Introduction
Prostate cancer is the second most commonly
diagnosed cancer among Chinese-American males, contributing to the
overall cancer burden in this population (1–3). Large
increases in both the incidence and mortality rates of prostate
cancer are noted for all countries including low-risk populations,
but substantial differences exist between countries. According to
previous research, genetic, dietary, lifestyle and environmental
factors are considered to play an influential role in the etiology
of prostate cancer development (2,4).
Lipopolysaccharide (LPS) is a representative strong
stimulator of inflammation and has been demonstrated to be
associated with various steps in chronic inflammation, such as
altering cytokine levels by stimulating inflammatory cells in the
tumor microenvironment (5). LPS
exposure can also lead to carcinogenesis, induce tumor cell
proliferation and survival, facilitate invasion and metastasis, and
promote angiogenesis (6–8). For example, LPS can promote prostate
cancer PC3 cell migration (9) by
inducing the secretion of tumor necrosis factor-α (TNF-α) and
interleukin-6 (IL-6) (10), serum
levels of which are correlated with the extent of disease in
patients with prostate cancer (11). TNF-α has been frequently detected in
biopsies from ovarian, renal and breast cancer (12,13)
and IL-6 also exhibits a strong association with many types of
cancer (14–18). Moreover, research suggests that LPS
plays an accelerative role in mediating epithelial-mesenchymal
transition (EMT), which has been proposed as a key process in
cancer progression with increased migration and invasiveness
(19), by modifying the nuclear
factor (NK)-κB signaling pathway in breast cancer cells (20). Hence, we speculated that LPS may
promote prostate cancer cell proliferation and invasion and tumor
progression.
The effect of dietary-derived inhibitory factors on
cancer cell invasion and progression have been investigated, such
as curcumin (20), anthocyanins
from fruits of Vitis coignetiae Pulliat (21), green tea polyphenols (22), and lycopene and β-carotene from
fruits and vegetables (23).
Sesamin, a lignan component from sesame oil, is considered as a
common but healthy food with antihypertensive and antioxidative
activities. According to previous studies, the potential anticancer
effects of food-derived sesamin on human cancer, including breast
and colon cancer, hepatocellular carcinoma, and lung cancer are
well documented (24–28).
Moreover, sesamin plays an inhibitory role in human
prostate cancer cell proliferation (29), implying its potential anticancer
application. However, the nature of the growth inhibitory mechanism
remains unknown, and the effect of sesamin on prostate cancer cell
invasion stimulated by LPS also remains unclear. In the present
study, we mainly explored the protective effect of sesamin on the
development of prostate cancer cell invasion and tumor progression
induced by LPS.
Materials and methods
Mouse treatment and cell culture
Male BALB/c nude mice, aged 6–8 weeks (20±2 g), were
purchased from Belda Biomedical Science and Technology Co., Ltd.
(Suzhou, China). The mice were maintained and used in strict
accordance with international standards of animal care guidelines.
All experimental procedures were carried out in accordance with the
regulations of the Zhengzhou University Committee on Ethics in the
Care and Use of Laboratory Animals and were approved by the
University Committee for Animal Experiments.
Human prostate cancer cell line PC3 was purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). PC3 cells were maintained in RPMI-1640 medium supplemented
with 10% heat-inactivated fetal bovine serum (FBS) (both from
Gibco, Rockville, MD, USA), 100 U/ml penicillin, and 100
μg/ml streptomycin (Life Technologies, Rockville, MD, USA),
in a humidified atmosphere of 5% CO2–95% air at 37°C.
Sesamin was dissolved initially in dimethyl sulfoxide (DMSO),
stored as small aliquots at −20°C, then thawed and diluted in a
cell culture medium as required to a final concentration of 0, 10,
50 or 100 μg/ml.
MTT assay
PC3 cells were seeded into 96-well plates (Corning
Inc., Corning, NY, USA) at a density of 5×103 cells/well
in RPMI-1640 medium with 10% FBS. After 24 h, the cells were
pre-incubated with 0, 10 (S10), 50 (S50) or 100 (S100) μg/ml
of sesamin, for 1 h before treatment with 1 μg/ml of LPS
(both from Sigma-Aldrich, St Louis, MO, USA) at 37°C in 5% of
CO2. Next, 24 or 48 h later, 20 μl of modified
tetrazolium salt
3-(4,5-dimethyl-2-thiazolyl)-2,5-dipheny-2H-tetrazolium-bromide
(MTT, 5 mg/ml; Sigma-Aldrich) was added to each well and samples
were incubated at 37°C for 4 h. Then the supernatant was carefully
removed and 100 μl of DMSO (Sigma-Aldrich) was added to lyse
the cells. After the dark-blue MTT crystals dissolved, the
absorbance was measured at 490 nm using a Benchmark microplate
reader (Bio-Rad, Hercules, CA, USA).
Invasion assay
In regards to the Transwell system, Matrigel-coated
24-well Transwell chambers with 8.0-μm polycarbonated
filters (Corning Inc.) were used for the in vitro invasion
assay. Here, 1×105 cells/300 μl were suspended in
serum-free RPMI-1640 medium in the upper chamber of each well, and
the lower well of each chamber was filled with 750 μl
RPMI-1640 medium supplemented with 15% of FBS. LPS (1 μg/ml)
and sesamin (0, 10, 50 and 100 μg/ml) were added to both the
upper and the lower chambers as described above. After 24 h of
treatment, the filters were fixed with 4% paraformaldehyde and
stained with 0.1% crystal violet (both from Sigma-Aldrich). Then,
the number of invasive cells in at least five randomly selected
microscope fields was counted and analyzed statistically.
Cytokine assay
PC3 cells were cultured in 6-well plates (Corning
Inc.) at a density of 4×105 cells/well in RPMI-1640
medium. LPS (1 μg/ml) and sesamin (0, 10, 50 and 100
μg/ml) were added as described previously. Conditioned
medium was collected over 48 h. Cytokines, including TNF-α and
IL-6, contained in the conditioned medium were analyzed in
triplicate using a sandwich-type enzyme-linked immunosorbent assay
(ELISA) kit (R&D Systems, Minneapolis, MN, USA) according to
the manufacturer’s instructions. The absorbance at 450 nm was
determined using a microplate reader (Bio-Rad).
p38-siRNA transfection
For siRNA transfection, PC3 cells were seeded in
6-well plates at a density of 4×105 cells/well and
transfected on the following day with p38-siRNA (25 nM; Cell
Signaling Technology, Beverly, MA, USA) or the non-silencing
control siRNA (si-NS, 25 nM), as indicated using Lipofectamine 2000
(both from Invitrogen, Carlsbad, CA, USA), according to the
manufacturer’s instructions. The transfection efficiency of
p38-siRNA was determined by western blotting.
