Introduction
Cardiovascular disease, a leading cause of mortality
in developed countries, is a chronic disease that remains
asymptomatic for decades. The incidence rate of this disease is
increasing worldwide. Hypercholesterolemia is a major risk factor
for the development of cardiovascular disease and modern
lifestyles, consisting of high-cholesterol diets and less physical
activity, contribute to hypercholesterolemia, which increases the
prevalence of cardiovascular disease (1). Epidemiological and interventional
studies have reported that high density lipoprotein (HDL) may be
protective by reversing cholesterol transport, inhibiting the
oxidation of low density lipoprotein (LDL) and neutralizing the
atherogenic effects of oxidized LDL (2). HDL has been hypothesized to protect
against atherosclerosis through its involvement in a reverse
cholesterol transport pathway (3,4). The
scavenger receptor B type I (SR-BI), a functional cell surface HDL
receptor, binds HDL and mediates efficient lipid uptake, and may be
important for HDL metabolism (5).
Progression in gene therapy has led to the
development of a novel treatment strategy for cardiovascular
diseases. Successful gene therapy depends on the design of
efficient, safe and stable gene delivery systems. Viral and
non-viral vectors have been used for DNA delivery with varying
levels of success, but have always been accompanied by
disadvantages, including insufficient expression levels or safety
concerns (6,7). In previous years, the use of
microbubbles and ultrasound (US) has been proposed for gene
delivery. Microbubble-enhanced US alters cell membrane permeability
for a short time due to sonoporation, which allows extracellular
macromolecules, including plasmid DNA, to instantaneously enter
cells without causing cytotoxicity (8–12).
This technique has been applied to the site-specific intracellular
delivery of macromolecules in vitro and in
vivo(13,14). Cationic lipids are associated with
specific advantages as gene delivery carriers (15,16).
Therefore, we hypothesized that cationic liposomal microbubbles
(CLMs) may represent novel gene delivery agents.
Based on cationic lipid technology, microbubbles
were developed using the sonication-lyophilization method. The
physiochemical properties of CLMs were analyzed to determine their
suitability as a contrast agent in medical imaging with US. In
addition, the efficiency of gene transfer and the hypolipidemic
effect of the SR-BI gene were evaluated using US in rats.
Materials and methods
Plasmid purification and microbubble
preparation
Full-length cDNAs of the rabbit SR-BI gene were
subcloned into the pcDNA3.1 vector (Invitrogen Life Technologies,
Carlsbad, CA, USA) as previously described (17). Plasmid DNA amplification was
performed using the Qiagen plasmid Giga kit (Qiagen, Germany).
Cationic liposomes composed of
1,2-dipalmitoyl-sn-glycero-3-phosphocholine (NOF Corporation,
Tokyo, Japan),
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene
glycol)-2000] (Avanti Polar Lipids, Inc., Alabaster, AL, USA) and
1,2-dioleoyl-3-trimethyl-ammonium-propane [94:6:100 (m/m)] were
constructed using the sonication-lyophilization method. Mixed
components were dissolved in an appropriate volume of tertiary
butyl alcohol. After the components were dissolved, sonication was
performed at 30°C (frequency, 40 kHz; 5 min). The microparticle
suspension solution was stored at 0°C for 30 min and −20°C for 1 h.
Next, the coagulated solution was lyophilized at 5×10−4
Pa pressure for 20 h (primary drying at −48°C for 15 h and gradual
increases in temperature to 10°C within 5 h). Lyophilized powder
was placed in 10-ml penicillin vials for subsequent analyses. Vials
were removed from the freeze dryer, flushed with perfluoropropane
gas and the vial stoppers were fully closed and sealed with crimp
aluminum caps. Following this, 5 ml PBS solution was added to the
lyophilized powder, followed by gentle shaking to form an
emulsion-like solution. The size distribution and Zeta potential
measurements of the microbubbles were measured with a Zetasizer
Nano S (Malvern Instruments, Malvern, UK) after the samples were
diluted with water. Each sample was measured four times and the
results were expressed as the average ± SD of three samples with a
corresponding polydispersity index (PDI) value that was indicated
for the size distribution measurements.
SR-BI DNA was added to the pre-formed microbubble
solutions and gently agitated. SR-BI DNA was then combined with
CLMs by electrostatic charge coupling.
