Introduction
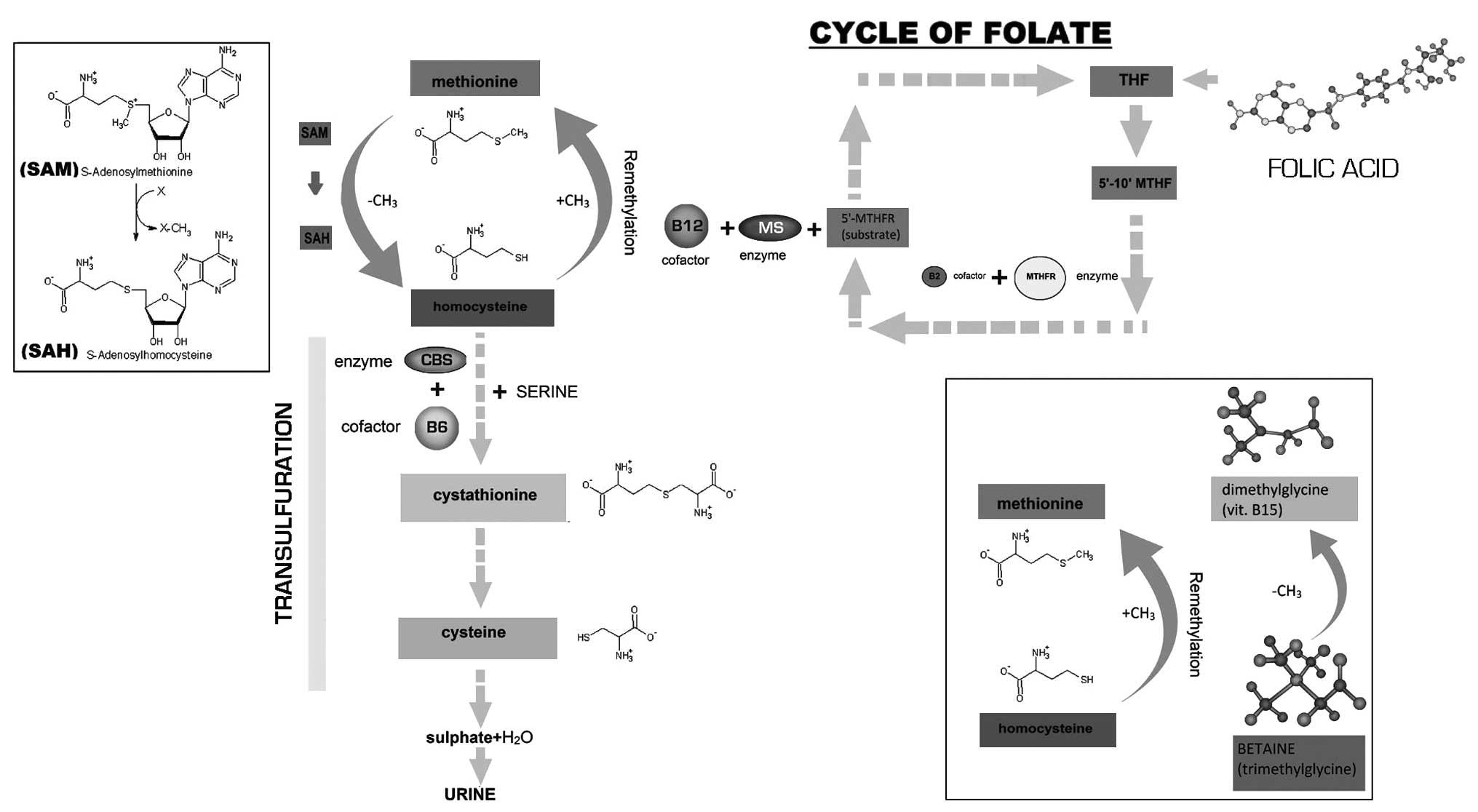
Homocysteine (Hcy) is a sulfur amino acid which is
not concerned with the composition of plasmatic proteins, therefore
there are no specific DNA base triplets which encode for this amino
acid. Hcy is formed as a result of the loss of a methyl group from
methionine, an essential amino acid that is introduced in the diet.
Hcy is an intermediate product of the metabolic pathway of
methionine (1).
Only 1–2% of total Hcy is free in the plasma, 70–80%
is combined with circulating proteins (mainly albumin) and the
remaining section is composed of disulfides, Hcy and a mix of
Hcy-cysteine disulfides. Hcy may transform itself through a
re-methylation process. This methionine-sparing process is
catalyzed by the methionine synthase enzyme (MS), which requires
5-methyltetrahydrofolate (5′-MTHF) as a substrate and cobalamin
(vitamin B12) as a cofactor in order to transfer the methyl group
of 5-MTHF to Hcy, thereby forming methionine and tetrahydrofolate
(THF).