Western blot analysis
Cultured PC3 cells were extracted using
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime,
Shanghai, China) after transfection and stimulation of LPS (1
μg/ml) and sesamin (0, 10, 50 and 100 μg/ml) or
SB203580 (10 nM; Calbiochem-Novabiochem Corp., San Diego, CA, USA)
as described above for 48 h. Protein concentrations of the cell
lysates were determined by the bicinchoninic acid assay (BCA;
Thermo Scientific, Rockford, IL, USA) and equal amounts of protein
were loaded onto sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), before being transferred to PVDF
membranes (Millipore Corp., Bedford, MA, USA), and then processed
according to the standard instructions. The antibodies used were
rabbit anti-human phospho-p38 mitogen-activated protein kinase
(p38-MAPK) (Thr180/Tyr182) monoclonal antibody (#4631) and rabbit
anti-p38-MAPK polyclonal antibody (#9212) (both from Cell Signaling
Technology); mouse anti-human cyclin D1 monoclonal antibody
(sc20044), goat anti-COX-2 polyclonal antibody (sc23983), mouse
anti-human Bcl-2 monoclonal antibody (sc509), rabbit anti-survivin
polyclonal antibody (sc10811), mouse anti-human matrix
metalloproteinase 9 (MMP-9) monoclonal antibody (sc21733), mouse
anti-human intercellular adhesion molecule-1 (ICAM-1) monoclonal
antibody (sc107), mouse anti-human vascular endothelial growth
factor (VEGF) monoclonal antibody (sc7269) and rabbit anti-β-actin
polyclonal antibody (sc130657) (dilution, 1:1,000) (all from Santa
Cruz Biotechnology, Santa Cruz, CA, USA). The secondary antibodies
were anti-rabbit or anti-mouse immunoglobulin G conjugated to
horseradish peroxidase (dilution, 1:5,000; Beyotime). Signals were
detected by enhanced chemiluminescence (ECL) reagent (Beyotime).
The absorbance values of target proteins and data analysis were
performed using Gel-Pro Analyzer version 4.0 software (Media
Cybernetics, Silver Spring, MD, USA).
NF-κB assay
Cultured PC3 cells were seeded in 6-well plates at a
density of 4×105 cells/well as indicated, respectively.
Stimulation of LPS (1 μg/ml), sesamin (100 μg/ml) or
SB203580 (10 nM) was performed as described above after
p38-siRNA/p38-siNS transfection or not. To prepare nuclear extracts
for the NF-κB assay, the cells were washed with cold PBS, collected
by centrifugation, resuspended in hypotonic lysis buffer, incubated
on ice for 15 min and then mixed with 0.75% Nonidet P-40 (NP-40)
solution. The nuclear extract was collected and centrifuged at
4,000 × g for 2 min, and stored at −80°C. Protein concentration was
determined by the BCA assay. Then, NF-κB activity in nuclear
extracts was analyzed by a NF-κB p65 ActivELISA kit (Imgenex, San
Diego, CA, USA) according to the manufacturer’s instructions. The
absorbance was determined using a microplate reader set at 405
nm.
In vivo assay
Cultured PC3 cells were washed 3 times with PBS. For
this, 1×107 cells/100 μl PBS were inoculated
subcutaneously at the right armpit of BALB/c nude mice. After 7
days, the mice were administered PBS (control) only, LPS (2 mg/kg)
only or sesamin (10 mg/kg) and SB203580 (10 mg/kg), respectively
before injection with LPS (n=4 per group). The sesamin and SB203580
were injected every 3 days, respectively. Twenty-one days later,
all mice from the different groups were sacrificed. Caliper
measurement of tumor volume was conducted every other day, and the
tumor volume (V) was calculated according to the following formula:
V = (largest diameter) x (smallest diameter)2/2.
Statistical analysis
Results are expressed as the mean ± SD
representative of three individual experiments performed in
triplicate. Statistical analysis was performed by the Student’s
t-test and ANOVA test. P<0.05 was considered to be statistically
significant compared to the respective control.
Results
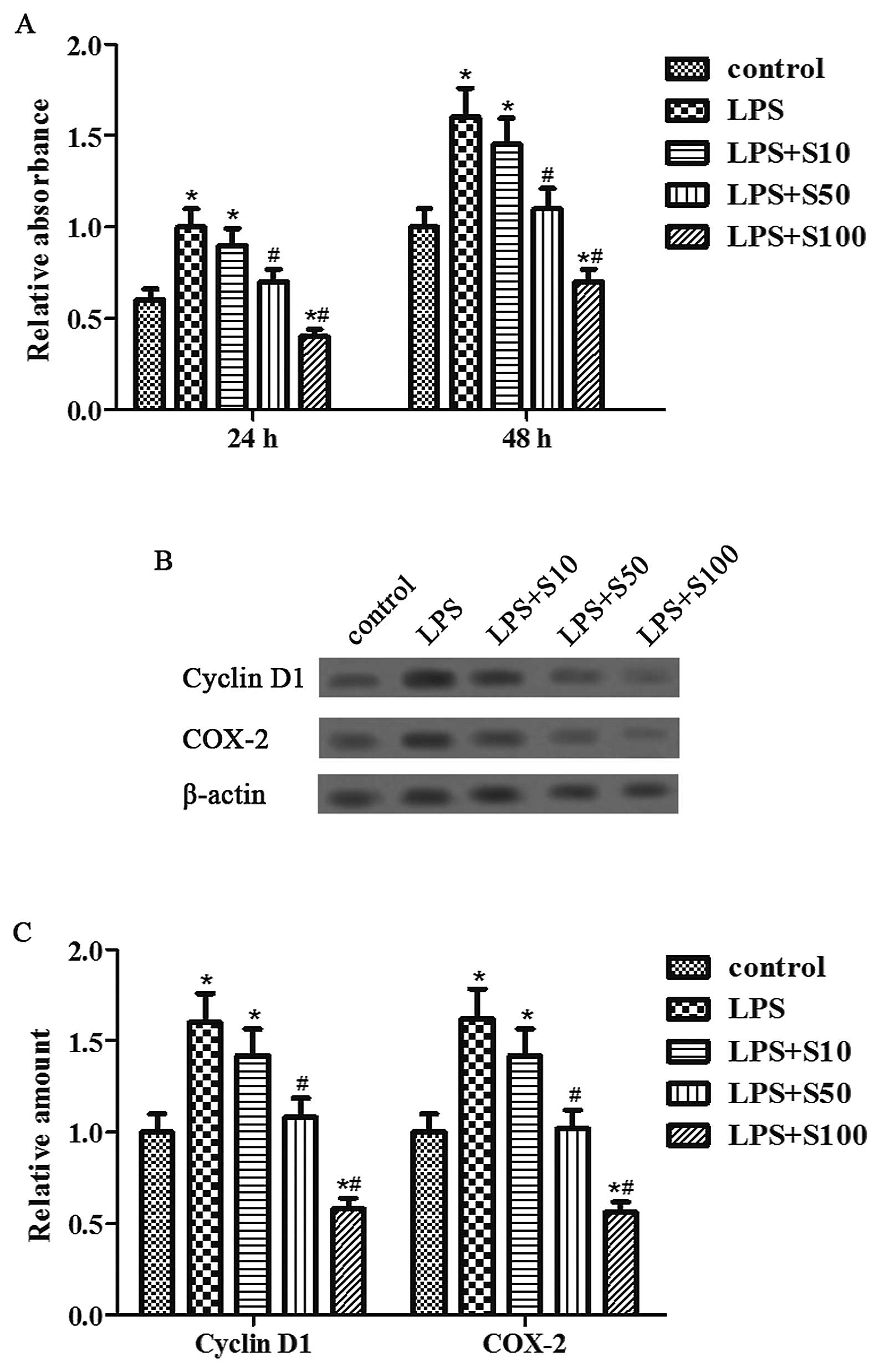
Sesamin inhibits LPS-induced cell
proliferation
To examine whether sesamin modulates prostate cancer
cell proliferation stimulated by LPS, we used the MTT assay and
further investigated the effect of sesamin (0, 10, 50 and 100
μg/ml) on the expression of proliferative-related proteins,
cyclin D1 and COX-2, in the LPS-stimulated PC3 cells. We found that
the proliferation rate of PC3 cells was distinctly promoted by LPS,
but was distinctly suppressed by sesamin pretreatment in a dose-
and time-dependent manner (Fig.