Experimental animals
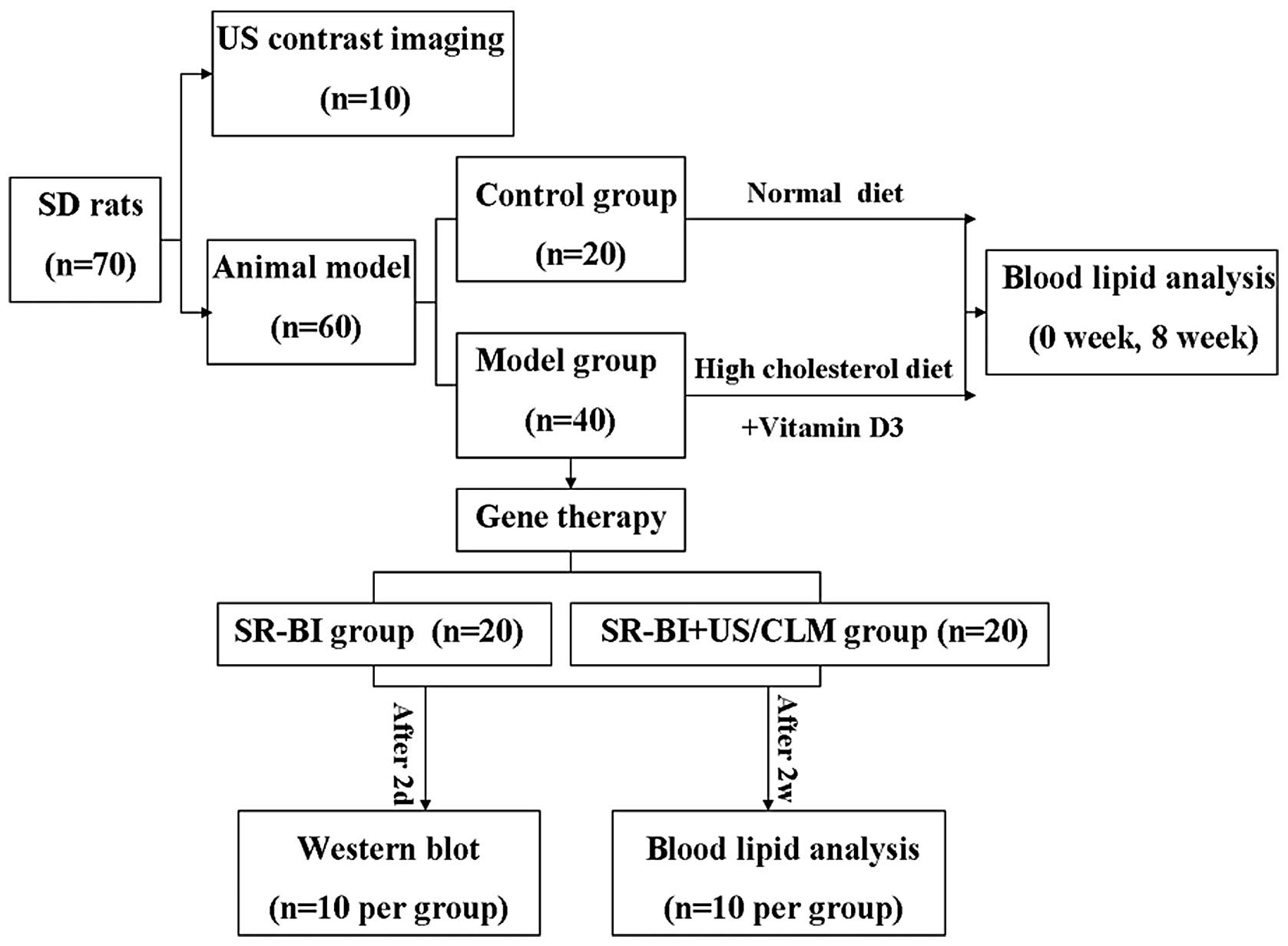
Seventy male Sprague-Dawley rats (weight, 250–300 g)
were used in this study. Rats were supplied by the Experimental
Animal Center of Shanghai Jiaotong University Affiliated Shanghai
Sixth People’s Hospital (Shanghai, China) and the experimental
protocols were approved by The Animal Care and Use Committee of the
Shanghai Jiaotong University Affiliated Shanghai Sixth People’s
Hospital.