In the liver, where methionine metabolism is
particularly active, in addition to the MS there is another enzyme
that produces methionine from Hcy by methylation. This enzyme is a
methyltransferase that uses betaine or trimethylglycine as a methyl
donor (trimethylglycine + Hcy → dimethylglycine + methionine).
If methionine is overintroduced, MS is inhibited in
order to reduce methyonine synthesis and the transsulfuration
pathway is activated by two vitamin B6 (pyridoxine)-dependent
enzymes, cystathionine-β-synthase (CBS) (2) and β-cistationase, in order to form
cystathionine and cysteine respectively (Fig. 1) (3).
N-acetyl cysteine (NAC) contributes to the
metabolism of Hcy due to the fact that it is a strong antioxidant
and the donation of sulfhydryl groups. NAC moves Hcy away from its
bond to plasmatic proteins thereby allowing it to be metabolized.
Furthermore, due to its antioxidant power, NAC benefits Hcy further
by inhibiting the production of reactive oxygen species (ROS)
during methionine degradation (4–7).
Balancing these metabolic pathways maintains a Hcy plasma
concentration of between 5–15 μmol/l.
The deficiency, or functional abnormality, of
methylenetetrahydrofolate reductase (MTHFR), MS, CBS and/or the
lack of their vitamin cofactors results in the defective metabolism
of Hcy and therefore it accumulates in the plasma causing mild
(15–30 μmol/l) or moderate (30–100 μmol/l) hyperhomocysteinemia
(HHcy). A recent meta-analysis of 26 cohort studies has concluded
that each 5 μmol/l increase in Hcy levels, compared with the normal
values, is associated with a 20% increase in the risk of a coronary
event, regardless of other risk factors (8).
MTHFR catalyzes the conversion of 5,10-MTHF to
5-MTHF, which is necessary in order for MS to convert Hcy to
methionine. MTHFR uses the B2 vitamin, riboflavin, as a cofactor.
Its key function in the metabolism of Hcy makes this enzyme a hot
point in the mechanism which deals with HHcy. The MTHFR gene is
polymorphic, with single nucleotide variants at codon 677 in exon 4
(C→T), which causes an alanine to valine substitution. The codon
677 variant encodes a thermolabile enzyme with reduced activity
which increases plasma Hcy levels. Individuals who are homozygous
for the codon 677 polymorphism (TT) demonstrate hypomethylation of
DNA in peripheral blood leukocytes, this is particularly pronounced
when folate levels are low.
The study aimed to demonstrate that the use of
multivitamins, administered in specific doses (riboflavin 2.1
mg/day, pyridoxine 2.1 mg/day; cyanocobalamin 3.75 μg/day;
pteroylmonoglutamic acid 0.3 mg/day; trimethylglycine: 250 mg/day
and NAC 300 mg/day) for 90 days, restores normal levels of plasma
Hcy, regardless of the MTHFR genotype, in a cohort of females of
reproductive age (30–42 years) with a TT (9) and Hcy levels ranging from 18 to 22
μmol/l (10).
This study demonstrates that it is unnecessary to
administer high doses of folate to reduce Hcy plasma levels. By
contrast, high doses may induce pro-inflammatory and proliferative
effects (11). Reduced folate
levels are a cardiovascular risk factor and HHcy is a biochemical
index of this deficiency (12).
Materials and methods
Patients
We enrolled 106 healthy females who were admitted to
the Madonna delle Grazie Hospital (Matera, Italy) from January,
2012 to June, 2012. The women were aged between 30–42 years and
were undergoing premonitory examinations prior to an assisted
reproductive technique (ART) cycle. Only females were enrolled in
order to rule out any bias due to the varying Hcy plasma
concentrations between males and females. All the females enrolled
were non-smokers and vegetarian, with no history of food abuse in
the previous months and with no history of hypertension. Patient
blood sampling was performed to measure Hcy, plasma folic acid and
vitamin B12 levels. Furthermore, molecular characterization of the
C677T polymorphism of MTHFR gene was also performed. Written
informed consent was obtained from the patient for publication of
this case report and accompanying images. Ethics approval was
obtained from the Ethics Committee of the Local Health Unit (LHU;
Prot. N. 1122/CE/P in November 5, 2011).
Laboratory examinations
Assay of Hcy
Blood was collected in the fasting state into tubes
containing sodium citrate as an anticoagulant; the tubes were
immediately centrifuged at 4°C in order to separate the plasma from
the corpuscular volume. Plasma samples were frozen at −20°C until
required for the Hcy assay. For the Hcy assay the ACL ELITE PRO
coagulometer manufactured by Instrumentation Laboratory
(Warrington, UK) (latex immunological test for the quantitative
determination of total L-Hcy of citrated plasma samples) was
used.