1A). Moreover, sesamin pretreatment dose-dependently
downregulated the expression of cyclin D1 and COX-2 gene products
induced by LPS (Fig. 1B and C).
Therefore, sesamin had a negative effect on the cell proliferation
and the expression of tumor cell-proliferative proteins induced by
LPS in the PC3 cells.
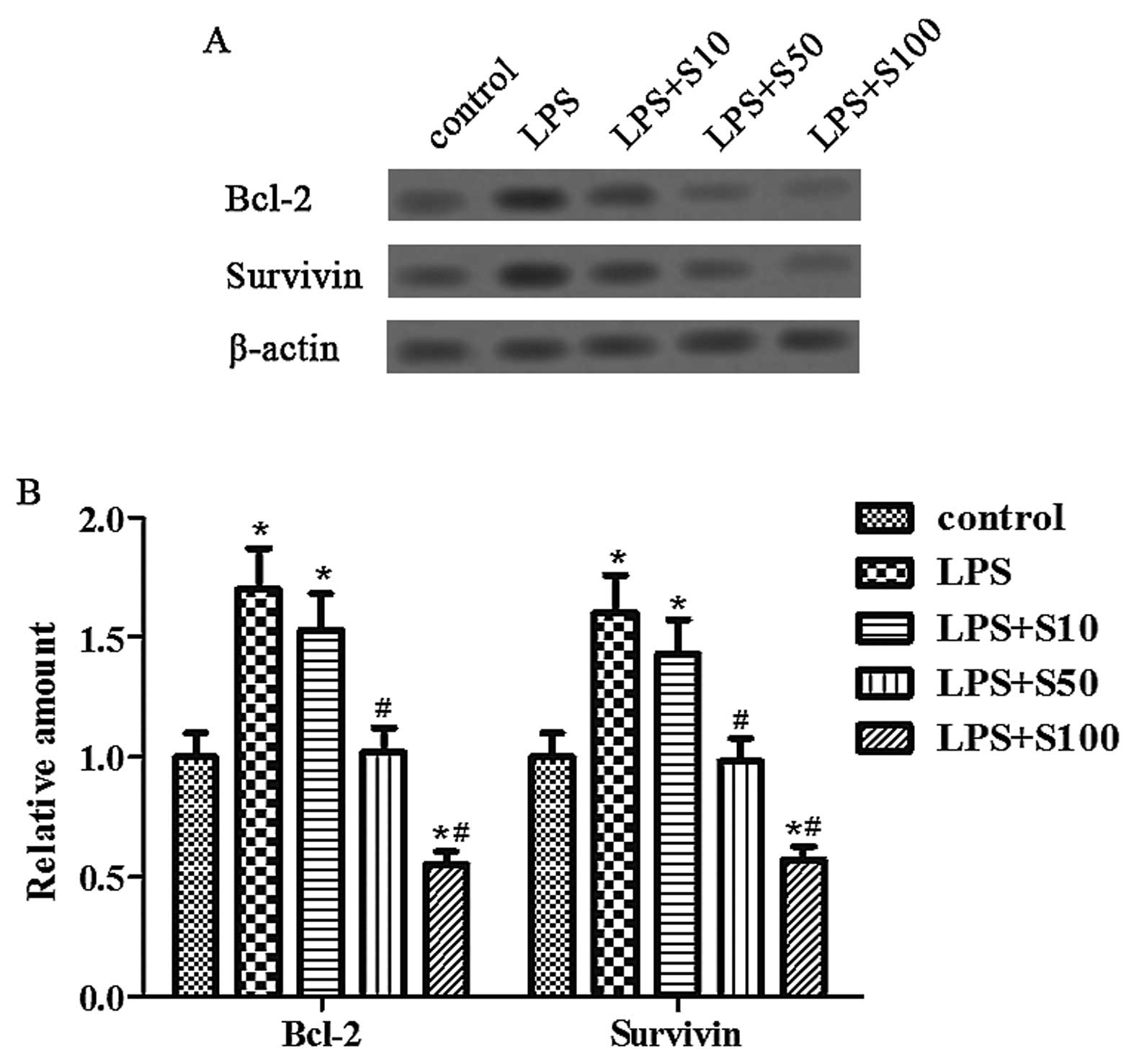
Sesamin inhibits the expression of Bcl-2
and survivin proteins induced by LPS
To evaluate the effect of LPS and sesamin on
prostate cancer cell apoptosis, we determined the expression of
Bcl-2 and survivin proteins related to tumor cell survival with
sesamin pretreatment under LPS stimulation. The results indicated
that sesamin pretreatment dose-dependently inhibited the expression
of Bcl-2 and survivin proteins induced by LPS (Fig. 2), thus implying that the possible
mechanism for the anti-apoptotic effect of sesamin may be through
the downregulation of cell survival proteins, including Bcl-2 and
survivin.
Sesamin inhibits LPS-induced cell
invasion
To determine the effect of LPS and sesamin on
prostate cancer cell invasion, we used the Matrigel invasion assay
and western blotting of invasion-related proteins, which are known
to be involved in cancer cell invasion, adhesion and angiogenesis.
The results revealed that the invasive ability of the PC3 cells was
markedly promoted by LPS and further markedly and dose-dependently
inhibited by sesamin pretreatment (Fig.
3A). More importantly, sesamin pretreatment dose-dependently
suppressed the increased expression of MMP-9, ICAM-1 and VEGF
proteins induced by LPS (Fig. 3B and
C). Thus, these data confirmed the inhibitory role of sesamin
in the invasiveness of prostate cancer and on the expression of
tumor cell metastatic proteins.