Hypercholesterolemic animal model
The experimental hypercholesterolemic model was
established in rats by administration of excessive vitamin D and
cholesterol. Rats were randomly divided into the control (n=20) and
model (n=40) groups. In the model group, rats were
intraperitoneally injected with 600,000 IU/kg vitamin D3, followed
by an additional 300,000-IU/kg dosage, which was repeated every 30
days. During model development, rats were fed a high-cholesterol
diet daily. Blood lipid analysis was performed at week 0 (baseline)
and 8 weeks following the high cholesterol diet (Fig. 1). Blood samples were collected in
chilled heparinized centrifuge tubes by cardiac puncture with
heparinized syringes and centrifuged at 4°C for 15 min. Collected
plasma samples were stored at −80°C until further analysis. The
total plasma cholesterol (TC), triglyceride (TG), LDL and HDL
levels were measured using reagents from Nanjing KeyGen Biotech
Co., Ltd. (Nanjing, China).
US contrast imaging following intravenous
injection of microbubbles
Ten rats were anesthetized with 4% pentobarbital
sodium (1 ml/kg) by intraperitoneal injection, placed on their
backs and restrained on an experimental table. Prior to the
experiments, the femoral vein was catheterized and the hair over
the neck was removed with depilatory cream.
An ultrasonic diagnostic instrument (Mylab. 90;
Esaote, Genoa, Italy) with 13-MHz linear-array transducers was
used. As the carotid artery was clearly depicted, all conditions
discussed were kept consistent during contrast imaging.
CLMs were injected via the femoral vein at a dose of
0.5 ml/kg, followed by normal saline to wash the tube. The bolus
injection of microbubbles was flushed into the circulation of the
animal and was favorable for improving the signal-to-noise imaging
ratio. Real-time imaging of the carotid artery was observed for 5
min following injection. Images were saved during enhanced
ultrasonography.
In vivo gene delivery and biochemical
assay
Forty rats were anesthetized and handled as
described. CLMs were injected slowly via the femoral vein within
the specified time.
Gene delivery was then performed according to the
assigned treatment group: SR-BI (local injection of 100 μg SR-BI);
SR-BI + US/CLM (intravenous push of 100 μg SR-BI); and CLM (1 ml)
by US (n=20/group). US parameters were as follows: frequency, 1
MHz; duty, 50%; intensity, 2.0 W/cm2; and time, 15 min.
Rats (n=10/group) were sacrificed 2 days post-transfection. Tissue
was obtained for western blot analysis from the rat carotid
arteries that were imaged by US. Western blot analysis was
performed as described previously (Fig. 1) (17).