Serum folate and vitamin B12
assays
Blood was collected in tubes without an
anticoagulant and the tubes were immediately centrifuged in order
to separate the serum from the corpuscular volume. Serum samples
were frozen at −20°C until they were required for the serum folate
and vitamin B12 assays. To do this we used the tool Unicel DXI 800
produced by Beckman Coulter (Miami, FL, USA). The normal ranges for
serum folates and vitamin B12 were 3.1–20 ng/μl and 211–911 pg/ml,
respectively.
Molecular probing to examine the
genetic polymorphism due to the substitution of cytosine to thymine
at nucleotide 677 of the MTHFR enzyme gene
Blood samples collected in EDTA-K3 were used.
Molecular analysis required the following steps: i) DNA isolation
from 25 μl of blood, using the extraction kit from Promega Italy
S.r.l. (DNA IQTM System, cod.C6701, Milan, Italy). ii)
Amplification of the gene sequences regarding the MTHFR gene. iii)
Reverse hybridization on a strip through the use of wild-type and
mutant probes and the colorimetric detection of hybrids. iv)
Amplifications and the reverse hybridizations on a strip were
obtained with the use of commercial kits produced by Nuclear Laser
Medicine (cod. AC012, Milan, Italy). Groups were assigned as
follows: Group 1 patients were homozygous for the C677T
polymorphism in the MTHFR gene (TT); Group 2 patients were
heterozygous for the C677T polymorphism in the MTHFR gene (CT);
Group 3 patients were wild-type in the C677T polymorphism in the
MTHFR gene (CC) (control group).
Statistical analysis
Results are expressed as the means ± standard
deviation (SD) and percentages.
Results and Discussion
Of the 106 women studied in this report, 16 (15.1%)
from Group 1 were TT, 46 (43.4%) from Group 2 were heterozygous for
the C677T polymorphism in the MTHFR gene (CT), and 44 (41.5%) from
Group 3 were wild type in the C677T polymorphism in the MTHFR gene
(CC) (control group).
With regards to the Hcy, vitamin B12 and folic acid
values found in the three groups are reported in Tables I and II. The plasma levels of folate and
vitamin B12 were in the normal range for Groups 2 and 3, but the
values were significantly higher in Group 3 (P<0.02). The
average Hcy plasma level for patients in Group 1 was 22±15 μmol/l,
while the average Hcy plasma level for Group 3 was 7.9±2.2 μmol/l
(P=0.005). The average level of plasma folates for Group 1 was
4.6±2.3 ng/ml, while in Group 3 (P<0.001) it was 15.2±4.9 ng/ml.
In Group 1, the mean vitamin B12 serum level was 342±350 pg/ml
compared with 417.0±115.0 pg/ml for Group 3.
 | Table IHomocysteine, folic acid and B12
vitamin levels found in the two groups of patients. |
Table I
Homocysteine, folic acid and B12
vitamin levels found in the two groups of patients.
| Group 2 MTHFR ‘CT’
(n=47) | Group 3 MTHFR ‘CC’
(n=41) | P-value |
|---|
| Homocysteinemia
(μmol/l) | 9.3±3.6 | 7.3±1.8 | |
| Mild HHcy:
15–30 | | | |
| Moderate HHcy:
31–100 | | | |
| Serum folates | 10.2±5.9 | 15.2±4.9 | <0.02 |
| Normal range: 3,1–20
ng/μl | | | |
| B12 vitamin | 303.0±116.1 | 417.0±115.0 | <0.02 |
| Normal range:
211–911 pg/ml | | | |
 | Table IIHomocysteine, folic acid and B12
vitamin levels found in the two groups of patients. |
Table II
Homocysteine, folic acid and B12
vitamin levels found in the two groups of patients.
| Group 1 MTHFR ‘TT’
(n=47) | Group 3 MTHFR ‘CC’
(n=41) | P-value |
|---|
| HHCy (μmol/l) | 22.0±15.0 | 7.3±1.8 | <0.005 |
| Mild HHcy: 15–30 | | | |
| Moderate HHcy:
31–100 | | | |
| Serum folates | 4.6±2.3 | 15.2±4.9 | <0.001 |
| Normal range: 3,1–20
ng/μl | | | |
| B12 vitamin | 342.0±350.0 | 417.0±115.0 | NS |
| Normal range:
211–911 pg/ml | | | |
To restore the Hcy plasma levels of patients in
Group 1, to the normal range, they were treated with multivitamins
in specific doses (riboflavin 2.1 mg/day, pyridoxine 2.1 mg/day;
cyanocobalamin 3.75 μg/day; pteroylmonoglutamic acid 0.3 mg/day;
trimethylglycine: 250 mg/day and NAC 300 mg/day) for 90 days.