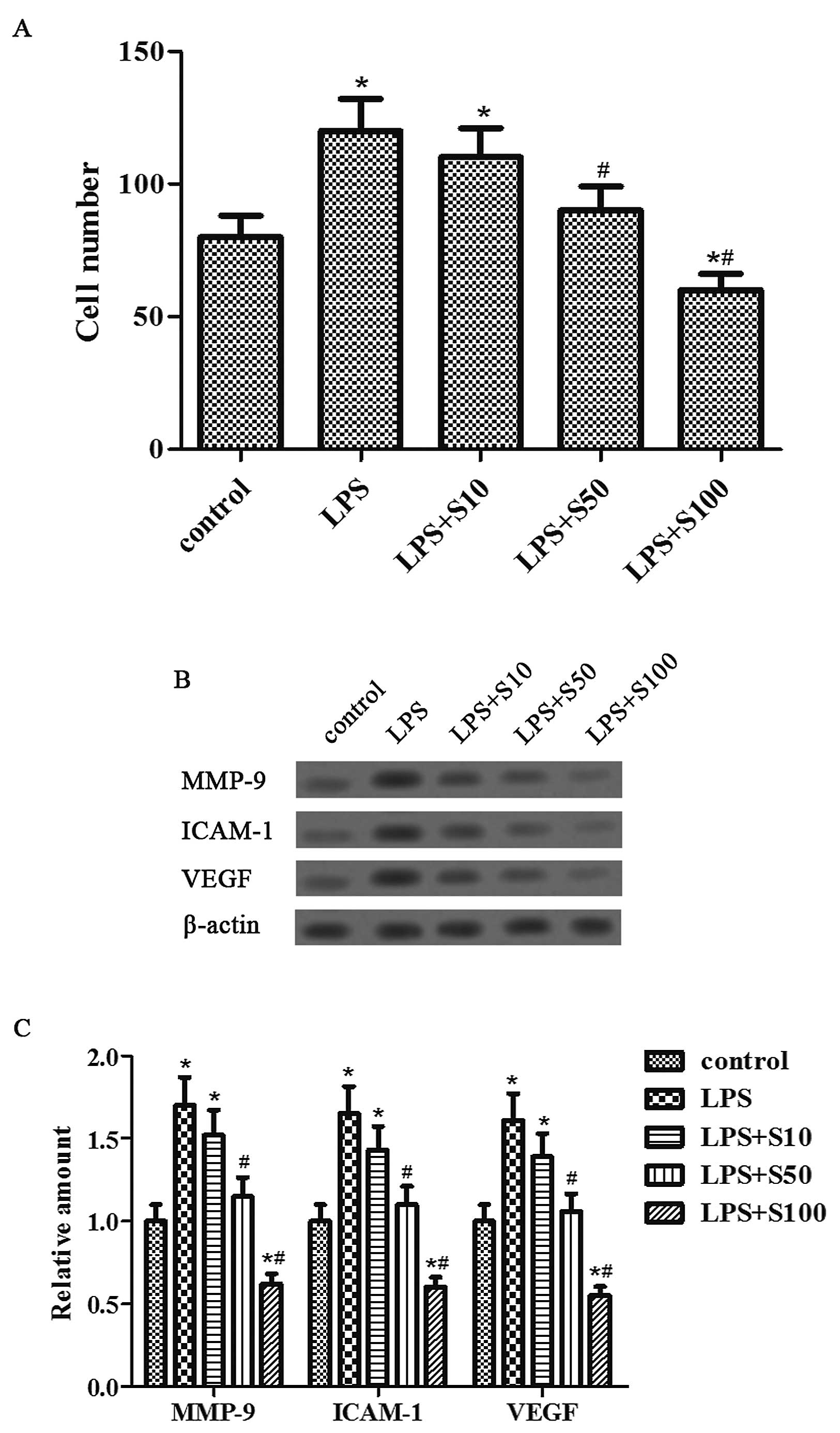 | Figure 3Sesamin attenuates cell invasion, as
well as downregulates the expression of MMP-9, ICAM-1 and VEGF
proteins induced by LPS. (A) PC3 cells were processed as described
in Materials and methods for 48 h for the Transwell assay. The
number of invasive cells was counted and analyzed statistically.
(B) PC3 cells were processed as described in Materials and methods
for 48 h; then MMP-9, ICAM-1, VEGF and β-actin proteins were
detected by western blotting. (C) Expression of MMP-9, ICAM-1 and
VEGF proteins was analyzed using Gel-Pro Analyzer version 4.0
software and normalized to β-actin. *P<0.05, compared
with the control group. #P<0.05, compared with the
LPS group. LPS, lipopolysaccharide; MMP-9, matrix metalloproteinase
9; ICAM-1, intercellular adhesion molecule-1; VEGF, vascular
endothelial growth factor. |
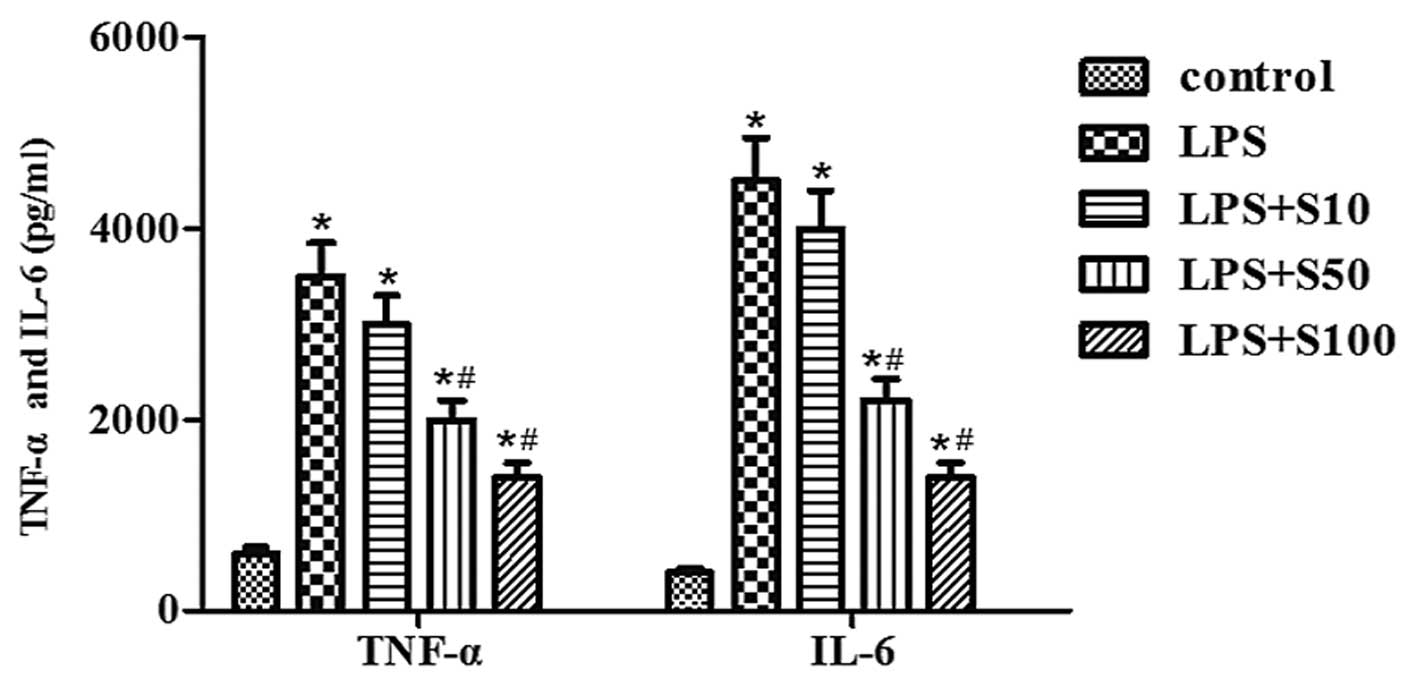
Sesamin inhibits LPS-induced cytokine
production
To investigate the effect of LPS and sesamin on the
secretion of TGF-α and IL-6 in PC3 cells, we detected the
concentration of TGF-α and IL-6 production in the culture
supernatant of PC3 cells under LPS stimulation with sesamin
pretreatment. The MTT assay revealed that there was no toxic impact
on cells following these processes for 48 h. The data revealed that
the accumulation of TGF-α and IL-6 production induced by LPS in
conditioned medium was decreased dose-dependently following sesamin
pretreatment in PC3 cells (Fig.