In the remaining rats (10/group), serum TC, TG, LDL
and HDL levels were measured 2 weeks post-transfection (Fig. 1).
Statistical analysis
All data are presented as the mean ± SD. Paired
t-tests were used to compare differences in the biochemical
variables between the control and model groups at baseline.
Multiple comparisons among the groups were performed with one-way
ANOVA analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Particle size and zeta potential
The lyophilized cake of CLMs retained a free powdery
appearance (Fig. 2). Rehydrated
formulations were clear dispersions with no visible particles. The
particle size was 1.17±0.06 μm and the PDI was 0.364±0.015.
Fig. 3 reveals a representative
image of CLMs captured by optical microscopy. The pH was 7.34 and
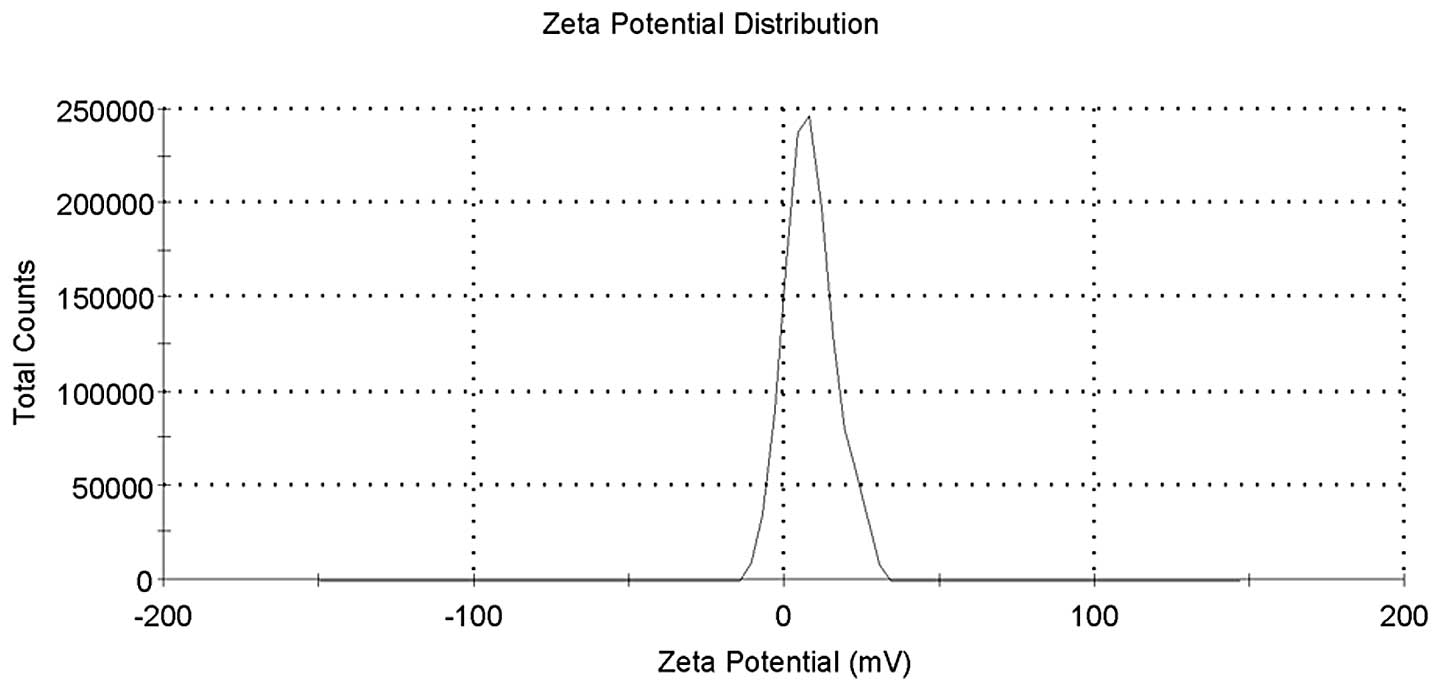
the Zeta potential value was 8.10±8.08 mV (Fig. 4).
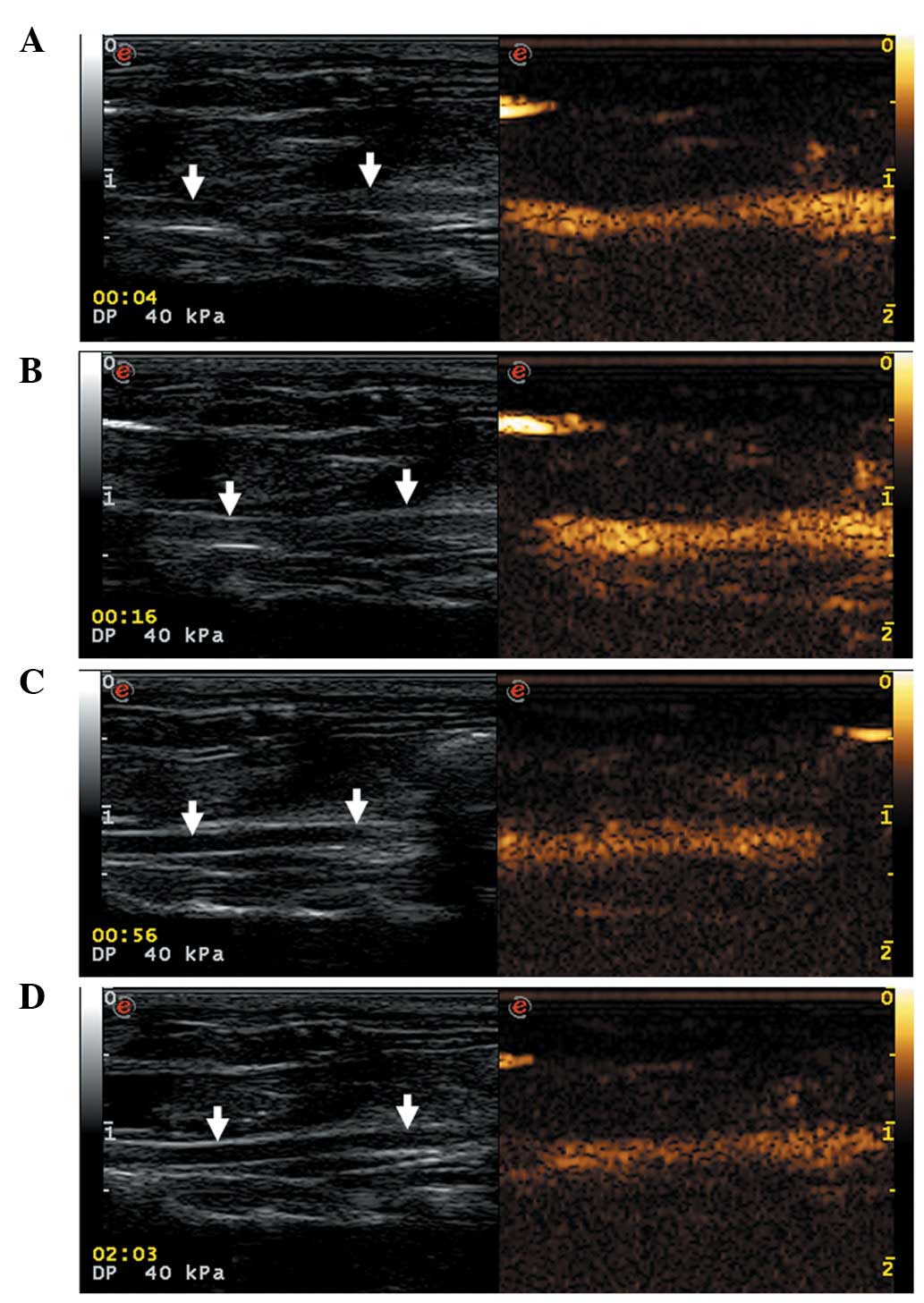
US contrast imaging of carotid
arteries
CLMs were found to markedly enhance carotid artery
imaging in all the rats. The echo intensities of normal carotid
arteries increased rapidly, peaking 3 sec after injection of the
CLMs. In addition, enhanced normal carotid artery echo intensity
was maintained for a longer duration of time (>3 min; Fig. 5).
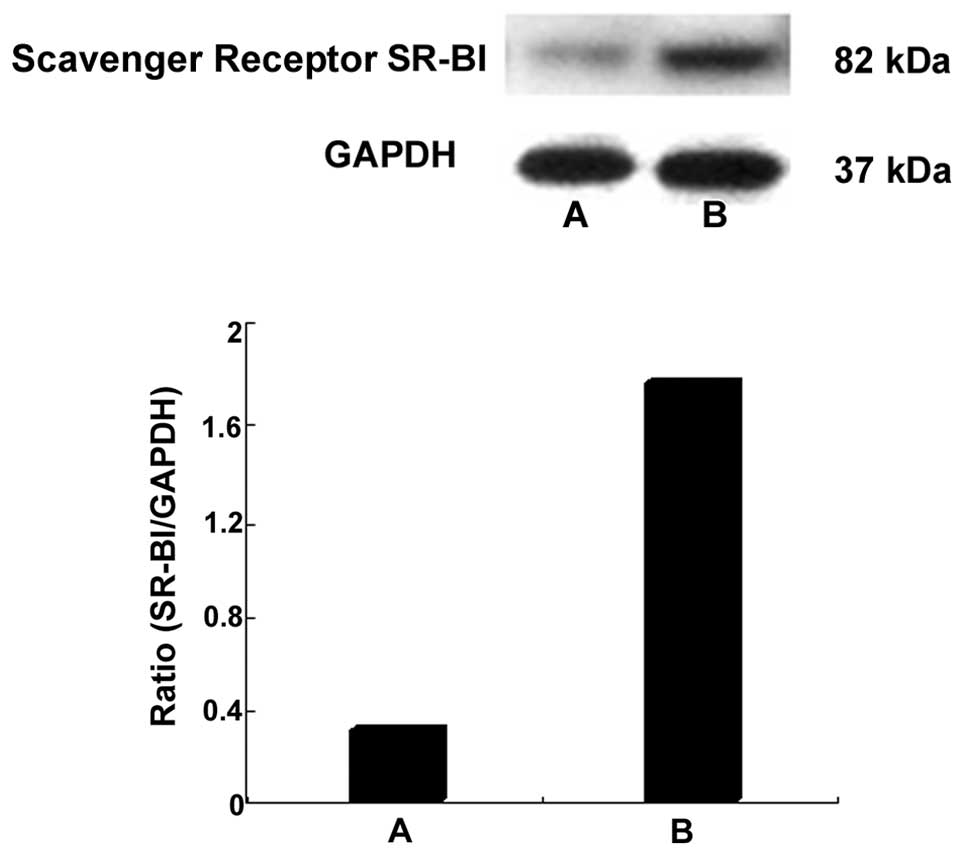
In vivo gene delivery
As demonstrated in Fig.
