F and
Streck EL: Oxidative stress and metabolism in animal model of
colitis induced by dextran sulfate sodium. J Gastroenterol Hepatol.
22:1846–1851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castillo-Muñoz N, Gómez-Alonso S,
García-Romero E and Hermosín-Gutiérrez I: Flavonol profiles of
Vitis vinifera red grapes and their single-cultivar wines. J
Agric Food Chem. 55:992–1002. 2007.
|
|
10
|
Lee YS and Choi EM: Myricetin inhibits
IL-1beta-induced inflammatory mediators in SW982 human synovial
sarcoma cells. Int Immunopharmacol. 10:812–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen W, Li Y, Li J, Han Q, Ye L and Li A:
Myricetin affords protection against peroxynitrite-mediated DNA
damage and hydroxyl radical formation. Food Chem Toxicol.
49:2439–2444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nirmala P and Ramanathan M: Effect of
myricetin on 1,2 dimethylhydrazine induced rat colon
carcinogenesis. J Exp Ther Oncol. 9:101–108. 2011.PubMed/NCBI
|
|
13
|
Kang NJ, Jung SK, Lee KW and Lee HJ:
Myricetin is a potent chemopreventive phytochemical in skin
carcinogenesis. Ann NY Acad Sci. 1229:124–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990.PubMed/NCBI
|
|
15
|
Suzuki K, Ota H, Sasagawa S, Sakatani T
and Fujikura T: Assay method for myeloperoxidase in human
polymorphonuclear leukocytes. Anal Biochem. 132:345–352. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miranda KM, Espey MG and Wink DA: A rapid,
simple spectrophotometric method for simultaneous detection of
nitrate and nitrite. Nitric Oxide. 5:62–71. 2001. View Article : Google Scholar
|
|
18
|
Keshavarzian A, Morgan G, Sedghi S, Gordon
JH and Doria M: Role of reactive oxygen metabolites in experimental
colitis. Gut. 31:786–790. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seril DN, Liao J, Yang GY and Yang CS:
Oxidative stress and ulcerative colitis-associated carcinogenesis:
studies in humans and animal models. Carcinogenesis. 24:353–362.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishihara T, Tanaka K, Tasaka Y, Namba T,
Suzuki J, Ishihara T, Okamoto S, Hibi T, Takenaga M, Igarashi R,
Sato K, Mizushima Y and Mizushima T: Therapeutic effect of
lecithinized superoxide dismutase against colitis. J Pharmacol Exp
Ther. 328:152–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sutton A, Imbert A, Igoudjil A, Descatoire
V, Cazanave S, Pessayre D and Degoul F: The manganese superoxide
dismutase Ala16Val dimorphism modulates both mitochondrial import
and mRNA stability. Pharmacogenet Genomics. 15:311–319. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Creveling CR: The role of
catechol-O-methyltransferase in the inactivation of
catecholestrogen. Cell Mol Neurobiol. 23:289–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rogler G and Andus T: Cytokines in
inflammatory bowel disease. World J Surg. 22:382–389. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arai Y, Takanashi H, Kitagawa H and
Okayasu I: Involvement of interleukin-1 in the development of
ulcerative colitis induced by dextran sulfate sodium in mice.
Cytokine. 10:890–896. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Naito Y, Takagi T, Uchiyama K, Kuroda M,
Kokura S, Ichikawa H, Yanagisawa R, Inoue K, Takano H, Satoh M,
Yoshida N, Okanoue T and Yoshikawa T: Reduced intestinal
inflammation induced by dextran sodium sulfate in
interleukin-6-deficient mice. Int J Mol Med. 14:191–196.
2004.PubMed/NCBI
|
|
26
|
Wang SJ, Tong Y, Lu S, Yang R, Liao X, Xu
YF and Li X: Anti-inflammatory activity of myricetin isolated from
Myrica rubra Sieb. et Zucc leaves. Planta Med. 76:1492–1496.
2010. View Article : Google Scholar : PubMed/NCBI
|