4).
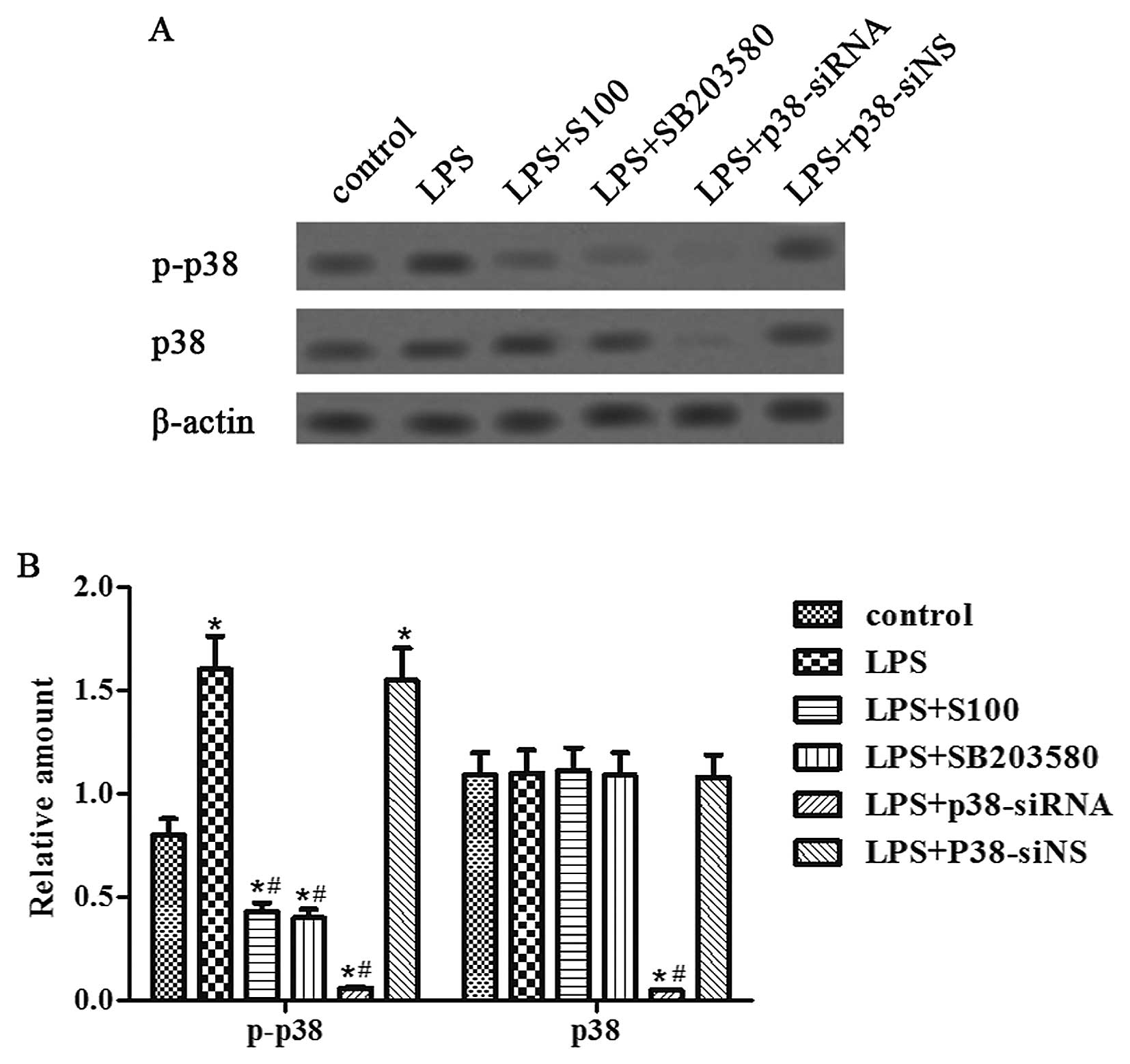
Sesamin inhibits LPS-induced p38-MAPK
activation
To examine whether the p38-MAPK signaling pathway is
involved in the enhanced cell proliferation and invasion in
response to TNF-α and IL-6 accumulation, we detected the p-p38 and
p38 proteins following either sesamin (100 μg/ml) or
SB203580 (10 nM) pretreatment or p38-siRNA/p38-siNS transfection in
the LPS-stimulated PC3 cells. The results revealed that sesamin
pretreatment inhibited the phosphorylation of the p38 protein
increased by LPS stimulation, and pretreatment with SB203580, a
specific inhibitor of p38-MAPK, showed the same degree of
inhibition (Fig. 5A and B). In
addition, compared to the control and p38-siNS transfection group,
pretreatment with p38-siRNA transfection obviously decreased both
p-p38 and p38 proteins under LPS stimulation.