6, a marked increase in SR-BI protein was observed 2 days after
transfection in the carotid arteries in the SR-BI + US/CLM group,
whereas no significant increase in SR-BI protein was detected in
the carotid arteries from the SR-BI group (P>0.05, compared with
the model group).
Biochemical assay
No significant difference in the serum lipid levels
between the control and model groups was found at baseline.
Following 8 weeks of a high cholesterol diet, serum TC, TG, LDL and
HDL levels were 2.91±0.05, 1.94±0.10, 0.72±0.09 and 0.48±0.02
mmol/l, respectively (Table I).
After 2 weeks of SR-BI gene transfection (combined CLMs and US),
the plasma lipid levels of the hypolipidemic rats were
significantly reduced compared with the untreated rats. However, no
significant difference in LDL levels was identified between the
model and SR-BI + US/CLM groups. In addition, no significant change
in TC levels was observed in the SR-BI group compared with the
SR-BI + US/CLM group (Table
II).
 | Table IPlasma lipid levels of hypolipidemic
rats induced by vitamin D3 administration and a high cholesterol
diet. |
Table I
Plasma lipid levels of hypolipidemic
rats induced by vitamin D3 administration and a high cholesterol
diet.
| TC (mmol/l) | TG (mmol/l) | LDL (mmol/l) | HDL (mmol/l) |
|---|
|
|
|
|
|
|---|
| Group | 0 weeks | 8 weeks | 0 weeks | 8 weeks | 0 weeks | 8 weeks | 0 weeks | 8 weeks |
|---|
| Control (n=20) | 0.96±0.05 | 1.90±0.02 | 0.31±0.02 | 0.33±0.05 | 0.28±0.05 | 0.34±0.01 | 1.12±0.03 | 1.09±0.02 |
| Model (n=40) | 1.12±0.05 | 2.91±0.05a | 0.30±0.01 | 1.94±0.10a | 0.29±0.07 | 0.72±0.09a | 1.10±0.05 | 0.48±0.02a |
 | Table IIPlasma lipid levels of rats in the
model, SR-BI and SR-BI + US/CLM groups. |
Table II
Plasma lipid levels of rats in the
model, SR-BI and SR-BI + US/CLM groups.
| Group | TC (mmol/l) | TG (mmol/l) | LDL (mmol/l) | HDL (mmol/l) |
|---|
| Model (n=40) | 2.91±0.05 | 1.94±0.10 | 0.72±0.09 | 0.48±0.02b |
| SR-BI (n=10) | 2.53±0.03 | 1.78±0.21 | 0.63±0.05 | 0.75±0.10 |
| SR-BI + US/CLM
(n=10) | 2.36±0.11a | 0.61±0.09a,b | 0.46±0.04b | 0.90±0.07a,b |
Discussion
A number of previous studies have indicated that
enhanced transfection with US and microbubble vehicles is caused by
the extravascular deposition of DNA, which results from the
disruption and dispersion of the microbubble shell (8–12).
In the present study, CLMs were developed using the thin-film
dispersion method as a chemical carrier for SR-BI DNA delivery.
When combined, US and CLMs were found to function synergistically
to increase SR-BI DNA transfection. Although a significant
improvement in gene expression activity and a longer metabolic
half-life were achieved, the long-term preservation of the
pcDNA/CLM solution remained a concern.
Due to several physical and chemical factors,
freshly prepared formulations demonstrated a loss of transfection
activity after 1 week of storage at room temperature.
Lyophilization has been employed widely to produce highly stable
pharmaceutical products (18,19).
More recently, lyophilization has also been investigated as a
practical technique to produce non-viral vectors with long-term
stability (20–22). Lyophilization significantly
improved the physical stability of CLMs compared with liquid
formulations. In our study, CLMs were developed using the
sonication-lyophilization method. The formulations are suitable for
long-term preservation at room temperature and microbubbles are
able to be used on a large scale.
The consumption of a high-cholesterol diet
contributes to cardiovascular disease, which is caused by
atherosclerosis, hyperlipidemia and abnormal lipid metabolism.