Following treatment, the plasma serum Hcy, serum
folic acid and vitamin B12 values were 7.4±1.7 ng/μl, 8.0±25 ng/μl
and 505±113 pg/ml, respectively. This demonstrated that using
specific doses of multivitamins restores plasma Hcy to normal
levels, regardless of the MTHFR genotype, due to the fact that TT
patients produce less CH3-THF when folate levels are
low. The reduced availability of CH3-THF leads to
reduced remethylation of Hcy with a consequent increase in plasma
Hcy (HHcy).
However, CC patients are unaffected by folate
deficiencies, as the synthesis of CH3-THF is preserved
for methylation reactions and for the conversion of Hcy to
methionine.
Therefore, the MTHFR C677T genotype does not alter
the availability of CH3-THF if there is adequate folate
intake. The present study demonstrates that it is unnecessary to
administer high doses of folate to reduce Hcy plasma levels and
high doses may have pro-inflammatory and pro-proliferative effects
(11).
In conclusion, regardless of any doubts as to the
role of HHcy as a pathogenic factor or a direct biochemical marker
for more complex metabolic abnormalities, there is evidence that
the administration of multivitamins, in appropriate doses, corrects
this alteration, with great, and not yet fully explored, benefits
for the cardiovascular system. The facility for correcting mild to
moderate HHcy creates an opportunity to prevent cardiovascular
events. Therefore, measuring Hcy is necessary, particularly for at
risk patients (12).
Acknowledgements
The authors thank ‘Association Gian Franco Lupo’
(ONLUS: non-profit organization of social utility).
References
|
1
|
Shiraiwa T, Nakagawa K, Kanemoto N, Kinda
T and Yamamoto H: Synthesis of optically active homocysteine from
methionine and its use in preparing four stereoisomers of
cystathionine. Chem Pharm Bull (Tokyo). 50:1081–1085. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Franchis R, Fermo I, Mazzola G,
Sebastio G, Di Minno G, Coppola A, Andria G and D’Angelo A:
Contribution of the cystathionine beta-synthase gene (844ins68)
polymorphism to the risk of early-onset venous and arterial
occlusive disease and of fasting hyperhomocysteinemia. Thromb
Haemost. 84:576–582. 2000.PubMed/NCBI
|
|
3
|
Mudd SH, Levy HL and Skovby F: Disorders
of transsulfuration. The Metabolic and Molecular Bases of Inherited
Diseases. Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B and
Vogelstein B: McGraw Hill; NY: 88. pp. 2008–2048. 2001
|
|
4
|
Hultberg B, Andersson A, Masson P, Larson
M and Tunek A: Plasma homocysteine and thiol compound fractions
after oral administration of N-acetylcysteine. Scand J Clin Lab
Invest. 54:417–422. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aćimović JM, Stanimirović BD, Todorović N,
Jovanović VB and Mandić LM: Influence of the microenvironment of
thiol groups in low molecular mass thiols and serum albumin on the
reaction with methylglyoxal. Chem Biol Interact. 188:21–30.
2010.PubMed/NCBI
|
|
6
|
Moshal KS, Sen U, Tyagi N, Henderson B,
Steed M, Ovechkin AV and Tyagi SC: Regulation of
homocysteine-induced MMP-9 by ERK1/2 pathway. Am J Physiol Cell
Physiol. 290:C883–C891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hultberg B: Elimination of high amounts of
extracellular homocysteine in human cell lines. Clin Chim Acta.
356:117–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Humphrey LL, Fu R, Rogers K, Freeman M and
Helfand M: Homocysteine level and coronary heart disease incidence:
a systematic review and meta-analysis. Mayo Clin Proc.
83:1203–1212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jacques PF, Bostom AG, Williams RR,
Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J and Rozen R:
Relation between folate status, a common mutation in
methylenetetrahydrofolate reductase, and plasma homocysteine
concentrations. Circulation. 93:7–9. 1996. View Article : Google Scholar
|
|
10
|
Guttormsen AB, Ueland PM, Nesthus I,
Nygård O, Schneede J, Vollset SE and Refsum H: Determinants and
vitamin responsiveness of intermediate hyperhomocysteinemia (>
or = 40 micromol/liter). The Hordaland Homocysteine Study. J Clin
Invest. 98:2174–2183. 1996.PubMed/NCBI
|
|
11
|
Smulders YM and Blom HJ: The homocysteine
controversy. J Inherit Metab Dis. 34:93–99. 2011. View Article : Google Scholar
|
|
12
|
Quéré I, Perneger TV, Zittoun J, et al:
Red blood cell methylfolate and plasma homocysteine as risk factors
for venous thromboembolism: a matched case-control study. Lancet.
359:747–752. 2002.PubMed/NCBI
|