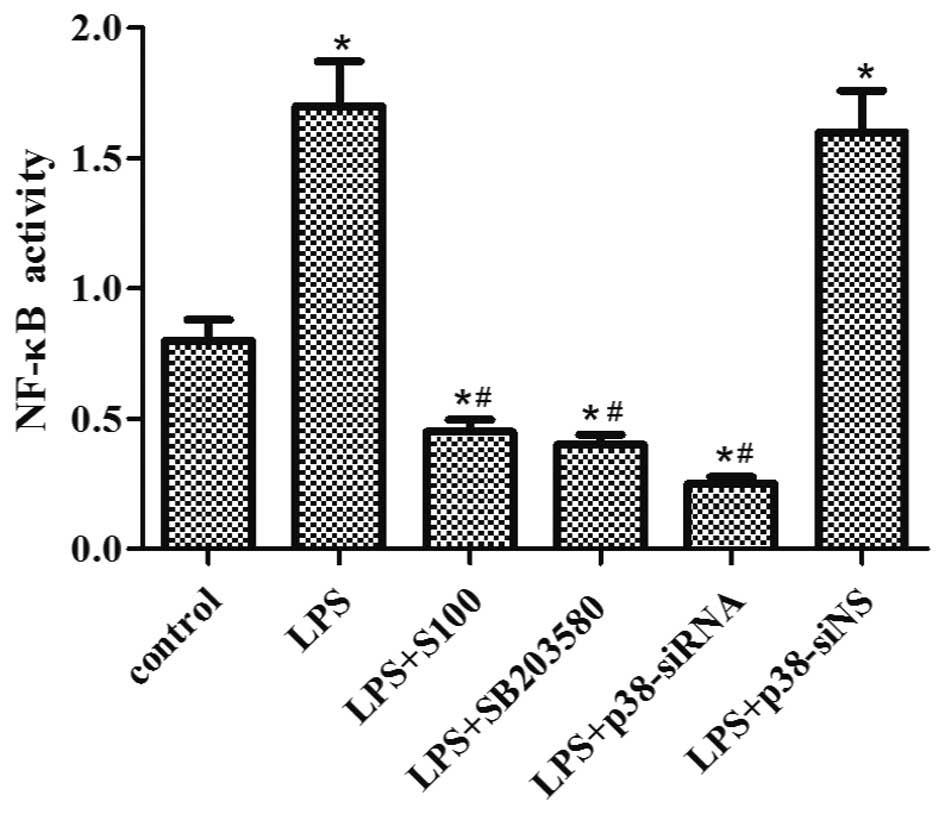
Sesamin inhibits LPS-induced NF-κB
activity
Importantly, to assess the effect of sesamin on
NF-κB activity induced by LPS, we used SB203580 pretreatment or
p38-siRNA/p38-siNS transfection to investigate the action mechanism
of sesamin on NF-κB activity in the LPS-stimulated PC3 cells.
Similar to the inhibitory effect of SB203580 and p38-siRNA, sesamin
pretreatment clearly suppressed the constitutive and inducible
NF-κB activity at a concentration of 100 μg/ml (Fig. 6). Therefore, it can be concluded
that LPS-induced translocation of the NF-κB p65 subunit to the
nucleus could be inhibited by sesamin to some extent.
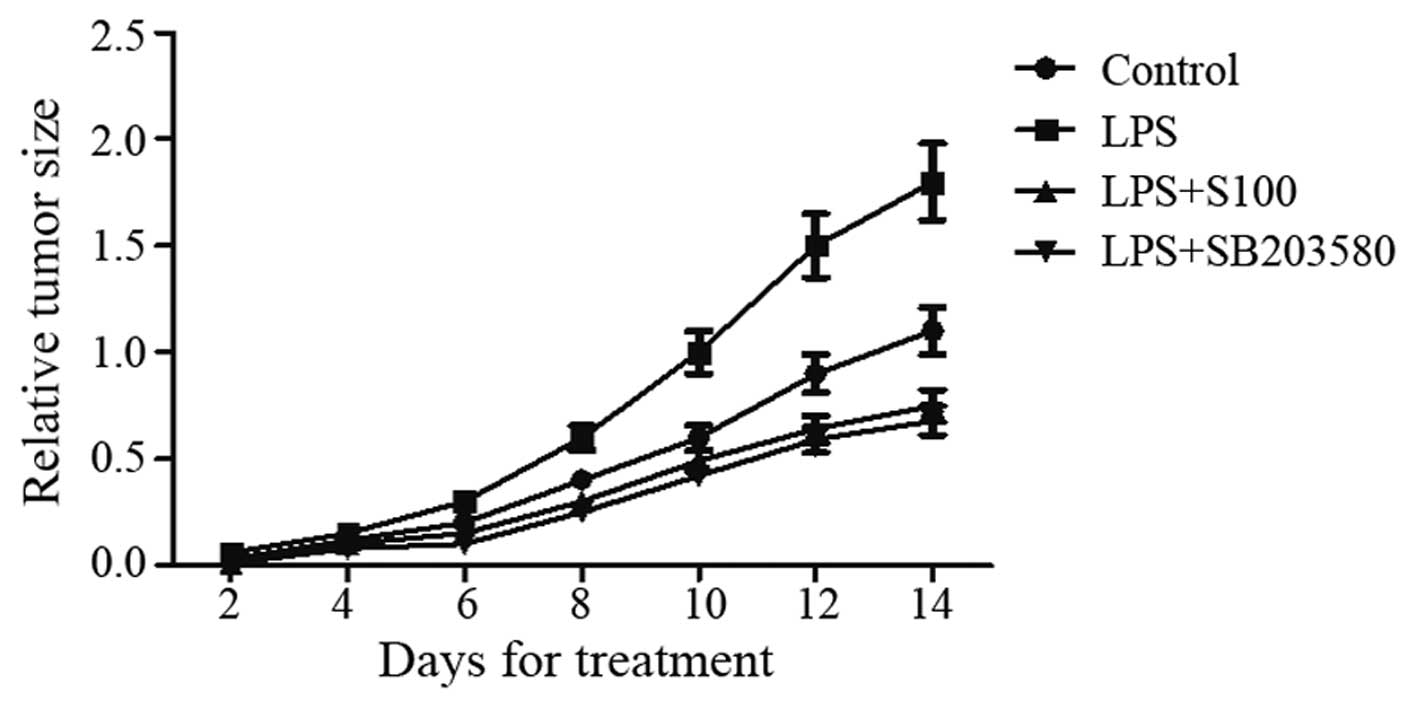
Sesamin reduces PC3 cell-derived tumor
growth enhanced by LPS in vivo
To evaluate the effect of sesamin on tumor growth
in vivo, we used the SB203580 pretreatment to determine the
LPS-stimulated tumor progression derived from PC3 cells in BALB/c
nude mice. Compared to the control group, the tumor volume in the
LPS pretreatment group was distinctly larger, and the tumor volume
in the LPS plus sesamin group was relatively smaller after 14 days
post-incubation (Fig. 7). The
results illustrated that either sesamin or SB203580 treatment could
obviously inhibit PC3 cell-derived tumor growth induced by LPS
in vivo.
Discussion
Prostate cancer is a relatively common cancer among
males worldwide, and an increasing number of men are at a high risk
due to various reasons, including dietary and environmental
factors. Sesamin, a type of phytoestrogen, is a lignan that is
found abundantly in sesame oil and potentially possesses multiple
functions, such as antioxidant, anti-inflammatory, and anticancer
activities. Considering this, the present study mainly focused on
the protective effect of sesamin on the development of prostate
cancer cell invasion and tumor progression under LPS
stimulation.