Vitamin D has been widely used to establish animal models of
atherosclerosis or calcific vasculopathy. There is a marked
correlation between vitamin D and atherosclerotic calcification or
aortic medial calcification, which are likely to involve multiple
mechanisms within the bone-vascular-renal endocrine axis (23). In animal models, vitamin D
treatment is markedly associated with increased arterial
calcification (24,25). Based on these studies, a rat model
of hyperlipidemia was established by administering rats with
vitamin D3 and a high-cholesterol diet. However, blood lipid
analysis results in the model group were inconsistent with a
previous study (26). Differences
in the components of the high-fat diet and the use of
intraperitoneal injection to administer vitamin D3 in the present
study may account for this discrepancy.
The marked correlation between HDL levels and the
incidence of cardiovascular disease is well established (27). SR-BI was the first HDL receptor to
be identified (28), mediating the
high affinity binding of HDL by facilitating the bidirectional flux
of cholesterol across the plasma membrane. SR-BI has been reported
to be significantly expressed in cells involved in reverse
cholesterol transport, including atherosclerotic plaque macrophages
and hepatocytes (29–31). To examine the feasibility of gene
therapy, serum TC, TG, LDL and HDL levels were analyzed in rats 2
weeks post-transfection with SR-BI. Treatment with SR-BI DNA by
combining CLMs and US was identified to significantly decrease
plasma lipid levels; however, no significant difference in the LDL
levels between the model and SR-BI + US/CLM groups was observed
(Table II). The lack of
significance between LDL levels in the two groups is most likely
due to the small sample size. In addition, the main limitation of
the present study was that histological identification was not
used. Further studies are required to overcome these
limitations.
Results of the current study highlight a novel
therapeutic strategy which utilizes SR-BI DNA delivered by
combining CLMs and US and indicate that this strategy may protect
against hypercholesterolemia.
Acknowledgements
The present study was supported grants from the
National Natural Science Foundation of China (no. 30970794) and the
Natural Science Foundation of Shanghai Science and Technology
Commission (no. 09ZR1424300).
References
|
1
|
Suanarunsawat T, Devakul Na Ayutthaya W,
Songsak T, Thirawarapan S and Poungshompoo S: Antioxidant activity
and lipid-lowering effect of essential oils extracted from
Ocimum sanctum L. leaves in rats fed with a high cholesterol
diet. J Clin Biochem Nutr. 46:52–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parthasarathy S, Barnett J and Fong LG:
High-density lipoprotein inhibits the oxidative modification of
low-density lipoprotein. Biochim Biophys Acta. 1044:275–283. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fielding CJ and Fielding PE: Molecular
physiology of reverse cholesterol transport. J Lipid Res.
36:211–228. 1995.PubMed/NCBI
|
|
4
|
Oram JF and Yokoyama S:
Apolipoprotein-mediated removal of cellular cholesterol and
phospholipids. J Lipid Res. 37:2473–2491. 1996.PubMed/NCBI
|
|
5
|
Han J, Parsons M, Zhou X, Nicholson AC,
Gotto AM Jr and Hajjar DP: Functional interplay between the
macrophage scavenger receptor class B type I and pitavastatin
(NK-104). Circulation. 110:3472–3479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hakkarainen T, Hemminki A, Curiel DT and
Wahlfors J: A conditionally replicative adenovirus that codes for a
TK-GFP fusion protein (Ad5Delta24TK-GFP) for evaluation of the
potency of oncolytic virotherapy combined with molecular
chemotherapy. Int J Mol Med. 18:751–759. 2006.
|
|
7
|
Iwamoto HS, Trapnell BC, McConnell CJ,
Daugherty C and Whitsett JA: Pulmonary inflammation associated with
repeated, prenatal exposure to an E1, E3-deleted adenoviral vector
in sheep. Gene Ther. 6:98–106. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taniyama Y, Tachibana K, Hiraoka K, et al:
Development of safe and efficient novel nonviral gene transfer
using ultrasound: enhancement of transfection efficiency of naked
plasmid DNA in skeletal muscle. Gene Ther. 9:372–380. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taniyama Y, Tachibana K, Hiraoka K, et al:
Local delivery of plasmid DNA into rat carotid artery using
ultrasound. Circulation. 105:1233–1239. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Christiansen JP, French BA, Klibanov AL,
Kaul S and Lindner JR: Targeted tissue transfection with ultrasound
destruction of plasmid-bearing cationic microbubbles. Ultrasound
Med Biol. 29:1759–1767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang SL: Liposomes in ultrasonic drug and
gene delivery. Adv Drug Deliv Rev. 60:1167–1176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki R, Takizawa T, Negishi Y, et al:
Gene delivery by combination of novel liposomal bubbles with
perfluoropropane and ultrasound. J Control Release. 117:130–136.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindner JR: Microbubbles in medical
imaging: current applications and future directions. Nat Rev Drug
Discov. 3:527–532. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li T, Tachibana K and Kuroki M and Kuroki
M: Gene transfer with echo-enhanced contrast agents: comparison
between Albunex, Optison and Levovist in mice - initial results.