Research has shown that sesamin induces arrest at
the G1 phase of the cell cycle in human breast cancer MCF-7 cells
(26) and inhibits cell
proliferation in various types of tumor cells (29). Sesamin also suppresses TNF-induced
expression of cyclin D1, COX-2 and cell survival gene products
including Bcl-2 and survivin proteins in human chronic myeloid
leukemia cells (29). In addition,
the COX-2 protein is reported to be overexpressed in lung tumor
tissues (30) and the inhibition of
COX-2 can arrest progression of the cell cycle in prostate cancer
cells (31). In addition, LPS
promotes cell survival and proliferation in hepatocellular
carcinoma cells (32) and
significantly activates the expression and activity of COX-2 in
human colon carcinoma cells (33).
In the present study, our data indicated that sesamin pretreatment
suppressed LPS-induced PC3 cell proliferation and the expression of
cyclin D1, COX-2, Bcl-2 and survivin proteins, which are correlated
with cell proliferation, the synthesis and secretion of
prostaglandin E2 and cell survival.
Moreover, sesamin was previously found to inhibit
the levels of TNF-α and IL-6 induced by macrophages, and reduced
the expression of VEGF and MMP-9 proteins, thus suppressing cell
migration and exhibiting anti-angiogenic activity in human breast
cancer MCF-7 cells (34). Our
results revealed that the accumulation of TNF-α and IL-6 was
enhanced by LPS and was suppressed to a lesser degree by sesamin
pretreatment in a dose-dependent manner in PC3 cells. Furthermore,
sesamin also showed a distinct negative effect on LPS-induced PC3
cell invasion and the expression of MMP-9, ICAM-1 and VEGF
proteins, thus implying the potential role of sesamin in the
suppression of human prostate cancer cell invasion, adhesion and
the prevention of tumor angiogenesis. Hence, we conclude that
sesamin downregulates the abnormal induction of related
inflammatory cytokines such as TNF-α and IL-6 induced by LPS, and
in turn, inhibits the reinforced induction of proteins which are
associated with cell invasion and progression in prostate cancer
PC3 cells.
In addition, an abnormality in levels of these
cytokines has also been shown as one of the major causes of
constitutive p38-MAPK signaling pathway and NF-κB activity in
cancer cells (34,35). Meanwhile, LPS cytotoxicity is
reported to be mediated through the activation of p38-MAPK and
NF-κB pathways in hepatocellular carcinoma cells (32). Upon LPS stimulation, the
phosphorylation of p38 was detected in human esophageal cancer
cells (36). NF-κB activity has
been commonly associated with inflammation, carcinogenesis, tumor
cell survival, proliferation, invasion and angiogenesis of cancer
(29). More importantly, both
nuclear levels of NF-κB and p38-MAPK activity were found to be
potently inhibited by sesamin via the downregulation of TNF-α and
IL-6 in human breast cancer cells (34). Likewise, our research found that
sesamin significantly suppressed phosphorylation of the p38 protein
and the NF-κB activity induced by LPS in prostate cancer PC3
cells.
Furthermore, SB203580, a specific inhibitor of
p38-MAPK, specifically reduces cell proliferation, migration and
survival in prostate cancer cells (37), and SB203580 pretreatment of head and
neck squamous cell carcinoma cells also reduced cancer growth in
tumor xenografts (38). Mice
treated with chronic inhibition of p38-MAPK with SB203580 showed
complete absence of osteoblastic growth in the intramedullary space
as well as significantly reduced tumor burden (39). In the present study, we used a nude
mouse model to investigate the role of the p38-MAPK and NF-κB
signaling pathways activated by LPS on inflammation-induced tumor
growth by an in vivo assay. Consistently, our research
showed that tumor growth was distinctly enhanced in response to LPS
and further significantly restrained by sesamin or SB203580
pretreatment in terms of tumor size. In addition, sesamin or
SB203580 pretreatment led to distinct reductions in the
phosphorylation of p38 protein and NF-κB activity induced by LPS.
Meanwhile, cells transfected with p38-siRNA showed the same
inhibitory effect, implying the potential role of the p38-MAPK and
NF-κB pathways in the sesamin-pretreated PC3 cells.
Collectively, these results demonstrated that
sesamin significantly attenuated the expression of TNF-α, IL-6,
cyclin D1, COX-2, Bcl-2 and survivin, as well as MMP-9, ICAM-1 and
VEGF proteins induced by LPS through the p38-MAPK signaling pathway
and NF-κB activation in PC3 cells under LPS stimulation and further
inhibited tumor growth in tumors derived from the inoculation of
PC3 cells. Thus, the present study showed the suppressive activity
of sesamin on prostate cancer cell proliferation and invasion
induced by LPS. These findings may assist in the better
understanding of the molecular mechanisms of the effects of sesamin
on cell invasiveness and tumorigenesis, thus providing a new
insight into the effect of sesamin during the progression of human
prostate cancer.
References
|
1
|
Lin SS, Clarke CA, Prehn AW, Glaser SL,
West DW and O’Malley CD: Survival differences among Asian
subpopulations in the United States after prostate, colorectal,
breast, and cervical carcinomas. Cancer. 94:1175–1182. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsing AW, Tsao L and Devesa SS:
International trends and patterns of prostate cancer incidence and
mortality. Int J Cancer. 85:60–67. 2000. View Article : Google Scholar
|
|
3
|
Hsing AW and Devesa SS: Trends and
patterns of prostate cancer: what do they suggest? Epidemiol Rev.
23:3–13. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sonn GA, Aronson W and Litwin MS: Impact
of diet on prostate cancer: a review. Prostate Cancer Prostatic
Dis. 8:304–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schetter AJ, Heegaard NH and Harris CC:
Inflammation and cancer: interweaving microRNA, free radical,
cytokine and p53 pathways. Carcinogenesis. 31:37–49. 2010.
View Article : Google Scholar :
|
|
6
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mantovani A: Cancer: inflammation by
remote control. Nature. 435:752–753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu RY, Chan CH, Spicer JD, Rousseau MC,
Giannias B, Rousseau S and Ferri LE: LPS-induced TLR4 signaling in
human colorectal cancer cells increases beta1 integrin-mediated
cell adhesion and liver metastasis. Cancer Res. 71:1989–1998. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Chong T, Wang Z, Chen H and Li H,
Cao J, Zhang P and Li H: A novel anti-cancer effect of resveratrol:
reversal of epithelial mesenchymal transition in prostate cancer
cells. Mol Med Rep. 10:1717–1724. 2014.PubMed/NCBI
|
|
10
|
Im HJ, Park NH, Kwon YJ, Shin S, Kim D and
Chun YJ: Bacterial lipopolysaccharides induce steroid sulfatase
expression and cell migration through IL-6 pathway in human
prostate cancer cells. Biomol Ther (Seoul). 20:556–561. 2012.