Radiology. 229:423–428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ilies MA, Seitz WA and Balaban AT:
Cationic lipids in gene delivery: principles, vector design and
therapeutical applications. Curr Pharm Des. 8:2441–2473. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirko A, Tang F and Hughes JA: Cationic
lipid vectors for plasmid DNA delivery. Curr Med Chem.
10:1185–1193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ritsch A, Tancevski I, Schgoer W, et al:
Molecular characterization of rabbit scavenger receptor class B
types I and II: portal to central vein gradient of expression in
the liver. J Lipid Res. 45:214–222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W: Lyophilization and development of
solid protein pharmaceuticals. Int J Pharm. 203:1–60. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Franks F: Freeze-drying of bioproducts:
putting principles into practice. Eur J Pharm Biopharm. 45:221–229.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Molina MD, Armstrong TK, Zhang Y, Patel
MM, Lentz YK and Anchordoquy TJ: The stability of lyophilized
lipid/DNA complexes during prolonged storage. J Pharm Sci.
93:2259–2273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
del Pozo-Rodríguez A, Solinís MA, Gascón
AR and Pedraz JL: Short- and long-term stability study of
lyophilized solid lipid nanoparticles for gene therapy. Eur J Pharm
Biopharm. 71:181–189. 2009.PubMed/NCBI
|
|
22
|
Kasper JC, Schaffert D, Ogris M, Wagner E
and Friess W: Development of a lyophilized plasmid/LPEI polyplex
formulation with long-term stability - A step closer from promising
technology to application. J Control Release. 151:246–255. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu JJ, Tintut Y and Demer LL: Vitamin D
and osteogenic differentiation in the artery wall. Clin J Am Soc
Nephrol. 3:1542–1547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hung CR, Chen WH and Wang PS: Protective
effect of lysozyme chloride on gastric oxidative stress and
hemorrhagic ulcers in severe atherosclerotic rats. Med Sci Monit.
13:BR271–BR279. 2007.PubMed/NCBI
|
|
25
|
Wu Y, Li J, Wang J, Si Q, Zhang J, Jiang Y
and Chu L: Anti-atherogenic effects of centipede acidic protein in
rats fed an atherogenic diet. J Ethnopharmacol. 122:509–516. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan CW, Wong CN, Pin WK, et al:
Chlorogenic acid exhibits cholesterol lowering and fatty liver
attenuating properties by up-regulating the gene expression of
PPAR-α in hypercholesterolemic rats induced with a high-cholesterol
diet. Phytother Res. 2012:Jun 6–2012.(Epub ahead of print).
|
|
27
|
Hong SC, Zhao SP and Wu ZH: Effect of
probucol on HDL metabolism and class B type I scavenger receptor
(SR-BI) expression in the liver of hypercholesterolemic rabbits.
Int J Cardiol. 115:29–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Acton S, Rigotti A, Landschulz KT, Xu S,
Hobbs HH and Krieger M: Identification of scavenger receptor SR-BI
as a high density lipoprotein receptor. Science. 271:518–520. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirano K, Yamashita S, Nakagawa Y, et al:
Expression of human scavenger receptor class B type I in cultured
human monocyte-derived macrophages and atherosclerotic lesions.
Circ Res. 85:108–116. 1999. View Article : Google Scholar
|
|
30
|
Trigatti BL, Krieger M and Rigotti A:
Influence of the HDL receptor SR-BI on lipoprotein metabolism and
atherosclerosis. Arterioscler Thromb Vasc Biol. 23:1732–1738. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chinetti G, Gbaguidi FG, Griglio S, et al:
CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and
regulated by activators of peroxisome proliferator-activated
receptors. Circulation. 101:2411–2417. 2000. View Article : Google Scholar : PubMed/NCBI
|