View Article : Google Scholar
|
|
11
|
Michalaki V, Syrigos K, Charles P and
Waxman J: Serum levels of IL-6 and TNF-alpha correlate with
clinicopathological features and patient survival in patients with
prostate cancer. Br J Cancer. 90:2312–2316. 2004.PubMed/NCBI
|
|
12
|
Balkwill F: Tumor necrosis factor or tumor
promoting factor? Cytokine Growth Factor Rev. 13:135–141. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szlosarek PW and Balkwill FR: Tumour
necrosis factor alpha: a potential target for the therapy of solid
tumours. Lancet Oncol. 4:565–573. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ayre JE: Cervical cancer: chronic
inflammation, stress and adaptation factors. Acta Unio Int Contra
Cancrum. 12:20–27. 1956.PubMed/NCBI
|
|
15
|
Dalgleish AG and O’Byrne KJ: Chronic
immune activation and inflammation in the pathogenesis of AIDS and
cancer. Adv Cancer Res. 84:231–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Farrow B and Evers BM: Inflammation and
the development of pancreatic cancer. Surg Oncol. 10:153–169. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nelson WG, De Marzo AM, DeWeese TL and
Isaacs WB: The role of inflammation in the pathogenesis of prostate
cancer. J Urol. 172:S6–S12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartsburd PM: Chronic inflammation as
inductor of pro-cancer microenvironment: pathogenesis of
dysregulated feedback control. Cancer Metastasis Rev. 22:95–102.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC
and Lee CH: Resveratrol inhibits LPS-induced epithelial-mesenchymal
transition in mouse melanoma model. Innate Immun. 18:685–693. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu JN, Lee WS, Kim MJ, Yun JW, Jung JH, Yi
SM, Jeong JH, Kim HJ, Choi YH, Kim GS, et al: The inhibitory effect
of anthocyanins on Akt on invasion and epithelial-mesenchymal
transition is not associated with the anti-EGFR effect of the
anthocyanins. Int J Oncol. 44:1756–1766. 2014.PubMed/NCBI
|
|
22
|
Yang CS and Wang X: Green tea and cancer
prevention. Nutr Cancer. 62:931–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gloria NF, Soares N, Brand C, Oliveira FL,
Borojevic R and Teodoro AJ: Lycopene and beta-carotene induce
cell-cycle arrest and apoptosis in human breast cancer cell lines.
Anticancer Res. 34:1377–1386. 2014.PubMed/NCBI
|
|
24
|
Akl MR, Ayoub NM, Abuasal BS, Kaddoumi A
and Sylvester PW: Sesamin synergistically potentiates the
anticancer effects of γ-tocotrienol in mammary cancer cell lines.
Fitoterapia. 84:347–359. 2013. View Article : Google Scholar
|
|
25
|
Tanabe H, Kuribayashi K, Tsuji N, Tanaka
M, Kobayashi D and Watanabe N: Sesamin induces autophagy in colon
cancer cells by reducing tyrosine phosphorylation of EphA1 and
EphB2. Int J Oncol. 39:33–40. 2011.PubMed/NCBI
|
|
26
|
Yokota T, Matsuzaki Y, Koyama M, Hitomi T,
Kawanaka M, Enoki-Konishi M, Okuyama Y, Takayasu J, Nishino H,
Nishikawa A, et al: Sesamin, a lignan of sesame, down-regulates
cyclin D1 protein expression in human tumor cells. Cancer Sci.
98:1447–1453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng P, Wang C, Chen L, Wang C, Du Y, Yan
X, Chen M, Yang G and He G: Sesamin induces cell cycle arrest and
apoptosis through the inhibition of signal transducer and activator
of transcription 3 signalling in human hepatocellular carcinoma
cell line HepG2. Biol Pharm Bull. 36:1540–1548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang HM, Cheng KC, Lin CJ, Hsu SW, Fang
WC, Hsu TF, Chiu CC, Chang HW, Hsu CH and Lee AY: Obtusilactone A
and (−)-sesamin induce apoptosis in human lung cancer cells by
inhibiting mitochondrial Lon protease and activating DNA damage
checkpoints. Cancer Sci. 101:2612–2620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harikumar KB, Sung B, Tharakan ST, Pandey
MK, Joy B, Guha S, Krishnan S and Aggarwal BB: Sesamin manifests
chemopreventive effects through the suppression of NF-kappa
B-regulated cell survival, proliferation, invasion, and angiogenic
gene products. Mol Cancer Res. 8:751–761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q,
Ren F, Liao H, Pu Q, Wang T, et al: Interleukin-17 and
prostaglandin E2 are involved in formation of an M2
macrophage-dominant microenvironment in lung cancer. J Thorac
Oncol. 7:1091–1100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bieniek J, Childress C, Swatski MD and
Yang W: COX-2 inhibitors arrest prostate cancer cell cycle
progression by down-regulation of kinetochore/centromere proteins.
Prostate. 74:999–1011. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Zhu R, Huang Z, Li H and Zhu H:
Lipopoly-saccharide-induced toll-like receptor 4 signaling in
cancer cells promotes cell survival and proliferation in
hepatocellular carcinoma. Dig Dis Sci. 58:2223–2236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Fan L, Sun Y, Zhang D, Yue Z, Niu Y,
Meng J, Yang T, Liu W and Mei Q: An apple oligogalactan suppresses
endotoxin-induced cyclooxygenase-2 expression by inhibition of LPS
pathways. Int J Biol Macromol. 61:75–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee CC, Liu KJ, Wu YC, Lin SJ, Chang CC
and Huang TS: Sesamin inhibits macrophage-induced vascular
endothelial growth factor and matrix metalloproteinase-9 expression
and proangiogenic activity in breast cancer cells. Inflammation.
34:209–221. 2011. View Article : Google Scholar
|
|
35
|
Lu T and Stark GR: Cytokine overexpression
and constitutive NFkappaB in cancer. Cell Cycle. 3:1114–1117. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rousseau MC, Hsu RY, Spicer JD, McDonald
B, Chan CH, Perera RM, Giannias B, Chow SC, Rousseau S, Law S, et
al: Lipopolysaccharide-induced toll-like receptor 4 signaling
enhances the migratory ability of human esophageal cancer cells in
a selectin-dependent manner. Surgery. 154:69–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Guo Z, Du T, Chen J, Wang W, Xu
K, Lin T and Huang H: Prostate specific membrane antigen (PSMA): a
novel modulator of p38 for proliferation, migration, and survival
in prostate cancer cells. Prostate. 73:835–841. 2013. View Article : Google Scholar
|
|
38
|
Leelahavanichkul K, Amornphimoltham P,
Molinolo AA, Basile JR, Koontongkaew S and Gutkind JS: A role for
p38 MAPK in head and neck cancer cell growth and tumor-induced
angiogenesis and lymphangiogenesis. Mol Oncol. 8:105–118. 2014.
View Article : Google Scholar :
|
|
39
|
Sukhtankar D, Okun A, Chandramouli A,
Nelson MA, Vanderah TW, Cress AE, Porreca F and King T: Inhibition
of p38-MAPK signaling pathway attenuates breast cancer induced bone
pain and disease progression in a murine model of cancer-induced
bone pain. Mol Pain. 7:812011. View Article : Google Scholar : PubMed/NCBI
|